- 1Department of Radiotherapy, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Oncology, Wenzhou Hospital of Traditional Chinese Medicine, Wenzhou, China
- 3Department of Radiotherapy, Quzhou People’s Hospital, Quzhou, China
- 4Department of Radiotherapy, Dongyang People’s Hospital, Jinhua, China
- 5Department of Radiotherapy Oncology, Lishui Central Hospital, Lishui, China
Background: This study aims to compare the efficacy and safety of neoadjuvant chemoradiotherapy (nCRT) with different radiotherapy doses (45Gy and 50.4Gy) in patients with locally advanced rectal cancer (LARC).
Methods: Herein, 120 patients with LARC were retrospectively enrolled between January 2016 and June 2021. All patients underwent two courses of induction chemotherapy (XELOX), chemoradiotherapy, and total mesorectum excision (TME). A total of 72 patients received a radiotherapy dose of 50.4 Gy, while 48 patients received a dose of 45 Gy. Surgery was then performed within 5-12 weeks following nCRT.
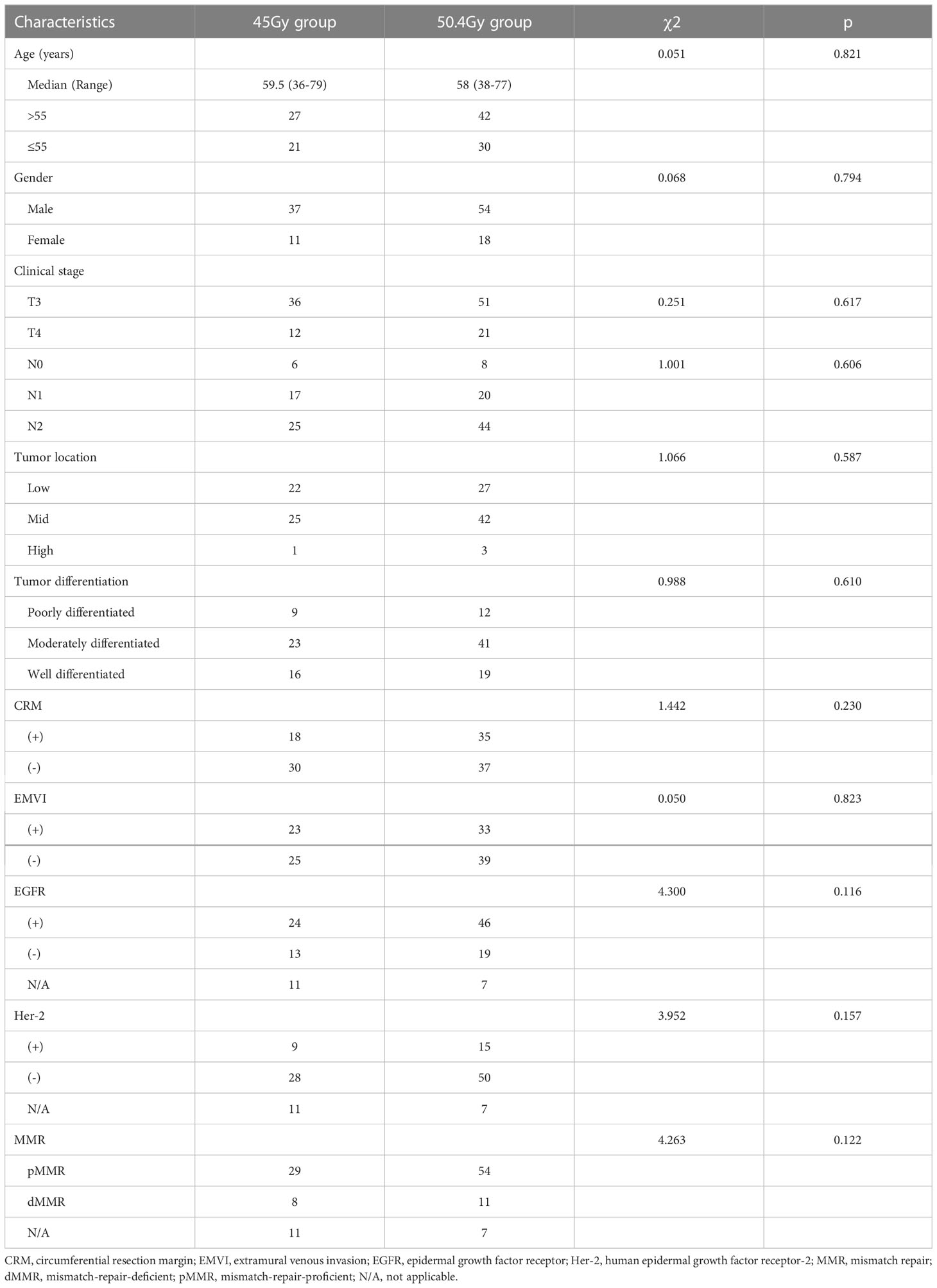
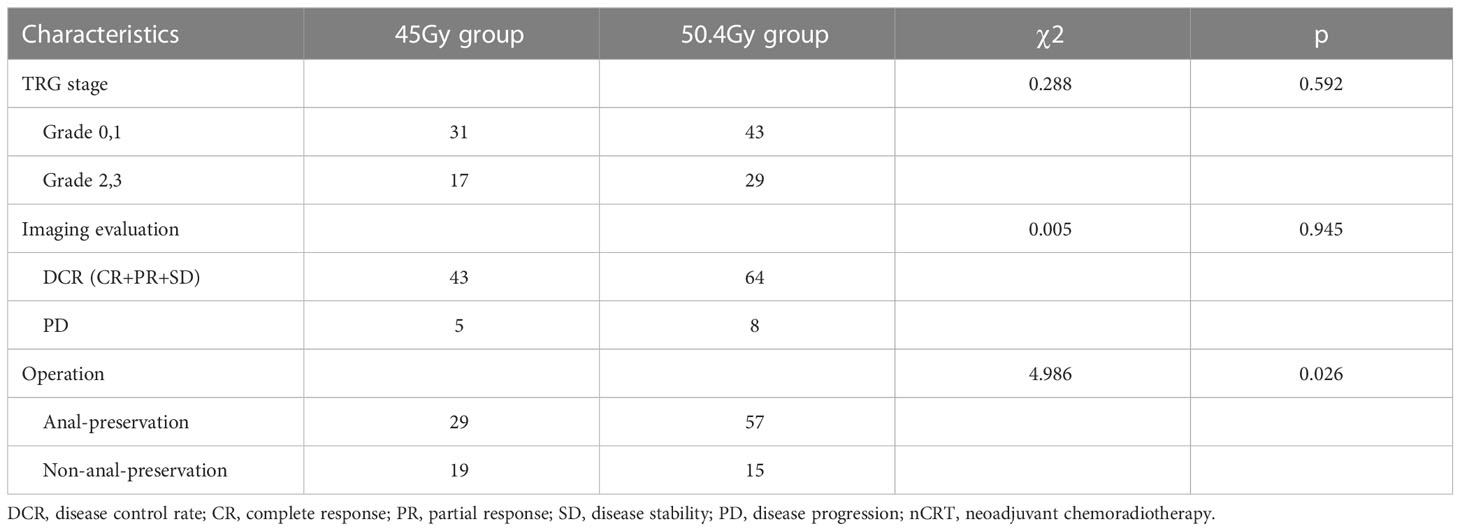
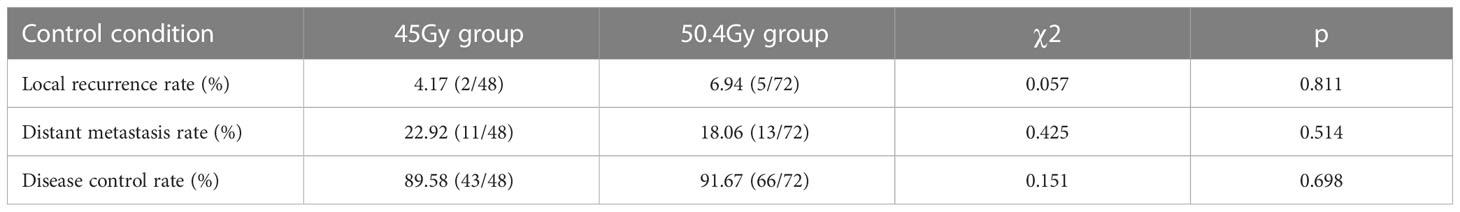
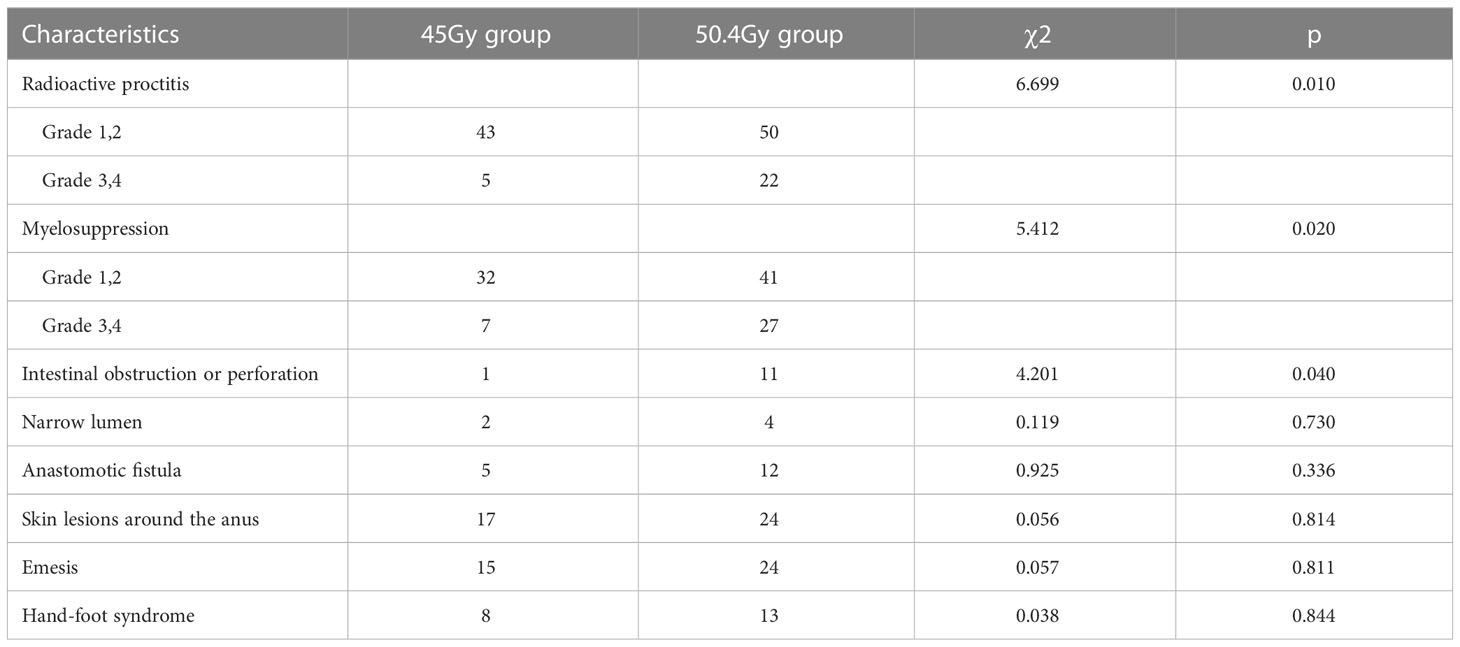
Results: There was no statistically significant difference between the baseline characteristics of the two groups. The rate of good pathological response in the 50.4Gy group was 59.72% (43/72), while in the 45Gy group achieved 64.58% (31/48) (P>0.05). The disease control rate (DCR) in the 50.4Gy group was 88.89% (64/72), compared to 89.58% (43/48) in the 45Gy group (P>0.05). The incidence of adverse reactions for radioactive proctitis, myelosuppression, and intestinal obstruction or perforation differed significantly between the two groups (P<0.05). The anal retention rate in the 50.4Gy group was significantly higher in contrast to the 45Gy group (P<0.05).
Conclusions: Patients receiving a radiotherapy dose of 50.4Gy have a better anal retention rate but also a higher incidence of adverse events such as radioactive proctitis, myelosuppression, and intestinal obstruction or perforation, and a comparable prognosis to patients treated with a radiotherapy dose of 45Gy.
Introduction
Colorectal cancer incidence and mortality ranked third and second overall in 2020, according to cancer statistics (1). It is common for patients with rectal cancer to be asymptomatic in the early stages, so many patients are already in an advanced stage upon diagnosis. Neoadjuvant chemoradiotherapy (nCRT) plays an important role for patients with locally advanced rectal cancer (LARC), and clinical trials like the CAO/ARO/AIO-94 Study, the Swedish Trial, and the CAO/ARO/AIO-04 Study have demonstrated its effectiveness in LARC patients (2–5). Neoadjuvant chemoradiotherapy combined with total mesorectum excision (TME) is the first-line treatment for LARC patients, and while it can reduce tumor burden, induce downstaging, and improve the local control rate, it does not improve the overall survival (OS) (4, 6–8).
