- 1Department of Pathology, University of Iowa Hospitals and Clinics, Iowa City, IA, United States
- 2Faculty of Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 3Department of Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 4Department of Otolaryngology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 5Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
Introduction: We investigated the clinicopathological features and prognoses of the new molecularly defined entities in latest edition of the World Health Organization (WHO) classification of sinonasal carcinoma (SNC)
Methods: Integrated data were combined into an individual patient data (IPD) meta-analysis.
Results: We included 61 studies with 278 SNCs including 25 IDH2-mutant, 41 NUT carcinoma, 187 SWI/SNF loss, and 25 triple negative SNCs (without IDH2 mutation, NUTM1 rearrangement, and SWI/SNF inactivation) for analyses. Compared to other molecular groups, NUT carcinoma was associated with a younger age at presentation and an inferior disease-specific survival. Among SNCs with SWI/SNF inactivation, SMARCB1-deficient tumors presented later in life and were associated with a higher rate of radiotherapy administration. SMARCA4-deficiency was mostly found in teratocarcinosarcoma while SMARCB1-deficient tumors were associated with undifferentiated carcinoma and non-keratinizing squamous cell carcinoma.
Conclusion: Our study facilitates our current understanding of this developing molecular-defined spectrum of tumors and their prognoses.
Introduction
Sinonasal carcinomas (SNC) are rare malignancies and are usually associated with poor outcomes. In the previous editions of the World Health Organization (WHO) Classification, sinonasal malignancies were mainly classified as conventional squamous cell carcinoma (SCC), non-keratinizing SCC, intestinal-type adenocarcinoma (ITAC), non-ITAC, neuroendocrine carcinoma (NEC), poorly differentiated carcinoma (PDCA), sinonasal undifferentiated carcinoma (SNUC), and other rare subtypes (1). The 2022 5th edition of the WHO Classification of the Head and Neck has made significant classification revisions, with newly added molecular groups for SNC as compared to previous versions (1).
SWI/SNF complex-deficient carcinomas, defined by loss of one of the SWI/SNF complex genes, include two major subtypes: SMARCB1- and SMARCA4-deficient sinonasal carcinoma (2–4). Most of these cases were previously misdiagnosed as PDCA, SNUC, NEC, or teratocarcinosarcoma (TCS). Mutations in IDH2 have also been recently described in a subset of PDCA and SNUC (5, 6). Tumors with these mutations are generally associated with better outcomes relative to those without IDH2 mutations (7, 8); however, results to the contrary have also been reported (9). Because of the rarity of these new entities, we lack a detailed understanding of the clinicopathological features and prognoses between them. This meta-analysis aimed to investigate the clinicopathological characteristics and survival patterns of SWI/SNF-deficient and IDH2-mutant tumors in comparison to the previously described NUT midline carcinoma of the sinonasal tract.
Materials and methods
Literature search and search term
Relevant articles were found by searching three electronic databases including PubMed, Web of Science, and Scopus from their inception to September 2022. We used the following search terms: (sinonasal OR nasal OR paranasal) AND (carcinoma OR cancer) AND (SMARCB1 OR SMARCB-1 OR SMARCB 1 OR INI1 OR INI 1 OR INI-1 OR SMARCA4 OR SMARCA-4 OR SMARCA 4 OR BRG1 OR BRG-1 OR BRG 1 OR SWI/SNF OR NUT OR isocitrate OR IDH1/2 OR IDH2). We carefully reviewed the reference list of potential articles to avoid missing important data. This study protocol strictly followed the recommendations of Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement (10).
Selection criteria, abstract/full text screening
For abstract screening, two independent teams (HGV, TL, TTBL, and HTL) reviewed the titles and abstracts of included articles. Studies were included if they are observational studies and report individual patient data (IPD) of SMARCB1-deficient, SMARCA4-deficient, NUT midline, and IDH2-mutant carcinoma of the sinonasal tract. We excluded studies if they are (i) reviews, (ii) conference abstracts or conference papers, (iii) books, (iv) without IPD, and (iv) duplicated data.
Following this step, two independent teams read all full texts of potential studies and extracted data into a standardized worksheet. The following data were collected: author names, institution, city, country, publication year, number of patients, age, gender, clinicopathological information (e.g., tumor location, largest diameter, tumor extension, nodal/distant metastases, TNM stage, original histological diagnosis, number of mitoses per 10 high-power filed, Ki67 index), treatments administered, progression-free survival (PFS), and disease-specific survival (DSS).
Statistical analysis
We divided data into four main groups: SWI/SNF loss, NUT carcinoma, IDH2-mutant, and those without SWI/INF deficiency, NUTM1 fusion, and IDH2 mutation (triple negative group). We excluded cases that were absent the NUTM1 rearrangement and SWI/SNF loss but missing information on IDH2 status. For SWI/INF-deficient tumors, we also compared the SMARCB1-deficient versus SMARCA4-deficient carcinomas. We used Chi-squared and Fisher’s exact test for comparison of categorical variables while t-test, Wilcoxon rank sum test, or analysis of variance (ANOVA) were utilized for continuous covariates, if applicable. The R program (The R Foundation, Vienna, Austria) was used for statistical analyses.
