- 1Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, China
- 2Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China
Background: To investigate the predictive value of controlling nutritional status (CONUT) score in Postoperative Recurrence and Metastasis of Breast Cancer Patients with HER2-Low Expression.
Methods: The clinicopathological data of 697 female breast cancer patients who pathology confirmed invasive ductal carcinoma and surgery in Harbin Medical University Tumor Hospital from January 2014 to January 2017 were retrospectively analyzed. The relationship between CONUT score and various clinicopathological factors as well as prognosis was evaluated.
Results: Based on the cut-off point of ROC curve, compared with the low CONUT score group, the high CONUT score group had worse 5-year RFS. In subgroup analysis, compared with the low CONUT group, the high CONUT group had worse prognosis at different TNM stages. Univariate and multivariate results showed that the low CONUT score group had better overall survival and recurrence-free survival than the high CONUT group.
Conclusion: CONUT score is an independent predictor of postoperative recurrence and metastasis in HER2-low breast cancer patients. It is may be used as an effective tool to predict the recurrence and metastasis of HER2-low breast cancer.
Introduction
The latest global cancer statistics show that breast cancer has become the most significant contributor to cancer-related morbidity and mortality among women worldwide (1). It is estimated that there are about 410,000 new cases of breast cancer and 110,000 deaths each year in China, making it the most important health problem for women (2). Many studies have now confirmed that breast cancer is a highly heterogeneous malignancy and that tumor development and progression is not a simple procedure, but a complex process involving multiple factors and stages (3–5). The combination of radiotherapy, chemotherapy, and targeted therapy has significantly improved the overall prognosis of breast cancer patients, but a large proportion of patients still experience local recurrence or distant metastases after the cure, or even death. Tailoring treatment to each patient will improve survival and prognosis (6).
As a result, the study of prognostic predictors of breast cancer has received high priority. Currently, the most commonly used serum tumor markers to predict the prognosis of breast cancer patients include carbohydrate antigen 153 (CA153) and carcinoembryonic antigen (CEA), but their specificity and sensitivity are limited (7, 8). In addition, studies have shown that immunity and inflammation play a crucial role in the development and prognosis of cancer (9). Neutrophil-lymphocyte ratio(NLR), Platelet-lymphocyte ratio(PLR), Prognostic nutritional index (PNI), and Controlling Nutritional Status(CONUT) score are also used to assess the response to adjuvant therapy and prognostic outcome of oncology patients (10–12). In particular, it has been shown that the CONUT score correlates with the prognosis of patients with malignancies such as gastric, lung, colorectal, liver, ovarian, cervical, and renal cell cancers (13–15). The prognostic role of the CONUT score in predicting breast cancer is also gaining attention.HER2-based targeted breast cancer therapy is more precise, and HER2-targeted breast cancer therapy is essential to our clinical practice (16). The 2022 CSCO guidelines classify the molecular staging of breast cancer as HER2-positive, HER2 low expression, or HER2-negative depending on HER2 status (17).
HER2 low-expressing breast cancers account for nearly half of all breast cancers and have unique biological and pathological characteristics (18). An in-depth study of this type of disease is our focus, which is of great importance for clinical guidance. Therefore, finding viable prognostic indicators will not only guide the selection and optimization of treatment options for this particular type of patient and predict the prognosis of the disease but will also fill a gap in the biomarker panel for the prediction of this particular type of breast cancer. Existing studies have not performed CONUT scoring between HER2 subgroups and expressed the prognostic value of specific HER2 low-expressing breast cancer patients. Therefore, this paper examines the predictive value of the control CONUT score in the recurrence of metastasis in patients with HER2 low-expressing breast cancer and the clinicopathological characteristics of the patients.
Material and methods
Clinical sample and data collection
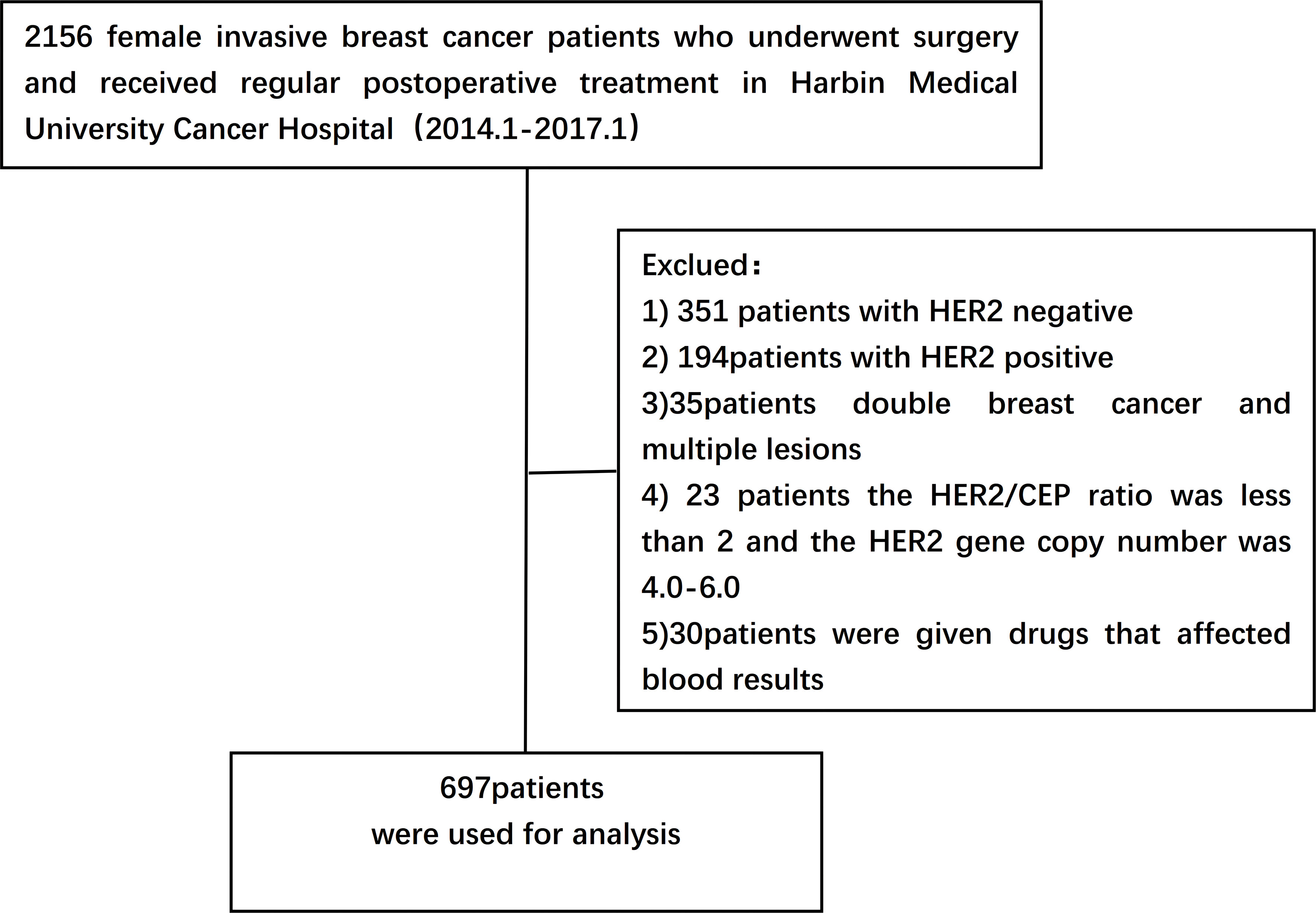
A retrospective study was performed on 2156 female breast cancer patients who underwent surgery and were pathologically diagnosed as having invasive ductal carcinoma after surgery from January 2014 to January 2017 in Harbin Medical University Cancer Hospital. Data collected included basic demographic characteristics of patients such as age, body mass index (BMI) and menopausal status, family history of tumors, date of diagnosis of breast cancer, and pathological features. All patients received standard treatment.