In recent years, the development of total neoadjuvant therapy (TNT) has also provided new options for LARC patients. CAO/ARO/AIO-12 Trial assessed the outcomes of 311 LARC patients treated with chemotherapy plus chemoradiotherapy (CRT) plus TME or CRT plus chemotherapy plus TME, and showed that if organ preservation is a priority, then TNT with consolidation chemotherapy (CNCT) after CRT is the preferred modality and this trial provides important evidence for the clinical use of TNT and has influenced the concept of organ preservation (9). RAPIDO trial looked at the efficacy of preoperative short-course radiotherapy (SCRT) plus nCRT compared to preoperative concurrent chemoradiotherapy (CCRT) and concluded that SCRT combined with nCRT reduced the probability of treatment failure in rectal cancer compared to standard treatment (10). The findings suggest that preoperative chemotherapy may be more effective than adjuvant chemotherapy and that this treatment modality may become the new standard of care for high-risk LARC (10). OPRA trial analyzed the outcomes of 324 LARC patients treated with induction chemotherapy (INCT) followed by CRT or CRT followed by CNCT, and concluded that half of the patients who received neoadjuvant treatment achieved organ preservation with no significant impairment in survival compared to previous controls who received radiotherapy, TME, and post-operative chemotherapy (11).
Notably, outcomes of nCRT vary widely among LARC patients, with more than one-third of patients experiencing recurrent or metastatic diseases (12). Moreover, some patients can achieve pathological complete remission (pCR) while others barely respond to nCRT. The proportion of patients who achieve pCR after nCRT is usually used as a reliable indicator of treatment response, Roh et al. reported that about 10-30% of patients achieved pCR (13), while Sanghera et al. concluded that 42% reached pCR (14) after nCRT. There is a strong correlation between clinical stage, tumor differentiation, and treatment regimens (6, 15, 16) in determining the outcomes of patients. Given that patients respond differently to nCRT, more research is needed to identify the most effective follow-up treatments.
Generally, surgery, chemotherapy, and radiotherapy are all crucial treatments for LARC, and each of these methods has made significant progress in recent years (17–23). The National Comprehensive Cancer Network (NCCN) guidelines and European Society for Medical Oncology (ESMO) guidelines recommend a total dose of 45-50.4Gy delivered in 25-28 fractions (24). In clinical practice, both radiotherapy doses are frequently used, but studies assessing their efficacy and safety are scarce. More studies focus on radiotherapy dose intensification versus conventional fractionation (25–28). Higher radiotherapy doses are known to be associated with improved efficacy but are also associated with an increased incidence of adverse events. A comparative analysis of radiotherapy doses of 45 Gy and 50.4 Gy was performed in the present study. Patients were divided into the 45Gy group and the 50.4Gy group according to the radiotherapy dose. Notably, pathological responses, imaging assessments, anal retention rate, local control, adverse reactions, and survival were analyzed across the two groups (45Gy and 50.4Gy).
Materials and methods
Patient selection
144 patients were recruited in total, but 24 were excluded due to loss of follow-up or distant metastases before treatment, and 120 patients were enrolled eventually. The patient flow diagram is shown in Figure 1.
