- 1Department of Medical Services, IQVIA Biotech, Houston, TX, United States
- 2Department of Emergency Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Background: Chimeric antigen receptor T cell infusion (CAR T) therapy has revolutionized the treatment of hematologic malignancies, but treatment-related toxicities are of concern. Understanding the timing and reasons for which patients present to the emergency department (ED) after CAR T therapy can assist with the early recognition and management of toxicities.
Methods: A retrospective observational cohort study was conducted for patients who had undergone CAR T therapy in the past 6 months and visited the ED of The University of Texas MD Anderson Cancer Center between 04/01/2018 and 08/01/2022. The timing of presentation after CAR T product infusion, patient characteristics, and outcomes of the ED visit were examined. Survival analyses were conducted using Cox proportional hazards regression and Kaplan-Meier estimates.
Results: During the period studied, there were 276 ED visits by 168 unique patients. Most patients had diffuse large B-cell lymphoma (103/168; 61.3%), multiple myeloma (21/168; 12.5%), or mantle cell lymphoma (16/168; 9.5%). Almost all 276 visits required urgent (60.5%) or emergent (37.7%) care, and 73.5% of visits led to admission to the hospital or observation unit. Fever was the most frequent presenting complaint, reported in 19.6% of the visits. The 30-day and 90-day mortality rates after the index ED visits were 17.0% and 32.2%, respectively. Patients who had their first ED visit >14 days after CAR T product infusion had significantly worse overall survival (multivariable hazard ratio 3.27; 95% confidence interval 1.29–8.27; P=0.012) than patients who first visited the ED within 14 days of CAR T product infusion.
Conclusion: Cancer patients who receive CAR T therapy commonly visit the ED, and most are admitted and/or require urgent or emergent care. During early ED visits patients mainly present with constitutional symptoms such as fever and fatigue, and these early visits are associated with better overall survival.
Introduction
Since the advent of chimeric antigen receptor T cell infusion (CAR T) therapy for hematologic malignancies, much has been learned and recognized about the risks and complications associated with CAR T therapy. Toxicities such as cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and infections in the days to months following the infusion of CAR T products have been recognized. The incidence rates of CRS and ICANS have been reported in the literature to range from 57% to 93% and 20% to 70%, respectively (1–3). These toxicities vary greatly and are thought to be influenced by multiple factors, including patient characteristics, tumor burden, CAR T cell dose, and differences in manufacturing processes, among others (4). The current treatment strategy of associated toxicities is focused on reduction of the overall inflammation by use of corticosteroids or cytokine inhibition, which is based on the grading of the toxicity as defined by the American Society for Transplantation and Cellular Therapy (ASTCT) guidelines on the management of CAR T related toxicities (5).
Although much is known about how clinicians should recognize, work up, and manage these toxicities (1), there is insufficient published literature on the use of the emergency department (ED) by this cohort of patients. CAR T therapy recipients are generally believed to have a potential for increased health care use after CAR T therapy, with high rates of intensive care unit (ICU) admissions and prolonged lengths of stay in the hospital, although re-hospitalization patterns appear to vary based on whether the patient’s CAR T therapy was an inpatient or outpatient event (6, 7). One study reported that hospital re-admission and ICU admission rates within the first 3 months after CAR T product infusion were 28.1% and 15.5%, respectively (7). Another study reported that nearly 40% were re-hospitalized and 21% visited the ED during the initial 12 months following CAR T product infusion (8). Reasons for re-hospitalizations or ED visits have been mostly related to the primary disease, pain, CAR T- related toxicities, and infection (6–8). However, despite these reports, much is still unknown, and more data is needed to better understand why patients who receive CAR T therapy visit the ED and what their outcomes are.
In the current study, we describe the reasons why patients at a single comprehensive cancer center visited the ED after CAR T product infusion, as well as their outcomes, including disposition (whether the patient was discharged, admitted, transferred, or other), hospitalization, and survival.
Methods
Population
A retrospective observational cohort study was conducted by identifying all cancer patients who visited the ED of The University of Texas MD Anderson Cancer Center (a comprehensive cancer center in Houston, Texas, USA) within 6 months after receiving any CAR T product infusion, using the institutional data warehouse. For each patient with multiple ED visits, the first ED visit after CAR T product infusion was identified as the index ED visit. The period studied was between 04/01/2018 and 08/31/2022 for the index ED visits. Patients who were less than 18 years of age at their index ED visit were excluded.
Study setting
Our institution is a comprehensive cancer center that established the first academic emergency medicine department in 2010. The ED is staffed by board certified emergency and internal medicine physicians and has 44 beds, serving approximately 26,000 patients annually. The patients that visit the ED are assessed and treated by the staff in the ED, in consultation with the patient’s oncologist. There is also an ED-run observation unit in the hospital, which serves patients projected to need in-hospital care for less than 2 midnights. This unit is functional 24-hours a day and is staffed by an emergency or internal medicine physician along with advanced practice providers. Most patients who are placed in the observation unit originate from the ED; however, patients may also come directly from clinics or procedure areas (9). Additionally, patients may also be admitted directly to the hospital by their oncologists, while bypassing the ED.
Variables and data collection
Thirty-day and 90-day mortality rates for the ED visits were calculated from the time of the index ED presentation to the reported time of death. ICU admission was reported as any ICU admission during the patient’s hospital stay associated with the index ED visit. In-hospital mortality was identified as a death during the ED visit or subsequently during the hospital admission associated with the index ED visit. The timing of the indexed ED visit was grouped based on the time from CAR T product infusion to the ED presentation, and was categorized as early (≤14 days after CAR T product infusion) or late (>14 days after CAR T infusion). CAR T product infusion was defined as the day the CAR T product was infused. Because the first 14 days after CAR T infusion are the most critical with regards to treatment-related toxicities (10, 11), we chose the time point of 14 days as the cut-off to define early versus late presentation. The acuity level assigned to the patient was based on the modified Emergency Severity Index (mESI) tool used to triage patients in our ED. Life-threatening is level 1, emergent level 2, urgent level 3, less urgent level 4, and non-urgent level 5. This five-level triage algorithm classifies patients based on disease severity at presentation and the expected resource utilization (12). The “presenting complaint” was defined as the patient’s reported reason for visiting the ED at the time of the triage assessment.
