
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 23 February 2023
Sec. Cancer Metabolism
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1116307
This article is part of the Research Topic Understanding patient nutrition and cancer progression View all 6 articles
 Yue Zhang1,2,3,4,5†
Yue Zhang1,2,3,4,5† Xiude Fan2,3,4,5
Xiude Fan2,3,4,5 Chunhui Zhao1,2,3,4,5
Chunhui Zhao1,2,3,4,5 Zinuo Yuan1,2,3,4,5
Zinuo Yuan1,2,3,4,5 Yiping Cheng1,2,3,4,5
Yiping Cheng1,2,3,4,5 Yafei Wu2,3,4,5
Yafei Wu2,3,4,5 Junming Han2,3,4,5
Junming Han2,3,4,5 Zhongshang Yuan6
Zhongshang Yuan6 Yuanfei Zhao7,8*†
Yuanfei Zhao7,8*† Keke Lu1,4*†
Keke Lu1,4*†Background & purpose: Obesity and metabolic disorders were associated with increased risk of MM, a disease characterized by high risk of relapsing and require frequent hospitalizations. In this study, we conducted a retrospective cohort study to explore the association of metabolic obesity phenotypes with the readmission risk of MM.
Patients & methods: We analyzed 34,852 patients diagnosed with MM from the Nationwide Readmissions Database (NRD), a nationally representative database from US. Hospitalization diagnosis of patients were obtained using ICD-10 diagnosis codes. According to obesity and metabolic status, the population was divided into four phenotypes: metabolically healthy non-obese (MHNO), metabolically unhealthy non-obese (MUNO), metabolically healthy obese (MHO), and metabolically unhealthy obese (MUO). The patients with different phenotypes were observed for hospital readmission at days 30-day, 60-day, 90-day and 180-day. Multivariate cox regression model was used to estimate the relationship between obesity metabolic phenotypes and readmissions risk.
Results: There were 5,400 (15.5%), 7,255 (22.4%), 8,025 (27.0%) and 7,839 (35.6%) unplanned readmissions within 30-day, 60-day, 90-day and 180-day follow-up, respectively. For 90-day and 180-day follow-up, compared with patients with the MHNO phenotype, those with metabolic unhealthy phenotypes MUNO (90-day: P = 0.004; 180-day: P = < 0.001) and MUO (90-day: P = 0.049; 180-day: P = 0.004) showed higher risk of readmission, while patients with only obesity phenotypes MHO (90-day: P = 0.170; 180-day: P = 0.090) experienced no higher risk. However, similar associations were not observed for 30-day and 60-day. Further analysis in 90-day follow-up revealed that, readmission risk elevated with the increase of the combined factor numbers, with aHR of 1.068 (CI: 1.002-1.137, P = 0.043, with one metabolic risk factor), 1.109 (CI: 1.038-1.184, P = 0.002, with two metabolic risk factors) and 1.125 (95% CI: 1.04-1.216, P = 0.003, with three metabolic risk factors), respectively.
Conclusion: Metabolic disorders, rather than obesity, were independently associated with higher readmission risk in patients with MM, whereas the risk elevated with the increase of the number of combined metabolic factors. However, the effect of metabolic disorders on MM readmission seems to be time-dependent. For MM patient combined with metabolic disorders, more attention should be paid to advance directives to reduce readmission rate and hospitalization burden.
Multiple myeloma (MM), the second most common hematological malignancy, accounts for 1% of all tumor diseases (1). Although the treatment of MM makes significant progress, MM remains a severe and incurable disease, and most patients relapse and require frequent hospitalizations (2, 3). Hospital readmission is a indicator of medical resource utilization and nursing quality evaluation, and also an important predictor of disease outcomes, such as increased morbidity, raised mortality, and the loss of functional independence (4, 5). The annual cost of readmissions within 30 days after discharge accounts for more than $17 billion of avoidable Medicare expenses in the United States (6). It is foreseeable that repeat hospitalization of MM patients will be a significant burden on the healthcare system and patients. Therefore, there is an urgent need to identify risk factors of readmission to reduce this significant and continuous burden.