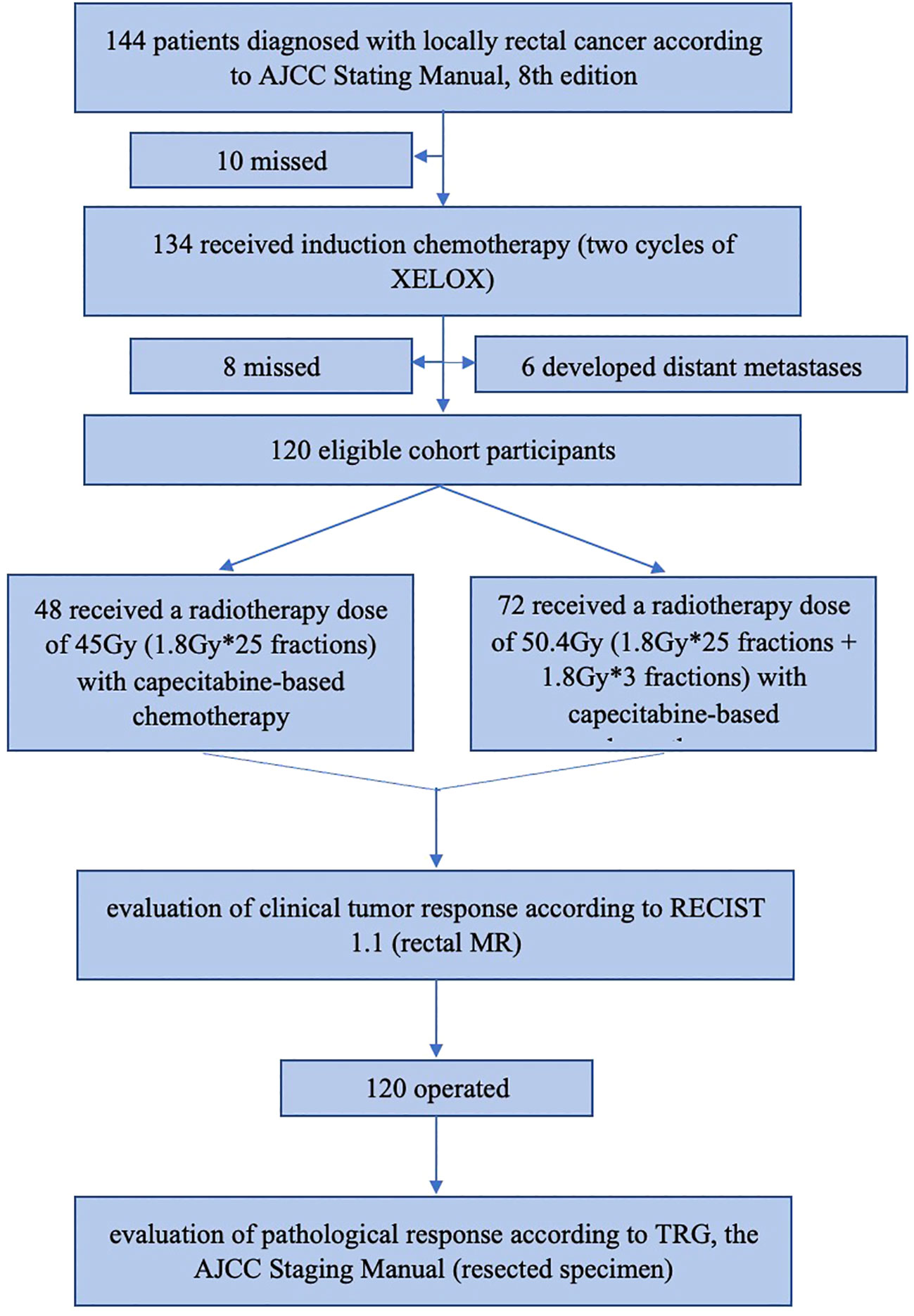
Figure 1 The patients flow diagram. AJCC, American Joint Committee on Cancer; XELOX regimen, Oxaliplatin in combination with capecitabine; MR, magnetic resonance.
The inclusion criteria were as follows: (I) patients aged 18 to 75 years old; (II) rectal adenocarcinoma was diagnosed by colonoscopy; (III) Eastern Cooperative Oncology Group performance status (ECOG PS) was 0 or 1; (IV) defined as stage II/III according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging; (V) no distant metastases or concurrent malignancy; (VI) normal heart, liver and kidney function; (VII) underwent complete chemoradiotherapy and radical surgical treatment.
The exclusion criteria were as follows: (I) diagnosed with distant metastasis; (II) prior chemoradiotherapy or targeted therapy or immunotherapy; (III) patients with other malignancy and incomplete clinical data.
Data collection
Patients baseline characteristics like age, gender, clinical stage, tumor location, tumor differentiation, circumferential resection margin (CRM) status, and extramural venous invasion (EMVI) status were collected on diagnosis. Adverse events, imaging assessments in pre- and post-nCRT, and pathological responses including epidermal growth factor receptor (EGFR) status, human epidermal growth factor receptor-2 (Her-2) status, and mismatch repair (MMR) status were recorded during follow-up observation. The time window for local recurrence rate, distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) was from the date of surgery to the date of final follow-up. The last follow-up date was in November 2021. This study employed the outpatient system, the inpatient system, and telephone consultations to collect accurate patient information and to check for gaps through various collection methods. Regarding tumor location, tumors less than 5cm from the anus were considered low, tumors between 5-10cm from the anus were considered median, and tumors 10-12cm from the anus were considered high. The research was approved by the local ethics committee of The First Affiliated Hospital of Wenzhou Medical University and the Hospital Reviewing Board.
Radiotherapy
Intensity-modulated radiation (IMRT) technology with the Elekta Synergy system (Elekta AB, Stockholm, Sweden) was utilized in this study. All patients were in the supine position. The CT scan range was from the upper boundary of the 2-3 lumbar vertebrae to the lower boundary of the upper 1/3 of the femur, with a thickness of 5mm. Contrast-enhanced venography was recommended if there were no contraindications. Calibration radiographs were taken for each patient at the first session and at regular intervals (once a week).
Radiotherapy targets for both groups (45Gy and 50.4Gy) were performed with the Monaco planning system according to the Radiation Therapy Oncology Group (RTOG) criteria. All patients were treated with 6 MV-X-rays. The specific target areas are outlined below. The gross tumor volume (GTV) was radiographically identified as gross lesions, including primary and metastatic lymph nodes. Clinical target volume (CTV) was defined as GTV + selective lymph node drainage area. CTV included the rectum and mesangial region, the presacral region, the internal iliac lymph nodes, and some obturator lymph nodes. The external iliac lymph nodes need to be irradiated when the tumor invades the bladder, prostate, and gynecological organs, and the external iliac and inguinal lymph nodes need to be irradiated when the tumor invades the anal canal or the lower 1/3 vagina. The upper boundary was the bifurcation of the common iliac artery, the lower boundary included the whole mesentery and was at least 2cm away from the lower edge of the tumor, the left and the right boundary was the inner edge of the true pelvis, the anterior boundary was 1cm in front of the posterior wall of the bladder or the anterior wall of the rectal organ; the internal iliac artery and vein were expanded by 0.7cm, and the posterior boundary was the front edge of the sacrum. Planning target volume (PTV) was defined as CTV+0.5-1.0cm.
The prescribed doses of PTV in the two groups were 45Gy and 50.4Gy, respectively. Patients in the 45Gy group received a total pelvic irradiation dose of 25×1.8Gy. Patients in the 50.4Gy group received a total pelvic irradiation dose of 45Gy (25×1.8Gy), and then the field was reduced to the mesenteric region for a supplement dose of 5.4Gy (3×1.8Gy). It should be pointed out that at least 95% of PTV received the specified dose. Radiotherapy was administered five times a week, from Monday to Friday. Organs at risk (OAR) mainly consisted of the femoral head, bladder and small intestine, the limited doses for each organ were as follows: femoral head Dmax <45Gy, bladder V50 <50Gy, and small intestine Dmax <50Gy.
Chemotherapy regimens
XELOX- oxaliplatin at 135 mg/m2 and capecitabine at 1,000 mg/m2-was administrated twice a day for 14 days, every 21 days for 2 cycles before chemoradiotherapy. Based on several clinical studies like STAR-01, ACCORD, NSABP R-04, and PETACC 6, the addition of oxaliplatin to capecitabine-based chemoradiotherapy failed to improve the rates of pathological complete response(pCR) and OS as expected. Furthermore, it could increase grade 3/4 side effects, thereby affecting patient tolerance (29–31). According to the results of the ACCORD trial, there was no significant difference between the groups in terms of 3-year local recurrence (4%, 6%), DFS (74%, 69%), and OS (both 88%). Fluorouracil-based chemotherapy is still considered to be the first-line regimen during radiotherapy in LARC patients. Therefore, capecitabine was given simultaneously during radiotherapy, twice a day, on weekdays. Patients were assessed 5-12 weeks following nCRT, and surgery was performed.
Adverse reactions monitoring
A variety of nCRT-related adverse reactions were evaluated, including bone marrow suppression, radioactive proctitis, intestinal obstruction or perforation, narrow lumen, anastomotic fistula, perianal skin injury, emesis, and hand-foot syndrome. Hand-foot syndrome was mainly associated with capecitabine treatment. During concurrent chemoradiotherapy, blood routine examinations and biochemical examinations were conducted weekly. RTOG radiation injury classification and Common Terminology Criteria for Adverse Events (CTCAE, Version 5) were adopted to assess adverse events. Grades 1 and 2 myelosuppression were considered mild, while grades 3 and 4 were considered moderate to severe. Similarly, grades 1 and 2 were defined as mild radiation proctitis, and grades 3 and 4 were defined as moderate to severe radiation proctitis. The remaining adverse reactions including intestinal obstruction or perforation, narrow lumen, anastomotic fistula, perianal skin injury, emesis, and hand-foot syndrome were evaluated by their occurrence or not.
Therapeutic effect evaluation
Clinical tumor response was determined by senior radiologists using rectal magnetic resonance (MR) imaging after nCRT and in keeping with RECIST 1.1. Complete response(CR) or clinical complete response (cCR) is defined as the disappearance of all targets lesions; partial response(PR) is achieved when the sum of the target diameter is reduced by at least 30% from baseline; progressive disease (PD) is characterized by an increase of at 20% in minimum diameter of all target lesions, while stable disease (SD) is the state between PR and PD. Disease control rate (DCR) is defined as a radiographic assessment of CR, PR, and SD. Postoperatively, the efficacy was evaluated by the pathological response. Pathological tumor response was evaluated by two experienced pathologists using resected specimens after TME and in accordance with TRG (the AJCC Staging Manual), with the four-tier AJCC Staging Manual being our study’s preferred evaluation method (32). Grade 0-pCR- is complete regression with the absence of cancer cells; grade 1 is moderate regression with single or few cancer cells remaining; grade 2 is mild regression and surplus tumor with extensive fibrotic stroma; grade 3 is no regression and extensive tumor residue accompanied by no or little tumor cell necrosis. Grades 0 and 1 are considered good pathological regression, while grades 2 and 3 are considered poor pathological regression.