Clinical and demographic information, ED visit-related data, and outcomes were collected from patients’ electronic health records and the institution’s data warehouse. Race and ethnicity groups were categorized according to the Office of Management and Budget standards for race and ethnicity (13). The diagnosis of CRS, ICANS, or active infection(s) was collected by reviewing the physician(s) and ED visit notes, reporting the grade for the CRS and the ICANS at the time of the ED visit if present.
Statistical analysis
Patient-level and visit-level data were reported using descriptive statistics. Medians and interquartile ranges were reported for continuous variables. Numerical data were evaluated for normality using quantile-quantile plots, histogram plots, and the Shapiro-Wilk test. Categorical variables were reported as counts and percentages. Statistical significance was appraised for proportions of categorical variables using the chi-square test or the Fisher exact test, as indicated. The Wilcoxon-Mann-Whitney test was used to determine significant differences for continuous variables (all data were not normally distributed).
For the survival analysis, survival time was defined as the time interval from the date of CAR T product administration onto the date of death or the end of the observation period, censoring patients who were lost to follow-up on the dates of their last recorded clinic visit or communication (email, video conference or phone call). We used the Kaplan-Meier method followed by the log-rank test to assess differences in overall survival between patients with early and late ED presentations. Univariate and multivariable Cox proportional hazards regression models were used to assess the association between different clinical factors and overall survival, reporting the hazard ratio and its 95% confidence interval. For the final model, the proportional hazards, the non-linearity, and the influential observations assumptions were evaluated by examining the Schoenfeld residuals, the Martingale residuals, and the Deviance residuals.
