
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 16 February 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1121140
This article is part of the Research TopicRecent Advances in the Mechanism and Treatment of Pituitary TumorsView all 8 articles
Background: Thyroid-stimulating hormone (TSH)-secreting pituitary adenomas (TSHomas) are rare and usually present with hyperthyroidism. Calcification in pituitary tumors is an infrequent finding. Herein, we report an extremely rare case of TSHoma with diffuse calcification.
Case description: A 43-year-old man was admitted to our department with a complaint of palpitations. An endocrinological examination revealed elevated serum levels of TSH, free triiodothyronine (FT3), and free thyroxin, whereas the physical examination revealed no obvious abnormality. Computerized tomography (CT) showed a sellar mass with diffuse calcification. Contrast-enhanced T1-weighted images revealed a less-enhancing tumor without obvious suprasellar or parasellar expansion. The tumor was completely removed via endoscopic transnasal-sphenoidal surgery. Microscopically, nests of cells were inconspicuous among the diffuse psammoma bodies. Expression of TSH was patchy, and only several TSH-positive cells were observed. Postoperatively, the serum levels of TSH, FT3, and FT4 decreased to their normal range. Follow-up MR images showed no evidence of residual tumor or regrowth after the resection.
Conclusions: Herein, we report a rare case of TSHoma with diffuse calcification that presented with hyperthyroidism. A correct and early diagnosis was made according to the European Thyroid Association guidelines. This tumor was completely removed via endoscopic transnasal-transsphenoidal surgery (eTSS), and thyroid function was normalized after the operation.
Among all functional pituitary adenomas, thyroid-stimulating hormone (TSH)-secreting pituitary adenomas (TSHomas) are rare and account for only 0.5%–3% of all pituitary tumors in surgical series (1, 2). TSHomas can produce excessive amounts of TSH, resulting in increased levels of thyroxine, and present with clinical features of hyperthyroidism (3). Calcification in the pituitary tumor is an infrequent finding and has been radiologically found in two forms: eggshell-like capsular calcification and intratumoral nodular calcification. Until now, only about 50 cases of calcification in pituitary adenomas have been reported, and diffuse calcification is rare (4, 5). Herein, we report an extremely rare case of TSHoma with diffuse calcification that presented with hyperthyroidism in our department.
A 43-year-old man visited the outpatient department complaining of palpitations. His height was 174 cm, and his body weight was 66 kg. He had continuously perspired profusely. His blood pressure was 160/95 mmHg. No abnormal findings were noted on physical examination, including the thyroid gland. Electrocardiogram showed atrial fibrillation, and the heart rate was 97 beats/min. Endocrinological examination revealed hypersecretion of TSH, free triiodothyronine (FT3), and free thyroxine (FT4); as a result, central hyperthyroidism was in consideration (Table 1). The thyroid iodine uptake was within the normal range. Other pituitary and adrenal hormones were within normal ranges. A sellar mass with calcification was then observed during computerized tomography (CT) scanning (Figure 1A). Magnetic resonance imaging (MRI) showed a sellar lesion without obvious suprasellar or parasellar extension. On enhanced scans, the tumor showed no enhancement after gadolinium administration (Figures 1B–D). The tumor size was 1.1 cm × 1.3 cm × 1.5 cm. The pituitary gland was compressed backward. The cavernous sinus and optic chiasma were not invaded. Short-term somatostatin analog test was performed (6), and the serum TSH concentration decreased by 53% after octreotide administration (Table 2). The examinations of autoantibodies and antineutrophilic cytoplasmic antibodies (ANCA) were all negative. Ultrasound detection of the thyroid revealed diffuse disorders; the thyroid was swelling without any mass detected, suggesting thyroid hypersecretion. To distinguish the syndrome of pituitary resistance to thyroid hormone (PRTH) (7, 8), the thyroid hormone receptor genes were examined, and there was no mutation.