Statistical analysis
SPSS 23.0 software (USA) was used for statistical analysis. Age, gender, clinical stage, tumor location, tumor differentiation, imaging reports, pathological response, imaging assessment, anal retention rate, disease control rate, and adverse events were compared using the χ2 test for the two groups. Univariate and multivariate Cox regression analyses were conducted to identify characteristics that related to survival in patients. Age, gender, clinical stage, tumor location, tumor differentiation, CRM status, EMVI status, EGFR status, MMR status, Her-2 status, and radiation dose were the included variable. The Kaplan-Meier method was used to estimate the survival curves, and the log-rank test was used for comparative analysis. A P-value<0.05 was considered statistically significant.
Results
Baseline characteristics
The baseline characteristics are shown in Table 1. The 45Gy group consisted of patients with a median age of 59.5years (from 36 to79 years) while the 50.4Gy group consisted of patients with a median age of 58 years (from 38 to 77 years), with a male predominance in both groups. No significant difference was observed between the 50.4Gy group and the 45Gy group in terms of clinical stage, tumor location, tumor differentiation, and several biological features.
Treatment outcomes
The rate of good pathological response (grade 0/1) was 59.72% in the 50.4Gy group (43/72), while it was 64.58% in the 45Gy group (31/48). The DCR in the 50.4Gy group was 91.67% (66/72), compared to 89.58% in the 45Gy group (43/48). The anal preservation rate in the 50.4Gy group was 79.17% (57/72), compared to 60.42% (29/48) in the 45Gy group (P<0.05). The efficacy of nCRT is shown in Table 2. Imaging evaluation in pre- and post-nCRT for patients is shown in Figure 2.
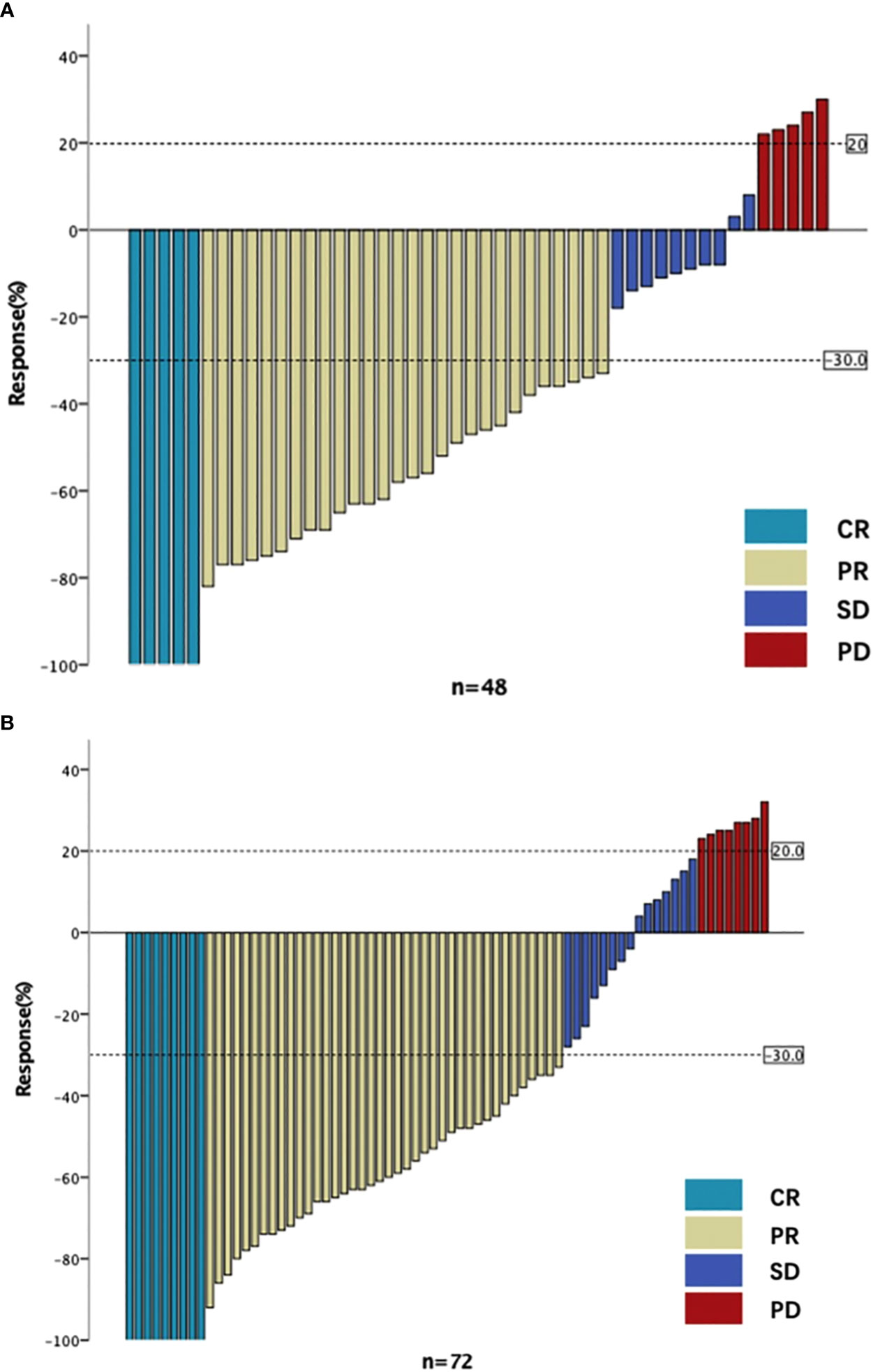
Figure 2 The imaging evaluation in two groups. (A) The imaging evaluation in 45Gy group. (B) The imaging evaluation in 50.4Gy group. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
As demonstrated in Table 3, the local recurrence rate in the 50.4Gy group was 6.94% (5/72), the distant metastasis rate was 18.06% (13/72), and the DCR was 88.89% (64/72), compared with 4.17% (2/48), 22.92% (11/48), and 89.58% (43/48) in the 45Gy group, respectively. No statistical difference was found between the two groups.
Univariate Cox regression analysis showed that tumor differentiation, Her-2 status, and MMR status were associated with DFS, HR=0.312 (tumor differentiated), 0.505 (Her-2), 0.344(MMR) (tumor differentiated 95% CI: 0.176–0.555, Her-2 95% CI: 0.263–0.968, MMR 95% CI: 0.147–0.804). Multivariate Cox regression analysis implied that tumor differentiation was an independent predictor for DFS (see Table 4). Univariate and multivariate Cox regression analysis revealed that only tumor differentiation was closely related to OS, HR=0.232 (95% CI: 0.068-0.794). However, Cox regression analysis showed that the radiation dose was not an independent predictor for DFS (HR=1.118, 95% CI: 0.559-2.525) or OS (HR=1.321, 95% CI: 0.293-5.945). Notably, the base variables of univariate and multivariate Cox regression analyses have been bolded and skewed in Tables 4, 5.