All statistical analyses were performed using R software (version 4.0.3, The R Foundation, http://www.r-project.org). Two-sided P values less than 0.05 were considered statistically significant.
Ethics
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved the study and granted a waiver of informed consent.
Results
Patient characteristics
During the period studied, 409 patients received CAR T therapy, and 171 (41.8%) had at least one ED visit within 6 months of CAR T product infusion (Figure 1). The clinical and demographic characteristics of the patients included in the analysis (n=168) are summarized in Table 1. The median age for the patients in our cohort was 63 years (interquartile range: 54–69). Most were male (60.7%), white (70.8%), and not of Hispanic or Latino ethnicity (72.6%). The most common cancer types for which CAR T therapy was initiated were diffuse large B-cell lymphoma (61.3%), multiple myeloma (12.5%), mantle cell lymphoma (9.5%), and acute lymphoblastic leukemia (6.0%). Eighteen patients (10.7%) had other cancer types. During the period studied, the median number of ED visits was 1 (interquartile range 1–2). For the type of CAR T product, 108 patients (64.3%) were treated with axicabtagene ciloleucel within 6 months prior to their ED visit (Table S1). The remaining ED visits were by patients who were treated with idecabtagene vicleucel (12.5%), brexucabtagene autoleucel (11.3%), tisagenlecleucel (7.1%), and lisocabtagene maraleucel (4.8%). All the patients had their CAR T product infusion administered in an inpatient setting. Table S3 summarizes the presentation and characteristics of the ED visits stratified by CAR T product type, while Table S4 summarizes the presentation and characteristics of the ED visits stratified by the underlying cancer type.
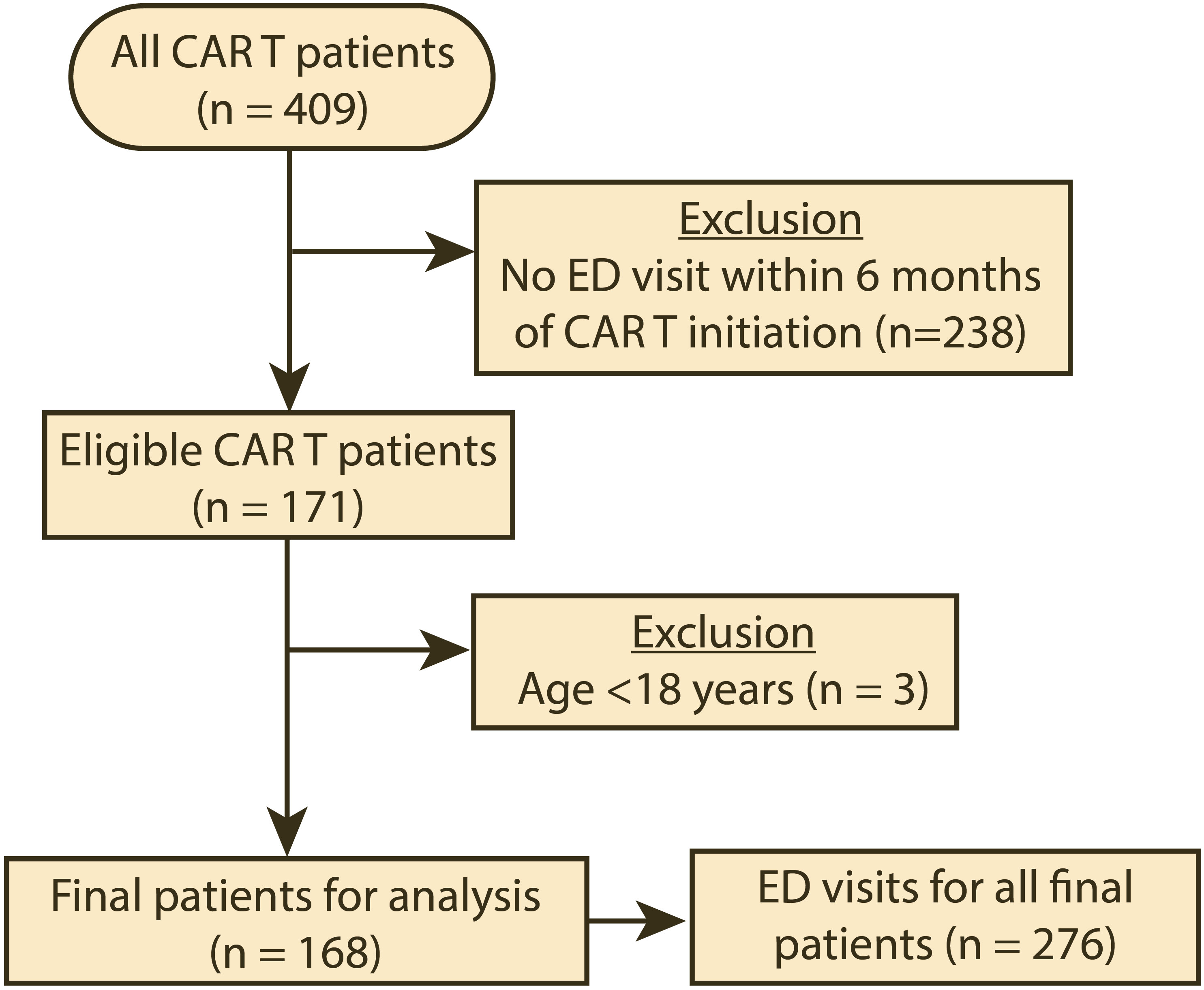
Figure 1 Flow diagram of the cohort selection for the period studied (04/01/2018–08/31/2022). CAR T, chimeric antigen receptor T-cell infusion therapy; ED, emergency department.
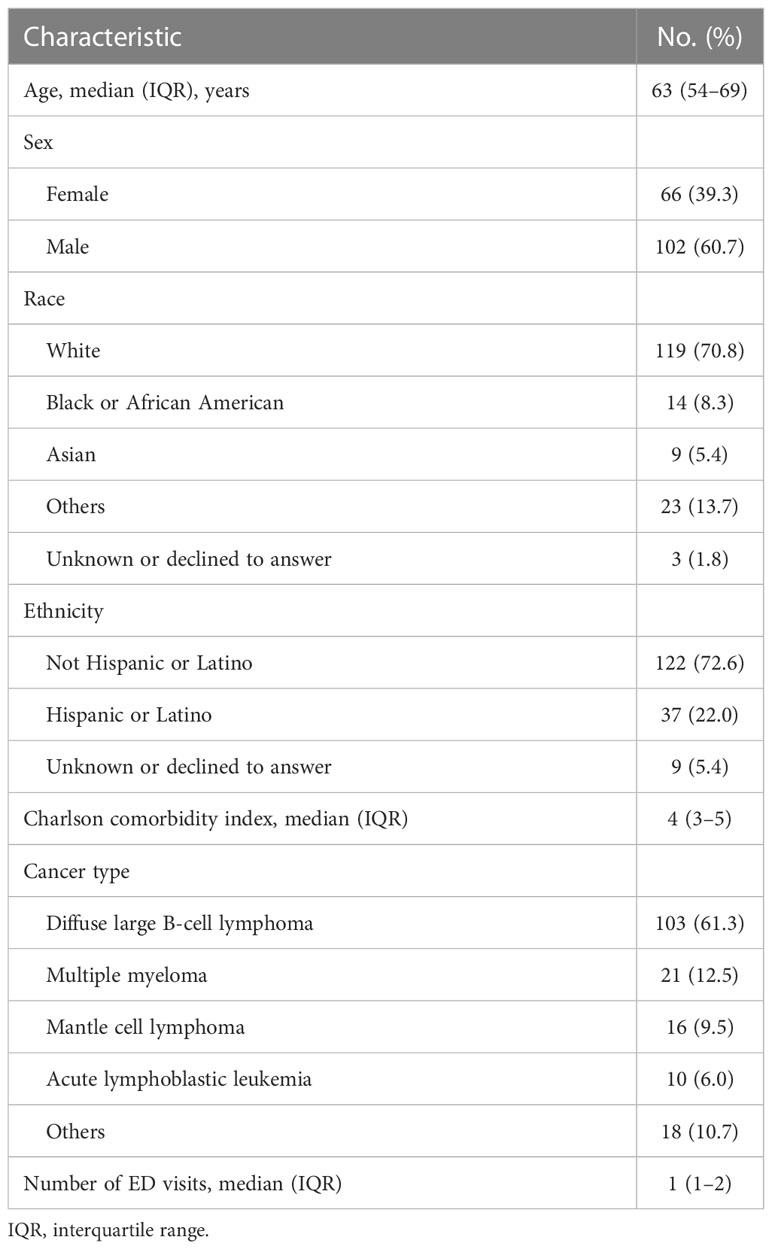
Table 1 Clinical and demographic characteristics of patients presenting to the emergency department (ED) after chimeric antigen receptor T-cell infusion therapy (n = 168).