Results
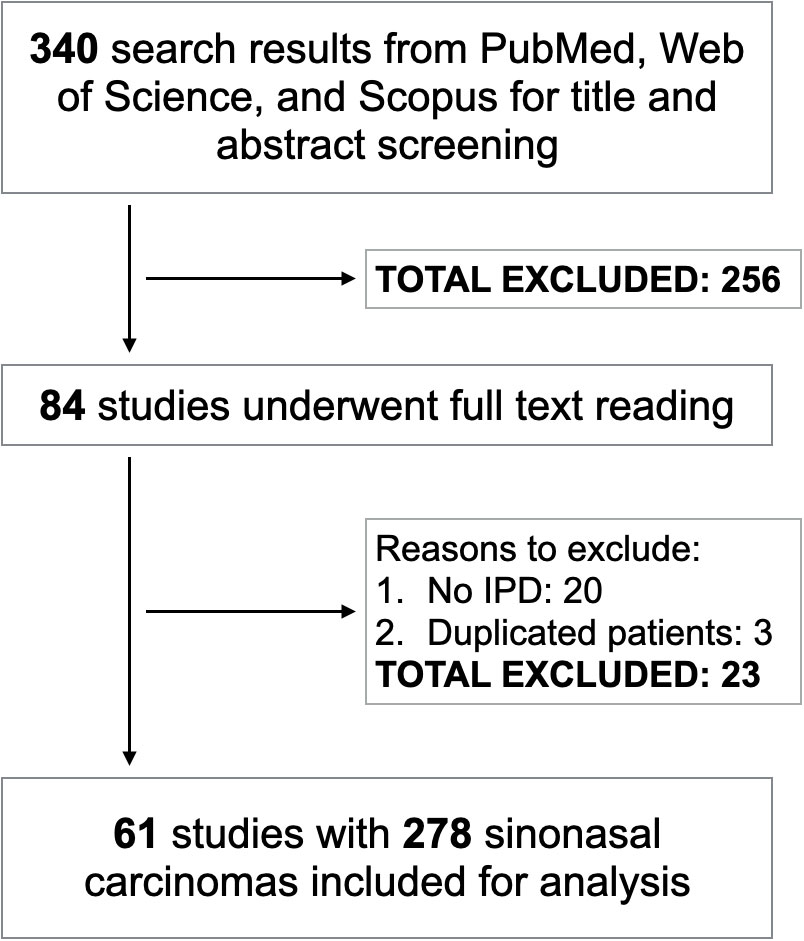
After merging search results from three electronic databases and removing the duplicates, we had 340 studies for title and abstract screening. Following this step, 84 articles were selected for full-text reading. Sixty-one of them met inclusion criteria corresponding to 278 SNCs which were included for analysis (2–5, 9, 11–66) (Figure 1). There were 25 IDH2-mutant, 41 NUT carcinoma, 187 SWI/SNF loss, and 25 triple negative SNCs. Among NUT carcinoma, BRD4:NUTM1 was the most common variant and only one case harbored BRD3:NUTM1 rearrangement. The R172 variant was the most predominant IDH2-mutant genotype. Regarding SNCs with inactivation of one of the SWI/SNF complex genes, SMARCB1-deficient carcinoma was the most frequent subtype followed by SMARCA4-deficient tumors. Loss of SMARCA1, SMARCA5, and SMARCE1 were also found in one SNC case each. IDH2 mutations, NUTM1 rearrangement, and inactivation of SWI/SNF complex were mutually exclusive with each other.
Clinicopathological features and treatment patterns of molecular groups of SNCs
Table 1 describes the clinicopathological and therapeutic parameters of different molecular groups of SNCs. Compared to IDH2-mutant, SWI/SNF loss, and triple negative groups, NUT carcinoma presented a significantly younger age (p < 0.001). Most IDH2-mutant SNCs were originally diagnosed as SNUC whereas the diagnosis of NUT carcinoma is usually more straightforward. SWI/SNF-loss SNCs were commonly misdiagnosed as SNUC, SCC, or TCS. Lymph node metastases were generally uncommon in SNCs whereas distant metastases were more frequently observed among all molecular groups.
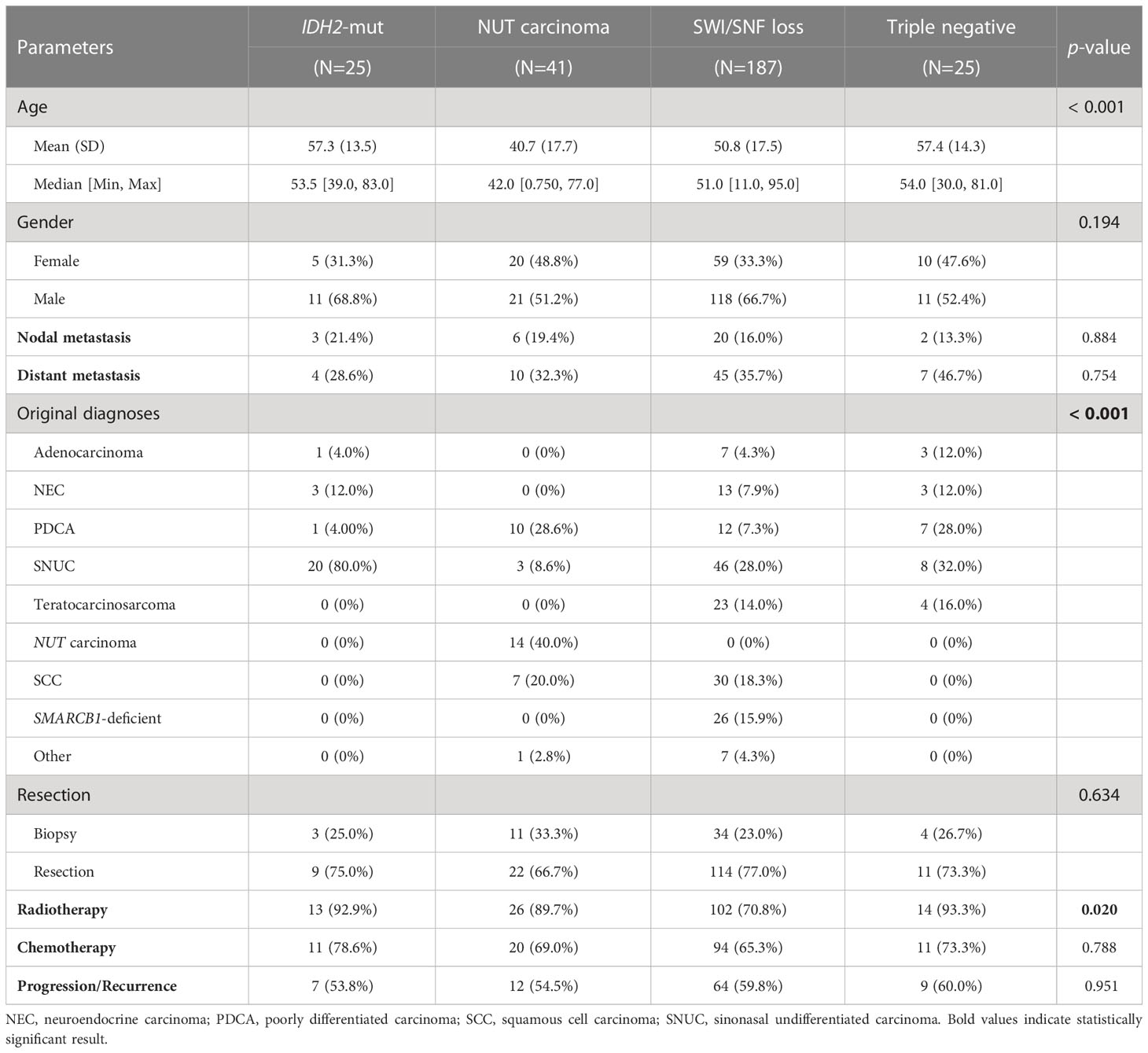
Table 1 Clinicopathological characteristics and treatment patterns of different molecular subgroups of SNCs.
Regarding treatments, SWI/SNF-loss SNCs were less likely to receive radiotherapy as compared to other groups (p = 0.020). The rate of nodal metastasis, distant metastasis, surgical resection, and chemotherapy administration were statistically comparable between the four groups.
