- 1Department of Oncology, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Hunan Normal University, Changsha, China
- 2Department of Nephrology and Laboratory of Kidney Disease, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Hunan Normal University, Hunan Clinical Research Center for Chronic Kidney Disease, Changsha, China
Background: Metabolic syndrome (MetS) has been related to a high incidence of hepatocellular carcinoma (HCC). However, the influence of MetS on survival of patients with HCC is still unclear. We performed a systematic review and meta-analysis to evaluate the association between MetS and survival of HCC patients.
Methods: A search of PubMed, Embase, and Web of Science retrieved relevant cohort studies from the inception of the databases to October 16, 2022. Data collection, literature search, and statistical analysis were carried out independently by two authors. We pooled the results using a random-effects model that incorporates heterogeneity.
Results: In the meta-analysis, 8080 patients with HCC were included from ten cohort studies, and 1166 patients (14.4%) had MetS. Eight studies included patients treated primarily with radical hepatectomy, one study with patients receiving sorafenib, and another study included patients who were treated with radical hepatectomy or non-surgical treatments. Pooled results showed that MetS was associated with poor overall survival (OS, risk ratio [RR]: 1.21, 95% confidence interval [CI]:1.08 to 1.37, p = 0.001; I2 = 32%) and progression-free survival (PFS, RR: 1.33, 95% CI: 1.18 to 1.49, p < 0.001, I2 = 14%). Influencing analysis by excluding one study at a time showed consistent results (p all < 0.05). Subgroup analyses showed similar results in studies with MetS diagnosed with the National Cholesterol Education Program Adult Treatment Panel III or International Diabetes Federal criteria, and in studies with mean follow-up durations < or ≥ 3.5 years (p for subgroup difference all > 0.05).
Conclusion: In patients with HCC, MetS may be a risk factor of poor OS and PFS, particularly for those after radical hepatectomy.
Introduction
As the sixth most commonly diagnosed cancer among adults and the third leading cause of cancer-related mortality globally, hepatocellular carcinoma (HCC) represents the most common type of liver cancer (1–3). In the current era of HCC treatment, surgical resection, transplantation, interventional chemoembolization, radiofrequency ablation, radiotherapy, and targeted drug therapy are the most common treatments available (4, 5). However, responses of HCC patients to these treatments are varying, and the survival of some patients with HCC remains poor despite of these comprehensive treatments (6, 7). Therefore, uncovering potential risk factors of poor prognosis in patients with HCC is important for risk stratification and development of adjunctive treatments in these patients (8).
Metabolic syndrome (MetS) refers to a cluster of metabolic disorders which involve central obesity, insulin resistance, high blood pressure, and dyslipidemia (9–11). Pathophysiologically, MetS is characterized by insulin resistance and systemic low-degree inflammation, which have been both related to carcinogenesis (12). Epidemiological studies have confirmed the role of MetS as a risk factor for the incidence of various cancers (13, 14), including HCC (15–17). However, for patients with diagnosed HCC, the influence of MetS on their survival remains unclear (18). Some previous studies showed that MetS may be a risk factor of poor survival of patients with HCC (19–23), while other studies did not show similar results (24–28). In this study, we performed a systematic review and meta-analysis to comprehensively investigate the association between MetS and survival of patients with HCC. In addition, the effect of different diagnostic criteria on the association was also explored in subgroup analyses.
Materials and methods
We followed the instructions of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (29, 30) and the Cochrane’s Handbook (31) through the meta-analysis. The protocol of the meta-analysis has been registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY, No. 2022120113).
Literature retrieving
The electronic databases PubMed, Embase, and Web of Science were searched from the inception of the databases until October 16, 2022. A combined search term was used, including (1) “metabolic syndrome” OR “insulin resistance syndrome” OR “syndrome X”; (2) “hepatocellular” OR “liver” OR “hepatic”; (3) “carcinoma” OR “cancer” OR “tumor” OR “malignancy” OR “malignant” OR “neoplasm”; and (4) “survival” OR “death” OR “mortality” OR “prognosis” OR “recurrence” OR “recurrent”. The search was limited to human studies published in full-length articles. No restriction was applied regarding the language of publication. To supplement our search, we manually reviewed the citations of relevant original articles and review articles.
Study selection
A PICOS-based inclusion criterion was used for this study.
P (patients): Adult patient with confirmed diagnosis of HCC, regardless of the cancer stage or treatments.
I (exposure): Patients with MetS at baseline.
C (control): Patients without MetS at baseline.
O (outcomes): A primary outcome was overall survival (OS), and a secondary outcome was progression-free survival (PFS), compared between HCC patients with and without MetS. Generally, OS was defined as the time elapsed from treatment and to the date of death from any cause, while a PFS is the interval between the initiation of treatment and the first recurrence or progression of the disease.
S (study design): Cohort studies, including prospective and retrospective cohorts;
The diagnosis of MetS was consistent with the criteria used within the included studies. The meta-analysis excluded reviews, preclinical studies, studies involving non-HCC patients, studies lacking the evaluation of MetS, and studies without survival outcomes.