ED visits and outcomes
During the period studied, patients included in our analysis made 276 unique visits to our ED. Most of the visits were of high acuity; 167 (60.5%) were urgent, and 104 (37.7%) were emergent or life-threatening. Only 5 visits (1.8%) had an acuity level of “less urgent” or “non-urgent”. In terms of presenting complaints, around one-fifth of the visits (19.6%) were for a fever, and fatigue was reported in 9.1% of the visits. Altered mental status was reported in 5.1% of the visits, hypotension in 4.7%, and suspected sepsis in 4.3%. Abdominal pain (6.5%), shortness of breath (6.2%), cough (5.4%), nausea and/or vomiting (4.3%), and dizziness (1.8%) were also commonly reported as a presenting complaint. Other complaints are reported in Table 2. CRS was reported in 21 patients (7.6%) at the time of the ED visit, with the majority (19/21) being grade 1, while ICANS was reported in only 9 ED visits, 4 of which were higher than grade 1 (Table 2). Tocilizumab and corticosteroids were administered during the ED stay in 1.1% and 5.8% of the visits, respectively (Table 3). In addition, infection was identified in 81 (29.3%) visits (Table 2), of which 18.5% (15/81) tested positive for COVID-19 at the ED visit. Within 14 days before the ED visit, COVID-19 was reported in 17 (6.2%) of the visits, including the aforementioned 15 (5.4%) active cases. Fever (≥38°C) was recorded in 9.8% of the ED visits. Antibiotics were administered in the ED in 50.7% of the visits (Table 3). Of significance, infections were higher in very late (>90 days after CAR T product infusion) visits compared to the early (≤14 days) visits (36.2% vs.13.8%, respectively; Table S2). Patients presented with severe neutropenia (<0.5 x 10^9/L) in 12.0% of the visits; while 48.6% of the ED visits were associated with severe thrombocytopenia (<50 x 10^9/L; Table 2).
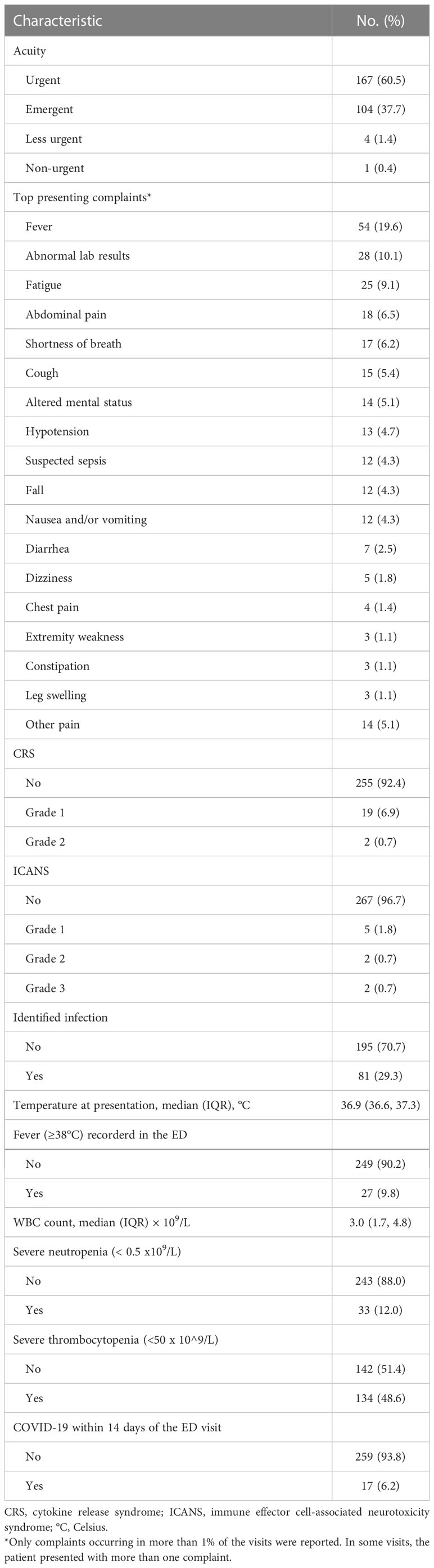
Table 2 Characteristics of emergency department visits by cancer patients in our analysis who had initiated chimeric antigen receptor T-cell infusion therapy within the past 6 months (total number of visits = 276).
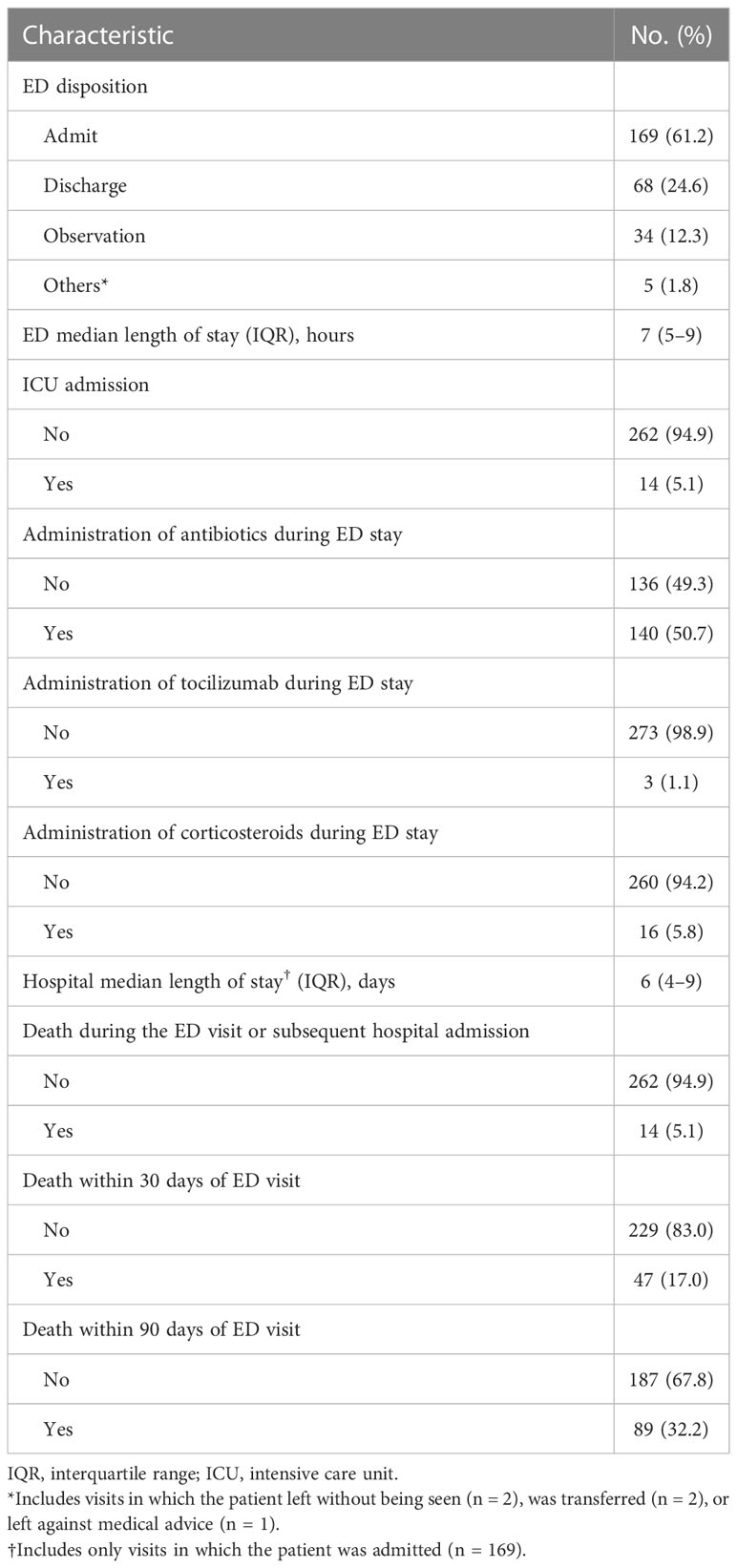
Table 3 Outcomes of emergency department (ED) visits for cancer patients in our analysis who had received chimeric antigen receptor T-cell infusion therapy within the past 6 months (total number of visits = 276).
As for the ED visit outcomes, 169 visits (61.2%) resulted in the patient being admitted to the hospital, and 68 (24.6%) resulted in discharge home (Table 3). Thirty-four visits resulted in the patient being placed in the observation unit. For patients who were admitted to the hospital, the median hospital length of stay was 6 days (interquartile range 4–9). When stratified by timing of the ED visit, visits that occurred within 14 days of CAR T product infusion had significantly higher rates of fever and fatigue as presenting complaints than those that occurred more than 90 days after CAR T product infusion (fever: 41.4% compared with 19.1%, P = 0.006; fatigue: 13.8% compared with 3.2%, P = 0.030; Table S2). For almost one-third of the visits (32.2%), the patient died within 90 days of the ED visit (Table 3). The thirty-day mortality rate for these ED visits was 17.0%.
Overall survival