We also sought to investigate the similarities and differences between SMARCA4-deficient versus SMARCB1-deficient SNCs (Table 2). SMARCA4-deficient SNCs presented at a significantly younger age compared to SMARCB1-deficient (median, 42.0 versus 53.0). A subset of SMARCA4-deficient SNCs had overlapping histopathological findings with TCS whereas SMARCB1-deficient were distributed in more diverse histological diagnoses. Radiotherapy administration was more commonly used for SMARCB1-deficient tumors.
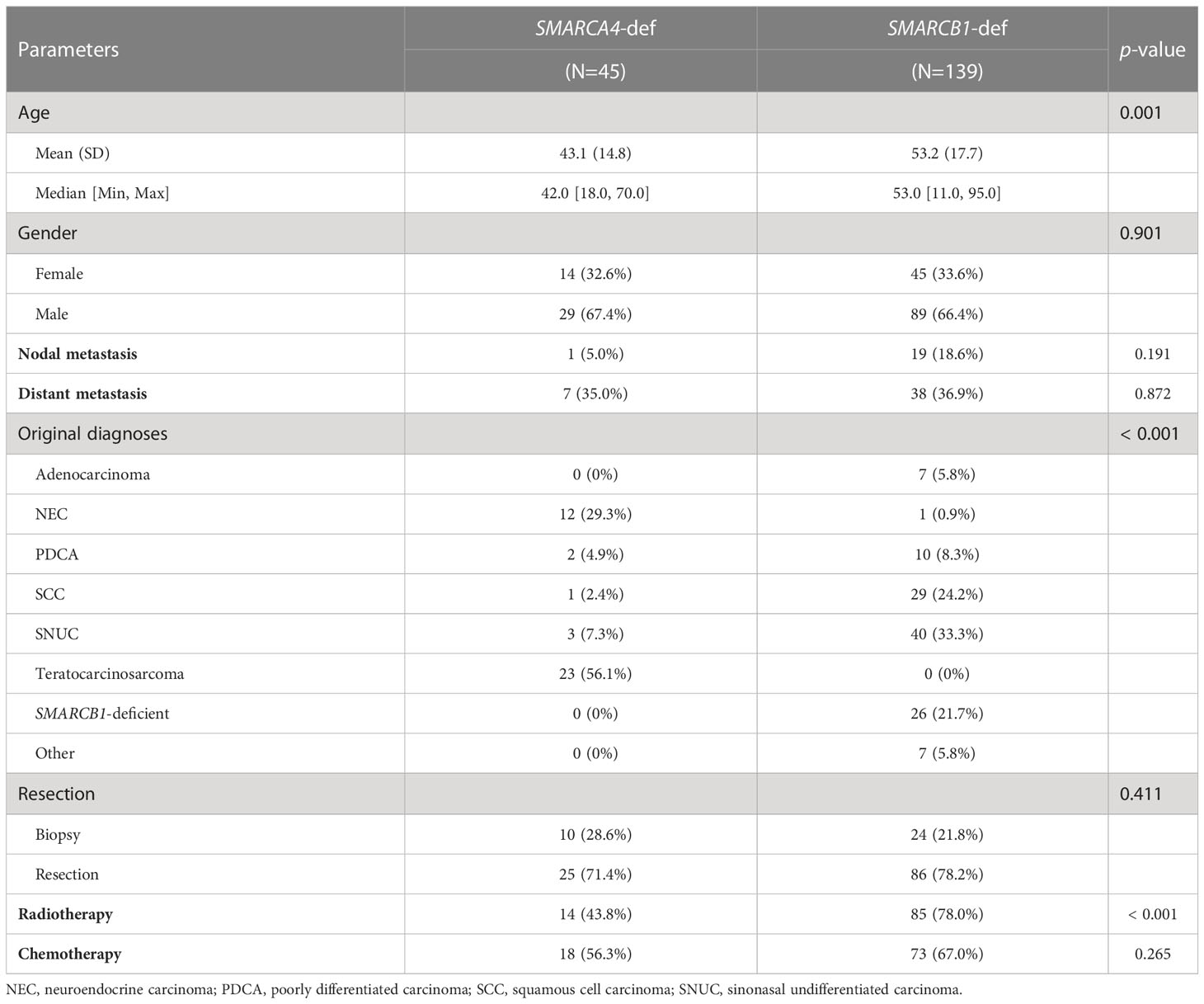
Table 2 Clinicopathological features and treatment patterns of SMARCA4-deficient versus SMARCB1-deficient SNCs.
Metastatic patterns of SNCs
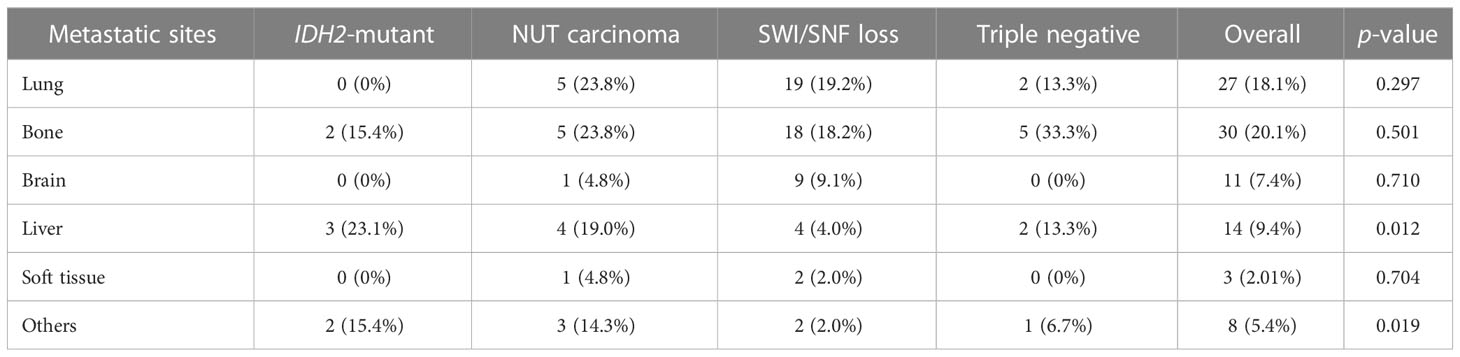
Bone and lung were the two most common metastatic sites for SNCs. We found significant different metastatic patterns of IDH2-mutant SNCs as compared to other groups. No IDH2-mutant SNCs metastasized to lung and most of these tumors had a metastatic preference to liver and other rare organs (e.g., adrenal glands, mediastinum) (Table 3).