Obesity, an urgent and growing global public health threat, is a significant risk factor for chronic diseases such as cardiovascular disease (CVD), diabetes, and certain types of cancer (7, 8). Although evidence suggests that obesity is associated with higher incidence and mortality in MM (9), the effect of obesity on MM is still controversial (10, 11). Studies suggested that metabolically healthy obese (MHO) individuals are at lower risk of CVD, cancers and mortality than those metabolically unhealthy obese (MUO) (12, 13). Similarly, normal-weight individuals with unhealthy metabolic characteristics (metabolically unhealthy non-obese [MUNO]) have increased risk of CVD than those with metabolically healthy status (metabolically healthy non-obese [MHNO]) (13). These differences implied that in addition to obesity, taking into account the coexistence of metabolic abnormalities can more effective in identifying risk factors for obesity-related diseases (9–11). Given the conflicting evidence on the relationship between obesity and MM, it seems to be more appropriate to consider the obesity and metabolic jointly to study the risk factors for MM.
Therefore, to accurately and systematically explore the potential modifiable risk factors for MM, we conducted a retrospective cohort study using the Nationwide Readmissions Database (NRD), a large contemporary nationwide database from the United States. We evaluated the differences in readmission risk among patients with different combinations of obesity and metabolic status to identify risk factor of readmission, and provide a clinical reference for optimizing patient care and minimizing the medical burden.
We conducted a retrospective cohort study using 2018 data from the NRD database (https://hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp). The NRD is a longitudinal database developed and maintained by the Agency for Healthcare Research and Quality from the US. The NRD 2018 contains data from 28 states including clinical and non-clinical information at the hospital and patient levels, representing 60 percent of total population and 58.7 percent of hospitalizations in the United States (14). Rehabilitation and long-term care hospitals are not included. Based on patient linkage numbers, the NRD can track individuals across hospitals within a state. Because of the de-identified nature of the data, this study was determined to be exempt from ethics board review by the local ethics committee, Biomedical Research Ethic Committee of Shandong Provincial Hospital.
We used the International Classification of Diseases-Tenth Revision-Clinical Modification (ICD-10-CM) codes (c90.x based) to identify patients with the first 30 diagnoses including MM on the index discharge. Due to data limitations, we did not consider the clinical stage, karyotype, oncogenic mutations and treatment strategies of MM patients in this study. The codes used to diagnose disease were listed in Supplementary Table 1. Patients with missing important hospitalization information, aged under 18 years old, pregnant, died during the index hospitalization, and low body weight [body mass index (BMI) ≤ 19.9 kg/m2] were excluded. Because the NRD cannot track admissions for the following year, we excluded those discharged in December for the 30-day follow-up study; for 60-day, we excluded those who were discharged in November and December; for 90-day, we excluded those who were discharged from October to December, and for the 180-day, we excluded those who were discharged from July to December. Detailed inclusion and exclusion criteria were shown in Figure 1.
For each patient, demographic variables and hospitalization characteristics were collected, which included age, gender, primary payer status (Medicare, Medicaid, private insurance, self-pay, free, and other types), median household income by ZIP code, discharge disposition, location, length of stay (LOS), total charges and comorbidities. Detailed information was provided in Table 1.
The readmission was defined as unplanned readmission to the hospital due to any diagnosis within 30 days, 60 days, 90 days, and 180 days from index discharge. If there were more than one readmission, only the first readmission was counted. Over-weight and obesity was defined as BMI ≥ 25 kg/m2 (15, 16). There was still lack of consensus on the definition of metabolic health status. However, many studies defined metabolic unhealthy status as presence of ≥2 metabolic risk factors (17, 18). According to the Adult Treatment Panel III (ATP-III) criteria and the International Diabetes Federation (IDF) consensus (19, 20), we defined metabolic risk factors including (1) hypertension: primary hypertension or secondary hypertension or undiagnosed elevated blood pressure; (2) dyslipidemia: high serum triglyceride (TGs) levels or high high-density lipoprotein (HDL)-cholesterol levels, etc.; (3) hyperglycemia: pre-diabetes or diabetes mellitus or other specific diabetes. Abdominal obesity was not included in the models because of the collinearity of waist circumference and BMI. Metabolically unhealthy status was defined as with two or more of the above metabolic risk factors. The codes used were listed in Supplementary Table 1. Based on the obesity and metabolic status, individuals were classified into four different phenotypes; (1) metabolically healthy non-obesity (MHNO); (2) metabolically unhealthy non-obesity (MUNO); (3) metabolically healthy obesity (MHO); and (4) metabolically unhealthy obesity (MUO). For 90-day analysis, based on combined metabolic risk factor type only, individuals were further divided into: (1) no metabolic risk factor; (2) only with hyperglycemia; (3) only with dyslipidemia; (4) only with hypertension. In addition, based on the number of combined metabolic risk factors, individuals were further divided into: (1) no metabolic risk factor; (2) one metabolic risk factor; (3) two metabolic risk factors; (4) three metabolic risk factors.