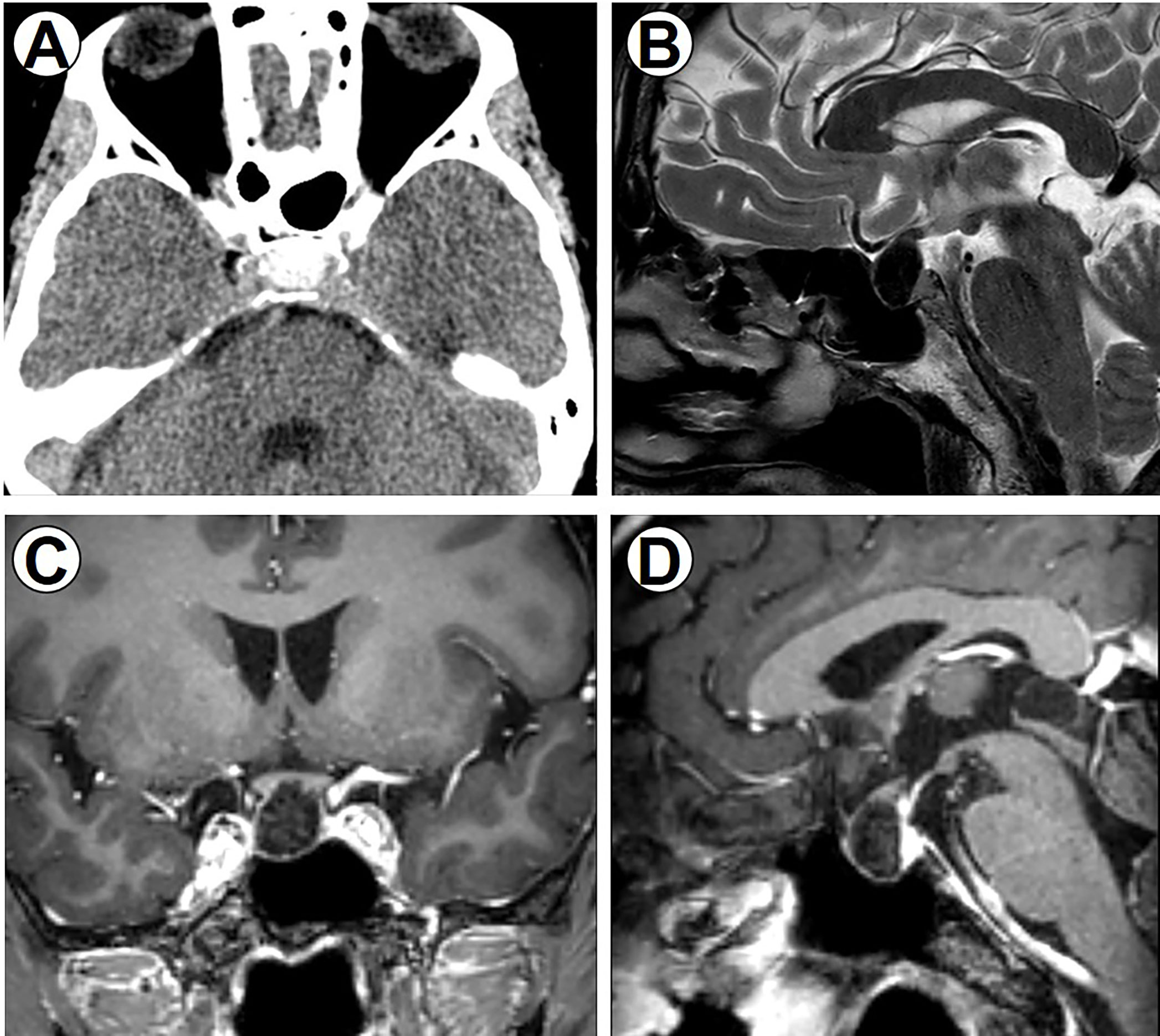
Figure 1 Perioperative imaging data. (A) Preoperative CT scan of the brain, axial section showing a sellar tumor with obvious calcification. (B) T2-weighted sagittal image showing hypointense sellar lesion. (C) Coronal T1-weighted image. (D) Sagittal T1-weighted image with gadolinium contrast showing hypointense sellar lesion anterior to the pituitary gland without enhancement.