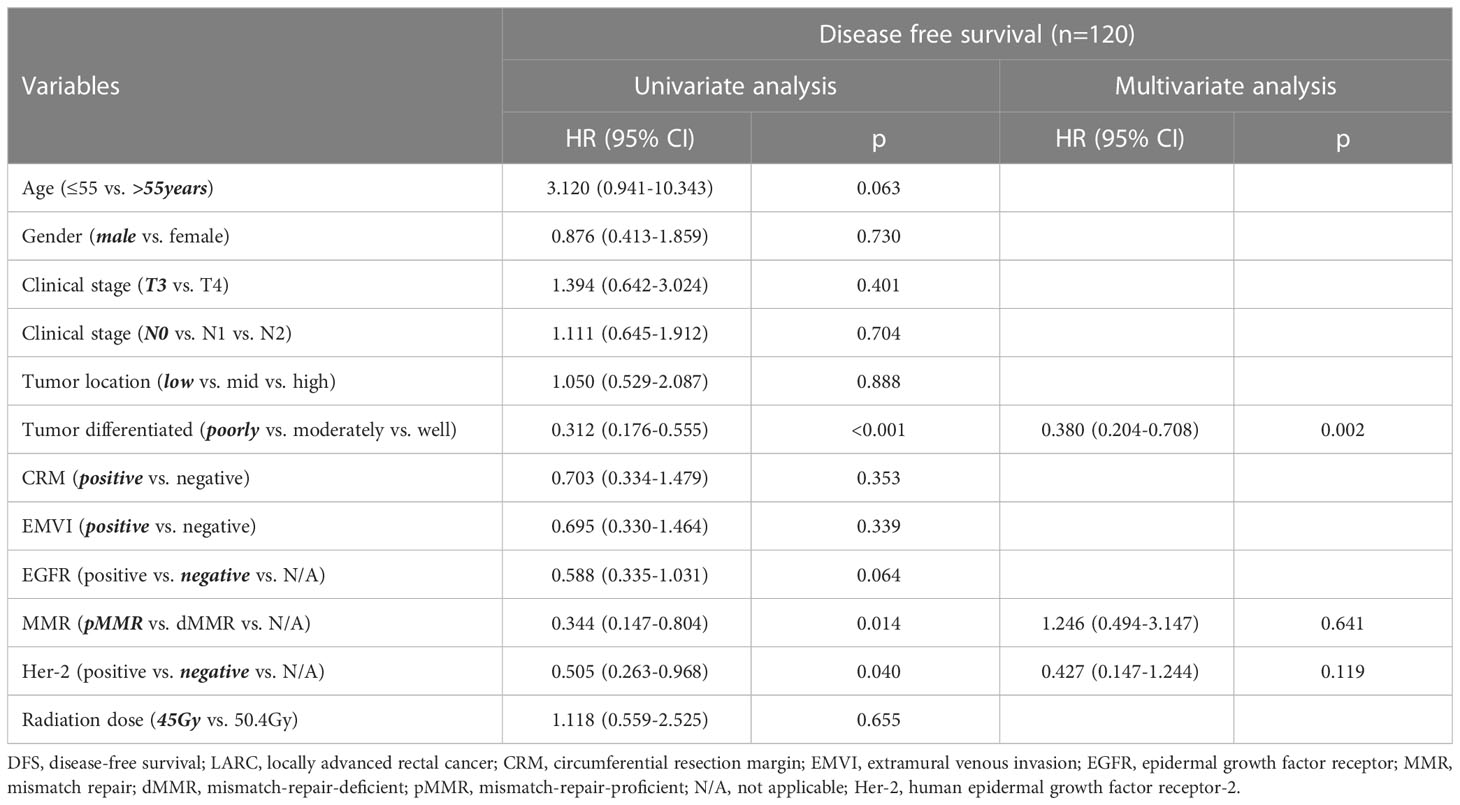
Table 4 Univariate and multivariate Cox regression analyses for different variables and DFS in LARC patients.
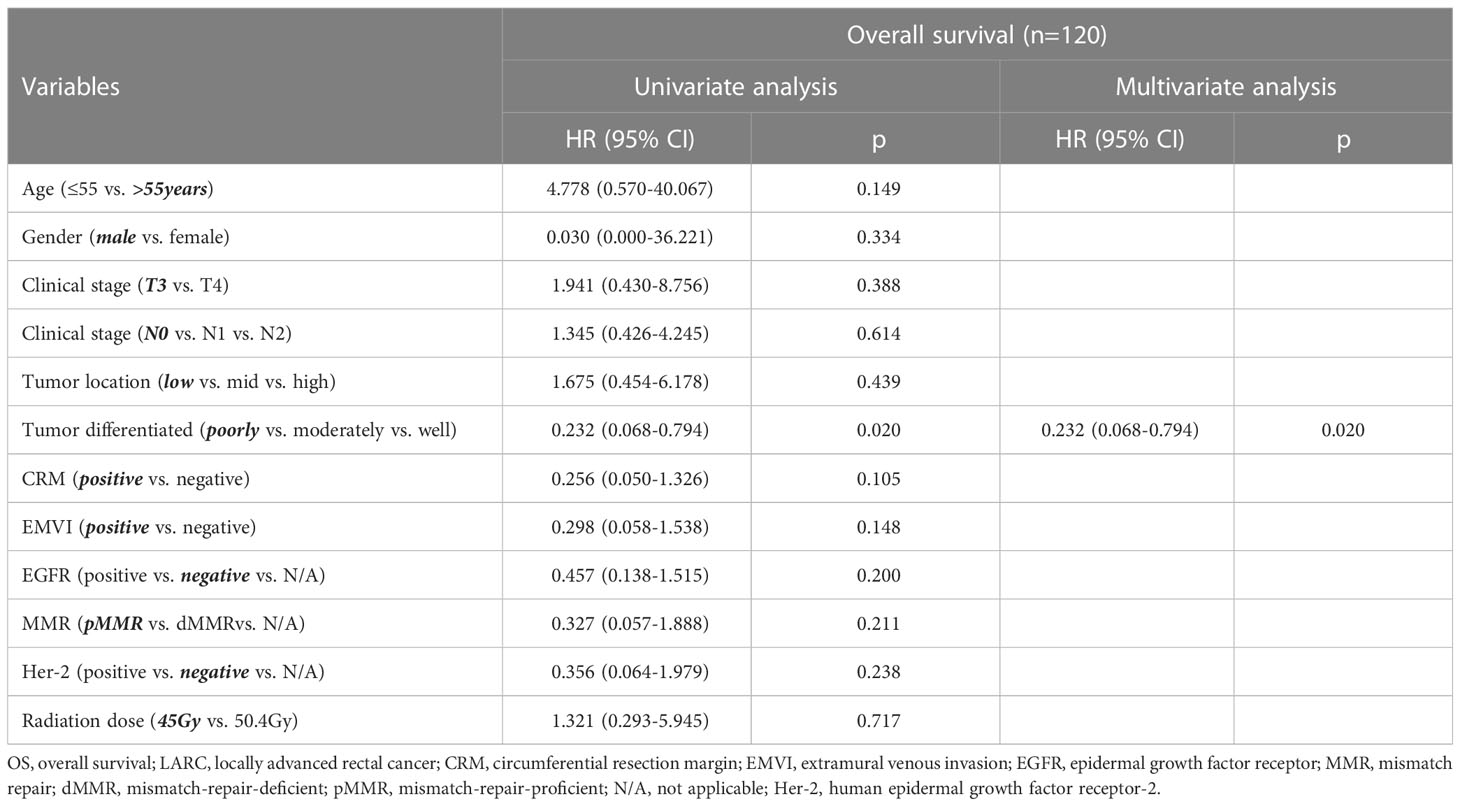
Table 5 Univariate and multivariate Cox regression analyses for different variables and OS in LARC patients.
Herein, higher radiation doses did not confer longer DFS (see Figure 3). As shown in Figure 4, there was no statistical difference between the two groups regarding OS. Radiotherapy dose intensification was not significant in this study, the long-term survival outcomes in the 45Gy group were comparable to outcomes in the 50.4Gy group.
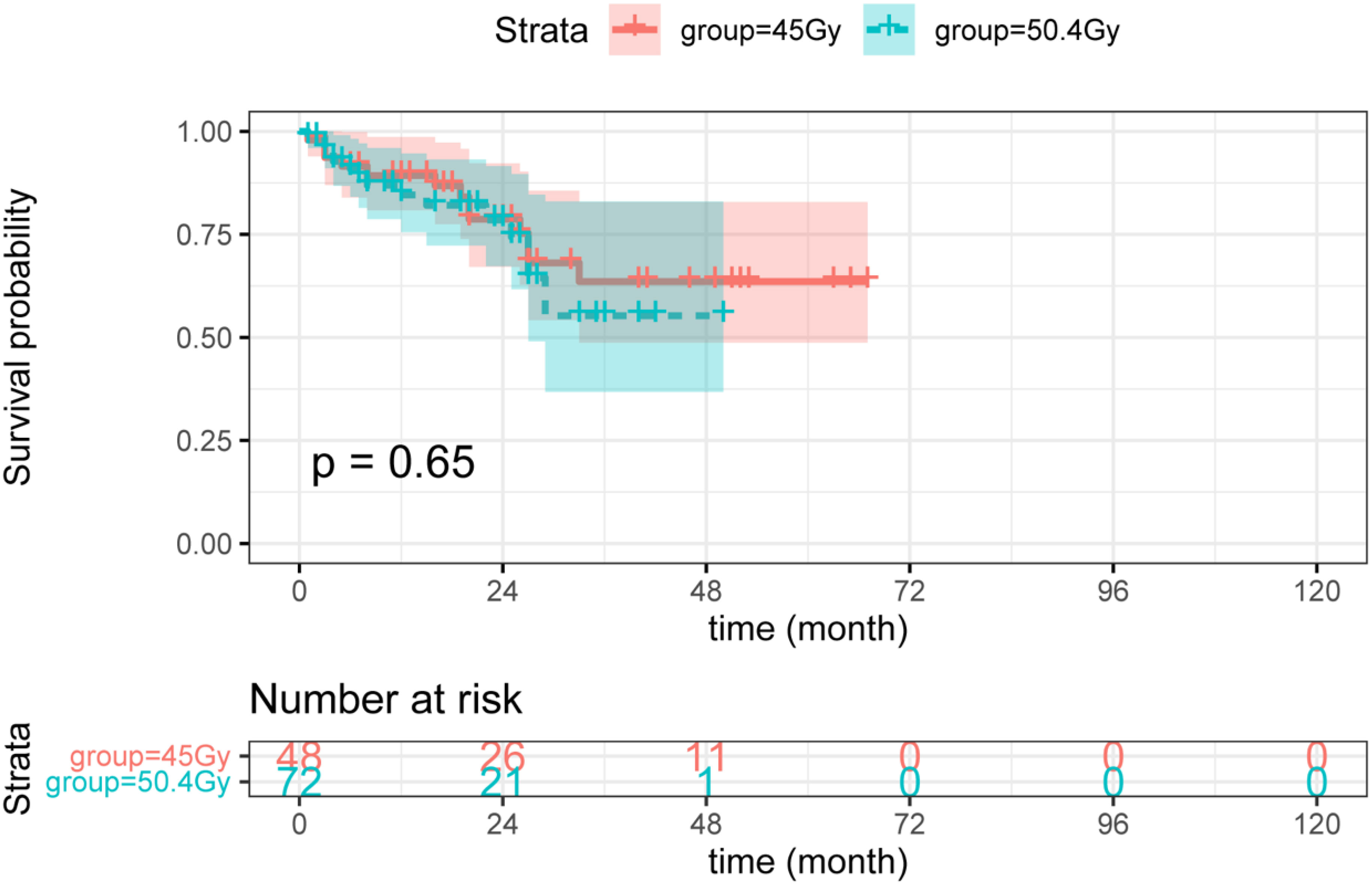
Figure 3 The DFS between LARC patients with different radiation doses. DFS, disease-free survival; LARC, locally advanced rectal cancer.