The primary outcome of the study was all-cause unplanned readmissions of different metabolic obesity phenotypes for four follow-up days among patients with MM. The secondary outcomes were the LOS, mean total hospitalization charges, and readmission mortality during the readmission.
We used descriptive statistics to compare the demographic and admission characteristics of patients in different metabolic obesity phenotypes. The Chi-square test was used to analyze categorical variables, and the ANOVA was used to analyze continuous variables. Categorical variables were presented as counts with percentages. Continuous variables were presented as means and standard deviation (SD). We estimated the unadjusted hazard ratio (HR) with the univariate Cox proportional hazards model. In addition, the multivariate Cox regression model was used to analyze adjusted HR (aHR) value including the potential confounding factors. Based on the associations found from the literature and univariate analysis, we adjusted factors such as age, gender, elective versus non-elective admission, primary payer, disposition of patient, resident, length of stay, total charges, emergency record, same day events, patient location, antineoplastic chemotherapy, and stem cells transplant status. All calculated P-values were two-sided, and the threshold for significance was set at P < 0.05. All statistical analyses were performed by SPSS software (version 26.0; SPSS, Chicago, IL).
From 17,686,511 discharge records in NRD 2018, we included 34,852 patients diagnosed with MM at index discharge in the cohort study (Figure 1). For 30-day, 60-day, 90-day and 180-day follow-ups, we analyzed 34,852, 32,334, 29,747 and 22,032 participants, respectively (Figure 1). Table 1 provided the demographic characteristics and common comorbidities of patients with different metabolic obesity phenotypes.
The mean age of the population was 69.8 years old, and the elderly aged 65 or above accounted for 69.4% (Table 1). The majority of patients were male (55.3%) and used privately insured (70.0%). There were 4718 (13.5%) patients with obesity and 15417 (44.2%) patients with metabolically unhealthy status.
The MHNO group was the largest of the four phenotypes, and the MHO had the longest mean LOS (9.5 days) and highest mean total charges ($112913.9). Compared with MHNO and MHO groups, MUNO and MUO groups had more elder patients (>65 years) and higher prevalence of heart failure, renal failure, and coronary heart disease. MUNO, MHO, and MUO groups had higher prevalence of depression than the MHNO group (Table 1).
For 30-day, 60-day, 90-day and 180-day follow-up, we observed that 5,400 (15.5%), 7,255 (22.4%), 8,025 (27.0%) and 7,839 (35.6%) patients experienced unplanned readmissions (Supplementary Table 2), respectively. Figure 2 showed the readmission rate among different metabolic obesity phenotypes in the four cohorts. The MUNO and MUO groups had higher readmission rate than MHNO and MHO groups in 60-day, 90-day, and 180-day studies, respectively (Figure 2).