The mortality rate during the study period was 37.5% (63/168). The cause and place of death are summarized in Table S5. Cancer progression (17.5%), infection/sepsis (14.3%), and organ failure (17.5%) were the most frequent causes of death in our cohort. Twenty-seven (42.9%) patients had their cause of death reported as unknown or not documented in the medical records. Only 42.9% deaths occurred in hospitals. For the subset of patients who died during the ED visit or subsequent hospital admission, infection was the most common cause of death (42.9%) for these patients (Table S6). Patients whose first ED visit occurred early (within 14 days of CAR T product infusion) had significantly (P=0.008) better overall survival than those whose first ED visit occurred late (more than 14 days after product infusion; Figure 2). Similar results were observed when a two-year survival was examined (Figure S1). As for the Cox regression analyses, non-linearity was detected for age and the Charlson comorbidity index, and we therefore categorized these variables into two groups. When compared with an early first ED visit (reference), patients whose first ED visit occurred >14 days after CAR T product infusion had significantly worse overall survival in both the univariate Cox regression analysis (hazard ratio 3.23, 95% confidence interval 1.29–8.10, P = 0.013) and the multivariable Cox regression analysis (hazard ratio 3.27, 95% confidence interval 1.29–8.27, P = 0.012; Table 4).
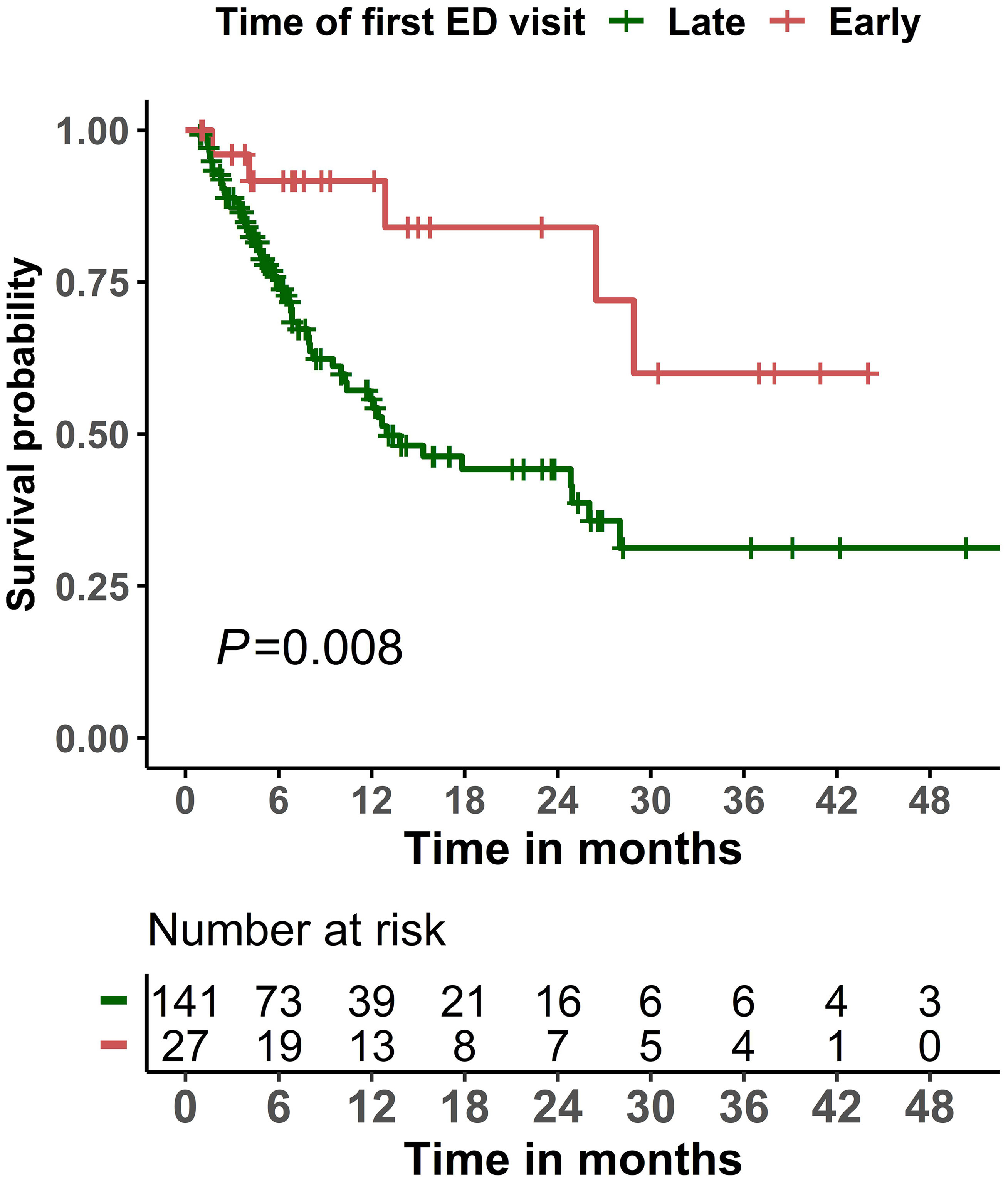
Figure 2 Kaplan-Meier overall survival curves among patients who visited the emergency department (ED) within 6 months of initiating chimeric antigen receptor T-cell infusion therapy, stratified by timing of the first ED visit (early: ≤14 days; late: >14 days).

Table 4 Univariate and multivariable Cox proportional hazards analyses of overall survival in cancer patients who visited the emergency department within 6 months after CAR T product infusion (n = 168).
Discussion
Integrating CAR T therapy into the treatment of hematologic malignancies paved the way for better survival outcomes (14–16). However, as with every other cancer therapy, adverse events are a concern. In the current study, we examined the reasons for ED use by patients who received CAR T therapy and their outcomes. We found that when patients visited the ED after CAR T therapy, they mainly complained of constitutional symptoms, including fever and fatigue, and for most of these visits (73.5%), the patient was admitted to either the hospital or placed in the observation unit. It has been previously reported that the presence of fever does not seem to affect the safety and efficacy of CAR T therapy, however, the same study suggested that the absence of fever indicates a poor response to CAR T therapy (17). Although 19.6% of the presenting complaints included fever, an infectious etiology for the visit was found in over 29% of the cases. Of these cases, 5.4% were due to COVID-19; however, only two patients died as a direct cause of COVID-19, and 80.0% were admitted. While the outcomes of COVID-19 in patients treated with CAR T therapy remain unclear, one study reported a prevalence of COVID-19 of 4.8% and a mortality rate of nearly 50% in patients who had received CAR T therapy (18). This is likely due to the immunocompromised state after CAR T product infusion but is also affected by other factors, such as malignant disease state and comorbidities. ED clinicians must recognize that these patients have a much higher rate of complications from COVID-19 and should have a lower threshold for admitting them.