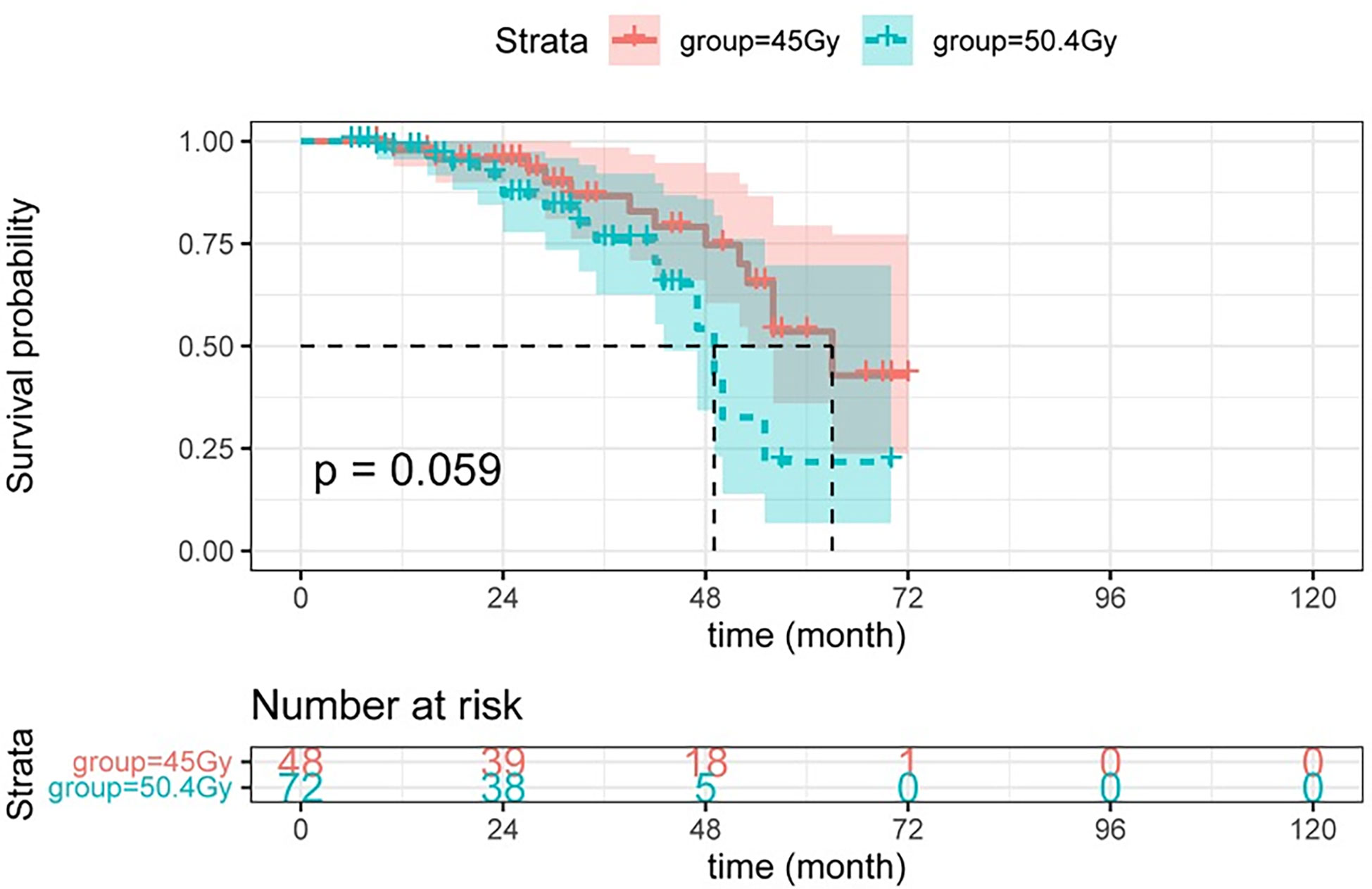
Figure 4 The OS between LARC patients with different radiation doses. OS, overall survival; LARC, locally advanced rectal cancer.
Adverse reactions
CTCAE assessment of myelosuppression and radiation proctitis was evaluated by the RTOG radiation injury classification. The incidence of myelosuppression, radiation proctitis, and intestinal obstruction or perforation in the high-dose group was higher than that in the low-dose group, and the differences were statistically significant. Incidence of narrow lumen, anastomotic fistula, perianal skin injury, emesis, and the hand-foot syndrome showed no significant difference between the two groups. The incidence of specific adverse events is listed in Table 6.
Discussion
In our retrospective study, we found that the survival outcomes of LARC patients treated with 50.4Gy were similar to those of patients treated with 45Gy. In the 50.4Gy group, the rates of pCR and cCR were 19.4% (14 of 72), and 12.5% (9 of 72), respectively, while in the 45Gy group, the rates were 22.9% (11 of 48), and 10.4% (5 of 48), showing no statistical significance. After radiotherapy dose transmutation from 45Gy to 50.4Gy, we observed no improvement in the rate of pCR, while there was a slight improvement in the rate of cCR. The rate of cCR in our study in question was partly based on data from a randomized phase 2 trial, where the rate of clinical complete/near-complete tumor response at MR did not increase after dose escalation from 50Gy to 65Gy (27). Radiotherapy dose intensification was not significant in this study. The statistics from a randomized trial showed that brachytherapy boost supplementation to conventional radiotherapy dose could improve the rate of near-complete response, but not that of pCR (33). Previously reported dose-response relationships may largely be directed by grade 0 and grade 1 (TRG, the AJCC Staging Manual), which could partly explain the rates of pCR in our study.
A radiotherapy dose of 50.4Gy was associated with higher rates of adverse reactions such as radioactive proctitis, myelosuppression, and intestinal obstruction or perforation. In the 45Gy group, 2.1% (1 of 48) of patients experienced intestinal obstruction, while 6.9% (5 of 72) of patients had intestinal obstruction, and 8.3% (6 of 72) had intestinal perforation in the 50.4Gy group. Due to a significant disadvantage relative to the rate of radioactive proctitis, the intestinal perforation rate was higher in the 50.4Gy group than in the 45Gy group. Among the patients in the 45Gy group, 14.6% (7 of 48) had severe myelosuppression compared with 37.5% (27 of 72) in the 50.4Gy (P<0.05). The districts of radiotherapy for LARC generally include the pelvis and pelvic lymph node areas, which exposes to hematological toxicity in the range of 30%-70% and there is a dose-likelihood efficiency (34, 35).
Colorectal cancer is an intractable worldwide public health issue due to its huge disease burden. The prevalence of western lifestyles, dietary changes, and reduced physical activity are the main reasons for the continued rise in colorectal cancer incidence worldwide (36). Multidisciplinary-based treatment is strongly recommended since advancements in diagnostic imaging and an evidence-based combination of chemotherapy, radiotherapy, and TME can markedly improve the prognosis of LARC patients. Especially in the case of a resectable lesion, the integration of chemotherapy and radiotherapy can achieve favorable tumor downstaging and local control rate (37, 38). However, owing to metastasis to other organs or local recurrence, the long-term survival of LARC is unsatisfactory (4, 17). To achieve a better prognosis, imaging (39, 40), carcinoembryonic antigen (CEA) combined with carbohydrate antigen 19-9 (CA19-9) (41), platelet-associated biomarkers (42), and circulating tumor DNA (ctDNA) (43) must be dynamically estimated and promptly evaluated. A more personalized treatment regimen is preferable for high risk patients, and novel combination regimens should be further investigated.