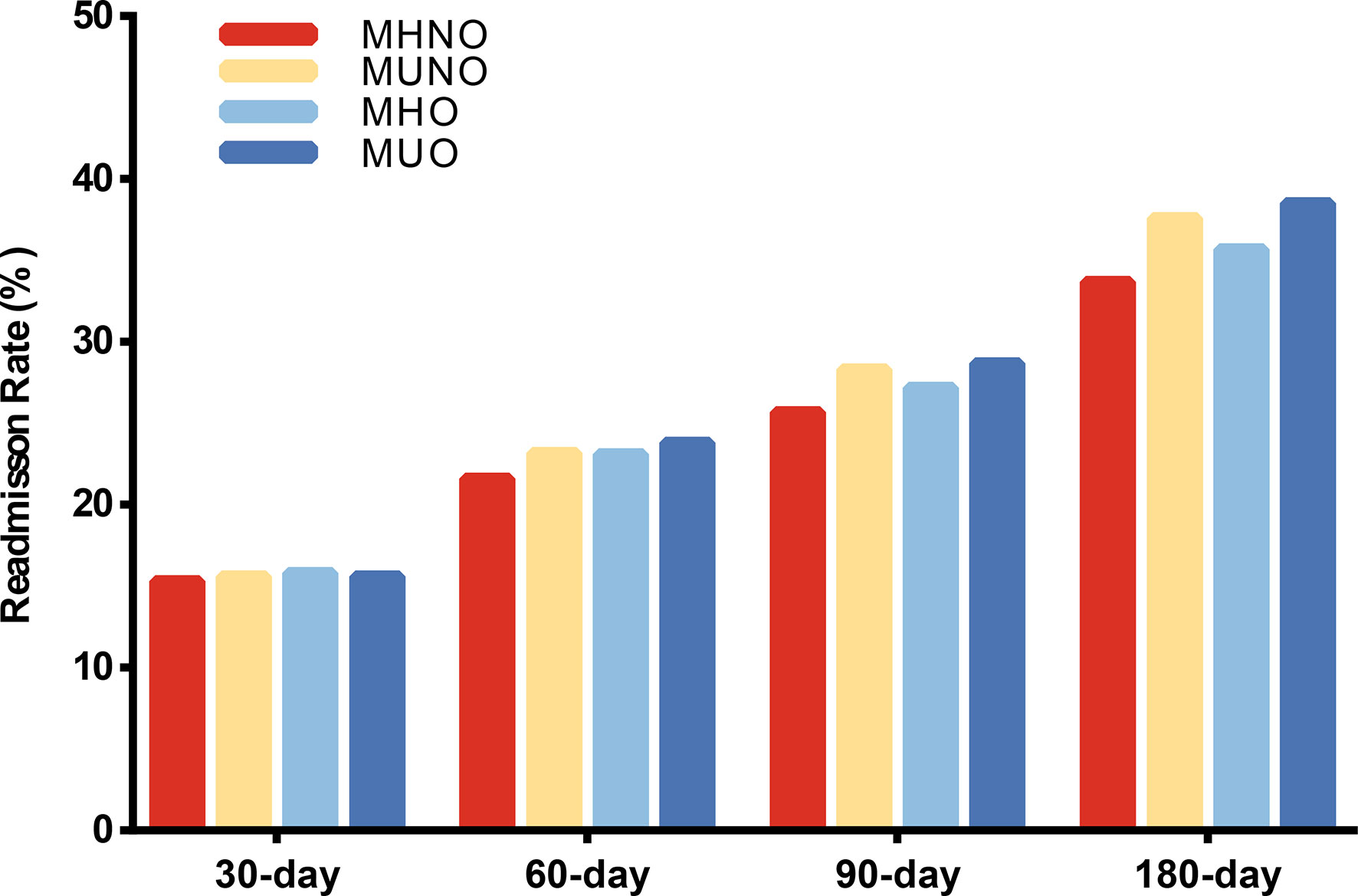
Figure 2 Readmission rate among different metabolic obesity phenotypes in four follow-up days. MHNO, metabolically healthy non-obese; MUNO, metabolically unhealthy non-obese; MHO, metabolically healthy obese; MUO, metabolically unhealthy obese.