Inclusion criteria:
(1) Newly diagnosed female patients who underwent surgery and received regular postoperative treatment
(2) Clinical staging from stage I to III
(3) IHC results were defined as HER2 1+ or 2+ and negative FISH according to ASCO/CAP guidelines at diagnosis
(4) Pathological findings were invasive ductal carcinoma
(5) Not received any anti-HER2 medication
(6) Patients with sufficient detailed clinicopathological data
Exclusion criteria:
(1) Patients receiving neoadjuvant therapy
(2) Bilateral breast cancer and multiple lesions
(3) HER2/CEP ratio <2 and HER2 gene copy number of 4.0-6.0
(4) Patients who have inflammatory diseases, autoimmune diseases, immune deficiency diseases, infectious diseases or other diseases which affect blood components (such as blood diseases, liver dysfunction, chronic kidney diseases, etc.)
(5) Patients taking drugs that have obvious effects on blood cells(such as Chloramphenicol, Phenobarbital, Chlorpromazine, etc.) (Figure 1).
Definition of data
Pathological features and molecular subtypes
Tumor stage according to the AJCC Cancer Staging Manual, Edition 8. ER, PR, HER2 and Ki67 states are assessed by immunohistochemistry (IHC) staining or in situ hybridization (ISH), and ER and PR nuclear ≥1% are defined as positive. HER2 immunohistochemical staining was divided into HER2 positive, HER2-low expression and HER2 negative. IHC 3+ or IHC 2+ and ISH positive was defined as HER2-positive;IHC 2+ and ISH negative or IHC 1+ was defined as HER2-low expression;IHC 0 was defined as HER2-negative (19–21). Ki-67 positive nuclear ≥14% was defined as high expression, <14% as low expression (22). Tumor markers CEA and CA153 were considered positive if they exceeded the upper limit of the normal range.
Controlling nutritional status score
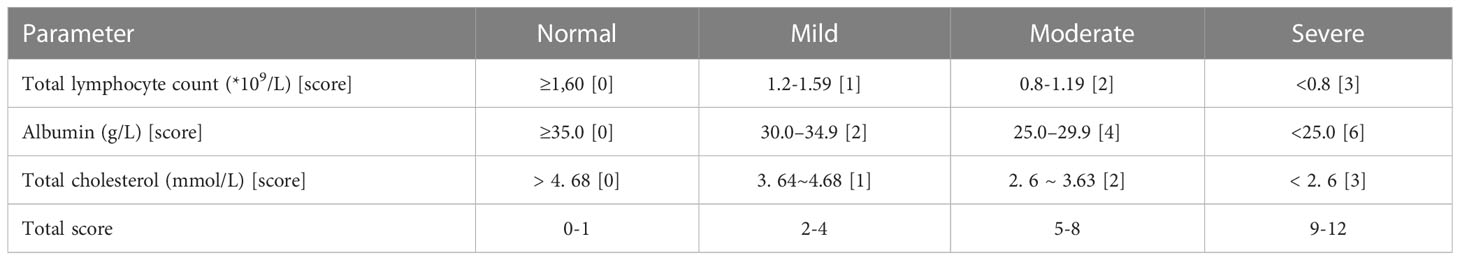
All patients had to fast peripheral venous blood collected early in the morning within two days of admission, and preoperative serum albumin (g/L), peripheral blood lymphocyte count (×109cells/L) and total serum cholesterol (mmol/L) data were collected retrospectively to calculate the CONUT score. The specific criteria for calculating the CONUT score are as follows: (1) Serum albumin was graded as ≥35.0 g/L, 30.0 ~ 34.9 g/L, 25.0 ~ 29.9 g/L and <25.0 g/L, with scores of 0, 2, 4 and 6, respectively;(2) Total peripheral blood lymphocytes were calculated as ≥ 1.60× 109/L, 1.20~1.59×109/L, 0.80~1.19×109/L and <0.80×109/L, respectively, with scores of 0, 1, 2 and 3;(3) Total serum cholesterol was scored as 0, 1, 2 and 3 in four classes: > 4.68 mmol/L, 3.64~4.68 mmol/L, 2.6~3.63 mmol/L and < 2.6 mmol/L, respectively. The scores of the above three parameters were added together to calculate the total CONUT score of the patients, where 0~1 is a normal, 2~4 is a mild abnormality, 5~8 is a moderate abnormality, and 9 and above is a severe abnormality (Table 1).
Follow-up and outcome
The survival status and treatment information of all patients were obtained through telephone follow-up and outpatient review in Harbin Medical University Cancer Hospital. The primary endpoint of this study was recurrence free survival (RFS), which was defined as the period from the date of surgery to the date of disease recurrence and metastasis or the end of follow-up. The status of recurrence and metastasis (including local recurrence, regional recurrence, isolated recurrence and distant metastasis) of patients was mainly judged by imaging examination (breast ultrasound, molybdenum target, chest CT, bone scan, etc.) or pathological results of tissue biopsy. The secondary endpoint was overall survival (OS), defined as the time from the date of surgery to any date of death or the date of the last follow-up. The follow-up period was until December 30, 2021.
Statistics and analysis
Statistical analysis was performed using SPSS 26.0 software. The optimal cut-off point of the CONUT score for predicting recurrence, metastasis and death was determined by the receiver operating characteristics curve (ROC curve) and maximum Youden index. CONUT score and clinicopathological parameters of breast cancer patients were evaluated by Pearson’s χ2 test or Fisher exact test method. Kaplan-Meier method was used to draw a survival curve. The Cox regression model was used to conduct univariate and multivariate analysis on the relationship between clinicopathological features and patients’ OS and RFS. P<0.05 for a statistically significant difference.
Results
Relationship between CONUT score and baseline characteristics of patients
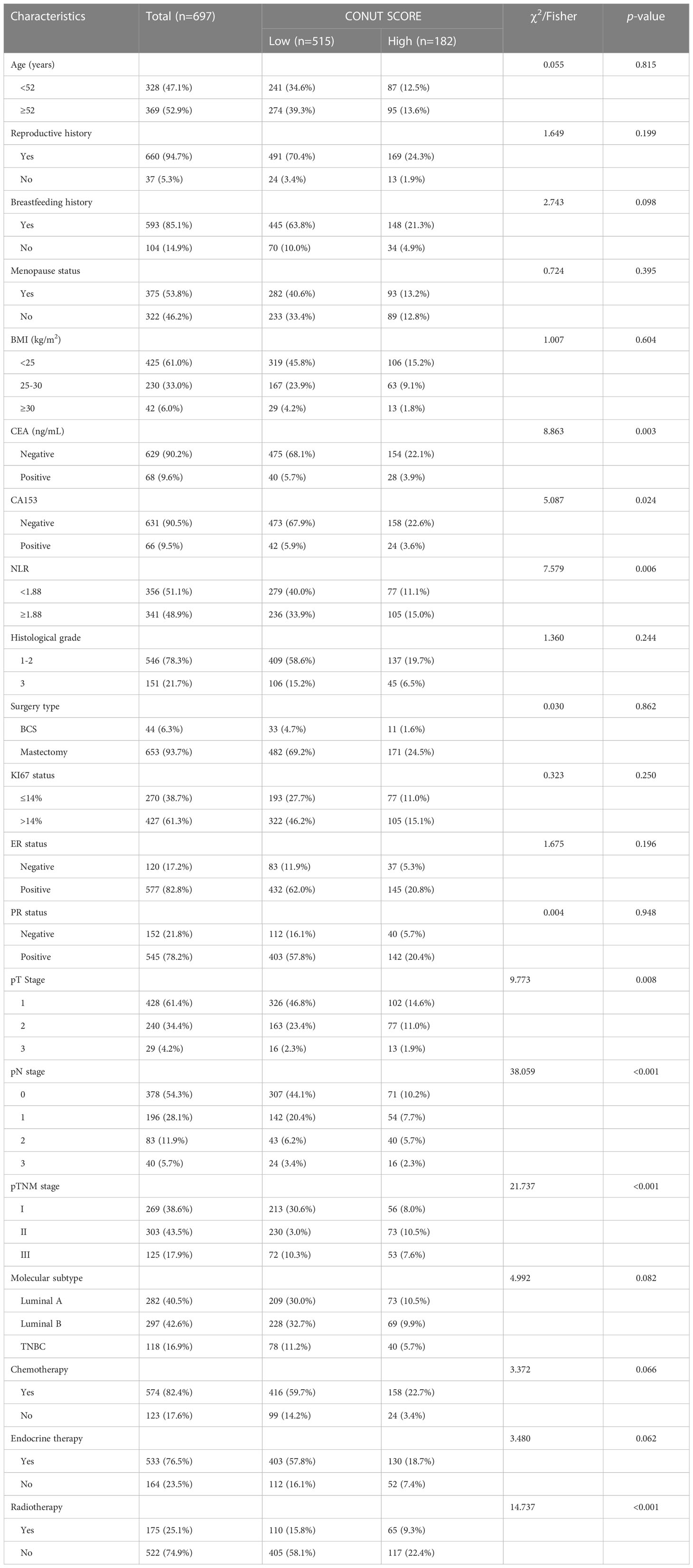
A total of 1216 women with breast cancer after surgery was finally eligible, of which 697 (57.3%) were confirmed to have HER2-low expression and were included in this study. The mean age of the patients was (52.0 ± 9.5) years The median age was 52 years (range: 19-79 years). The age of enrolled patients was divided into the <52 years old group and the ≥52 years old group. Total mastectomy was performed in 93.7%(653/697)patients. 104(19.4%)patients had no history of breastfeeding; 53.8%(375/697)were menopausal at the time of diagnosis; about 39% were overweight, of whom 6% were severely obese. There were 428(61.4%) patients in stage T1, 240(34.4%)patients in stage T2, and 29(4.2%)patients in stage T3; the positive rate of lymph nodes was 45.7%(319/697); The postoperative TNM stage was 269 (38.6%) for stage I patients, 303 (43.5%) for stage II patients and 125 (17.9%) for stage III patients; 546 (78.3%) for histological grade I-II and 152 (21.7%) for grade III; 577 (82.8%) for ER positive patients and 545 (78.2%) for PR positive patients. There were 118 cases of triple-negative breast cancer, accounting for 16.9% of all patients, 282 cases of Luminal A breast cancer (40.5%), and 297 cases of Luminal B breast cancer (42.6%). The baseline characteristics of the patients are shown in Table 2.