SCRT, which is the conventional treatment in European countries, is developing rapidly. Generally, patients receive pelvic radiotherapy at a dose of 5×5Gy during the first week, followed by surgical intervention and six sessions of adjuvant chemotherapy. Interestingly, no significant difference in recurrence rates, distant metastasis, or late adverse events compared to long-term radiotherapy was observed (44). Moreover, a single-arm phase II clinical research (45) concluded that SCRT followed by chemotherapy plus immunotherapy and surgery demonstrated an impressive pCR rate with good tolerance in patients. Furthermore, SCRT treatment resulted in fewer late adverse events and rectal injury (46, 47). However, another randomized trial concluded that SCRT with delayed surgery was associated with an increased risk of local recurrence after a 10-year follow-up period (48).
Given the association between treatment intensification-tumor response and tumor prognosis, more consolidated treatment options are needed. TNT-chemotherapy combined with chemoradiotherapy before surgery-is a novel treatment approach for LARC patients, achieving improved downstaging, patient compliance, and micrometastases elimination rate (49–51). TNT is a promising systemic strategy to target micrometastases, especially for patients unfit for surgery (49). The pCR rate in patients treated with TNT (36%) was found to be higher compared to patients receiving nCRT (21%) (49). Patients who achieved pCR may choose non-operative treatment, sphincter-sparing surgery, or observation and periodic review. Nowadays, chemotherapy is administrated before or after radiotherapy, and the sequence of radiotherapy, chemotherapy and surgery has been extensively explored. In general, INCT combined with CRT is a preferred method since it is associated with better compliance and fewer acute adverse reactions (13, 50, 52). In our study, all patients underwent two cycles of induction chemotherapy and fluorouracil-based chemoradiotherapy.
Cancer is more averse to becoming a “chronic disease” as medical technology advances and therapeutic methods evolve. Functional needs and survival needs (46) are two central issues we should aim to address in future treatment prospects of rectal cancer. Our goal at the moment is to achieve a complete resection of the lesion while sparing the functioning sphincter complex of the anus, thereby improving the quality of life of patients. Notably, surgical improvements have helped reduce the local recurrence rate from above 50% to below 10% (53, 54). Herein, we noticed a significantly higher rate of anal retention in the 50.4Gy group compared to the 45Gy group (79.2% (57 of 72) vs. 60.4% (29 of 48)).
CRM and EMVI are important factors that predict survival outcomes and contribute to clinical treatment planning (55, 56). As a result, no significant difference between the two groups was observed. The survival curve in this study was not statistically significant, but the 45Gy group had a higher 80-month survival rate, which may be related to the higher incidence of adverse reactions in the 50.4Gy group, particularly intestinal obstruction or perforation and myelosuppression. Furthermore, according to our findings, a radiotherapy dose of 50.4Gy resulted in a favorable anal retention rate but at the expense of increased rates of several adverse reactions, with no improvement in the rate of good pathological response, DFS or OS.
Nevertheless, our study has several limitations. We included a small number of patients from a single center, and the follow-up time was not long enough to obtain long-term survival statistics. Moreover, our study was retrospective in nature, which may have resulted in bias to some extent. Nonetheless, to our knowledge, this is the first study that concluded the rate of pCR in the 45Gy group was higher than that of the 50.4Gy group. This study has clinical guiding significance and, to some extent, provides the basis for choosing radiotherapy doses in LARC patients. A radiotherapy dose of 50.4Gy is preferred for a higher likelihood of anal retention if a patient-centered outcome is prioritized. Meanwhile, if an efficacy-centered outcome is preferred, a radiotherapy dose of 45Gy is desired for a greater degree of pCR and a lower likelihood of adverse reactions. Combined with the trend of individualized treatment of the tumor, the radiotherapy dose needs to be considered according to the tolerance of the patient, which includes age, ECOG PS, and underlying disease. In the follow-up treatment, the efficacy and quality of life are the focus of doctors.
Conclusion
A radiation dose of 50.4Gy contributes to a better anal retention rate but at the cost of serious adverse events and failure to improve the rate of good pathological response, imaging remission, DFS or OS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
(I) Conception and design: XZ, CZ; (II) Administrative support: HZ; (III) Provision of study materials or patients: YX, ZS; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: XR; (VI) Manuscript writing: All authors. (VII) All authors contributed to the article and approved the submitted version.
Funding
The research was funded by Wenzhou Science Technology Bureau (grant number Y20180220) and Zhejiang Medical Association (grant number 2022ZYC-Z27).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LARC, Locally advanced rectal cancer; nCRT, Neoadjuvant chemoradiotherapy; TME, Total mesorectum excision; OS, Overall survival; NCCN, National Comprehensive Cancer Network; AJCC, American Joint Committee on Cancer; DMFS, Distant metastasis free survival; PFS, progression-free survival; IMRT, Intensity-modulated radiation; RTOG, Radiation Therapy Oncology Group; GTV, Gross tumor volume; CTV, Clinical target volume; PTV, Planning target volume; XELOX regimen, Oxaliplatin in combination with capecitabine; pCR, Pathological complete response; DFS, Disease-free survival; CTCAE, Common Terminology Criteria for Adverse Events; MR, magnetic resonance; CR, Complete response; PR, Partial response; SD, Stable disease; PD, Progressive disease; DCR, Disease control rate; CRM, Circumferential resection margin; EMVI, Extramural venous invasion; SCRT, Short-course radiotherapy.
References
1. Ferlay J, Colombet M, Soerjomataram I, Parkin D, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer (2021). doi: 10.1002/ijc.33588
2. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal cancer trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol (2005) 23(24):5644–50. doi: 10.1200/JCO.2005.08.144
3. Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2015) 16(8):979–89. doi: 10.1016/S1470-2045(15)00159-X
4. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol (2012) 30(16):1926–33. doi: 10.1200/JCO.2011.40.1836
5. van der Valk M, Marijnen C, van Etten B, Dijkstra E, Hilling D, Kranenbarg E, et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer - results of the international randomized RAPIDO-trial. Radiother Oncol (2020) 147:75–83. doi: 10.1016/j.radonc.2020.03.011
6. Bosset J, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results–EORTC 22921. J Clin Oncol (2005) 23(24):5620–7. doi: 10.1200/JCO.2005.02.113
7. Bosset J, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P, et al. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. report of the 22921 randomised trial conducted by the EORTC radiotherapy group. Eur J Cancer (Oxford Engl 1990) (2004) 40(2):219–24. doi: 10.1016/j.ejca.2003.09.032
8. Gérard J, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin M, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol (2006) 24(28):4620–5. doi: 10.1200/JCO.2006.06.7629
9. Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: Long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol (2022) 8(1):e215445. doi: 10.1001/jamaoncol.2021.5445
10. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6
11. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol (2022) 40(23):2546–56. doi: 10.1200/JCO.22.00032
12. Rahma O, Yothers G, Hong T, Russell M, You Y, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: Initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol (2021) 7(8):1225–30. doi: 10.1001/jamaoncol.2021.1683
13. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP r-03. J Clin Oncol (2009) 27(31):5124–30. doi: 10.1200/JCO.2009.22.0467
14. Sanghera P, Wong DW, McConkey CC, Geh JI, Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol) (2008) 20(2):176–83. doi: 10.1016/j.clon.2007.11.013
15. Mo S, Dai W, Xiang W, Huang B, Li Y, Feng Y, et al. Survival contradiction between stage IIA and stage IIIA rectal cancer: A retrospective study. J Cancer (2018) 9(8):1466–75. doi: 10.7150/jca.23311
16. Huang Q, Qin H, Xiao J, He X, Xie M, He X, et al. Association of tumor differentiation and prognosis in patients with rectal cancer undergoing neoadjuvant chemoradiation therapy. Gastroenterol Rep (Oxf) (2019) 7(4):283–90. doi: 10.1093/gastro/goy045
17. Glimelius B. Multidisciplinary treatment of patients with rectal cancer: Development during the past decades and plans for the future. Upsala J Med Sci (2012) 117(2):225–36. doi: 10.3109/03009734.2012.658974