In the 30-day and 60-day research, we observed that there were no differences in readmission risk among different metabolic obesity phenotypes (Figures 3A, B, Supplementary Table 2). In the 90-day and 180-day research, patients in the metabolically unhealthy groups (MUNO and MUO) had a higher readmission rate than those in the metabolically healthy group (MHNO and MHO), respectively (Supplementary Table 2). In 90-day analysis, patients in the MUNO group [aHR = 1.07 (1.023-1.128), P = 0.004] and the MUO group [aHR = 1.089 (1.000-1.186), P = 0.049] had higher readmission risk than those in the MHNO group (Figure 3C, Supplementary Table 2). In 180-day analysis, we also observed higher risk of readmission in the MUNO group [aHR = 1.092 (1.039-1.147), P = < 0.001] and the MUO group [aHR = 1.133 (1.040-1.234), P = 0.004] than MHNO (Figure 3D, Supplementary Table 2), respectively.
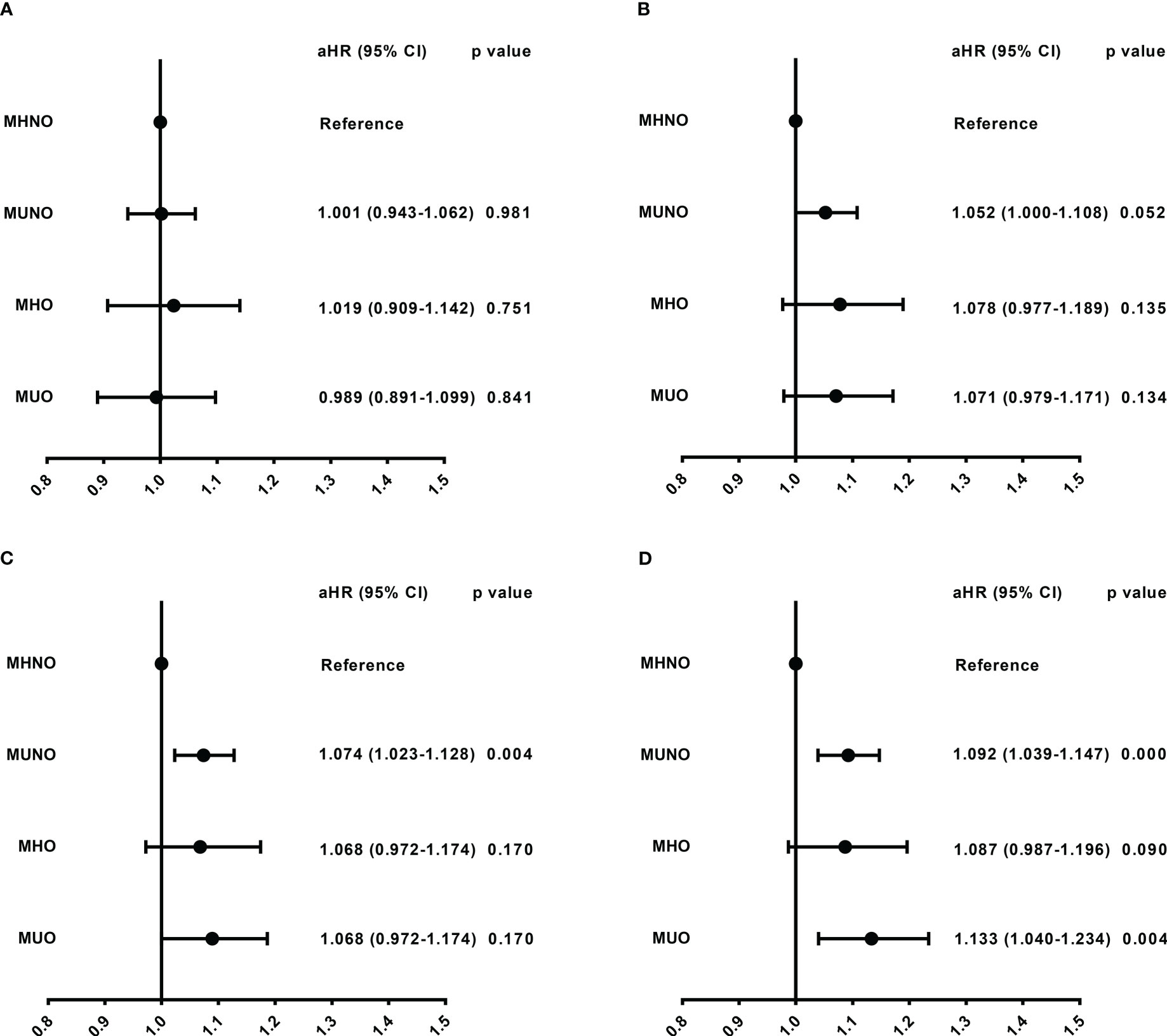
Figure 3 Readmission risk among different metabolic obesity phenotypes in four follow-up days. (A) Readmission risk in 30-day. (B) Readmission risk in 60-day. (C) Readmission risk in 90-day. (D) Readmission risk in 180-day. The model was adjusted for age, sex, elective admission, primary payer, disposition of patient, resident, length of stay, total charges, emergency record, same day events, patient location, antineoplastic chemotherapy, and stem cells transplant status. aHR, adjusted hazard ratio; CI, confidence interval; MHNO, metabolically healthy non-obese; MUNO, metabolically unhealthy non-obese; MHO, metabolically healthy obese; MUO, metabolically unhealthy obese.