Data collection and quality assessment
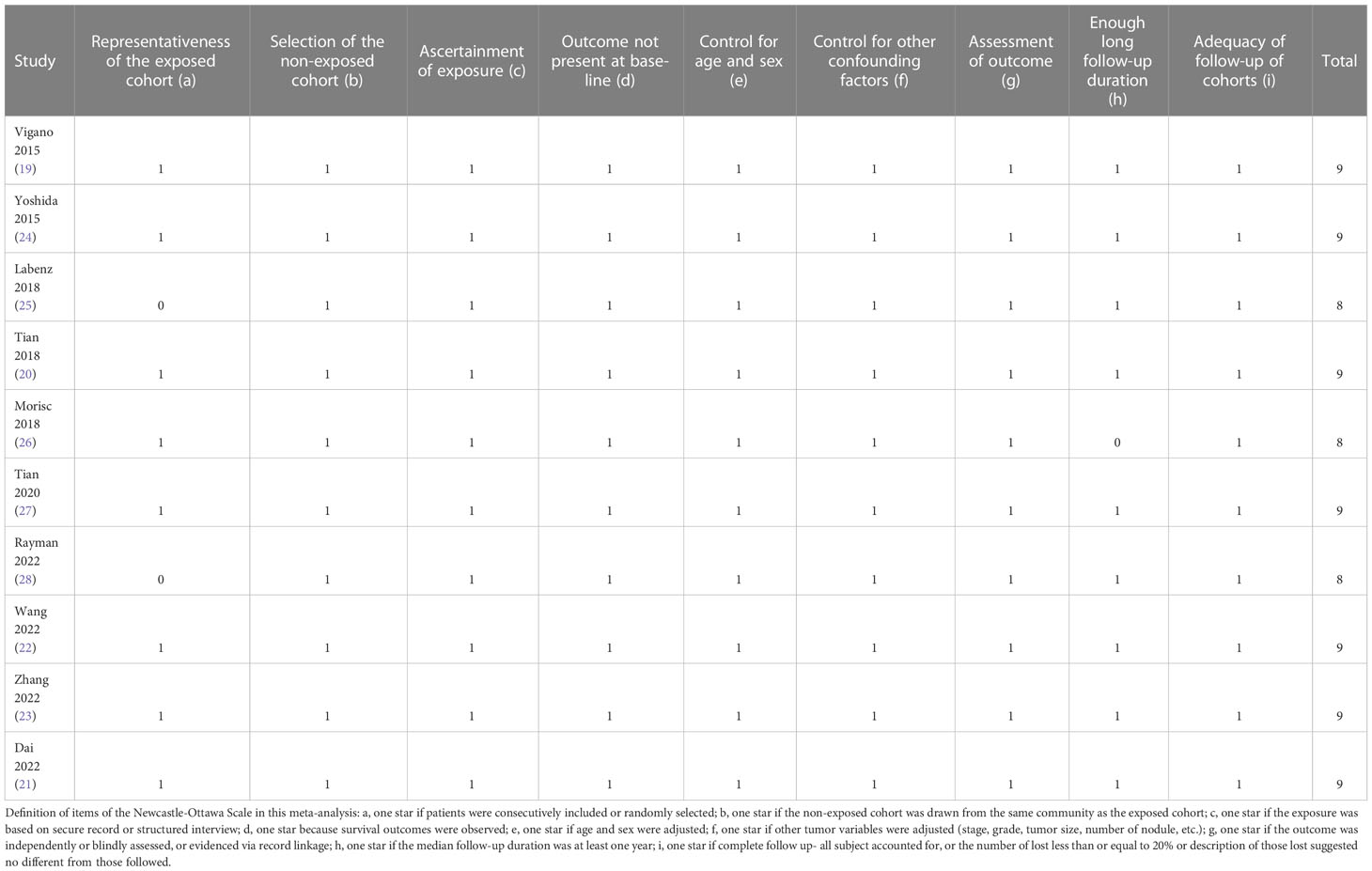
Separately, two authors searched and analyzed literature, collected data, and assessed study quality. A third author was consulted if discrepancies were encountered. Data of study information, patient demographic factors, main treatments, diagnostic criteria of MetS, follow-up durations, outcomes reported, and variables adjusted in the regression model for the analysis of the association between MetS and survival outcomes were collected. An assessment of study quality was done using the Newcastle-Ottawa Scale (32) based on criteria for participant selection, comparability of groups, and validity of results. A study’s quality was determined by the number of stars between 1 and 9, with more stars representing a better study quality.
Statistical analyses
The main objective was to determine the relative risks of OS and PFS comparing between HCC patients with and without MetS, which were presented as risk ratios (RRs) and the confidence intervals (CIs). By using 95% CIs or p-values, RRs and standard errors (SEs) could be calculated, and a subsequent logarithmical transformation kept the variance stabilized and normalized. Cochrane’s Q test and I2 statistics were used to estimate study heterogeneity (33), and the significant heterogeneity is reflected by an I2 > 50%. The results were combined using a random-effects model incorporating heterogeneity’s influence (31). A sensitivity analysis that omitted one study at a time was conducted to observe what effect each study has on the overall results (34). Besides, sensitivity analyses were also performed in studies of patients after radical hepatectomy for HCC and in patients who are positive of hepatitis B virus (HBV). In addition, subgroup analyses were conducted to examine how study characteristics influenced the results. Medians of the continuous variables were used as the cutoffs for defining subgroups. An estimate of publication bias was made by constructing funnel plots and applying Egger’s regression asymmetry test to the visual judgment of their symmetry (35). Our analyses were done using RevMan (version 5.1; Cochrane Collaboration, Oxford, UK) and Stata (version 12.0; Stata Corporation, College Station, TX).
Results
Studies obtained
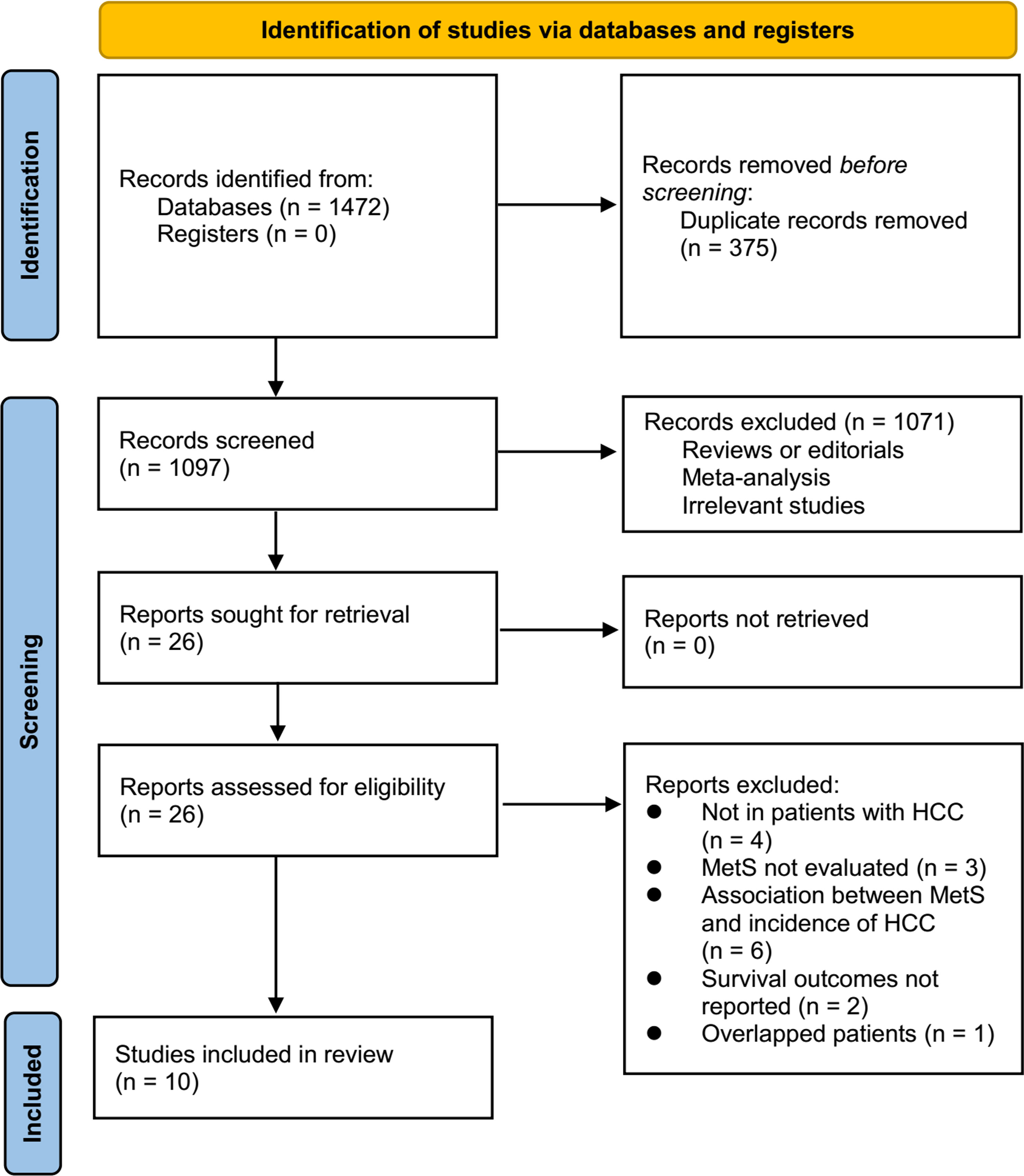
Figure 1 displays the procedure of literature analysis. In short, the initial search of the databases retrieved 1472 articles, and 1097 were left after excluding the duplicated records. In addition, 1071 articles were excluded since their titles and abstracts were not relevant to the meta-analysis, leaving 26 studies in total for the full-text review. Finally, after excluding 16 studies through full-text review, ten studies (19–28) were included. The reasons for the removing of the 16 studies are also presented in Figure 1.