18. Lichthardt S, Wagner J, Löb S, Matthes N, Kastner C, Anger F, et al. Pathological complete response due to a prolonged time interval between preoperative chemoradiation and surgery in locally advanced rectal cancer: analysis from the German StuDoQ|Rectalcarcinoma registry. BMC cancer (2020) 20(1):49. doi: 10.1186/s12885-020-6538-8
19. Stijns R, de Graaf E, Punt C, Nagtegaal I, Nuyttens J, van Meerten E, et al. Long-term oncological and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer: The CARTS study. JAMA surg (2019) 154(1):47–54. doi: 10.1001/jamasurg.2018.3752
20. Hofheinz R, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann J, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol (2012) 13(6):579–88. doi: 10.1016/S1470-2045(12)70116-X
21. Ng S, Chu J, Chander S, Bressel M, McKendrick J, Wong R, et al. Results of phase II trial of intensified neoadjuvant treatment with interdigitating radiotherapy and chemotherapy with oxaliplatin, 5-fluorouracil and folinic acid in patients with locally advanced rectal cancer (PROARCT trial). Radiother Oncol (2021) 155:27–32. doi: 10.1016/j.radonc.2020.10.012
22. Sun Y, Lin Y, Liu Z, Jiang W, Chi P. Combined laparoscopic lymphoadenectomy of lateral pelvic and inguinal nodal metastases using indocyanine green fluorescence imaging guidance in low rectal cancer after preoperative chemoradiotherapy: a case report. BMC gastroenterol (2022) 22(1):123. doi: 10.1186/s12876-022-02193-1
23. Schellenberg A, Moravan V, Christian F. A competing risk analysis of colorectal cancer recurrence after curative surgery. BMC gastroenterol (2022) 22(1):95. doi: 10.1186/s12876-022-02161-9
24. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(suppl_4):iv22–40. doi: 10.1093/annonc/mdx224
25. Delishaj D, Fumagalli IC, Ursino S, Cristaudo A, Colangelo F, Stefanelli A, et al. Neoadjuvant radiotherapy dose escalation for locally advanced rectal cancers in the new era of radiotherapy: A review of literature. World J Clin Cases (2021) 9(30):9077–89. doi: 10.12998/wjcc.v9.i30.9077
26. Alongi F, Fersino S, Mazzola R, Fiorentino A, Giaj-Levra N, Ricchetti F, et al. Radiation dose intensification in pre-operative chemo-radiotherapy for locally advanced rectal cancer. Clin Transl Oncol (2017) 19(2):189–96. doi: 10.1007/s12094-016-1522-0
27. Couwenberg AM, Burbach JPM, Berbee M, Lacle MM, Arensman R, Raicu MG, et al. Efficacy of dose-escalated chemoradiation on complete tumor response in patients with locally advanced rectal cancer (RECTAL-BOOST): A phase 2 randomized controlled trial. Int J Radiat Oncol Biol Phys (2020) 108(4):1008–18. doi: 10.1016/j.ijrobp.2020.06.013
28. Bertocchi E, Barugola G, Nicosia L, Mazzola R, Ricchetti F, Dell'Abate P, et al. A comparative analysis between radiation dose intensification and conventional fractionation in neoadjuvant locally advanced rectal cancer: A monocentric prospective observational study. Radiol Med (2020) 125(10):990–8. doi: 10.1007/s11547-020-01189-9
29. Schmoll H, Stein A, Van Cutsem E, Price T, Hofheinz R, Nordlinger B, et al. Pre- and postoperative capecitabine without or with oxaliplatin in locally advanced rectal cancer: PETACC 6 trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J Clin Oncol (2021) 39(1):17–29. doi: 10.1200/JCO.20.01740
30. Gérard J, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne P, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-prodige 2. J Clin Oncol (2010) 28(10):1638–44. doi: 10.1200/JCO.2009.25.8376
31. Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol (2011) 29(20):2773–80. doi: 10.1200/JCO.2010.34.4911
32. Trakarnsanga A, Gönen M, Shia J, Nash G, Temple L, Guillem J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Institute (2014) 106(10):2773–2780. doi: 10.1093/jnci/dju248
33. Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys (2012) 84(4):949–54. doi: 10.1016/j.ijrobp.2012.02.006
34. Kuntz L, Noel G. [Pelvic irradiation and hematopoietic toxicity: A review of the literature]. Cancer Radiother (2021) 25(1):77–91. doi: 10.1016/j.canrad.2020.05.018
35. Sini C, Fiorino C, Perna L, Noris Chiorda B, Deantoni CL, Bianchi M, et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol (2016) 118(1):79–84. doi: 10.1016/j.radonc.2015.11.020
36. Dekker E, Tanis P, Vleugels J, Kasi P, Wallace M. Colorectal cancer. Lancet (London England) (2019) 394(10207):1467–80. doi: 10.1016/S0140-6736(19)32319-0
37. Valentini V, Glimelius B, Haustermans K, Marijnen CA, Rödel C, Gambacorta MA, et al. EURECCA consensus conference highlights about rectal cancer clinical management: the radiation oncologist's expert review. Radiother Oncol (2014) 110(1):195–8. doi: 10.1016/j.radonc.2013.10.024
38. Lupattelli M, Matrone F, Gambacorta MA, Osti M, Macchia G, Palazzari E, et al. Preoperative intensity-modulated radiotherapy with a simultaneous integrated boost combined with capecitabine in locally advanced rectal cancer: short-term results of a multicentric study. Radiat Oncol (2017) 12(1):139. doi: 10.1186/s13014-017-0870-4
39. Wan L, Peng W, Zou S, Ye F, Geng Y, Ouyang H, et al. MRI-Based delta-radiomics are predictive of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Acad Radiol (2021) 28 Suppl 1:S95–s104. doi: 10.1016/j.acra.2020.10.026
40. Caruso R, Vicente E, Quijano Y, Duran H, Fabra I, Diaz E, et al. Role of 18F-PET-CT to predict pathological response after neoadjuvant treatment of rectal cancer. Discovery Oncol (2021) 12(1):16. doi: 10.1007/s12672-021-00405-w
41. Zheng Z, Wang X, Lu X, Huang Y, Chi P. Prognostic significance of carcinoembryonic antigen combined with carbohydrate antigen 19-9 following neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Colorectal Dis (2021) 23(9):2320–30. doi: 10.1111/codi.15694
42. Wang P, Wang Z, Liu Y, Xie J, Ren Y. Prognostic value of platelet-associated biomarkers in rectal cancer patients received neoadjuvant chemoradiation: A retrospective study. Cancer Radiother (2021) 25(2):147–54. doi: 10.1016/j.canrad.2020.06.030
43. McDuff SGR, Hardiman KM, Ulintz PJ, Parikh AR, Zheng H, Kim DW, et al. Circulating tumor DNA predicts pathologic and clinical outcomes following neoadjuvant chemoradiation and surgery for patients with locally advanced rectal cancer. JCO Precis Oncol (2021) 5. doi: 10.1200/PO.20.00220
44. Ngan S, Burmeister B, Fisher R, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman radiation oncology group trial 01.04. J Clin Oncol (2012) 30(31):3827–33. doi: 10.1200/JCO.2012.42.9597
45. Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J immunother Cancer (2021) 9(11):147–154. doi: 10.1136/jitc-2021-003554
46. Ahmed K, Correa C, Dilling T, Rao N, Shridhar R, Trotti A, et al. Altered fractionation schedules in radiation treatment: A review. Semin Oncol (2014) 41(6):730–50. doi: 10.1053/j.seminoncol.2014.09.012
47. Michalski J, Gay H, Jackson A, Tucker S, Deasy J. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat oncol biol phys (2010) 76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078
48. Pach R, Sierzega M, Szczepanik A, Popiela T, Richter P. Preoperative radiotherapy 5×5 gy and short versus long interval between surgery for resectable rectal cancer: 10-year follow-up of the randomised controlled trial. Radiother Oncol (2021) 9(11). doi: 10.1016/j.radonc.2021.10.006
49. Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol (2018) 4(6):e180071. doi: 10.1001/jamaoncol.2018.0071
50. Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol (2015) 26(8):1722–8. doi: 10.1093/annonc/mdv223
51. Cercek A, Goodman KA, Hajj C, Weisberger E, Segal NH, Reidy-Lagunes DL, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw (2014) 12(4):513–9. doi: 10.6004/jnccn.2014.0056
52. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol (2019) 37(34):3212–22. doi: 10.1200/JCO.19.00308
53. Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the county of Stockholm. Stockholm Colorectal cancer study group, Basingstoke bowel cancer research project. Lancet (2000) 356(9224):93–6. doi: 10.1016/S0140-6736(00)02469-7
54. Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: Anterior vs. abdominoperineal resection. Dis Colon Rectum (2004) 47(1):48–58. doi: 10.1007/s10350-003-0012-y
55. Taylor F, Quirke P, Heald R, Moran B, Blomqvist L, Swift I, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol (2014) 32(1):34–43. doi: 10.1200/JCO.2012.45.3258
Keywords: locally advanced rectal cancer, radiotherapy dose, intensity-modulated radiation therapy, neoadjuvant chemoradiotherapy, adverse reactions
Citation: Xu Y, Zou H, Shao Z, Zhang X, Ren X, He H, Zhang D, Du D and Zou C (2023) Efficacy and safety of different radiotherapy doses in neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: A retrospective study. Front. Oncol. 13:1119323. doi: 10.3389/fonc.2023.1119323
Received: 08 December 2022; Accepted: 06 February 2023;
Published: 21 February 2023.
Edited by:
Marco Rengo, Sapienza University of Rome, ItalyReviewed by:
Francesco Ricchetti, Sacro Cuore Don Calabria Hospital, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyJesus C. Fabregas, University of Florida, United States
Copyright © 2023 Xu, Zou, Shao, Zhang, Ren, He, Zhang, Du and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changlin Zou, emNsMTk2NzAxMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yuyan Xu
Yuyan Xu Haizhou Zou
Haizhou Zou Zhenyong Shao1
Zhenyong Shao1 Changlin Zou
Changlin Zou