The 90-day data was then processed for further analysis. When stratified by age, higher risk in the MUNO group [aHR = 1.063 (1.005-1.125), P = 0.032] and the MUO group [aHR = 1.12 (1.009-1.242), P = 0.033] than in the MHNO was only observed in the elderly population aged 65 or older (Supplementary Table 3). As shown in subgroups based on metabolic risk factor type (Table 2), patients with hyperglycemia only [HR = 1.193 (1.044-1.364), P = 0.01] and hypertension only [HR = 1.091 (1.022-1.164), P = 0.009] had increased risk of readmission compared with those without metabolic risk factors. However, similar result was not observed after correction for confounding factors. In subgroups based on the number of metabolic risk factors, we found that patients with metabolic risk factors had higher readmission risk than those without metabolic risk factors. Furthermore, the risk elevated with the increase of the number of combined factors (Table 2), with aHR of 1.068 (P = 0.043, combined with one metabolic risk factor), 1.109 (P = 0.002, combined with two metabolic risk factors), and 1.125 (P = 0.003, combined with three metabolic risk factors).
Of the patients discharged from their index hospitalization and followed for 30-days, 60-days, 90-days, and 180-days, 5400 (15.5%), 7255 (22.4%), 8025 (27.0%), 7839 (35.6%) were readmitted, respectively. During the readmission, the MUO group were more likely to have LOS over 7 days than those in the MHNO group at 30-days (36.3% vs. 28.5%, p < 0.05), 60-days (35.1% vs. 28.1%), p < 0.05), and 90-days (32.9% vs. 27.3%, p < 0.05) (Table 3). At 90-day readmission, patients in the MHO group had higher total charges than the MUNO group ($ 88752.3 vs. $ 73876.1, p < 0.05; Table 3). However, no significant difference in mortality was found among the four metabolic obesity phenotypes.
With the increasing prevalence of MM in elderly patients, it is important to focus on its risk factors to identify high-risk patients and reduce the burden of disease. In this retrospective cohort study based on a representative NRD database, we analyzed 34,852 patients diagnosed with MM from the National Readmission Database (NRD) in the United States. According to obesity and metabolic status, the population was divided into four phenotypes: metabolically healthy non-obese (MHNO), metabolically unhealthy non-obese (MUNO), metabolically healthy obese (MHO), and metabolically unhealthy obese (MUO). The patients with different phenotypes were observed for hospital readmission at days 30-day, 60-day, 90-day and 180-day. We found that the metabolically unhealthy (MUNO and MUO) individuals had higher risk of 90-day and 180-day readmission than MHNO individuals. In the age subgroup analysis of 90-day, we observed similar results only in the older group (≥ 65 years of age). Moreover, further analysis found that the risk of readmission increased significantly as the number of metabolic risk factors increased. Analysis of hospitalization characteristics of readmission patients showed that patients with metabolically unhealthy obesity (MUO) were at a higher risk of long-term hospitalization than patients in other groups.