Our study shows that of patients presenting to ED within 6 months of CAR T cell therapy, CRS and ICANS was present in 7.6% and 3.3%, respectively. This is likely because the CAR T product administration in all our patients was done in an inpatient setting, during which these toxicities were closely monitored and treated during their inpatient hospital stay. The CRS and ICANS diagnosed at their presentation to our ED were those cases with delayed or late occurrence. In our cohort, most of the CRS and the ICANS reported during the ED visit were of grades 1 or 2. Our institutional guidance recommends patients stay within 30 minutes of the hospital for 30 days after CAR T product infusion, therefore this might have prompted patients to present earlier in the course of their illness, however, as CAR T product administration is increasingly being done as an outpatient procedure, ED physicians need to be aware of these toxicities, including the optimal evaluation, grading, and management plans (1), as higher grades (grade 3 and grade 4) are to be expected with the shift to outpatient administration.
As for the timing of the ED visits, those that occurred within 14 days of CAR T product infusion had significantly higher rates of fever and/or fatigue as a presenting complaint, with CRS and ICANS reported in these visits. Patients who first visited the ED early after CAR T product infusion had better overall survival outcomes compared with those who initially visited the ED later. After CAR T therapy, some patients present to the ED for the management of inflammatory events associated with the CAR T product, with certain severe cases needing to be admitted to the ICU (8, 19, 20). Our study showed that only a minority of the ED visits (5.1%) resulted in an ICU admission. However, 26.2% of the patients that presented to the ED had an ICU admission at some point within three months after CAR T therapy, either through direct admission or transfer to the ICU from initial hospitalization for CAR T-cell infusion. While the ICU stays resulting from ED visits have not been explored before, the overall ICU admission rate is similar to a recent international multicenter report showing that up to 27% of patients required ICU admission after CAR T therapy. Other studies reported different ICU admission rates ranging from 10-47% (7, 19, 21). The overall differences in ICU admission rates may stem from differences in local practices, especially the use of in-hospital infusion versus outpatient infusion. In this study, all the patients had in-hospital CAR T therapy infusion; therefore, CAR T therapy toxicities were closely monitored during that patient’s inpatient stay prior to their ED presentation and were admitted to the ICU during that stay if needed. Additionally, our institutional guidance recommends patients stay within 30 minutes of the hospital for 30 days after CAR T product infusion; therefore, this might have prompted patients to present early in the course of their illness. Only 1 (1.6%) patient had CAR T therapy toxicity as the main cause of death.