To prevent a thyrotoxic crisis during the perioperative period, thiamazole was administered at a dosage of 30 mg/day, and the hyperthyroidism was significantly relieved. After careful preoperative evaluation, endoscopic transnasal-sphenoidal surgery (eTSS) was performed in December 2020. During the operation, a solid yellowish mass full of calcifications was identified in the sellar area (Figures 2A–C). The mass was limited, and it did not appear to invade the cavernous sinus or diaphragma sellae. Total removal of the mass was achieved after eTSS.
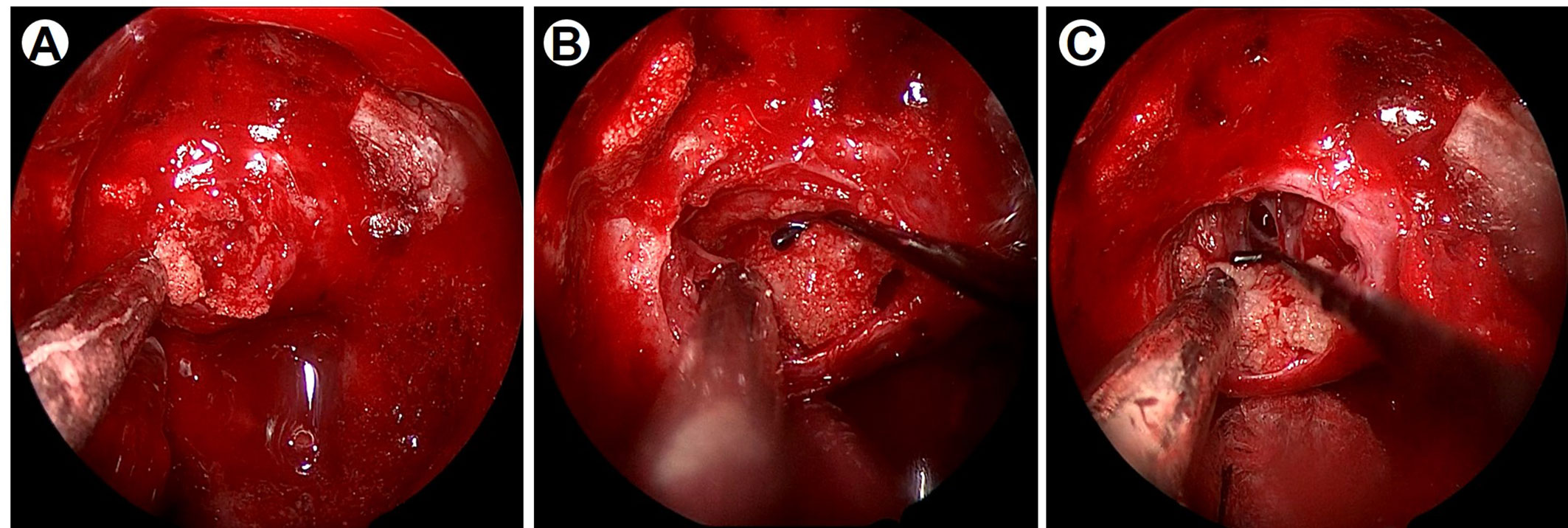
Figure 2 Intraoperative imaging data under neuroendoscopy. (A) Intraoperative imaging indicates the yellowish tumor was firm and consisted of psammoma bodies. (B) The lesion adhered closely to the diaphragma sellae. (C) After the tumor removal, the diaphragma sellae was broken and cerebrospinal fluid leakage was observed.
Histologic sections of the surgical specimen revealed an abundance of psammoma bodies, and nests of cells were inconspicuous among the psammoma bodies (Figure 3A). There was no evidence of pleomorphism, mitosis, or necrosis, suggesting a benign tumor. Upon immunohistochemical staining, only a few TSH-positive cells were detected. Moreover, the cells were negative for all other anterior pituitary hormones except follicle-stimulating hormone (FSH), epithelial membrane antigen (EMA), progesterone receptor (PR), P63, P40, and Ki67, but the cells were positive for chromogranin A (CgA), CD56, cytokeratin (CK), and synaptophysin (Syn) (Figures 3B–D).