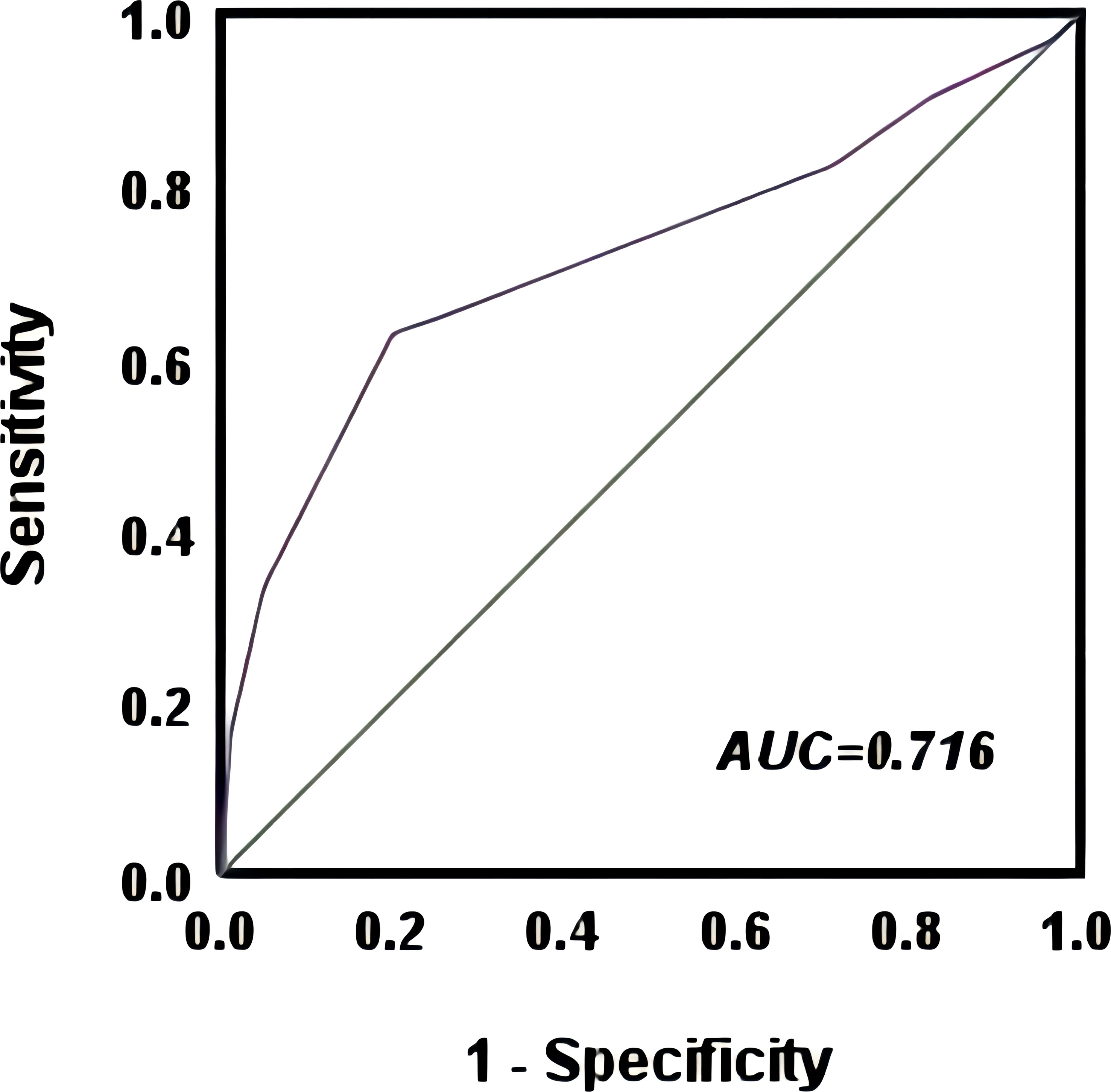
The optimal cut-off point for the CONUT score to predict postoperative recurrent metastasis in breast cancer patients was 2.5 with an AUC value of 0.716 (95% CI=0.660~0.771), as shown by the ROC curve. Based on this cut-off point, all patients were divided into a high CONUT group (≥3 points) and a low CONUT group (<3 points) (Figure 2) The results showed that CEA (χ2 = 8.863, P=0.003), CA153 (χ2 = 5.087, P=0.024), NLR(χ2 = 7.579, P=0.006), T-stage (χ2 = 9.773, P=0.008), lymph node infiltration (χ2 = 17.128, P=0.001), TNM stage (χ2 = 21.737, P<0.01), radiotherapy (χ2 = 14.737, P<0.01), and possibly CONUT high subgroup breast cancer were influential factors (Table 2).