Prognoses of molecular groups of SNCs
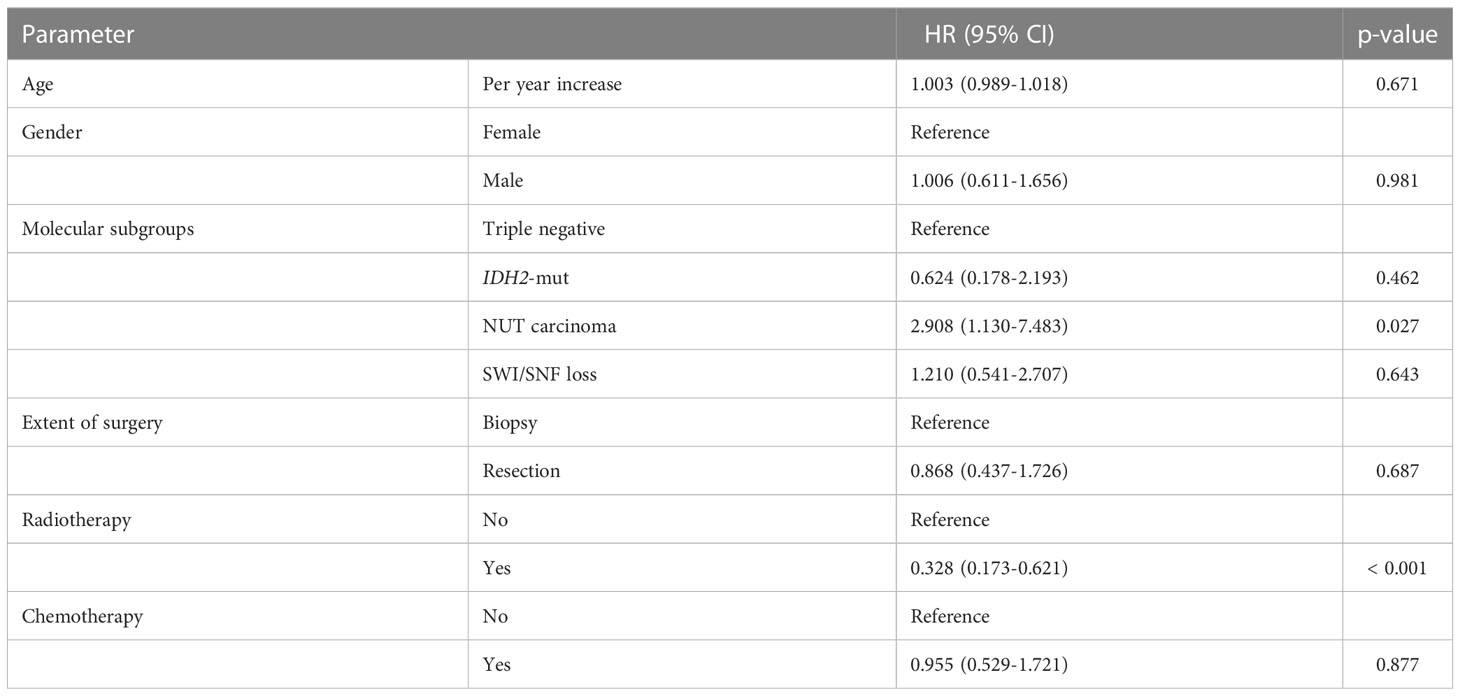
SNCs were associated with high-risk for local relapse and tumor progression during follow-up. We could not calculate and compare the PFS between the molecular groups due to high rate of missing data. Kaplan-Meier analyses demonstrated that IDH2-mutant and triple negative SNCs have a more favorable DSS compared to NUT carcinoma (p = 0.014) (Figure 2A). The DSS was not statistically different between SMARCA4-deficient versus SMARCB1- deficient SNCs (Figure 2B). In a multivariate Cox regression model, NUT carcinoma and no radiotherapy administration were prognostic indicators for poor prognosis (Table 4).
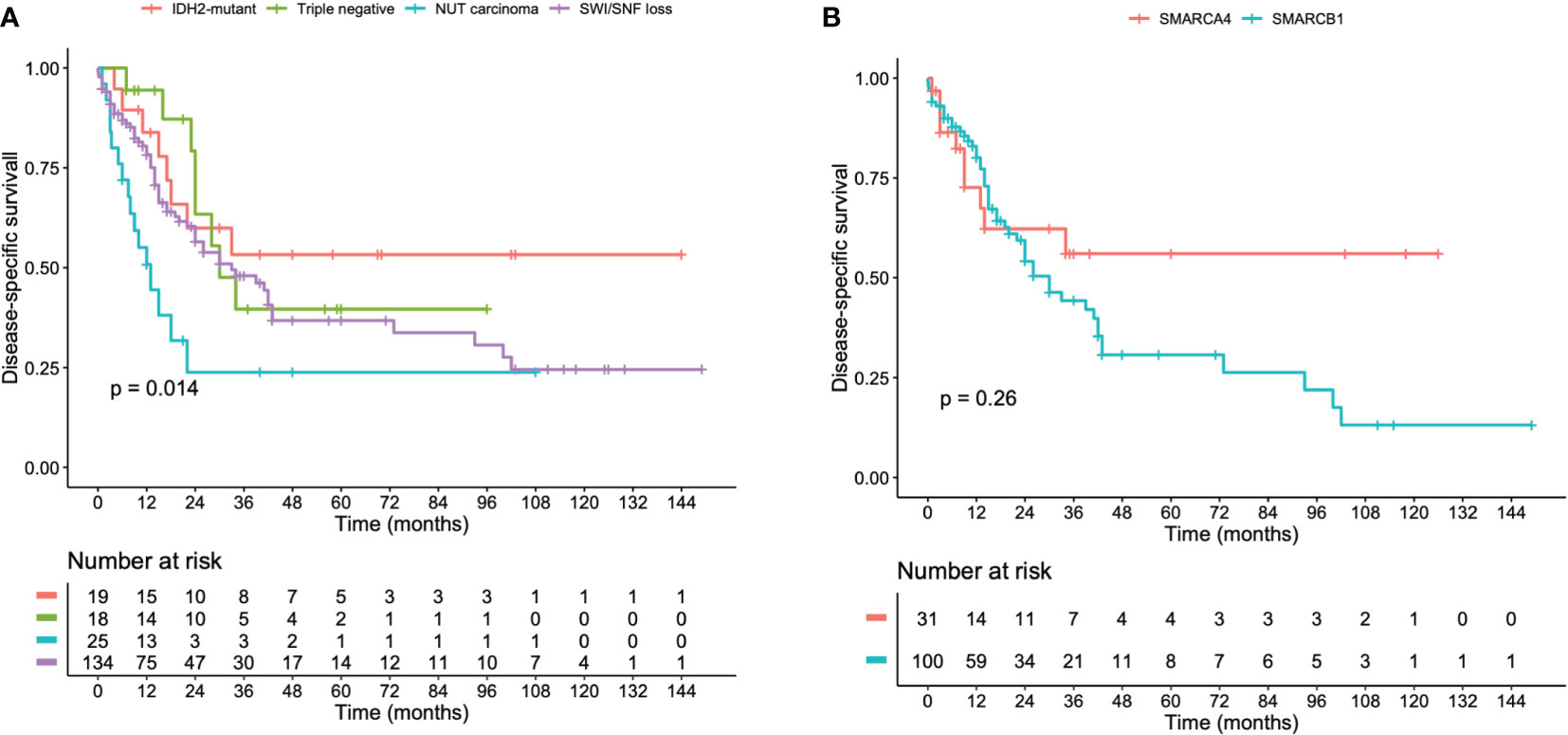
Figure 2 Kaplan-Meier curves illustrating the DSS of (A) IDH2-mutant, NUT midline, SWI/SNF-loss, and triple negative sinonasal carcinomas. (B) SMARCA4-deficient and SMARCB1-deficient sinonasal carcinomas.