Characteristics of the included studies
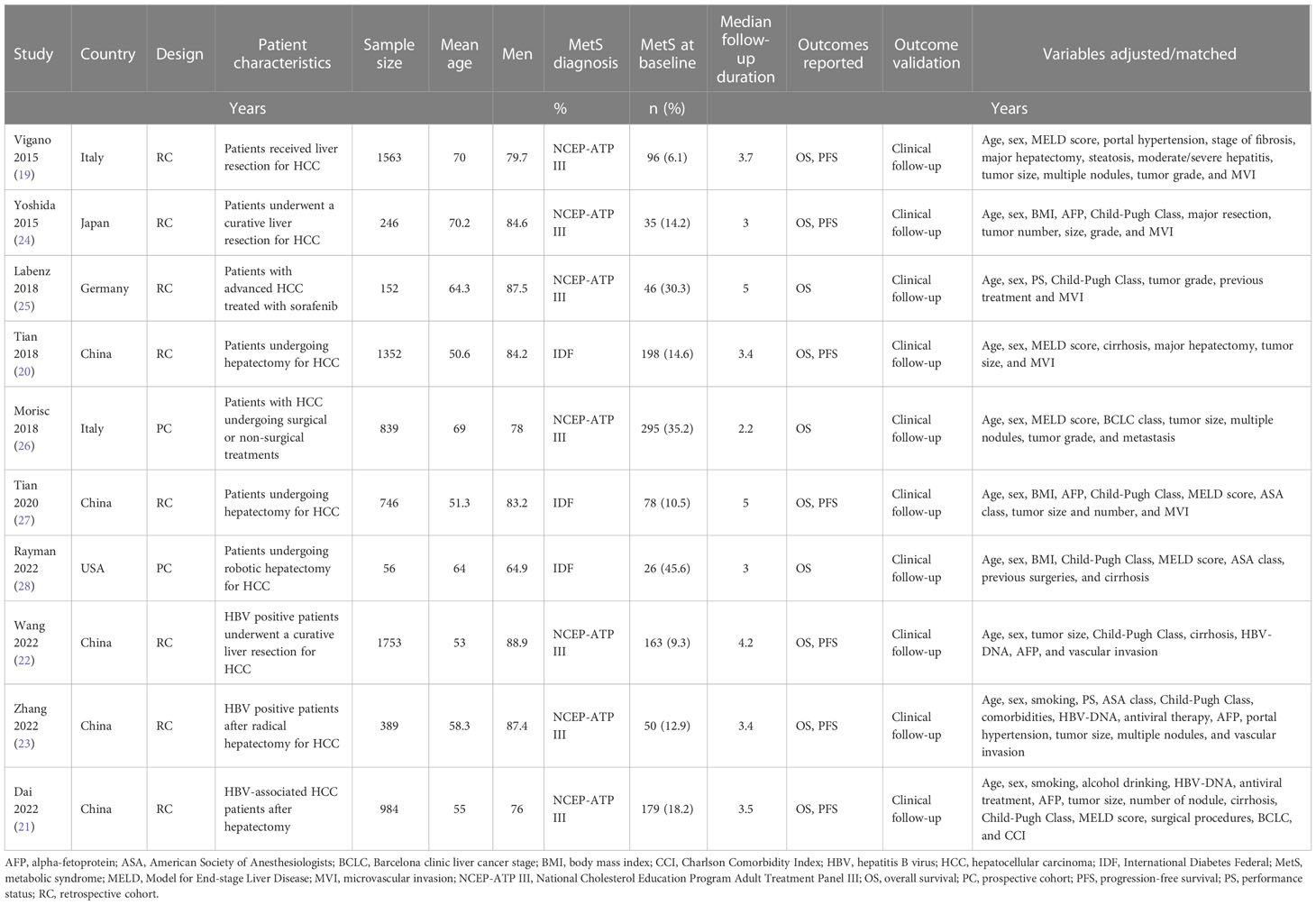
As shown in Table 1, ten cohort studies, eight retrospective studies (19–25, 27) and two prospective studies (26, 28), involving 8080 patients with HCC were included in the meta-analysis. These studies were published between 2015 and 2022, and performed Italy, Japan, Germany, China, and the United States. All of the studies included patients with HCC, with eight studies with patients treated primarily with surgical resection (19–24, 27, 28), one with sorafenib (25), and another one with surgical resection or non-surgical treatments (26). The sample size of the included studies varied between 56 and 1753. The mean ages of the patients were 50.6 to 70.2 years. The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria were used for the diagnosis of MetS in seven studies (19, 21–26), while the International Diabetes Federal (IDF) criteria were used in the other three studies (20, 27, 28). Accordingly, 1166 patients (14.4%) had MetS at baseline. The mean follow-up durations varied between 2.2 and 5.0 years. The outcome of OS was reported in all of the included ten studies (19–28), while the outcome of PFS was reported in seven studies (19–24, 27). Multivariate models were applied to analyze the association between MetS and survival outcomes in HCC, and possible confounding factors such as age, sex, hepatic function, tumor size, grade, and treatments etc. were adjusted. The NOS of the included studies were 8 to 9 stars, suggesting moderate to good study quality (Table 2).