Relationship between CONUT score and recurrence and metastasis in HER2-low breast cancer
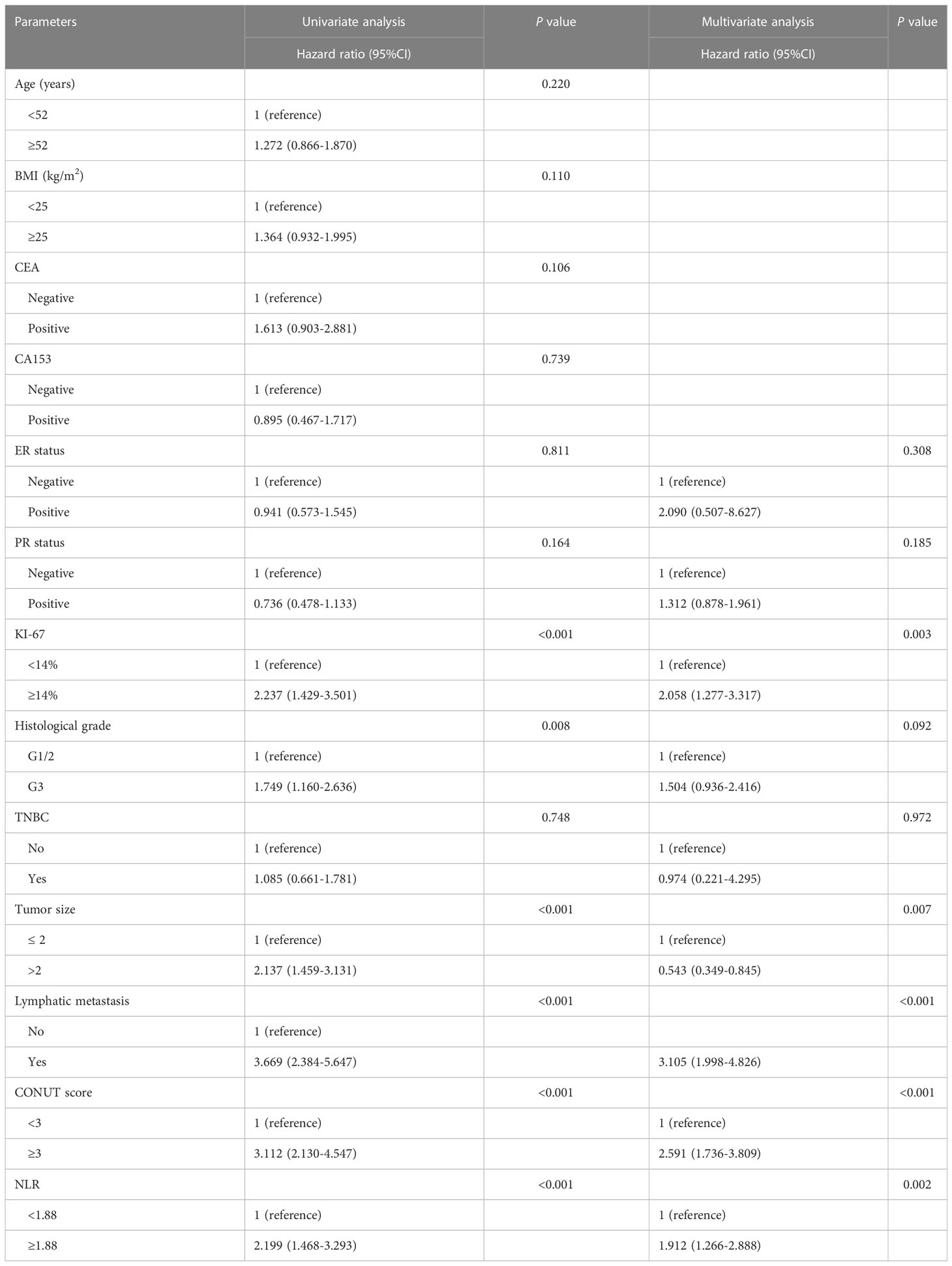
The median follow-up time for the whole group was 70 months (range: 9-96 months),107 (15.4%) patients developed recurrent metastases during the follow-up period, with RFS rates of 93.2%, 86.8% and 85.9% at 3, 5 and 7 years respectively. Kaplan-Meier curves demonstrated a significantly higher rate of recurrence and metastasis in the high CONUT subgroup compared to the low CONUT subgroup, with 5-year RFS rates of 78.9% and 89%, respectively, with statistically significant differences (χ2 = 38.384, P<0.01) (Figure 3A). Subgroup analysis further analyzed the prognosis of breast cancer patients in the CONUT score subgroup at different stages and showed that the TNM stage was a poor prognostic factor for breast cancer patients in the high CONUT subgroup. For stage I patients, the 5-year RFS rates in the high CONUT group and low CONUT group were 87.5% and 95.3% respectively, with a statistically significant difference (χ2 = 4.882, P=0.027)(Figure 3B); For stage II patients, the 5-year RFS rates in the two groups were 75.4% and 91.3% respectively, with a statistically significant difference (χ2 = 14.465, P<0.01) (Figure 3C); For stage III patients, the 5-year RFS rates for the two groups were 52.8% and 73.9%, respectively, with statistically significant differences (χ2 = 4.495, P=0.034) (Figure 3D).

Figure 3 Kaplan-Meier analysis predicts recurrent metastases in HER2-low breast cancer patients based on CONUT score (A) All breast cancer patients; (B) Stage I breast cancer patients; (C), Stage II breast cancer patients; (D) Stage III breast cancer patients.
Table 3 further analyzes the univariate and multivariate analysis affecting RFS, and the results show that the CONUT score is predictive of recurrent metastasis in breast cancer patients. Multivariate Cox regression analysis showed that lymph node metastasis (HR=3.105, 95%CI=1.998-4.826, P<0.001), tumor size (HR=0.543 95%CI=0.349-0.845, P=0.007), Ki67 status (HR=2.058, 95%CI=1.277-3.317, P=0.003) and CONUT score (HR=2.591, 95%CI=1.736-3.809, P<0.001) were independent predictors of recurrent metastasis in patients with early to mid-stage HER2 low expression breast cancer receiving regular treatment. The results showed that the CONUT score had a predictive effect on recurrence and metastasis in breast cancer patients (Table 3).
Association between CONUT score and overall survival in HER2-low breast cancer
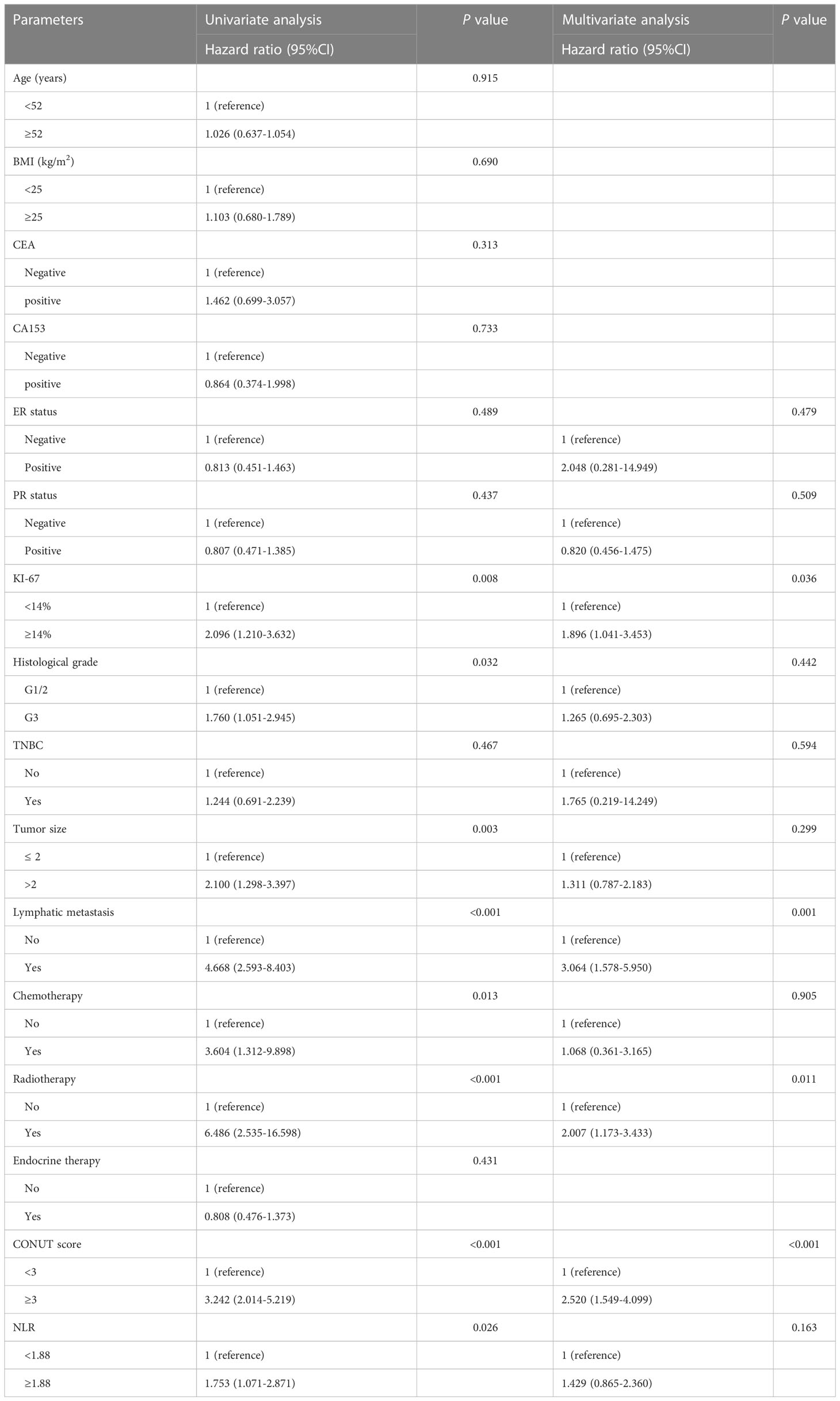
A total of 68 (9.8%) deaths occurred during the follow-up period, with overall patient survival rates of 99.2%, 96.5%, and 90.5% at 3, 5, and 7 years respectively. Kaplan-Meier curves demonstrated significantly higher mortality in the high CONUT group compared to the low CONUT group, with 5-year OS rates of 87.9% and 92.5%, respectively, with statistically significant differences (χ2 = 26.3215, P<0.01) (Figure 4A); Subgroup analysis further analyzed the prognosis of breast cancer patients in the CONUT score subgroup at different stages and showed that the TNM stage was a poor prognostic factor for breast cancer patients in the high CONUT subgroup.