Discussion
In recent years, new molecular profiles of SNCs have been further clarified and novel molecular groups have been incorporated into the latest WHO classification of SNCs (1–6). Prior to the molecular era, most IDH2-mutant, NUT midline, and SWI/SNF-deficient SNCs were categorized as SNUC, PDCA, TCS, or NEC (2, 3, 5, 33). In the latest WHO classification of head and neck tumors, NUT midline, SMARCA4-deficient, and SMARCB1-deficient SNCs have been recognized as separate entities. Given their distinct clinicopathological features and prognoses as compared to IDH2-wild type tumors (8), IDH2-mutant SNCs may nevertheless be regarded as a distinct molecular group in future WHO editions. Because of the rarity of SNCs, most data were presented as case reports or small- to medium-sized case series. The clinicopathological features and prognostic outcomes of new molecular groups of SNCs have been described. However, it is still controversial as to how these tumors are different from each other and in how clinicians can better assess patient outcomes. In this study, we integrated IPD of published studies into a meta-analysis to improve the statistical implication compared to cohort studies with limited sample size.
Our results showed that these tumors were uniformly high-grade and distributed in diverse histopathological spectrums with SNUC and PDCA being the most common variants. All molecular groups of SNCs had a relatively considerable risk for tumor metastases to distant organs with bone and lung being the most common sites. We found that IDH2-mutant SNCs were most likely to metastasize to liver and other rare organs compared to other groups. Like prior studies, our meta-analysis demonstrated improved survival of IDH2-mutant SNCs as compared to those without these mutations (5, 6, 8). The prognostic implication of IDH1/2 mutations in gliomas, chondrosarcoma, and cholangiocarcinoma have similarly been established (67–70). The discovery of IDH2 mutation in SNCs provides a promising opportunity for targeted therapy with IDH inhibitors. Most IDH2-mutant SNCs occur in codon 172 and can be diagnosed by immunohistochemistry assay which is an accessible, rapid, and inexpensive method (8, 31).
This meta-analysis also highlighted that SNCs usually present at older age except for NUT carcinoma, which is more commonly seen in young adults and pediatric patients. NUT carcinoma is exceedingly rare, typically occurs in the midline structures, and histopathologically resembles PDCA. This tumor is characterized by a chromosomal rearrangement involving NUTM1 gene (32). The availability of NUT immunohistochemistry antibody has improved the accuracy of NUT midline carcinoma diagnosis and differentiated them from other PDCA. NUT midline carcinoma is associated with high rates of mortality (71) and our study further confirmed the uniformly poor prognosis of these tumors compared to other genetic groups of SNCs.
The most common genetically defined group of SNCs involves the SWI/SNF complex genes with loss of SMARCB1 and SMARCA4 being the most common variants. It is still poorly understood regarding how these subtypes are different from each other. SMARCB1-loss SNC is associated with rhabdoid differentiation in SNUC (2), which is an important diagnostic parameter to differentiate them from other PDCA. On the other hand, recurrent loss of SMARCA4 is commonly observed in TCS (3) and SNCs with neuroendocrine differentiation (4). Our analyses further confirmed these histopathological associations. We also found that SMARCB1-deficient SNCs occur at a significantly older age and more likely to have radiotherapy administration in comparison to SMARCA4-deficient tumors. From our analysis, the DSS of these two new SNC entities were comparable. With the use of immunohistochemistry, it is easier to recognize these two rare entities and separate them from other sinonasal PDCAs.
This study is the first meta-analysis comparing the new molecular groups of SNCs in the new edition of WHO classification. It helps summarize and facilitate our current understanding about the clinicopathological behaviors and prognoses of these aggressive tumors. However, there are certain limitations. First, all included studies are retrospective cohort studies or case reports/series leading to inevitable selection bias. Next, we could not include other recently described molecular entities such as DEK::AFF2-rearranged non-keratinizing SCC and TP53-mutant ITAC due to limited data. In addition, we could not compare the effectiveness of treatment modalities in each molecularly defined SNC subgroup due to missing data. Finally, we could not assess PFS, an important prognostic value due to missing data in most included studies. Future large multicenter prospective studies are essential to validate the results of this study.
In summary, the evolution of molecular pathology alongside standard immunohistochemistry enables us to recognize and accurately diagnose novel molecular entities of SNCs. These tumors have distinct clinicopathological profiles and prognoses and should be distinguished from other SNCs to better understand their unique natural histories and treatment implications.