For patients who first visited the ED later (>14 days of CAR T product infusion), in whom we observed worse overall survival outcomes, it is known that cancer patients frequently visit the ED near the end of life, with reasons mainly related to cancer progression (22). Moreover, and as previously reported, post CAR T relapse can be observed early after infusion and is associated with poor overall survival, mainly due to the persistence or progression of the primary malignant disease (23, 24). In our study, cancer progression was a main cause of death especially for patients who died later after their ED visit and/or the subsequent hospital admission, suggesting the utilization of ED by these patients near the end of life and explaining the poor survival outcomes for these patients. However, the interpretation of these results should pay heed to the fact that all the patients in this study had at least one ED visit, and other patients who had CAR T infusions but never visited the ED after their treatment may have other characteristics and different survival outcomes.
Mortality rates after the ED visits in our study were high, with a 30-day mortality rate of 17.0% and a 90-day mortality rate of 32.2%. To the best of our knowledge, the current study is the first to evaluate mortality rates in patients who visited the ED after CAR T therapy. A systematic review reported that fatal toxic side effects may occur with up to a 5% mortality rate within the first 30 days of CAR T therapy, but the risk of death varied depending on the product administered and other disease-specific factors (25). Additionally, others have reported non-relapse mortality associated with CAR T therapy to be as high as 15% overall, with infections and neurologic toxicities being major contributors to mortality (26). For this reason, it is important that ED clinicians are able to recognize these toxicities quickly when patients present to the ED after CAR T therapy.
Certain limitations accompanied our study, mainly due to the retrospective nature of the study. First, our cohort consisted of only patients who presented to the ED within 6 months of initiating CAR T therapy. The characteristics of patients who did not come to the ED could be different and need to be further investigated. In this study, interpretation of the reported toxicities needs to take into consideration that the prevalence rates reported in our study are limited to those reported at the time of the ED visit, which happened after the patients were discharged from their inpatient stay for CAR T product infusion, where we anticipate most of the toxicities occurred. Second, in the current study, we reported specifically the reasons for presentation to the ED. Such an approach limited the integration of important clinical variables during pre- or post-ED visits, including adverse events that were presented elsewhere or events that occurred before or after the ED visit. Similarly, we could have possibly missed patients who presented to another ED or were admitted directly to the hospital. Finally, the cause of death was unknown or undocumented in 42.9% of the patients, for which a prospective study is needed to have a better and a complete understanding of the causes of death in these patients.
In conclusion, we found that cancer patients who receive CAR T therapy commonly visit the ED, and the timing of the visit is associated with different survival outcomes in these patients. Early ED visits appear to be related to the early systemic inflammatory response resulting from CAR T therapy and are associated with better overall survival. Most of the patients were admitted, and the 90-day mortality rate after these visits was high (32.2%). Additional future studies are needed to further investigate and identify characteristics of early presentation to the ED that can be used as a predictor of response to the treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The University of Texas MD Anderson Cancer Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DL, AQ, and S-CY conceived and designed the study and developed the methods. AQ and PC collected the data. AQ was responsible for content analysis. S-CY supervised the statistical analysis. DL and AQ created the figures and tables. DL, AQ, PC, MW, PK, CR-G, and S-CY interpreted the results and drafted the first version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Erica Goodoff from the Research Medical Library at The University of Texas MD Anderson Cancer Center for editing this manuscript.
Conflict of interest
Author DL was employed by company IQVIA Biotech. S-CY was a member of an expert panel for Celgene, Inc. S-CYhad funding support from Bristol-Myer Squibb, Inc. and DepoMed, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1122329/full#supplementary-material
References
1. Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: Asco guideline. J Clin Oncol (2021) 39(35):3978–92. doi: 10.1200/JCO.21.01992
2. Sheth VS, Gauthier J. Taming the beast: Crs and icans after car T-cell therapy for all. Bone Marrow Transplant (2021) 56(3):552–66. doi: 10.1038/s41409-020-01134-4
3. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel car T-cell therapy in refractory Large b-cell lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
4. Fischer JW, Bhattarai N. Car-T cell therapy: Mechanism, management, and mitigation of inflammatory toxicities. Front Immunol (2021) 12:693016. doi: 10.3389/fimmu.2021.693016
5. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. Astct consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant (2019) 25(4):625–38. doi: 10.1016/j.bbmt.2018.12.758
6. Fowler NH, Dickinson M, Ghosh M, Chen AI, Andreadis C, Tiwari R, et al. Assessment of healthcare resource utilization and hospitalization costs in patients with relapsed or refractory follicular lymphoma undergoing car-T cell therapy with tisagenlecleucel: Results from the elara study. Transplant Cell Ther (2022) S2666-6367(22):01660–8. doi: 10.1016/j.jtct.2022.09.022
7. Johnson PC, Jacobson C, Yi A, Saucier A, Dhawale TM, Nelson A, et al. Healthcare utilization and end-of-Life outcomes in patients receiving car T-cell therapy. J Natl Compr Canc Netw (2021) 19(8):928–34. doi: 10.6004/jnccn.2020.7678
8. Kenzik KM, Johnson PC, Bhatia R, Bhatia S. Assessment of hospitalizations and emergency department visits after chimeric antigen receptor T-cell therapy among commercially insured patients. JAMA Oncol (2022) 8(7):1068–70. doi: 10.1001/jamaoncol.2022.1044
9. Chaftari P, Lipe DN, Wattana MK, Qdaisat A, Krishnamani PP, Thomas J, et al. Outcomes of patients placed in an emergency department observation unit of a comprehensive cancer center. JCO Oncol Pract (2022) 18(4):e574–e85. doi: 10.1200/OP.21.00478
10. Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the car T cell therapy tisagenlecleucel. J Hematol Oncol (2018) 11(1):35. doi: 10.1186/s13045-018-0571-y
11. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med (2011) 365(8):725–33. doi: 10.1056/NEJMoa1103849
12. Lipe DN, Bourenane SS, Wattana MK, Gaeta S, Chaftari P, Cruz Carreras MT, et al. A modified emergency severity index level is associated with outcomes in cancer patients with covid-19. Am J Emerg Med (2022) 54:111–6. doi: 10.1016/j.ajem.2022.02.002
13. Revisions to the standards for the classification of federal data on race and ethnicity. (1997, October 30). Office of management and budget. Available at: https://obamawhitehouse.archives.gov/omb/fedreg_1997standards. Accessed on February 15, 2023.