Figure 4 Kaplan-Meier analysis predicts overall survival in HER2-Low breast cancer patients based on CONUT score (A), All breast cancer patients; (B), Stage I breast cancer patients; (C), Stage II breast cancer patients; (D), Stage III breast cancer patients.
For stage I patients, the 5-year OS rates for the high CONUT group and low CONUT group were 96.4% and 98.5%, respectively, with a statistically significant difference (χ2 = 9.200, P=0.002)(Figure 4B); For stage II patients, the 5-year OS rates for the two groups were 93.1% and 96.0%, respectively, with statistically significant differences (χ2 = 5.088, P=0.024) (Figure 4C); For stage III patients, the 5-year OS rates for the two groups were 80.6% and 90.1%, respectively, with a statistically significant difference (χ2 = 4.845, P=0.028) (Figure 4D).
Table 4 incorporates relevant clinicopathological variables for further univariate and multivariate analyses affecting OS, and the results indicate that the CONUT score is also predictive of overall survival in breast cancer patients. Multivariate Cox regression analysis showed that radiotherapy (HR=2.111, 95%CI=1.243~3.587, P=0.006), lymph node infiltration (HR=3.064, 95%CI=1.578-5.950, P=0.001), Ki67 status (HR=1.896, 95%CI=1.041-3.453, P=0.036) and CONUT score (HR=2.520, 95%CI=1.549-4.099, P<0.001) were independent factors affecting the survival prognosis of patients with HER2 low expression breast cancer. The above findings further illustrate the feasibility of the CONUT score in predicting survival and recurrence in breast cancer patients.
Relationship between ER status and CONUT scores
We analyzed the relationship between strong ER positivity and CONUT scores. Using 10% as the cut-off point for strong ER positivity, 513 of 697 patients were strongly ER-positive, of which 129 were in the high CONUT group and 384 were in the low CONUT group. Strongly positive ER(χ2 = 1.884, P=0.170) by chi-squared test, not statistically significantly different from the CONUT group.
However, we analyzed the relationship between ER strongly positive patients in different CONUT score groups and the use of endocrine drugs by Kaplan-Meier survival curves. 114 patients in the high CONUT subgroup used endocrine drugs, and RFS (χ2 = 13.606, P<0.001) and OS (χ2 = 16.151, P<0.001) were statistically different. Thirty-five patients in the low CONUT group were not on endocrine medication and although there was no statistically significant difference in RFS(χ2 = 3.841, P=0.050) and OS(χ2 = 2.060, P=0.151), the curves changed significantly. It was concluded from the Kaplan-Meier survival curves that patients using endocrine drugs had longer RFS and OS than those not using endocrine drugs, both in the high CONUT subgroup and in the low CONUT subgroup (Figure 5).
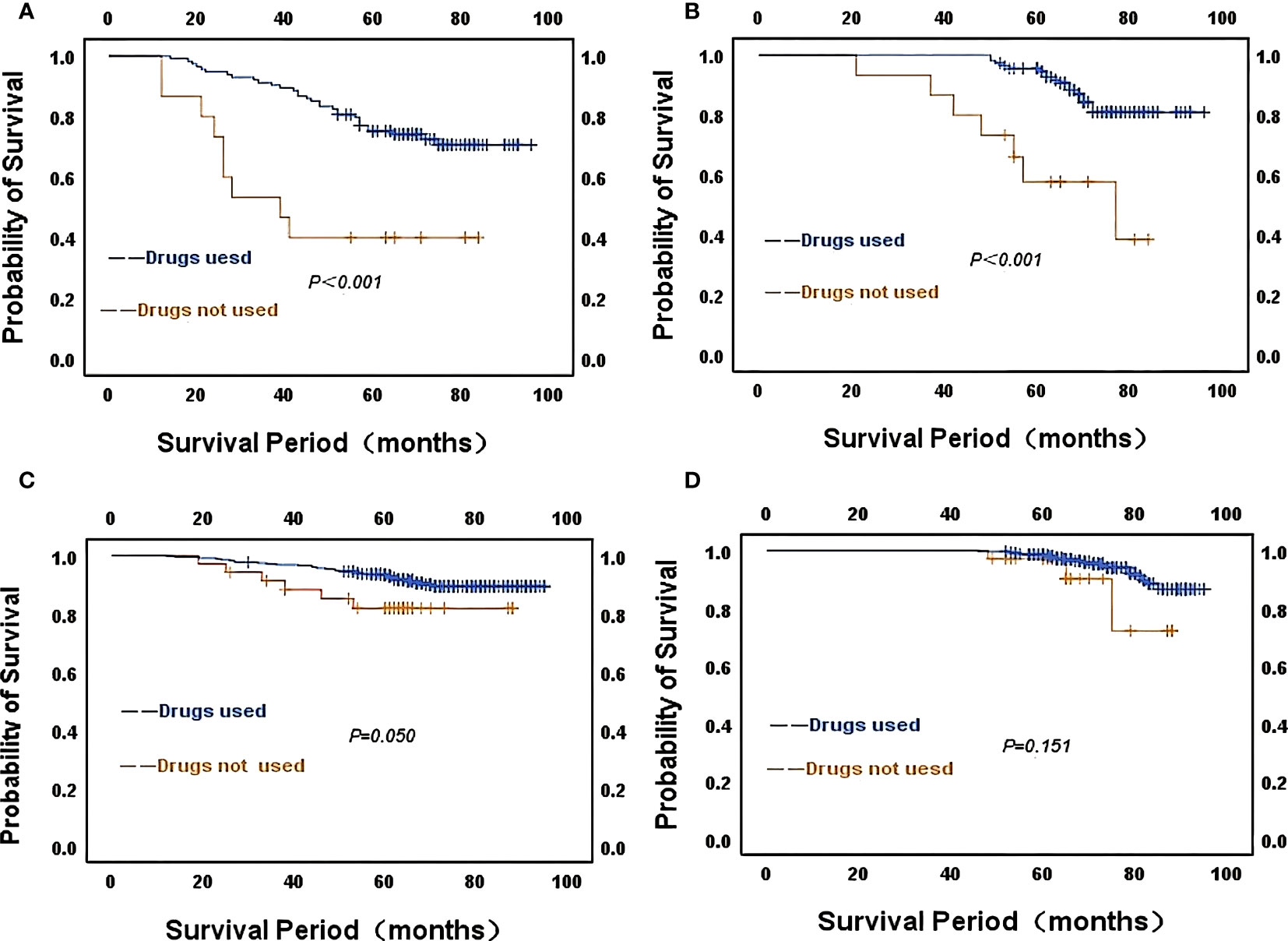
Figure 5 Kaplan-Meier survival curves of strongly ER-positive patients in different CONUT score groups about the use of endocrine drugs (A) CONUT high score for RFS; (B) CONUT high score for OS; (C) CONUT low score for RFS; (D) CONUT low score for OS.