Author contributions
HV: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, writing original, review, and editing. TL: data curation, formal analysis, investigation, methodology, review, and editing. TTBL: data curation, formal analysis, investigation, methodology, review, and editing. HL: data curation, formal analysis, investigation, methodology, review, and editing. EE-R: data curation, formal analysis, investigation, methodology, review, and editing. KM: data curation, formal analysis, investigation, methodology, review, and editing. ID: conceptualization, project administration, validation, review, editing, and supervisions. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thompson LDR, Bishop JA. Update from the 5th edition of the world health organization classification of head and neck tumors: Nasal cavity, paranasal sinuses and skull base. Head Neck Pathol (2022) 16:1–18. doi: 10.1007/s12105-021-01406-5
2. Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol (2014) 38:1282–9. doi: 10.1097/pas.0000000000000285
3. Rooper LM, Uddin N, Gagan J, Brosens LAA, Magliocca KR, Edgar MA, et al. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol (2020) 44:1331–9. doi: 10.1097/pas.0000000000001508
4. Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma: A series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol (2020) 44:703–10. doi: 10.1097/pas.0000000000001428
5. Dogan S, Chute DJ, Xu B, Ptashkin RN, Chandramohan R, Casanova-Murphy J, et al. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol (2017) 242:400–8. doi: 10.1002/path.4915
6. Riobello C, López-Hernández A, Cabal VN, García-Marín R, Suárez-Fernández L, Sánchez-Fernández P, et al. IDH2 mutation analysis in undifferentiated and poorly differentiated sinonasal carcinomas for diagnosis and clinical management. Am J Surg Pathol (2020) 44:396–405. doi: 10.1097/pas.0000000000001420
7. Dogan S, Vasudevaraja V, Xu B, Serrano J, Ptashkin RN, Jung HJ, et al. DNA Methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol (2019) 32:1447–59. doi: 10.1038/s41379-019-0285-x
8. Glöss S, Jurmeister P, Thieme A, Schmid S, Cai WY, Serrette RN, et al. IDH2 R172 mutations across poorly differentiated sinonasal tract malignancies: Forty molecularly homogenous and histologically variable cases with favorable outcome. Am J Surg Pathol (2021) 45:1190–204. doi: 10.1097/pas.0000000000001697
9. Libera L, Ottini G, Sahnane N, Pettenon F, Turri-Zanoni M, Lambertoni A, et al. Methylation drivers and prognostic implications in sinonasal poorly differentiated carcinomas. Cancers (2021) 13. doi: 10.3390/cancers13195030
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
11. Agaimy A, Hartmann A, Antonescu CR, Chiosea S, El-Mofty SK, Lewis J, et al. SMARCB1 (INI-1)-Deficient sinonasal carcinoma: A series of 33 cases expanding the morphological and clinicopathological spectrum of a recently described entity. Lab Invest (2017) 97:319A–A. doi: 10.1097/PAS.0000000000000797
12. Albrecht T, Harms A, Roessler S, Goeppert B. NUT carcinoma in a nutshell: A diagnosis to be considered more frequently. Pathol Res Pract (2019) 215:152347. doi: 10.1016/j.prp.2019.01.043
13. Allard FD, Bell D, Stelow EB. Cytopathologic features of SMARCB1 (INI-1)-deficient sinonasal carcinoma. Cancer Cytopathol (2018) 126:567–74. doi: 10.1002/cncy.22020
14. Allison DB, Bishop JA, Ali SZ. Cytopathologic characteristics of SMARCB1 (INI-1) deficient sinonasal carcinoma: A potential diagnostic pitfall. Diagn Cytopathol (2016) 44:700–3. doi: 10.1002/dc.23503
15. Alsayed AM, Aljufairi EA, Alshammari AO, Alsindi KA, Sabra OA. INI-1-Deficient sinonasal carcinoma: Case report with emphasis on differential diagnosis. Case Rep Pathol (2022), 2022, 5629984. doi: 10.1155/2022/5629984
16. Arimizu K, Hirano G, Makiyama C, Matsuo M, Sasaguri T, Makiyama A. NUT carcinoma of the nasal cavity that responded to a chemotherapy regimen for ewing’s sarcoma family of tumors: a case report. BMC Cancer (2018) 18:1134. doi: 10.1186/s12885-018-5087-x
17. Ayyanar P, Mishra P, Preetam C, Adhya AK. SMARCB1/INI1 deficient sino-nasal carcinoma: Extending the histomorphological features. Head Neck Pathol (2021) 15:555–65. doi: 10.1007/s12105-020-01246-9
18. Bell D, Bell A, Ferrarotto R, Glisson B, Takahashi Y, Fuller G, et al. High-grade sinonasal carcinomas and surveillance of differential expression in immune related transcriptome. Ann Diagn Pathol (2020) 49:151622. doi: 10.1016/j.anndiagpath.2020.151622
19. Crocetta FM, Botti C, Fornaciari M, Castellucci A, Murri D, Santandrea G, et al. Sinonasal NUT carcinoma: Delayed diagnosis due to the COVID-19 pandemic and a review of the literature. Head Neck Pathol (2021) 15:1409–14. doi: 10.1007/s12105-021-01311-x
20. da Silva MA, Bentes RGL, Teixeira HS, Carvalho BM, Queiroz ALG, Schmid MF, et al. Diffuse spinal cord metastasis after resection of SMARCB1 sinonasal carcinoma manifesting with a right foot drop-a case report. Spinal Cord Ser cases (2022) 8:64. doi: 10.1038/s41394-022-00532-8
21. Edgar M, Caruso AM, Kim E, Foss RD. NUT midline carcinoma of the nasal cavity. Head Neck Pathol (2017) 11:389–92. doi: 10.1007/s12105-016-0763-0
22. Fang W, French CA, Cameron MJ, Han Y, Liu H. Clinicopathological significance of NUT rearrangements in poorly differentiated malignant tumors of the upper respiratory tract. Int J Surg Pathol (2013) 21:102–10. doi: 10.1177/1066896912451651
23. Gomez-Acevedo H, Patterson JD, Sardar S, Gokden M, Das BC, Ussery DW, et al. SMARC-B1 deficient sinonasal carcinoma metastasis to the brain with next generation sequencing data: a case report of perineural invasion progressing to leptomeningeal invasion. BMC Cancer (2019) 19:827. doi: 10.1186/s12885-019-6043-0
24. Gonçalves JM, Scarini JF, Gondak R, Altemani A, Mariano FV. Oral involvement of sinonasal undifferentiated carcinoma: A case report and immunohistochemical study of a challenging case. Oral Oncol (2022) 126:105779. doi: 10.1016/j.oraloncology.2022.105779