MetS and OS of patients with HCC
Ten studies reported the association between MetS and OS in patients with HCC (19–28). Pooled results showed that MetS was associated with poor OS of HCC (RR: 1.21, 95% CI: 1.08 to 1.37, p = 0.001; Figure 2A) with mild heterogeneity (p for Cochrane’s Q test = 0.15; I2 = 32%). Sensitivity analysis by excluding one study at a time showed consistent results (RR: 1.18 to 1.25, p all < 0.05). Sensitivity limited to the eight studies (19–24, 27, 28) only included patients after hepatectomy for HCC showed similar results (RR: 1.25, 95% CI: 1.13 to 1.38, p < 0.001; I2 = 0%). Similarly, sensitivity analyses limited to the three studies (21–23) including only HBV-positive patients also showed similar results (RR: 1.41, 95% CI: 1.17 to 1.71, p < 0.001; I2 = 0%). Subgroup analyses according to study country (Figure 2B), diagnostic criteria of MetS (Figure 2C), and follow-up durations (Figure 3A) showed similar results (p for subgroup difference all > 0.05). However, subgroup analysis according to study quality showed that MetS was associated with poor OS in studies of 9 points in NOS (RR: 1.28, 95% CI: 1.15 to 1.43, p < 0.001; I2 = 0%), but not in studies of 8 points in NOS (RR: 1.00, 95% CI: 0.86 to 1.18, p = 0.96; I2 = 0%; p for subgroup difference = 0.01; Figure 3B).
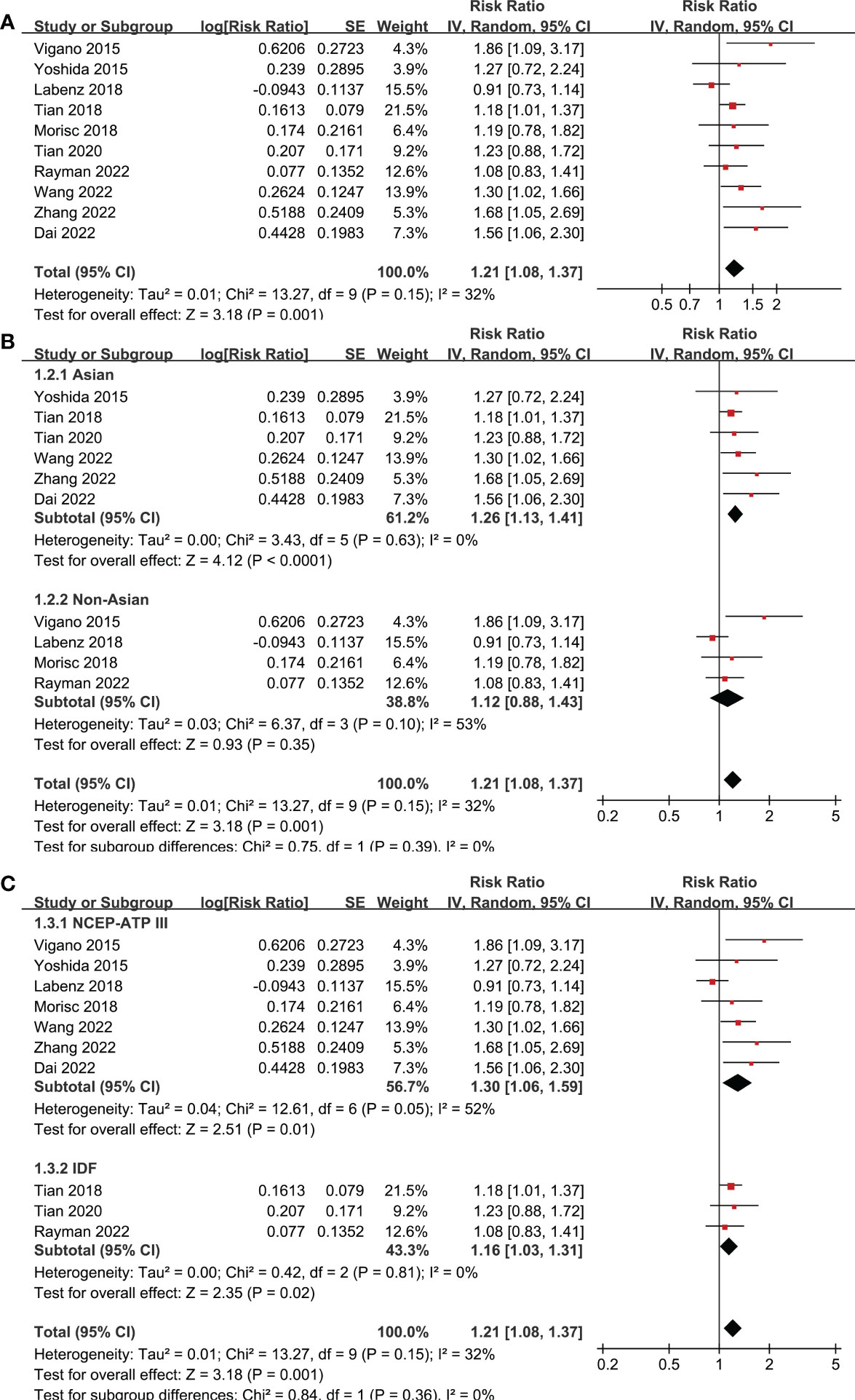
Figure 2 Forest plots for the meta-analyses regarding the association between MetS and OS in patients with HCC. (A) overall meta-analysis; (B) subgroup analysis according to the study country; and (C) subgroup analysis according to the diagnostic criteria for MetS.