Kaplan-Meier survival curves to statistically analyze the duration of endocrine drug use in patients in different CONUT score groups. Of the 114 patients using endocrine drugs in the CONUT high subgroup, 63 patients had been on endocrine drugs for at least 5 years, with better overall survival for patients on drugs compared to those on drugs for less than 5 years. There was no statistical difference between RFS(χ2 = 1.572, P=0.210) and OS(χ2 = 2.725, P=0.099). Of the 349 patients using endocrine medication in the low CONUT subgroup, 113 used medication for less than 5 years and 236 patients used endocrine medication for no less than 5 years. The analysis concluded that patients with longer duration of drug use had better survival compared to those with shorter duration of use. No statistical difference between RFS(χ2 = 0.307, P=0.579) and OS(χ2 = 3.573, P=0.059). The above analysis concluded that patients who had been on endocrine drugs for a longer period had relatively better overall and relapse-free survival in both the high CONUT and low CONUT subgroups.
Kaplan-Meier survival curves to statistically analyze the duration of endocrine drug use in patients in different CONUT score groups. Of the 114 patients using endocrine drugs in the CONUT high subgroup, 63 had been on endocrine drugs for at least 5 years. The analysis yielded no statistical difference in the effect of the duration of drug use in the CONUT high group on patients’ RFS(χ2 = 1.572, P=0.210) and OS(χ2 = 2.725, P=0.099). Of the 349 patients using endocrine drugs in the low CONUT subgroup, 113 had been using the drugs for less than 5 years and 236 patients had been using endocrine drugs for no less than 5 years. There was also no statistical difference in RFS (χ2 = 0.307, P=0.579) and OS(χ2 = 3.573, P=0.059) for patients by the length of drug use in the lower subgroups. However, the curves show that patients with longer duration of endocrine drug use had relatively better overall survival and relapse-free survival in both the CONUT high and CONUT low subgroups (Figure 6).
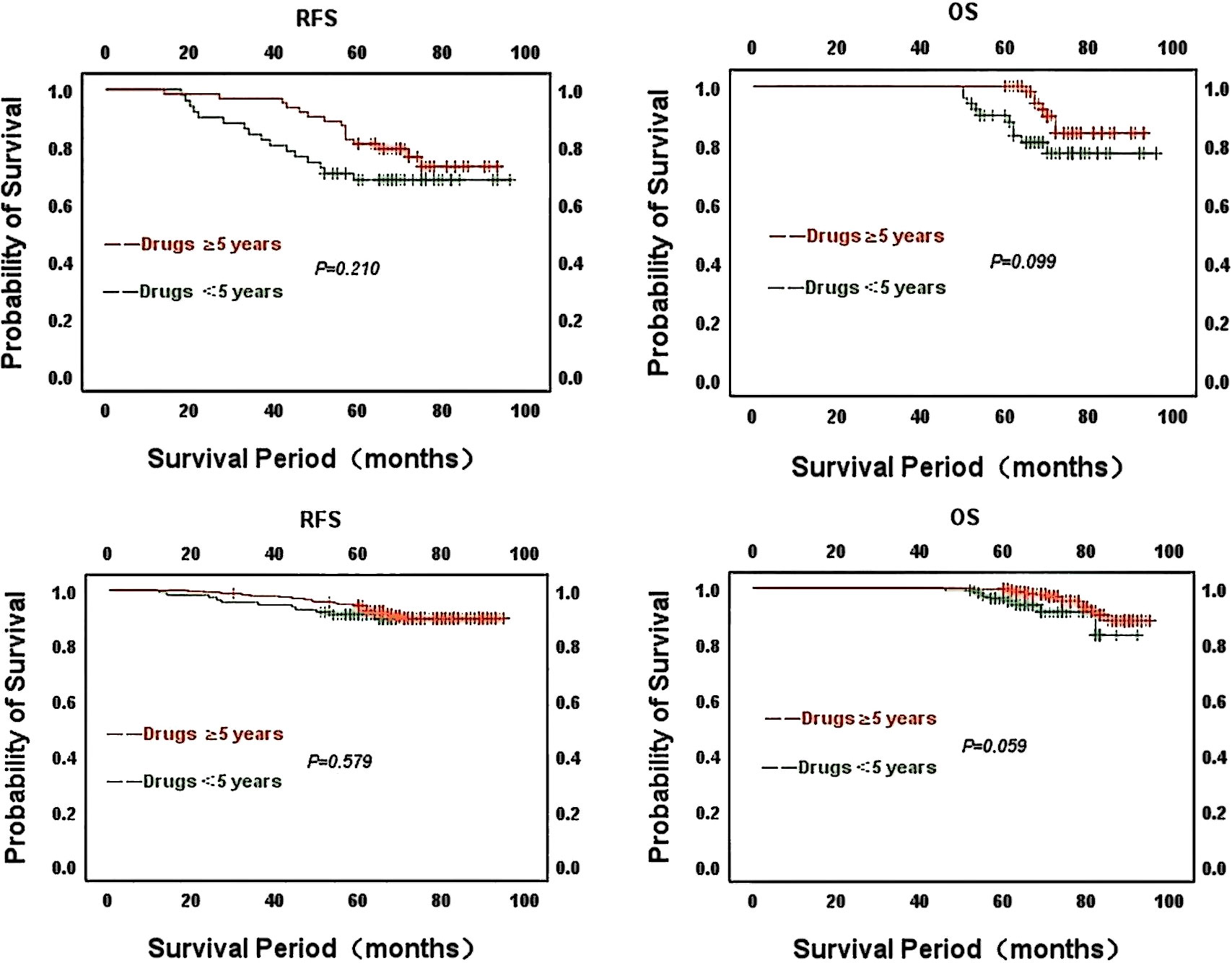
Figure 6 Kaplan-Meier survival curves for the duration of endocrine drug use in patients with strong ER positivity in different CONUT score groups Above: CONUT high group; Below: CONUT low group.
The predictive role of CONUT scores in HER2-positive patients
Relationship between CONUT scores and patient characteristics at baseline
The median age of patients was 55 years, 124 (61.4%) were not younger than 55 years and 155 (76.7%) had a history of breastfeeding before diagnosis; 13.9% of breast cancer patients had no children before diagnosis; 50.5% of breast cancer patients were menopausal at diagnosis; about 38% of breast cancer patients were overweight; The positive rate of lymph nodes was 51.0% (103/202); postoperative TNM staging, 43 (21.3%) patients with stage I, 109 (54.0%) patients with stage II and 50 (24.7%) patients with stage III; 144 (71.3%) patients positive for ER and 122 (60.4%) patients positive for PR. The baseline characteristics of breast cancer patients are shown in Supplementary Table 1.
Relationship between CONUT score and survival in HER2-positive breast cancer patients
The median follow-up time for the whole group was 72 months (range: 12-98 months), with a total of 45 cases (22.2%) of recurrent metastases during the follow-up period and RFS rates of 88.2%, 76.8%, and 71.4% at 3, 5 and 7 years, respectively. The 5-year RFS rates were 66.7% and 80.3%, respectively, with statistically significant differences (χ2 = 5.455, P=0.020). Supplementary Image 1
Univariate and multifactorial analyses of factors affecting RFS showed that lymph node metastasis (HR=7.900, 95% CI=3.315-18.829, P<0.001), tumor size (HR=5.331, 95% CI=2.083-13.641, P<0.001) and CONUT score (HR=2.127, 95% CI= 1.153-3.925, P=0.016) were independent predictors of recurrent metastasis in patients with HER2-positive early to mid-stage breast cancer receiving regular treatment, see Supplementary Table 2.
The Kaplan-Meier curve demonstrated a significantly higher rate of recurrence and metastasis in the high CONUT group compared to the low CONUT group, with 5-year RFS rates of 91.7% and 78.3% respectively, a statistically significant difference (χ2 = 5.455, P=0.020). The difference was statistically significant (χ2 = 5.455, P=0.020).