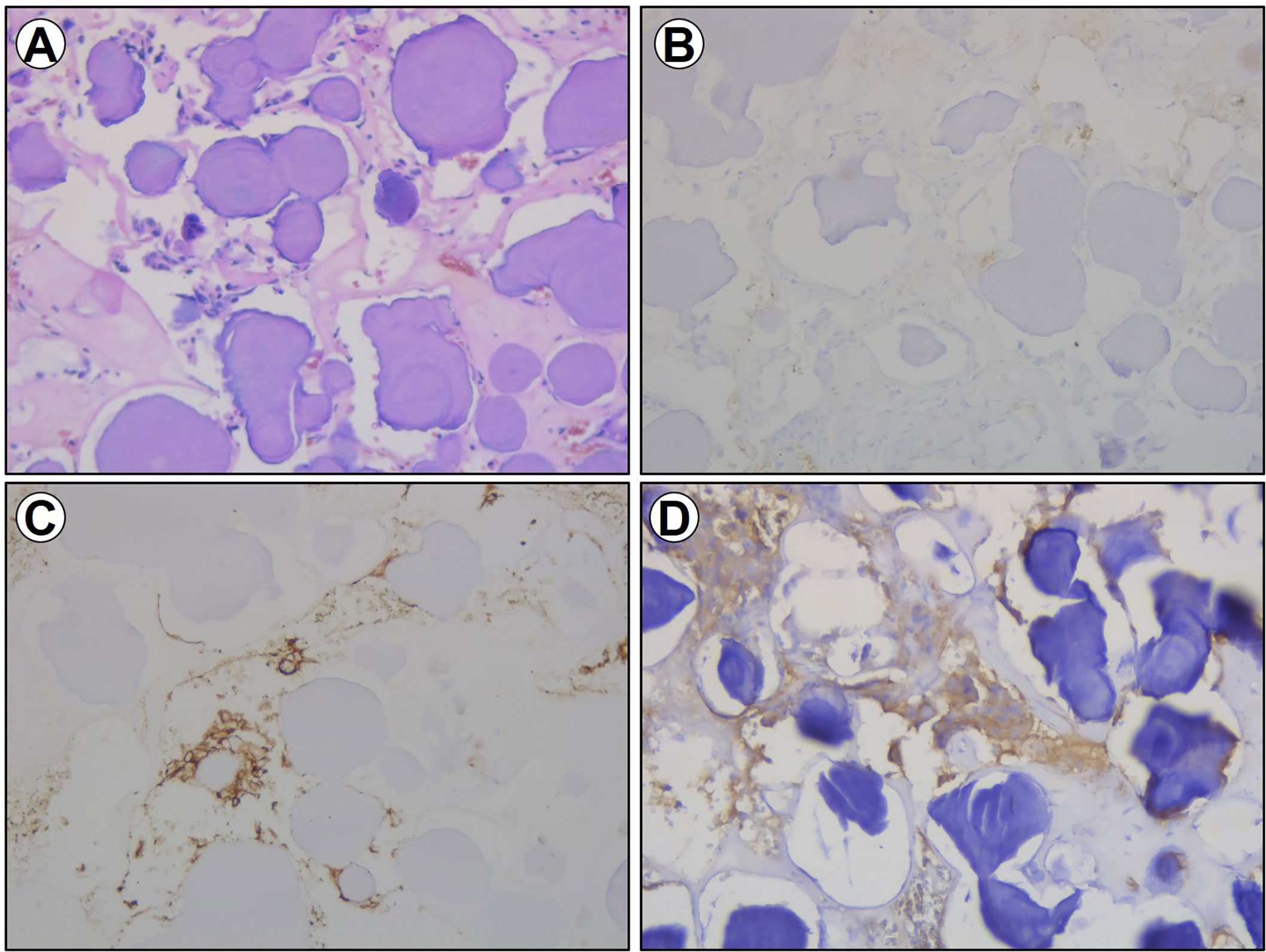
Figure 3 Section imaging data of the excised specimen. (A) HE staining for the excised specimen. (B) Immunohistochemical staining of TSH for the excised specimen. (C) Immunohistochemical staining of CD56 for the excised specimen. (D) Immunohistochemical staining of Syn for the excised specimen.