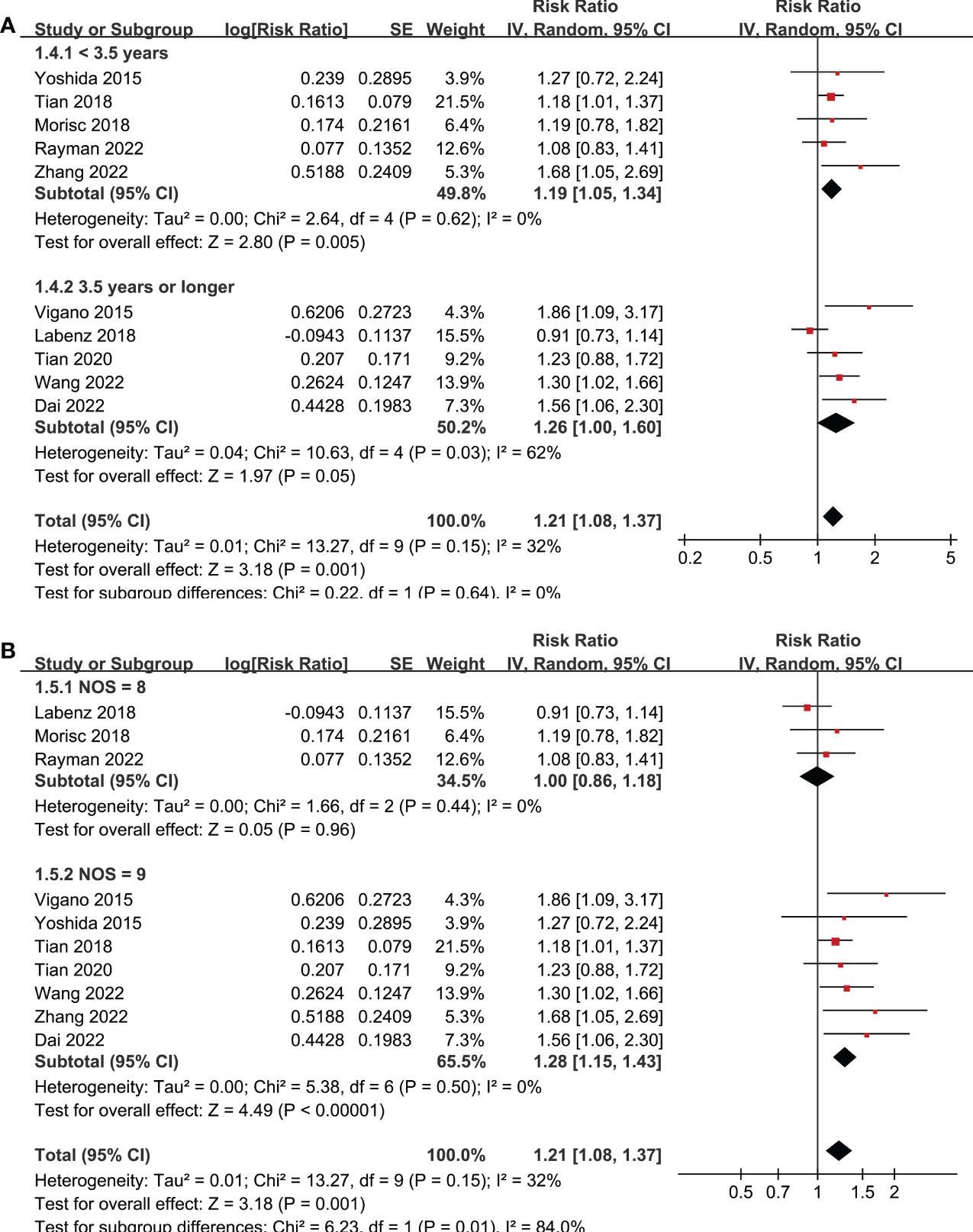
Figure 3 Forest plots for the subgroup-analyses regarding the association between MetS and OS in patients with HCC. (A) subgroup analysis according to the follow-up durations; and (B) subgroup analysis according to the study quality scores.
MetS and PFS of patients with HCC
The outcome of PFS was reported in seven studies (19–24, 27), which all included patients after hepatectomy for HCC. Pooled results of seven studies, all with NOS of 9 points, showed that MetS was associated with poor PFS (RR: 1.33, 95% CI: 1.18 to 1.49, p < 0.001, I2 = 14%; Figure 4A) in patients with HCC. Sensitivity analysis by omitting one study at a time also showed consistent results (RR: 1.27 to 1.42, p all < 0.05). Sensitivity analyses limited to the three studies (21–23) including only HBV-positive patients also showed similar results (RR: 1.41, 95% CI: 1.21 to 1.65, p < 0.001; I2 = 0%). Subgroup analyses according to the diagnostic criteria of MetS and follow-up durations showed similar results (p for subgroup difference all > 0.05; Figures 4B, C).

Figure 4 Forest plots for the meta-analyses regarding the association between MetS and PFS in patients with HCC. (A) overall meta-analysis; (B) subgroup analysis according to the diagnostic criteria for MetS; and (C) subgroup analysis according to the follow-up durations.
Publication bias
According to Figures 5A, B, funnel plots for OS and PFS outcomes show symmetry, indicating low risks of publication bias. Results of Egger’s regression tests also indicated low risks of publication bias underlying the meta-analyses for the associations between MetS with OS and PFS of HCC patients (p = 0.29 and 0.37, respectively).
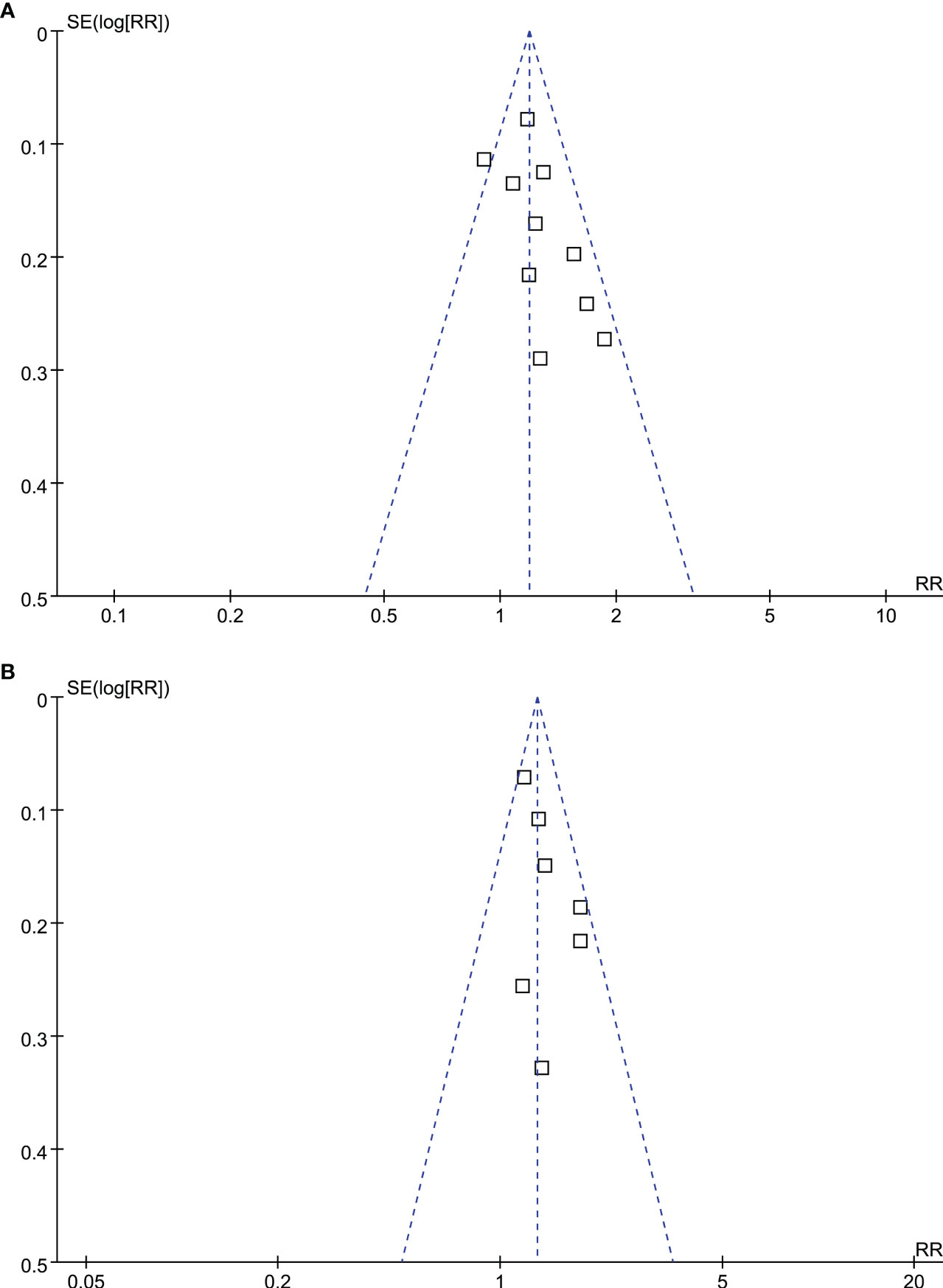
Figure 5 Funnel plots for the publication bias underlying the meta-analyses; (A) funnel plots for the meta-analysis of OS; and (B) funnel plots for the meta-analysis of PFS.