Univariate and multifactorial analyses of factors affecting OS showed that lymph node metastasis (HR=5.822, 95% CI=1.687-20.093, P=0.005), tumor size (HR=9.292, 95% CI=1.230-70.197, P=0.031) and CONUT score (HR=2.127, 95%CI= 1.153-3.925, P=0.016) were independent predictors of overall survival in patients with HER2-positive early to mid-stage breast cancer receiving regular treatment, see Supplementary Table 3.
Discussions
Breast cancer is a heterogeneous disease and precise treatment is chosen according to the individual patient’s condition. In recent years, there is increasing evidence that the immune-nutritional status of the body plays an important role in tumor development and treatment tolerance. The CONUT score reflects the nutritional status of patients and the immune defense function of the body through the combination of peripheral lymphocyte count, serum albumin and total cholesterol level. Similarly, tumor progression and treatment tolerance are closely related to nutritional and inflammatory status (23).
Daisuke et al. (24) demonstrated that CONUT can be used not only to assess the nutritional status of patients with gastric cancer and help guide the selection of appropriate preoperative nutritional interventions but also to predict long-term OS after radical gastrectomy for gastric cancer. Yuki Nemoto et al. (25) studied the effect of CONUT score on tumor outcome after radical cystectomy for advanced bladder cancer and showed that recurrence-free survival, specific survival, and overall survival were significantly shorter in the high CONUT group than in the low CONUT group. Huang et al. (26) also studied and discussed the correlation between CONUT scores and breast. Yi et al. used the CONUT score in a predictive study of HER2-positive breast cancer and showed that the CONUT score was an independent risk factor for recurrence and metastasis in patients with HER2-positive early breast cancer.
Based on previous studies, the CONUT score has shown some promise for clinical application. Our study focused on a unique subtype of all breast cancers for data analysis, which has unique pathological features and biological characteristics and is the most represented molecular subtype of all breast cancer patients, and it is more clinically relevant to understand the association of CONUT scores with this type of breast cancer. Therefore, this study retrospectively collected patients with surgically treated HER2 low-expression breast cancer to assess the relationship between CONUT scores and recurrence, metastasis, and survival in patients with this particular type of breast cancer.
Our study showed that higher CONUT scores correlated with tumor size, lymph node infiltration, and blood markers CEA and CA153 in all patients with low HER2 expression. Among the pathological variables, we found higher CONUT scores with higher Ki-67 status apparent value-added scores. Analysis of univariate and multifactorial Cox proportional hazards regression models showed that the CONUT score was an independent prognostic factor for OS and RFS. Our results are similar to those of previous studies (27), with patients with lower CONUT scores exhibiting longer RFS and OS compared to the high CONUT subgroup. but our study was conducted in a separate HER2 subtype, which not only brings more nuance to breast cancer but is also an important complementary part of the role of such predictors in breast cancer. At the same time, the role of the CONUT score in HER2-positive subtypes was further analyzed in our additional data and the results are similar to previous studies, confirming that the CONUT score is an independent prognostic indicator for this type of breast cancer and reinforcing the use of the CONUT score in the prognosis of HER2 subtypes of breast cancer. High CONUT scores not only suggest a high risk of recurrence and metastasis but also suggest relatively poor overall survival for patients.
Considering the possible influence of ER expression on this type, we compared the correlation between patients with strong positive ER and CONUT scores, but the chi-square test showed no statistically significant difference. Comparing the association with the use of endocrine drugs and the different CONUT score groups showed that patients using endocrine drugs had better survival than those not using endocrine drugs, regardless of whether they were in the high or low group. Patients who used endocrine drugs for more than 5 years had better survival than those who used them for less than 5 years. We also explored the predictive role of the CONUT score in HER2-positive patients. The results were similar to those in HER2 low expressing breast cancer, with the CONUT high subgroup having worse RFS and OS than the CONUT low subgroup.
Among the 3 evaluation parameters of the CONUT score, serum albumin level is the most important influencing factor. It not only reflects the nutritional status of patients but also correlates with the incidence of postoperative complications, mortality and overall survival of oncology patients (28–31). A decrease in serum albumin weakens cellular and humoral immunity, phagocytosis, and other defense mechanisms in cancer patients, leading to an inflammatory response, and conversely, serum albumin levels are reduced by inflammation (32, 33). Many studies have confirmed that low serum albumin levels predict poorer morbidity and mortality in solid and hematologic tumors (34). As the most commonly used inflammatory indicator in the clinic, peripheral blood lymphocytes play a crucial role in tumor immunity, such as cytotoxic cell death, tumor cell proliferation, migration inhibition, etc. Its decrease reflects the suppression of the body’s tumor immune function, which puts the growth of tumor cells in an infinite proliferation state, which in turn leads to tumor metastasis and poor prognosis (35–38). Likewise, cholesterol is an essential lipid for maintaining cellular homeostasis and is involved in the acquired and adaptive immune responses of the body, playing a critical role in cell membrane formation and various biochemical pathways that are essential for normal biological functions. The relationship between total serum cholesterol levels and tumorigenesis, prognostic outcome, and chemotherapy resistance has been reported in various malignancies (39–41).
The above evidence may explain the relationship between CONUT scores and the poor prognosis of tumor patients. The results of this paper and the above studies suggest that the CONUT score may also be an important indicator for the prognosis of breast cancer patients and that the three categories of blood indicators included in the CONUT score can reflect the immune-nutritional status of individuals more accurately and comprehensively than previous predictors, helping clinicians to accurately identify patients at high risk and thus provide more individualized and comprehensive management plans for cancer patients. This study has some limitations in that the patient sample size was limited and the data collected were from a single-center retrospective pilot study of only one specific type of breast cancer patient, which increases the risk of bias. At the same time, this article lacks external data to validate the cohort, and the prognostic value of the CONUT score for breast cancer patients with different molecular subtypes still needs to be confirmed by large, multicenter, prospective studies.
Conclusion
The preoperative CONUT score may be a useful predictor of postoperative recurrence and metastasis in patients with HER2-low breast cancer, monitoring oncology patients and providing clinical guidance for more individualized treatment.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Harbin Medical University Cancer Hospital. All procedures in this study comply with the Commission’s standards, as well as the 2013 Declaration of Helsinki and other ethical standards. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL and YZ: Conceptualization, Data curation, Formal analysis, Writing-original draft. ZZ: Data curation, Formal analysis. LS: Data curation, Formal analysis. YH: Data curation, Formal analysis. XL: Data curation, Formal analysis. SC: Data curation, Formal analysis, Writing-review & editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Petrel Foundation of Harbin Medical University Cancer Hospital (JJZD2020-10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1116631/full#supplementary-material
Supplementary Image 1 | Kaplan-Meier survival curves for recurrence-free survival and overall survival in HER2-positive breast cancer with CONUT score.