14. Atrash S, Bano K, Harrison B, Abdallah AO. Car-T treatment for hematological malignancies. J Investig Med (2020) 68(5):956–64. doi: 10.1136/jim-2020-001290
15. Han D, Xu Z, Zhuang Y, Ye Z, Qian Q. Current progress in car-T cell therapy for hematological malignancies. J Cancer (2021) 12(2):326–34. doi: 10.7150/jca.48976
16. Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M. The application of car-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm Sin B (2018) 8(4):539–51. doi: 10.1016/j.apsb.2018.03.001
17. Davis JA, Gaffney KJ, McGann M, Smith D, Edwards K, Baldino E, et al. Fever characteristics and impact on safety and efficacy of chimeric antigen receptor T-cell therapy. Clin Lymphoma Myeloma Leuk (2022) S2152-2650(22):01689–5. doi: 10.1016/j.clml.2022.09.005
18. Busca A, Salmanton-Garcia J, Corradini P, Marchesi F, Cabirta A, Di Blasi R, et al. Covid-19 and car T cells: A report on current challenges and future directions from the epicovideha survey by eha-idwp. Blood Adv (2022) 6(7):2427–33. doi: 10.1182/bloodadvances.2021005616
19. Azoulay E, Castro P, Maamar A, Metaxa V, de Moraes AG, Voigt L, et al. Outcomes in patients treated with chimeric antigen receptor T-cell therapy who were admitted to intensive care (Carttas): An international, multicentre, observational cohort study. Lancet Haematol (2021) 8(5):e355–e64. doi: 10.1016/S2352-3026(21)00060-0
20. Long B, Yoo MJ, Brady WJ, Holian A, Sudhir A, Gottlieb M. Chimeric antigen receptor T-cell therapy: An emergency medicine focused review. Am J Emerg Med (2021) 50:369–75. doi: 10.1016/j.ajem.2021.08.042
21. Li L, Liu J, Xu M, Yu H, Lv C, Cao F, et al. Treatment response, survival, safety, and predictive factors to chimeric antigen receptor T cell therapy in Chinese relapsed or refractory b cell acute lymphoblast leukemia patients. Cell Death Dis (2020) 11(3):207. doi: 10.1038/s41419-020-2388-1
22. Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ (2010) 182(6):563–8. doi: 10.1503/cmaj.091187
23. John S, Pulsipher MA, Moskop A, Hu Z-H, Phillips CL, Hall EM, et al. Real-world outcomes for pediatric and young adult patients with relapsed or refractory (R/R) b-cell acute lymphoblastic leukemia (All) treated with tisagenlecleucel: Update from the center for international blood and marrow transplant research (Cibmtr) registry. Blood (2021) 138(Supplement 1):428–. doi: 10.1182/blood-2021-146393
24. Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D'Aveni-Piney M, et al. Predictive factors of early progression after car T-cell therapy in Relapsed/Refractory diffuse Large b-cell lymphoma. Blood Adv (2020) 4(22):5607–15. doi: 10.1182/bloodadvances.2020003001
25. Cai C, Tang D, Han Y, Shen E, Abdihamid O, Guo C, et al. A comprehensive analysis of the fatal toxic effects associated with Cd19 car-T cell therapy. Aging (Albany NY) (2020) 12(18):18741–53. doi: 10.18632/aging.104058
Keywords: chimeric antigen receptor T cells, emergency department, CAR T cells, utilization, Mortality, disposition, length of stay, oncologic emergency
Citation: Lipe DN, Qdaisat A, Chaftari P, Wattana MK, Krishnamani PP, Reyes-Gibby C and Yeung S-CJ (2023) Emergency department use by patients who received chimeric antigen receptor T cell infusion therapy. Front. Oncol. 13:1122329. doi: 10.3389/fonc.2023.1122329
Received: 12 December 2022; Accepted: 08 March 2023;
Published: 17 March 2023.
Edited by:
Anand Rotte, Arcellx Inc, United StatesReviewed by:
Amandeep Godara, The University of Utah, United StatesRobert Weinkove, Victoria University of Wellington, New Zealand
Copyright © 2023 Lipe, Qdaisat, Chaftari, Wattana, Krishnamani, Reyes-Gibby and Yeung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sai-Ching J. Yeung, c3lldW5nQG1kYW5kZXJzb24ub3Jn
 Demis N. Lipe
Demis N. Lipe Aiham Qdaisat
Aiham Qdaisat Patrick Chaftari
Patrick Chaftari Monica K. Wattana2
Monica K. Wattana2 Cielito Reyes-Gibby
Cielito Reyes-Gibby Sai-Ching J. Yeung
Sai-Ching J. Yeung