Postoperatively, normalization of thyroid hormones was achieved with no deficiency of other pituitary hormones. The patient was discharged from the hospital on the 12th postoperative day. A follow-up MRI was performed 12 months after eTSS (Figure 4); no regrowth of the tumor was noted, and the patient’s thyroid function tests were within normal limits.
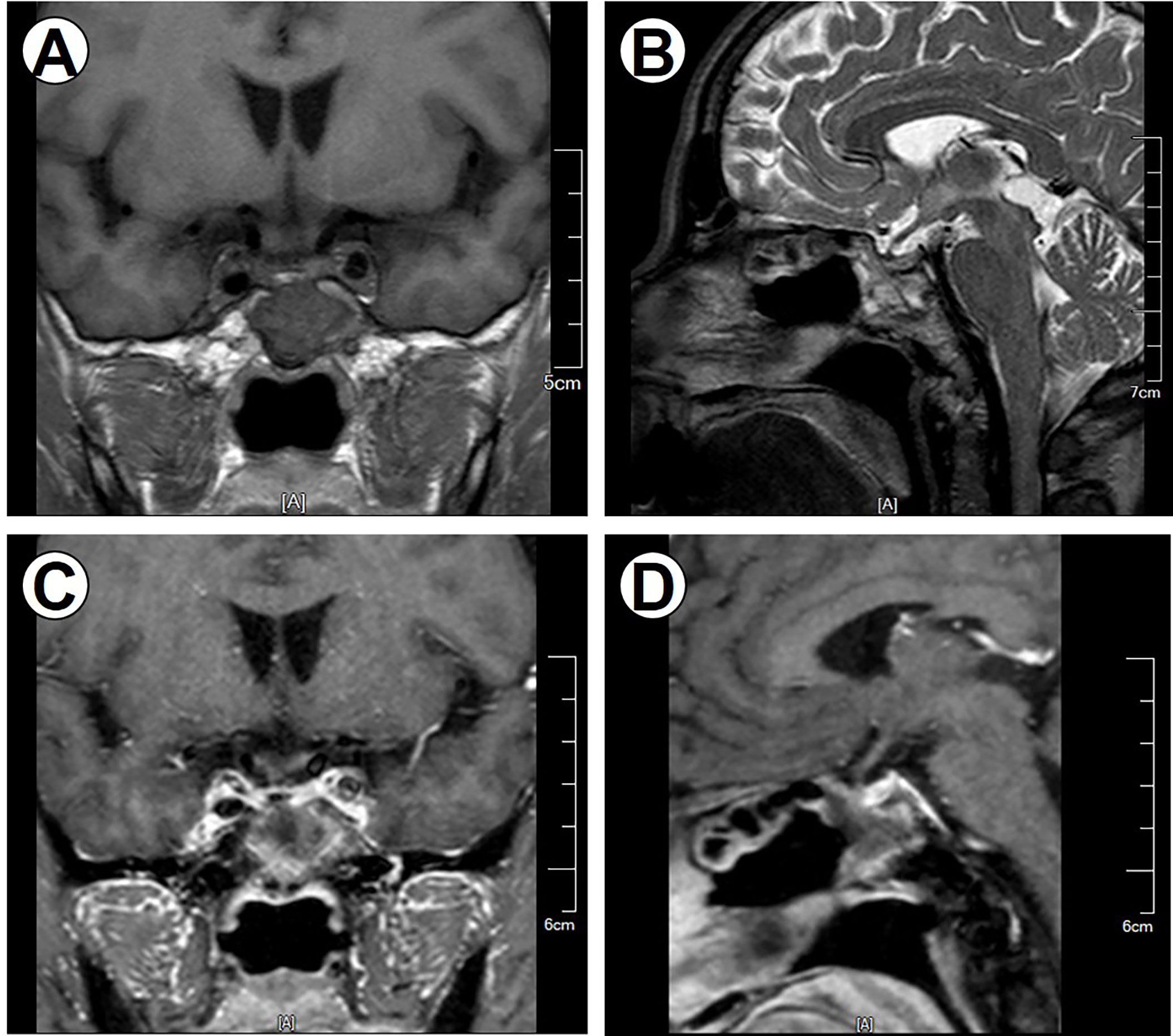
Figure 4 Postoperative MRI imaging data. Postoperative MR images demonstrate no regrowth of the tumor. (A) Coronal noncontrast T1-weighted image. (B) Sagittal T2-weighted image. (C) Coronal contrast T1-weighted image. (D) Sagittal contrast T1-weighted image.