25. Gradecki SE, Kelting SM, Stelow EB. SMARCB1 (INI1) deficient sinonasal carcinoma with yolk sac tumor differentiation: Case report and review of the literature. Ajsp-Reviews Rep (2021) 26:259–63. doi: 10.1097/pcr.0000000000000456
26. Gurung N, Kapoor N, Mukherjee U, Khurana A. Reappraisal of SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily b member 1/INI-1 deficient tumor: Report of two cases. J Cancer Res Ther (2022) 18:780–3. doi: 10.4103/jcrt.JCRT_577_20
27. Hazir B, Şímşek B, Erdemír A, Gürler F, Yazici O, Kizil Y, et al. Sinonasal SMARCB1 (INI1) deficient carcinoma with yolk sac tumor differentiation: A case report and treatment options. Head Neck Pathol (2022) 16:596–601. doi: 10.1007/s12105-021-01375-9
28. Heft Neal ME, Birkeland AC, Bhangale AD, Zhai J, Kulkarni A, Foltin SK, et al. Genetic analysis of sinonasal undifferentiated carcinoma discovers recurrent SWI/SNF alterations and a novel PGAP3-SRPK1 fusion gene. BMC Cancer (2021) 21:636. doi: 10.1186/s12885-021-08370-x
29. Hsieh MS, French CA, Liang CW, Hsiao CH. NUT midline carcinoma: case report and review of the literature. Int J Surg Pathol (2011) 19:808–12. doi: 10.1177/1066896909353600
30. Agaimy A, Hartmann A, Antonescu CR, Chiosea SI, El-Mofty SK, Geddert H, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma: A series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol (2017) 41:458–71. doi: 10.1097/pas.0000000000000797
31. Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol (2017) 30:650–9. doi: 10.1038/modpathol.2016.239
32. Kakkar A, Antony VM, Irugu DVK, Adhikari N, Jain D. NUT midline carcinoma: A series of five cases, including one with unusual clinical course. Head Neck Pathol (2018) 12:230–6. doi: 10.1007/s12105-017-0858-2
33. Kakkar A, Antony VM, Pramanik R, Sakthivel P, Singh CA, Jain D. SMARCB1 (INI1)-deficient sinonasal carcinoma: a series of 13 cases with assessment of histologic patterns. Hum Pathol (2019) 83:59–67. doi: 10.1016/j.humpath.2018.08.008
34. Kakkar A, Ashraf SF, Rathor A, Adhya AK, Mani S, Sikka K, et al. SMARCA4/BRG1-deficient sinonasal carcinoma: Morphologic spectrum of an evolving entity. Arch Pathol Lab Med (2022) 146(9):1122–30. doi: 10.5858/arpa.2021-0001-OA
35. Klijanienko J, Le Tourneau C, Rodriguez J, Caly M, Theocharis S. Cytological features of NUT midline carcinoma arising in sino-nasal tract and parotid gland: Report of two new cases and review of the literature. Diagn Cytopathol (2016) 44:753–6. doi: 10.1002/dc.23506
36. Laco J, Chmelařová M, Vošmiková H, Sieglová K, Bubancová I, Dundr P, et al. SMARCB1/INI1-deficient sinonasal carcinoma shows methylation of RASSF1 gene: A clinicopathological, immunohistochemical and molecular genetic study of a recently described entity. Pathol Res Pract (2017) 213:133–42. doi: 10.1016/j.prp.2016.10.012
37. Laco J, Kovarikova H, Chmelarova M, Vosmikova H, Sieglova K, Bubancova I, et al. Analysis of DNA methylation and microRNA expression in NUT (nuclear protein in testis) midline carcinoma of the sinonasal tract: a clinicopathological, immunohistochemical and molecular genetic study. Neoplasma (2018) 65:113–23. doi: 10.4149/neo_2018_161122N581
38. Levitan I, Fichman S, Laviv Y. Fulminant presentation of a SMARCB1-deficient, anterior cranial fossa tumor in adult. Surg Neurol Int (2020) 11:195. doi: 10.25259/sni_171_2020
39. Li CY, Han YM, Xu K, Wu SY, Lin XY, Cao HY. Case report: SMARCB1 (INI-1)-Deficient carcinoma of the nasal cavity with pure yolk sac tumor differentiation and elevated serum AFP levels. Onco Targets Ther (2021) 14:2227–33. doi: 10.2147/ott.S302613
40. Li C, Wang YM, Zhang J. [SMARCA4-deficient sinonasal carcinoma: report of a case]. Zhonghua Bing Li Xue Za Zhi (2022) 51:157–9. doi: 10.3760/cma.j.cn112151-20210809-00559
41. McHugh KE, Policarpio-Nicolas MLC. Metastatic SMARCB1 (INI-1)-Deficient sinonasal carcinoma diagnosed by endobronchial ultrasound-guided fine-needle aspiration (EBUS-FNA): A potential diagnostic pitfall and review of the literature. Acta Cytol (2019) 63:431–7. doi: 10.1159/000500351
42. Minato H, Kobayashi E, Nakada S, Kurose N, Tanaka M, Tanaka Y, et al. Sinonasal NUT carcinoma: clinicopathological and cytogenetic analysis with autopsy findings. Hum Pathol (2018) 71:157–65. doi: 10.1016/j.humpath.2017.10.011
43. Ng JKM, Chan JYK, Li JJX, Tang K, Yeung DCM, Chan ABW. SMARCB1 (INI1)-deficient sinonasal carcinoma with yolk sac differentiation showing Co-loss of SMARCA4 immunostaining - a case report and literature review. Head Neck Pathol (2022) 16:934–41. doi: 10.1007/s12105-022-01423-y
44. Parsel SM, Jawad BA, McCoul ED. SMARCB1-deficient sinonasal carcinoma: Systematic review and case report. World Neurosurg (2020) 136:305–10. doi: 10.1016/j.wneu.2020.01.130
45. Patel SA, Singer B, Shen C, Zanation AM, Yarbrough WG, Weiss J. A case of metastatic NUT carcinoma with prolonged response on gemcitabine and nab-paclitaxel. Clin Case Rep (2021) 9:e04616. doi: 10.1002/ccr3.4616
46. Shaikh F, Pagedar N, Awan O, McNeely P. Sinonasal NUT-midline carcinoma - a multimodality approach to diagnosis, staging and post-surgical restaging. Cureus (2015) 7:e288. doi: 10.7759/cureus.288
47. Shanti RM, Farahi A, Curry JM, Alawi F. SMARCB1 (Integrase interactor 1)-deficient sinonasal carcinoma of the maxillary sinus: A newly described sinonasal neoplasm. J Oral Maxillofac Surg (2020) 78:1870.e1871–1870.e1876. doi: 10.1016/j.joms.2020.05.033
48. Shatzkes DR, Ginsberg LE, Wong M, Aiken AH, Branstetter B, Michel MA, et al. Imaging appearance of SMARCB1 (INI1)-deficient sinonasal carcinoma: A newly described sinonasal malignancy. AJNR Am J Neuroradiol (2016) 37:1925–9. doi: 10.3174/ajnr.A4841