Discussion
Researches regarding the roles of metabolic disorders in the pathogenesis and progression of cancer have become a hotspot in recent decades. As a syndrome integrating multiple common metabolic disorders, MetS has been shown to be closely related to the incidence of multiple cancers (36–40), but not all (41). Besides, in view of the high prevalence of MetS in patients with confirmed diagnosis of cancer (42), it is also important to determine the influence of MetS on survival of these patients. Previous studies have shown that MetS may be a predictor of poor survival in patients with breast cancer (43), prostate cancer (44), colorectal cancer (45), and gastric cancer (46). In this meta-analysis, by combining the results of ten available cohort studies, we further confirmed that MetS was also independently associated with poor OS and PFS in patients with HCC, particularly in patients after radical hepatectomy for HCC. These findings further expanded the role of MetS in HCC, which suggested that besides of being a risk factor of high incidence of HCC (15, 16) MetS may also be a predictor of poor survival of patients with confirmed diagnosis of HCC.
To the best of our knowledge, this study may be the first meta-analysis which evaluated the association between MetS and survival in patients with HCC. The strengths of the meta-analysis included the followings. First, extensive literature search was performed in three electronic databases, which retrieved ten up-to-date studies relevant to the aim of the meta-analysis. Second, all of the included studies were cohort studies, which could provide a longitudinal relationship between MetS and poor survival of patients with HCC. Third, multivariate regression analyses were used in all of the included studies when the association between MetS and survival outcome of patients with HCC was investigated. After adjustment of potential confounding factors such as age, sex, tumor characteristics, and therapy, results of the meta-analysis may suggest an independent association between MetS and poor survival in patients with HCC. Finally, consistent results were obtained in sensitivity analyses by excluding one study at a time and in subgroup analyses according to predefined study characteristics such as definition of MetS and follow-up durations. These findings may indicate the robustness of the meta-analysis results. Interestingly, subgroup analysis according to study quality showed that MetS was associated with poor OS in studies of 9 points in NOS, but not in studies of 8 points in NOS. These findings may further reflect the reliability of the meta-analysis results regarding OS, which were supported by high-quality studies. For the three studies with 8 points of quality score (21–23), they were either exposed to patient selection bias or short follow-up duration, which may lead to bias of the results.
The mechanisms underlying the association between MetS and poor survival of patients with MetS may be multifactorial. Pathophysiologically, MetS is characterized by low-grade systemic inflammation and insulin resistance, both of which may adversely affect the progress of HCC. For example, MetS has been related to a variety of inflammatory signaling pathways, such as tumor necrosis factor alpha, interleukin-1, toll-like receptors, C-type lectin receptors, and nuclear factor kappa-light-chain-enhancer of activated B cells etc., all of which have been documented to be involved in the development of the malignant properties of HCC, including promoting proliferative and survival signaling, inducing angiogenesis, evading immune surveillance, activating invasion and metastasis, and inducing genome instability (47, 48). Insulin resistance has been related to the generation of multidrug resistance of HCC in some experimental studies (49). In addition, hyperinsulinemia and subsequent insulin resistance has been revealed as a promoting factor for maintaining the homeostasis in the endoplasmic reticulum by regulating autophagy, enhancing the survival of HCC (50). Moreover, a recent study showed that insulin resistance as indicated by a higher level of Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), was a significant predictor for HCC recurrence after curative treatment (51). Finally, some components of MetS have also been suggested to adversely affect the survival of patients with HCC, including visceral adiposity (52), diabetes (53), and dyslipidemia (54), which may also partly explain the association between MetS and poor survival in patients with HCC.
Results of our meta-analysis also have limitations. Firstly, eight of the ten included studies were retrospective cohort studies, which may be associated with the risks of selection and recall biases. Accordingly, results of the meta-analysis should be validated in large-scale prospective studies. Secondly, because all of the included studies focused on the tumor-related outcome of the patients, and the results of the meta-analysis showed that MetS was associated with both poor OS and PFS in patients with HCC, it could be concluded that tumor progression and related death was likely to increase in HCC patients with MetS. It could not be determined at current stage whether non-cancer related death (such as cardiovascular death) was also increased in HCC patients with MetS. Further studies are needed in this regard. Thirdly, eight of the studies included HCC patients after surgical resection. We could not determine whether the association between MetS and prognosis of HCC was consistent in HCC patients who received other non-surgical treatments, such as transarterial chemoembolization. Large-scale prospective studies are needed to determine the influences of different treatment strategies on the association between MetS and survival of patients with HCC. To the best of our knowledge, no study to date has observed the potential influence of MetS on survival of liver transplantation (LT) solely in HCC patients. A recent study (55) showed that for patients receiving LT (47% with HCC), MetS at baseline is associated with a higher risk of post-LT morbidity yet without affecting mortality. Large prospective studies are also needed to determine if MetS may affect the OS and PFS in HCC patients after LT, which may interesting in the debate of LT versus hepatectomy for HCC on MetS. Moreover, the etiologies of HCC are multifactorial, and the different etiologies to HCC may affect the association between MetS and prognosis of HCC. Although our sensitivity analyses showed consistent association between MetS and poor survival of HCC in HBV-positive patients, it remains unclear whether the association was similar for patients with other risk factors/etiologies for HCC, such as those with HCV-positivity, alcoholic liver disease, nonalcoholic fatty liver disease, aflflatoxin exposure, hemochromatosis, and others (56). Studies are needed in the future for further investigation. In addition, although results of the meta-analysis were obtained on the basis of studies with multivariate analyses, we could not exclude the residual confounding factors that may affect the association between MetS and survival of HCC patients, such as the concurrent medications. For example, satin use has been related to reduced mortality and recurrence of HCC (57). Finally, as a meta-analysis of observational studies, we could not determine if the association between MetS and poor survival of HCC is causative. Clinical studies may be considered to investigate whether interventions targeting the metabolic disorders in MetS could favorably influence the clinical outcome of patients with HCC.