TSHomas are the least frequent pituitary adenomas; they occur in all age groups and exhibit no gender predilection (2). TSHomas can be defined by serum hormone levels suggestive of central hyperthyroidism before surgery or by immunohistochemical profiles for TSH on postoperative pathological analysis. Some tumors that stain immunochemically for TSH are clinically silent or are associated with hypopituitarism (9, 10). The diagnosis of TSHomas is challenging. A correct and early diagnosis of TSHomas is essential to avoid misdiagnosis, and thereby, proper treatment could be taken immediately (11). Clinical manifestations of hypopituitarism, including sinus tachycardia, hypertension, and weight loss, were observed in this case. His endocrinological examination revealed hypersecretion of TSH, FT3, and FT4, suggesting the possibility of central hyperthyroidism (12). According to the European Thyroid Association guidelines (6, 13), a pituitary MRI/CT scan, short-term somatostatin analog test, and examination of thyroid hormone receptor genes were performed, and the results supported the diagnosis of TSHoma.
Surgical resection is considered the first-line therapy for TSHomas (1, 11), aiming at removing tumor mass and normalizing thyroid function. According to a recent meta-analysis, biochemical remission is achieved in about 70% of the TSHomas; however, total resection is slightly lower, at only 54% (14). This might due to the fact that most TSHomas are invasive macroadenomas (15). After careful evaluation and preoperative preparation, the eTSS was performed. In this case, TSHoma was noninvasive. During the surgery, we found that this tumor was hard and fixated. Moreover, the tumor adhered closely to the diaphragma sellae, and after the tumor removal, cerebrospinal fluid leakage was observed. Without considering endocrine abnormalities, this mass was more like a diaphragma sellae meningioma, especially a psammomatous meningioma (16). Fortunately, total resection was achieved, and thyroid function was normalized in this patient.
In accordance with practice, a histopathological examination was performed after surgery. Frequently, TSHomas are chromophobic and the tumors are usually composed of irregular or elongated angular cells possessing long cytoplasmic processes, and TSH immunoreactivity is variable (10, 17). Among pituitary stones, most of them are presented in the form of psammoma bodies scattered between adenoma cells. In the current case, diffuse psammoma bodies were observed at light microscopy, and nests of cells were inconspicuous around them. After decalcification, immunohistochemistry of the resected specimen showed expression of TSH was patchy and only several TSH-positive cells were observed. Moreover, TSH is also frequently expressed in plurihormonal secretory adenomas. In our case, the cells were positive for FSH, while other circulating hormones were in the normal range.
The sellar region, one of the most complex neoplastic areas of the brain, can give rise to a wide variety of pathologically distinct tumors, including pituitary adenoma, germ cell tumor, Rathke’s cleft cyst, meningioma, chordoma, craniopharyngioma, etc. (18). Most of these tumors, especially craniopharyngiomas, are often accompanied by calcification; however, pituitary adenomas with calcification are scarcely rare (19). According to previous reports, calcification in pituitary adenomas could be radiologically observed in two forms: eggshell-like capsular calcification and intratumoral nodular calcification. The latter, also known as “pituitary stones,” are relatively rare. Furthermore, calcification in pituitary adenoma raises special diagnostic and therapeutic challenges (20). In this case, the tumor was diffusely calcified on a CT scan, and it was hard to get the correct diagnosis of pituitary adenoma just according to imaging studies. Generally, gross total resection via eTSS approach is considered difficult to accomplish in pituitary adenomas with extensive calcification because of the hardness. With the development of neuroendoscopic surgery, some calcified pituitary adenomas could be completely removed via the eTSS approach, even when large, as in the current case. The eTSS approach might be an effective and feasible option for removing such tumors after careful evaluation.