49. Shaverdashvili K, Azimi-Nekoo E, Cohen P, Akbar N, Ow TJ, Halmos B, et al. INI-1 (SMARCB1)-deficient undifferentiated sinonasal carcinoma: Novel paradigm of molecular testing in the diagnosis and management of sinonasal malignancies. Oncologist (2020) 25:738–44. doi: 10.1634/theoncologist.2019-0830
50. Solomon LW, Magliocca KR, Cohen C, Müller S. Retrospective analysis of nuclear protein in testis (NUT) midline carcinoma in the upper aerodigestive tract and mediastinum. Oral Surg Oral Med Oral Pathol Oral Radiol (2015) 119:213–20. doi: 10.1016/j.oooo.2014.09.031
51. Srivastava P, Husain N, Anand N. SMARCB1/INI-1 deficient sinonasal carcinoma: An emerging entity. J Oral Maxillofac Surg Med Pathol (2020) 32:563–7. doi: 10.1016/j.ajoms.2020.06.004
52. Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol (2008) 32:828–34. doi: 10.1097/PAS.0b013e31815a3900
53. Stirnweiss A, McCarthy K, Oommen J, Crook ML, Hardy K, Kees UR, et al. A novel BRD4-NUT fusion in an undifferentiated sinonasal tumor highlights alternative splicing as a contributing oncogenic factor in NUT midline carcinoma. Oncogenesis (2015) 4:e174. doi: 10.1038/oncsis.2015.33
54. Suzuki S, Kurabe N, Minato H, Ohkubo A, Ohnishi I, Tanioka F, et al. A rare Japanese case with a NUT midline carcinoma in the nasal cavity: a case report with immunohistochemical and genetic analyses. Pathol Res Pract (2014) 210:383–8. doi: 10.1016/j.prp.2014.01.013
55. Tasche KK, Samuelson MI, Pagedar NA. Unilateral nasal mass in a woman in her 20s. JAMA Otolaryngology-Head Neck Surg (2019) 145:862–3. doi: 10.1001/jamaoto.2019.1671
56. Trieu V, Aulet RM, Ciolino A, Rimash T. SMARCB1-deficient sinonasal carcinoma: A case report and discussion of the clinical implications. Ann Otol Rhinol Laryngol (2019) 128:676–80. doi: 10.1177/0003489419836668
57. Vakani PN, Maheshwari J, Maheshwari M, Shah B. Sinonasal NUT midline carcinoma: A new histological entity. Indian J Pathol Microbiol (2020) 63:103–5. doi: 10.4103/ijpm.Ijpm_373_19
58. Vaziri Fard E, Zhang S, Cai Z, Ding J, Sun Q, Saluja K, et al. Sinonasal undifferentiated carcinoma: clinicopathological spectrums and diagnosis reappraisal. Hum Pathol (2019) 89:62–70. doi: 10.1016/j.humpath.2019.04.008
59. Wang X, Wang J, Luo X, Lu J, Wang L, Li Q, et al. Diagnosis of NUT carcinoma despite false-negative next-generation sequencing results: A case report and literature review. Onco Targets Ther (2021) 14:4621–33. doi: 10.2147/ott.S327722
60. Wang R, Wang L, Fang J, Zhong Q, Hou L, Ma H, et al. Clinical diagnosis and treatment analyses on SMARCB1 (Integrase interactor 1)-deficient sinonasal carcinoma: Case series with systematic review of the literature. World Neurosurg (2022) 161:e229–43. doi: 10.1016/j.wneu.2022.01.114
61. Wasserman JK, Dickson BC, Perez-Ordonez B, de Almeida JR, Irish JC, Weinreb I. INI1 (SMARCB1)-deficient sinonasal carcinoma: A clinicopathologic report of 2 cases. Head Neck Pathol (2017) 11:256–61. doi: 10.1007/s12105-016-0752-3
62. Wei X, Teng X, Zhang Y, Cheng M, Chen G. Case report: NUT carcinoma in an elderly woman with unique morphology and immunophenotype highlights a diagnostic pitfall. Transl Cancer Res (2022) 11:1850–60. doi: 10.21037/tcr-22-364
63. Yanagawa N, Suzuki M, Sugimoto R, Osakabe M, Uesugi N, Shiga K, et al. SMARCB1-deficient sinonasal carcinoma: a case report and literature review. J Surg Case Rep 2021 (4):rjab161 doi: 10.1093/jscr/rjab161
64. Yang H, Zhou L, Zhong G, Li X, Wang Y. SMARCB1 (INI-1)-Deficient sinonasal carcinoma: A case report and literature review. World Neurosurgery (2020), 136:305–10 1455613221082622. doi: 10.1177/01455613221082622
65. Zamecnik M, Rychnovsky J, Syrovatka J. Sinonasal SMARCB1 (INI1) deficient carcinoma with yolk sac tumor differentiation: Report of a case and comparison with INI1 expression in gonadal germ cell tumors. Int J Surg Pathol (2018) 26:245–9. doi: 10.1177/1066896917741549
66. Zelman B, Chen H, Pambuccian S, Massarani-Wafai R, Mehrotra S, Ananthanarayanan V. A rare case of NUT carcinoma and review of the literature. Ajsp-Reviews Rep (2021) 26:264–8. doi: 10.1097/pcr.0000000000000464
67. Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. New Engl J Med (2015) 372:2499–508. doi: 10.1056/NEJMoa1407279
68. Vuong HG, Altibi AM, Duong UN, Ngo HT, Pham TQ, Chan AK-Y, et al. TERT promoter mutation and its interaction with IDH mutations in glioma: Combined TERT promoter and IDH mutations stratifies lower-grade glioma into distinct survival subgroups–a meta-analysis of aggregate data. Crit Rev oncology/hematology (2017) 120:1–9. doi: 10.1016/j.critrevonc.2017.09.013
69. Farshidfar F, Zheng S, Gingras M-C, Newton Y, Shih J, Robertson AG, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep (2017) 18:2780–94. doi: 10.1016/j.celrep.2017.06.008
70. Vuong HG, Ngo TN, Dunn IF. Prognostic importance of IDH mutations in chondrosarcoma: An individual patient data meta-analysis. Cancer Med (2021) 10:4415–23. doi: 10.1002/cam4.4019
Keywords: sinonasal carcinoma, sinonasal undifferentiated carcinoma, SMARCB1, SMARCA4, IDH2, nut
Citation: Vuong HG, Le T, Le TTB, Le HT, El-Rassi ET, McKinney KA and Dunn IF (2023) Clinicopathological features and prognostic outcomes of molecularly defined entities in the new edition of the WHO classification of sinonasal carcinoma. Front. Oncol. 13:1117865. doi: 10.3389/fonc.2023.1117865
Received: 07 December 2022; Accepted: 20 February 2023;
Published: 01 March 2023.
Edited by:
Raymond Tsang, National University of Singapore, SingaporeReviewed by:
Bingcheng Wu, National University Hospital, SingaporeStephanie Nga Sze Wong, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2023 Vuong, Le, Le, Le, El-Rassi, McKinney and Dunn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian F. Dunn, aWFuLWR1bm5Ab3Voc2MuZWR1
 Huy Gia Vuong
Huy Gia Vuong Thoa Le
Thoa Le Trang T.B. Le2
Trang T.B. Le2 Kibwei A. McKinney
Kibwei A. McKinney Ian F. Dunn
Ian F. Dunn