Abbreviations
CONUT, Controlling Nutritional Status; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor; CI, Confidence interval; FISH, Fluorescence in situ; IHC, Immunohistochemistry; HR, hazard ratio; BMI, body mass index; TNBC, triple negative breast cancer; BCS, Breast-Conserving Surgery; CA153, carbohydrate antigen153; CEA, carcinoembryonic antigen; OS, Overall survival; RFS, Recurrence free survival; NLR, Neutrophil to lymphocyte ratio; PLR, Platelet to lymphocyte ratio; PNI, Prognostic nutritional index.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal. A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
3. Burstein HJ, Curigliano G, Thurlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the st. gallen international consensus guidelines for treatment of early breast cancer 2021. Ann Oncol (2021) 32(10):1216–35. doi: 10.1016/j.annonc.2021.06.023
4. Kalinowski L, Saunus JM, McCart Reed AE, Lakhani SR. Breast cancer heterogeneity in primary and metastatic disease. Adv Exp Med Biol (2019) 1152:75–104. doi: 10.1007/978-3-030-20301-6_6
5. Curigliano G, Burstein HJ, P.W. E, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the st. gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol (2019) 30(7):1181. doi: 10.1093/annonc/mdy537
6. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. NCCN Guidelines(R) insights: breast cancer, version 4.2021. J Natl Compr Canc Netw (2021) 19(5):484–93. doi: 10.6004/jnccn.2021.0023
7. Tang S, Zhou F, Sun Y, Wei L, Zhu S, Yang R, et al. CEA in breast ductal secretions as a promising biomarker for the diagnosis of breast cancer: a systematic review and meta-analysis. Breast Cancer (2016) 23(6):813–9. doi: 10.1007/s12282-016-0680-9
8. Uygur MM, Gumus M. The utility of serum tumor markers CEA and CA 15-3 for breast cancer prognosis and their association with clinicopathological parameters. Cancer Treat Res Commun (2021) 28:100402. doi: 10.1016/j.ctarc.2021.100402
9. Faldoni FLC, Rainho CA, Rogatto. SR. Epigenetics in inflammatory breast cancer: biological features and therapeutic perspectives. Cells (2020) 9(5). doi: 10.3390/cells9051164
10. Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res (2020) 2020:9146042. doi: 10.1155/2020/9146042
11. Chen L, Bai P, Kong X, Huang S, Wang Z, Wang X, et al. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Dev Biol (2021) 9:656741. doi: 10.3389/fcell.2021.656741
12. Corbeau I, Jacot W, Guiu S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers (Basel) (2020) 12(4):20–21. doi: 10.3390/cancers12040958
13. Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer (2018) 21(2):204–12. doi: 10.1007/s10120-017-0744-3
14. Lin ZX, Ruan DY, Jia CC, Wang TT, Cheng JT, Huang HQ, et al. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with HBV-associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol (2020) 22(3):370–80. doi: 10.1007/s12094-019-02137-4
15. Mao YS, Hao SJ, Zou CF, Xie ZB, Fu DL. Controlling nutritional status score is superior to prognostic nutritional index score in predicting survival and complications in pancreatic ductal adenocarcinoma: a Chinese propensity score matching study. Br J Nutr (2020) 124(11):1190–7. doi: 10.1017/S0007114520002299
16. Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers (Basel) (2018) 10(10):10. doi: 10.3390/cancers10100342
17. Li J, Jiang Z. Chinese Society of clinical oncology breast cancer (CSCO BC) guidelines in 2022: stratification and classification. Cancer Biol Med (2022) 19(6):769–73. doi: 10.20892/j.issn.2095-3941.2022.0277
18. de Moura Leite L, Cesca MG, Tavares MC, Santana DM, Saldanha EF, Guimaraes PT, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat (2021) 190(1):155–63. doi: 10.1007/s10549-021-06365-7
19. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med (2022) 387(1):9–20. doi: 10.1056/NEJMoa2203690
20. Chick RC, Clifton GT, Hale DF, Vreeland TJ, Hickerson AT, Kemp Bohan PM, et al. Subgroup analysis of nelipepimut-s plus GM-CSF combined with trastuzumab versus trastuzumab alone to prevent recurrences in patients with high-risk, HER2 low-expressing breast cancer. Clin Immunol (2021) 225:108679. doi: 10.1016/j.clim.2021.108679
21. Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean breast cancer society. Breast Cancer Res (2022) 24(1):22. doi: 10.1186/s13058-022-01519-x
22. Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology (2017) 49(2):166–71. doi: 10.1016/j.pathol.2016.11.006
23. Andrejeva G, Rathmell. JC. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab (2017) 26(1):49–70. doi: 10.1016/j.cmet.2017.06.004
24. Aoyama T, Komori K, Nakazano M, Hara K, Tamagawa H, Kazama K, et al. The clinical influence of the CONUT score on survival of patients with gastric cancer receiving curative treatment. In Vivo (2022) 36(2):942–8. doi: 10.21873/invivo.12784
25. Nemoto Y, Kondo T, Ishihara H, Takagi T, Fukuda H, Yoshida K, et al. The controlling nutritional status CONUT score in patients with advanced bladder cancer after radical cystectomy. In Vivo (2021) 35(2):999–1006. doi: 10.21873/invivo.12343
26. Huang ZZ, Song CG, Huang JJ, Xia W, Bi XW, Hua X, et al. Prognostic significance of the controlling nutritional status (CONUT) score in surgically treated breast cancer patients. Gland Surg (2020) 9(5):1370–9. doi: 10.21037/gs-20-294
27. Li W, Li M, Wang T, Ma G, Deng Y, Pu D, et al. Controlling nutritional status (CONUT) score is a prognostic factor in patients with resected breast cancer. Sci Rep (2020) 10(1):6633. doi: 10.1038/s41598-020-63610-7
28. Hu WH, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J (2019) 18(1):33. doi: 10.1186/s12937-019-0458-y
29. Sofic A, Rasic I, Halilovic E, Mujic A, Muslic D. Is preoperative hypoproteinemia associated with colorectal cancer stage and postoperative complications? Med Glas (Zenica) (2021) 18(2):450–5. doi: 10.17392/1353-21
30. Bekos C, Polterauer S, Seebacher V, Bartl T, Joura E, Reinthaller A, et al. Pre-operative hypoalbuminemia is associated with complication rate and overall survival in patients with vulvar cancer undergoing surgery. Arch Gynecol Obstet (2019) 300(4):1015–22. doi: 10.1007/s00404-019-05278-7
31. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J (2010) 9:69. doi: 10.1186/1475-2891-9-69
32. Fujii T, Tokuda S, Nakazawa Y, Kurozumi S, Obayashi S, Yajima R, et al. Implications of low serum albumin as a prognostic factor of long-term outcomes in patients with breast cancer. In Vivo (2020) 34(4):2033–6. doi: 10.21873/invivo.12003
33. Ouyang X, Dang Y, Zhang F, Huang. Q. Low serum albumin correlates with poor survival in gastric cancer patients. Clin Lab (2018) 64(3):239–45. doi: 10.7754/Clin.Lab.2017.170804
34. Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, et al. The therapeutic use of human albumin in cancer patients’ management. Crit Rev Oncol Hematol (2017) 120:203–9. doi: 10.1016/j.critrevonc.2017.11.008
35. Greten FR, Grivennikov. SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
36. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer (2011) 105(1):93–103. doi: 10.1038/bjc.2011.189
37. Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer (2016) 4. doi: 10.1186/s40425-016-0165-6
38. Demaria O, Vivier E. Immuno-oncology beyond TILs: unleashing TILCs. Cancer Cell (2020) 37(4):428–30. doi: 10.1016/j.ccell.2020.03.021
39. Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol (2021) 14(6):101043. doi: 10.1016/j.tranon.2021.101043
40. Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab (2020) 2(2):132–41. doi: 10.1038/s42255-020-0174-0
Keywords: controlling nutritional status, breast cancer, HER-2 low, recurrence, metastasis, prognosis
Citation: Li Y, Zhang Y, Zhou Z, Shang L, Huang Y, Lu X and Cheng S (2023) Predictive value of controlling nutritional status score in postoperative recurrence and metastasis of breast cancer patients with HER2-low expression. Front. Oncol. 13:1116631. doi: 10.3389/fonc.2023.1116631
Received: 05 December 2022; Accepted: 19 June 2023;
Published: 10 July 2023.
Edited by:
Antonio Macciò, Ospedale Oncologico Armando Businco, ItalyReviewed by:
Govind Babu Kanakasetty, HCG Cancer Hospital, IndiaGazi Nurun Nahar Sultana, University of Dhaka, Bangladesh
Copyright © 2023 Li, Zhang, Zhou, Shang, Huang, Lu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoqiang Cheng, c2hhb3FpYW5nY2hlbmdASG90bWFpbC5jb20=; Xiangshi Lu, bHV4aWFuZ3NoaUAxNjMuY29t
†These authors have contributed equally to this work
 Yue Li1†
Yue Li1† Zhaoyue Zhou
Zhaoyue Zhou Lingmin Shang
Lingmin Shang Yuanxi Huang
Yuanxi Huang Shaoqiang Cheng
Shaoqiang Cheng