The real mechanisms of calcification formation in pituitary adenomas were still unclear. Previous studies suggested that pituitary apoplexy, hyperprolactinemia, regressive processes of pituitary adenoma, and treatment by dopamine receptor agonists or irradiation might be the possible causes (4). These hypotheses were based on cases in which the persistence of prolactin granules was detected in the calcified adenomatous tissue (21). However, it also appears that calcification can occur in any type of pituitary adenoma, including prolactinoma, suggesting that hormone secretion might not be the main mechanism of pituitary stone formation. Apart from endocrine abnormalities, calcification was also found in some cases with intratumoral hemorrhages or degenerative changes during surgery or pathological examination (21). Nevertheless, without these findings or in the absence of the medical histories described above, calcified pituitary adenomas have also been reported. In this case, no evidence suggesting the mechanism of calcification formation was found. This result suggested that other causes might be involved in the calcification formation in pituitary adenomas.
TSHoma is a rare type of functional pituitary adenoma and often presents with hyperthyroidism. A correct and early diagnosis of TSHomas is challenging; it can tell the surgeons to provide proper treatment. Calcification in pituitary tumors is an infrequent finding and identification of calcification before the operation is essential as it helps in surgical planning. Herein, we report a rare case of TSHoma with diffuse calcification that presented with hyperthyroidism and was completely removed via an eTSS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HY and CY wrote and revised the manuscript together. JM participated in the surgery and handled the pictures. WJ gave some important advice for the revision of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer ZC declared a shared parent affiliation with the authors to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ANCA, anti-neutrophilic cytoplasmic antibodies; CgA, chromogranin A; CK, cytokeratin; CT, computerized tomography; EMA, epithelial membrane antigen; eTSS, endoscopic transnasal-sphenoidal surgery; FSH, follicle-stimulating hormone; FT3, free triiodothyronine; MRI, magnetic resonance imaging; PA, pituitary adenoma; PR, progesterone receptor; PRTH, pituitary resistance to thyroid hormone; Syn, synaptophysin; TSH, thyroid-stimulating hormone.
1. Beck-Peccoz P, Giavoli C, Lania A. A 2019 update on TSH-secreting pituitary adenomas. J Endocrinol Invest (2019) 42(12):1401–6. doi: 10.1007/s40618-019-01066-x
2. Cossu G, Daniel RT, Pierzchala K, Berhouma M, Pitteloud N, Lamine F, et al. Thyrotropin-secreting pituitary adenomas: A systematic review and meta-analysis of postoperative outcomes and management. Pituitary (2019) 22(1):79–88. doi: 10.1007/s11102-018-0921-3
3. Razvi S, Hostalek U. Therapeutic challenges in the application of serum thyroid stimulating hormone testing in the management of patients with hypothyroidism on replacement thyroid hormone therapy: A review. Curr Med Res Opin (2019) 35(7):1215–20. doi: 10.1080/03007995.2019.1570769
4. Murase M, Toda M, Ohara K, Ishihara E, Yoshida K. Endoscopic endonasal removal of Large calcified pituitary adenoma: Case report and review of the literature. World Neurosurg (2019) 123:239–43. doi: 10.1016/j.wneu.2018.11.255
5. Xie Z, Wang Q, Lu X. Endoscopic endonasal resection of nonfunctioning pituitary adenoma with radiological calcification. Pituitary. (2019) 22(4):381–6. doi: 10.1007/s11102-019-00967-7