In conclusion, results of the meta-analysis indicate that MetS may be independently associated with poor OS and PFS in patients with HCC, particularly for patients after radical hepatectomy for HCC. Future studies are needed to determine the underlying mechanisms, and to investigate the potential benefits of interventions targeting the metabolic disorders in patients with HCC.
Author contributions
JF and KL designed the study. JF and JJ performed database search, literature review, data collection, and study quality evaluation. JF and KL performed statistical analyses and interpreted the results. JF drafted the manuscript. All authors revised the manuscript and approved the submission.
Funding
This study was supported by the Subject of Health Commission of Hunan Province (202203102958).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin (2022) 72(5):409–36. doi: 10.3322/caac.21731
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: Profiles, trends, and determinants. Chin Med J (Engl) (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
4. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
5. Renne SL, Sarcognato S, Sacchi D, Guido M, Roncalli M, Terracciano L, et al. Hepatocellular carcinoma: A clinical and pathological overview. Pathologica. (2021) 113(3):203–17. doi: 10.32074/1591-951X-295
6. Natu A, Singh A, Gupta S. Hepatocellular carcinoma: Understanding molecular mechanisms for defining potential clinical modalities. World J Hepatol (2021) 13(11):1568–83. doi: 10.4254/wjh.v13.i11.1568
7. Raoul JL, Frenel JS, Raimbourg J, Gilabert M. Current options and future possibilities for the systemic treatment of hepatocellular carcinoma. Hepat Oncol (2019) 6(1):HEP11. doi: 10.2217/hep-2019-0001
8. Jiri T, Igor K. Hepatocellular carcinoma future treatment options. Klin Onkol (2020) 33(Supplementum 3):26–9.
9. Ahmed M, Kumari N, Mirgani Z, Saeed A, Ramadan A, Ahmed MH, et al. Metabolic syndrome; definition, pathogenesis, elements, and the effects of medicinal plants on it's elements. J Diabetes Metab Disord (2022) 21(1):1011–22. doi: 10.1007/s40200-021-00965-2
10. Marzoog BA, Vlasova TI. The metabolic syndrome puzzles; possible pathogenesis and management. Curr Diabetes Rev (2022) 29. doi: 10.2174/1573399818666220429100411
11. Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr (2020) 23(3):189–230. doi: 10.5223/pghn.2020.23.3.189
12. Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med (2021) 42(3):199–214. doi: 10.1055/a-1263-0898
13. Karra P, Winn M, Pauleck S, Bulsiewicz-Jacobsen A, Peterson L, Coletta A, et al. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obes (Silver Spring) (2022) 30(7):1323–34. doi: 10.1002/oby.23444
14. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: Pathophysiological and therapeutic associations. Endocrine. (2021) 74(3):478–97. doi: 10.1007/s12020-021-02884-x
15. Li Y, Shi J, Liu X, Deng Q, Huang Y, Yang Z. Metabolic syndrome relates to high risk in hepatocellular carcinoma: A meta-analysis. Discov Med (2018) 26(144):185–96.
16. Chen Y, Li X, Wu S, Ye W, Lou L. Metabolic syndrome and the incidence of hepatocellular carcinoma: A meta-analysis of cohort studies. Onco Targets Ther (2018) 11:6277–85. doi: 10.2147/OTT.S154848
17. Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: Systemic review and meta-analysis. J Clin Gastroenterol (2014) 48(2):172–7. doi: 10.1097/MCG.0b013e3182a030c4
18. Satriano L, Lewinska M, Rodrigues PM, Banales JM, Andersen JB. Metabolic rearrangements in primary liver cancers: Cause and consequences. Nat Rev Gastroenterol Hepatol (2019) 16(12):748–66. doi: 10.1038/s41575-019-0217-8
19. Vigano L, Conci S, Cescon M, Fava C, Capelli P, D'Errico A, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. J Hepatol (2015) 63(1):93–101. doi: 10.1016/j.jhep.2015.01.024
20. Tian Y, Lyu H, He Y, Xia Y, Li J, Shen F. Comparison of hepatectomy for patients with metabolic syndrome-related HCC and HBV-related HCC. J Gastrointest Surg (2018) 22(4):615–23. doi: 10.1007/s11605-017-3629-1
21. Dai J, Zhu X, Shen J, Zhang Y, Xie F, Yu Y, et al. The effect of metabolic syndrome on the outcome of hepatitis b-associated hepatocellular carcinoma patients after hepatectomy: A multicenter study. Front Oncol (2022) 12:811084. doi: 10.3389/fonc.2022.811084
22. Wang MD, Tang SC, Li C, Sun LY, Xu X, Liang YJ, et al. Association of concurrent metabolic syndrome with long-term oncological prognosis following liver resection for hepatocellular carcinoma among patients with chronic hepatitis b virus infection: A multicenter study of 1753 patients. Ann Surg Oncol (2022) 30(1):346–58. doi: 10.1245/s10434-022-12529-6
23. Zhang KJ, Ye TW, Lu WF, Xu FQ, Xie YM, Wang DD, et al. Impact of metabolic syndrome on the long-term prognosis of patients with hepatitis b virus-related hepatocellular carcinoma after hepatectomy. Front Oncol (2022) 12:1042869. doi: 10.3389/fonc.2022.1042869
24. Yoshida N, Takayama T, Midorikawa Y, Higaki T, Nakayama H, Moriguchi M, et al. Surgical outcomes in patients with hepatocellular carcinoma associated with metabolic syndrome. World J Surg (2015) 39(2):471–7. doi: 10.1007/s00268-014-2828-0
25. Labenz C, Prenosil V, Koch S, Huber Y, Marquardt JU, Schattenberg JM, et al. Impact of individual components of the metabolic syndrome on the outcome of patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Dis (2018) 36(1):78–88. doi: 10.1159/000477578
26. Morisco F, Guarino M, Valvano MR, Auriemma F, Farinati F, Giannini EG, et al. Metabolic disorders across hepatocellular carcinoma in Italy. Liver Int (2018) 38(11):2028–39. doi: 10.1111/liv.13877
27. Tian Y, Li T, Qi S, Alhourani H, Luo B, Chenqin J, et al. The impact of metabolic syndrome (MetS) on surgical outcome for patients with mostly HBV-related hepatocellular carcinoma (HCC) underwent hepatectomy. J Surg Oncol (2020) 5. doi: 10.1002/jso.26055
28. Rayman S, Sucandy I, Ross SB, Crespo K, Syblis C, App S, et al. Does metabolic syndrome effect the perioperative course and costs of patients with hepatocellular carcinoma undergoing robotic hepatectomy? A propensity score-matched analysis. Am Surg (2022) 88(9):2108–14. doi: 10.1177/00031348221091476
29. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
30. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71.
31. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane Collab (2021). Available at: www.training.cochrane.org/handbook.
32. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2010). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
33. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58.
34. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int J Epidemiol (2008) 37(5):1148–57.
35. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34.
36. Zhang J, Wu H, Wang R. Metabolic syndrome and esophageal cancer risk: A systematic review and metaanalysis. Diabetol Metab Syndr (2021) 13(1):8.
37. Shen X, Wang Y, Zhao R, Wan Q, Wu Y, Zhao L, et al. Metabolic syndrome and the risk of colorectal cancer: A systematic review and meta-analysis. Int J Colorectal Dis (2021) 36(10):2215–25. doi: 10.1007/s00384-021-03974-y
38. Wang L, Du ZH, Qiao JM, Gao S. Association between metabolic syndrome and endometrial cancer risk: A systematic review and meta-analysis of observational studies. Aging (Albany NY) (2020) 12(10):9825–39. doi: 10.18632/aging.103247
39. Guo M, Liu T, Li P, Wang T, Zeng C, Yang M, et al. Association between metabolic syndrome and breast cancer risk: An updated meta-analysis of follow-up studies. Front Oncol (2019) 9:1290. doi: 10.3389/fonc.2019.01290
40. Du W, Guo K, Jin H, Sun L, Ruan S, Song Q. Association between metabolic syndrome and risk of renal cell cancer: A meta-analysis. Front Oncol (2022) 12:928619. doi: 10.3389/fonc.2022.928619
41. Qiao L, Ma D, Lv H, Shi D, Fei M, Chen Y, et al. Metabolic syndrome and the incidence of lung cancer: a meta-analysis of cohort studies. Diabetol Metab Syndr (2020) 12:95. doi: 10.1186/s13098-020-00598-0
42. Uzunlulu M, Telci Caklili O, Oguz A. Association between metabolic syndrome and cancer. Ann Nutr Metab (2016) 68(3):173–9.
43. Li P, Wang T, Zeng C, Yang M, Li G, Han J, et al. Association between metabolic syndrome and prognosis of breast cancer: A meta-analysis of follow-up studies. Diabetol Metab Syndr (2020) 12:10.
44. Gacci M, Russo GI, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis (2017) 20(2):146–55.
45. Lu B, Qian JM, Li JN. The metabolic syndrome and its components as prognostic factors in colorectal cancer: A meta-analysis and systematic review. J Gastroenterol Hepatol (2022) 26. doi: 10.1111/jgh.16042
46. Luo Y, Liu JS, Dai B, Qian K. The influence of metabolic syndrome on gastric cancer: A meta-analysis. Asian J Surg (2021) 44(12):1596–7.
47. Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol (2018) 2(1):6. doi: 10.1038/s41698-018-0048-z
48. Chen H, Zhou XH, Li JR, Zheng TH, Yao FB, Gao B, et al. Neutrophils: Driving inflammation during the development of hepatocellular carcinoma. Cancer Lett (2021) 522:22–31. doi: 10.1016/j.canlet.2021.09.011
49. Montesi L, Mazzotti A, Moscatiello S, Forlani G, Marchesini G. Insulin resistance: mechanism and implications for carcinogenesis and hepatocellular carcinoma in NASH. Hepatol Int (2013) 7 Suppl 2:814–22. doi: 10.1007/s12072-013-9451-2
50. Li L, Liu X, Zhou L, Wang W, Liu Z, Cheng Y, et al. Autophagy plays a critical role in insulin resistance- mediated chemoresistance in hepatocellular carcinoma cells by regulating the ER stress. J Cancer (2018) 9(23):4314–24. doi: 10.7150/jca.27943
51. Imai K, Takai K, Hanai T, Suetsugu A, Shiraki M, Shimizu M. Homeostatic model assessment of insulin resistance for predicting the recurrence of hepatocellular carcinoma after curative treatment. Int J Mol Sci (2019) 20(3):605. doi: 10.3390/ijms20030605
52. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol (2015) 63(1):131–40. doi: 10.1016/j.jhep.2015.02.031
53. Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: A systematic review and meta-analysis. PLos One (2014) 9(5):e95485. doi: 10.1371/journal.pone.0095485
54. Cross TJ. Diabetes, dyslipidemia, and death from hepatocellular carcinoma in the middle-aged and elderly. Hepatology (2015) 61(5):1763. doi: 10.1002/hep.27419
55. Herreras Lopez J, Puchades L, Di Maira T, Canada AJ, Maupoey J, Lopez-Andujar R, et al. Metabolic syndrome before liver transplantation: Does it have an impact on post liver transplantation outcome? Rev Esp Enferm Dig (2022) 114(10):586–91. doi: 10.17235/reed.2022.8384/2021
56. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol (2019) 16(10):589–604. doi: 10.1038/s41575-019-0186-y
Keywords: metabolic syndrome, hepatocellular carcinoma, survival, meta-analysis, predictor
Citation: Fu J, Jiang J and Liu K (2023) Metabolic syndrome and survival of patients with hepatocellular carcinoma: A meta-analysis. Front. Oncol. 13:1117846. doi: 10.3389/fonc.2023.1117846
Received: 06 December 2022; Accepted: 04 January 2023;
Published: 23 February 2023.
Edited by:
Nicolas Golse, Hôpital Paul Brousse, FranceReviewed by:
Chao Li, Eastern Hepatobiliary Surgery Hospital, ChinaLea Duhaut, Assistance Publique Hopitaux De Paris, France
Copyright © 2023 Fu, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanghan Liu, liukanghan@163.com
 Jia Fu1
Jia Fu1 Kanghan Liu
Kanghan Liu