6. Han R, Shen L, Zhang J, Xie J, Fang W, Sun Q, et al. Diagnosing thyrotropin-secreting pituitary adenomas by short-term somatostatin analogue test. Thyroid (2020) 30(9):1236–44. doi: 10.1089/thy.2019.0470
7. Suzuki S, Shigematsu S, Inaba H, Takei M, Takeda T, Komatsu M. Pituitary resistance to thyroid hormones: Pathophysiology and therapeutic options. Endocrine. (2011) 40(3):366–71. doi: 10.1007/s12020-011-9538-2
8. Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol (2014) 10(10):582–91. doi: 10.1038/nrendo.2014.143
9. Teramoto A, Sanno N, Tahara S, Osamura YR. Pathological study of thyrotropin-secreting pituitary adenoma: Plurihormonality and medical treatment. Acta Neuropathol. (2004) 108(2):147–53. doi: 10.1007/s00401-004-0863-x
10. Cyprich J, Donoho DA, Brunswick A, Hurth K, Carmichael JD, Weiss MH, et al. Surgical management of clinically silent thyrotropin pituitary adenomas: A single center series of 20 patients. J Clin Neurosci (2020) 71:70–5. doi: 10.1016/j.jocn.2019.10.013
11. Amlashi FG, Tritos NA. Thyrotropin-secreting pituitary adenomas: Epidemiology, diagnosis, and management. Endocrine (2016) 52(3):427–40. doi: 10.1007/s12020-016-0863-3
12. McDermott MT, Ridgway EC. Central hyperthyroidism. Endocrinol Metab Clin North Am (1998) 27(1):187–203. doi: 10.1016/S0889-8529(05)70306-6
13. Beck-Peccoz P, Lania A, Beckers A, Chatterjee K, Wemeau JL. 2013 European Thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur Thyroid J (2013) 2(2):76–82. doi: 10.1159/000351007
14. Herguido NG, Fuentes ED, Venegas-Moreno E, Maorad LB, Flores-Martinez A, Ruiz PR, et al. Surgical outcome and treatment of thyrotropin-secreting pituitary tumors in a tertiary referral center. World Neurosurg (2019) 130:e634–9. doi: 10.1016/j.wneu.2019.06.180
15. Wang EL, Qian ZR, Yamada S, Rahman MM, Inosita N, Kageji T, et al. Clinicopathological characterization of TSH-producing adenomas: special reference to TSH-immunoreactive but clinically non-functioning adenomas. Endocr Pathol (2009) 20(4):209–20. doi: 10.1007/s12022-009-9094-y
16. Nanda A, Ambekar S, Javalkar V, Sharma M. Technical nuances in the management of tuberculum sellae and diaphragma sellae meningiomas. Neurosurg Focus. (2013) 35(6):E7. doi: 10.3171/2013.10.FOCUS13350
17. Lopes M. The 2017 world health organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. (2017) 134(4):521–35. doi: 10.1007/s00401-017-1769-8
18. Wen J, Yin R, Chen Y, Chang J, Ma B, Zuo W, et al. Hypothalamus-pituitary dysfunction as an independent risk factor for postoperative central nervous system infections in patients with sellar region tumors. Front Endocrinol (2021) 12:661305. doi: 10.3389/fendo.2021.661305
19. Ibrahim R, Kalhan A, Lammie A, Kotonya C, Nannapanenni R, Rees A. Dense calcification in a GH-secreting pituitary macroadenoma. Endocrinology Diabetes Metab Case Rep (2014) 2014:130079. doi: 10.1530/EDM-13-0079
20. Khan Y, Malik N, Awan SI, Khalid SH, Laghari AA. Pituitary adenoma with calcifications: A case report. Curēus (2019) 11:e5542. doi: 10.7759/cureus.5542
Keywords: pituitary adenoma (PA), TSHoma, hyperthyroidism, calcification, macroadenoma
Citation: Yan H, Yan C, Mao J and Jin W (2023) Case report: A rare case of thyrotropin-secreting pituitary macroadenoma with diffuse calcification presenting with hyperthyroidism and literature review. Front. Oncol. 13:1121140. doi: 10.3389/fonc.2023.1121140
Received: 11 December 2022; Accepted: 27 January 2023;
Published: 16 February 2023.
Edited by:
Wei Shi, Affiliated Hospital of Nantong University, ChinaCopyright © 2023 Yan, Yan, Mao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jin, bmpuZXVyb3N1cmdlcnlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.