MM is a hematological malignancy with genetic abnormalities. Patients with MM had higher incidence of some modifiable risk factors, such as obesity, hyperglycemia, dyslipidemia, and hypertension (21–23). This is more likely due to the metabolic dysregulation of MM. To ensure energy demands for rapid cell proliferation and tumor growth, myeloma cells reprogram the metabolic pathways, involving metabolic reorganization of glycolysis and oxidative phosphorylation, abnormal fatty acid metabolism, and chronic inflammation (24, 25). Overweight and obesity are associated with increased morbidity and mortality risk of MM through inflammatory cytokines, leptin, insulin, and insulin-like growth factor levels (26). Previous research found that obesity could increase the number and size of bone marrow adipocytes to obtain energy and induce the overexpression of protumor cytokines (27). For MM patients with diabetes, tumor cells can evade apoptosis by insulin resistance, hyperinsulinemia, and overproduction of insulin-like growth factor 1 (25). Dyslipidemia in MM has also been widely reported. The prevailing view is that the binding of paraproteins to serum lipoproteins and related tissue may result in reduced lipoproteins clearance (21, 28). However, some studies have also pointed out that myeloma cells are dependent upon exogenous cholesterol for survival, such as low density lipoprotein that is an important antiapoptotic drug and may prevent myeloma cell apoptosis and promote myeloma cell survival (29). In addition, the higher incidence of hypertension is related to the increased cardiovascular complications in MM patients (23).
However, previous studies did not compare the effects of obesity and metabolic disorders on MM. Our analyses indicated that metabolic status rather than obesity was the determinant risk of MM readmissions. This seems to confirm that metabolic disorders have a stronger effect on MM than obesity in some respects. We found that the prevalence of cardiovascular complications was significantly higher in the metabolically unhealthy (MUNO and MUO) groups than in the metabolically healthy (MHNO and MHO) groups, which suggests that the readmission risk of metabolically unhealthy patients may be related to a higher burden of comorbidities. This was consistent with the conclusions of some previous studies (13, 30–32). In a prospective cohort study with 30 years follow-up, metabolically unhealthy individuals are at higher cardiovascular disease risk across all BMI categories, and the transition from metabolically healthy status to unhealthy phenotypes is also associated with increased cardiovascular disease risk (33). MUO is more likely to show insulin resistance, adverse fat distribution, higher inflammation markers, and adipose tissue dysfunction than MHO (13, 34). In fact, these factors are associated with higher risk of atherosclerosis and cardiovascular disease (35, 36). Furthermore, as an elderly disease, MM is more likely to be affected by severe comorbidities. Additionally, metabolic disorders may affect the clinical manifestations of MM in terms of the drug efficacy and tumor cell activity (25). Metabolically unhealthy patients may face a lower intensity of treatment (21, 34) and poorly executed care (25), which will affect the treatment efficacy of the disease.
Although there has been substantial evidence to show that obesity is the only modifiable risk factor for MM (26, 37), other studies have observed different phenomena. Increased BMI was not significantly associated with adverse outcomes in MM patients (10, 38) and was even associated with lower morbidity and longer overall survival (11). As suggested in recent literature, the influence of BMI on MM may depend on different disease stages (26). For normal individuals or patients in the early stages of the disease, elevated BMI was associated with higher mortality and disease progression, while for MM patients in a transplant or relapsing state, a higher BMI meant patients could tolerate better treatment and experience less disease-related weight loss (26). In summary, the role of obesity in MM is complex. However, previous studies did not consider the correlation between obesity and metabolic disorders. Our study found that the MM-related readmission risk, as a result of disease progression or unexpected complications, was affected by metabolic disorders, which may imply that modifiable metabolic risk factors play a more significant role than obesity in the short-term disease progression of MM patients.
As mentioned above, all metabolic risk factors can induce adverse outcomes in MM patients through multiple pathways and mechanisms. Our analysis demonstrated the cumulative effect of metabolic risk factors numbers on the risk of MM readmission, which was supported by previous studies (39). This result is worrying for MM patients with multiple metabolic risk factors, especially for the elderly patients. Interestingly, we also observed that the association between metabolically unhealthy status and readmission risk appeared in 90-day and 180-day studies, rather than in 30-day and 60-day studies. Disease progression within 60 days after treatment is commonly referred to as refractory MM (40), which is often related to cytogenetic risk (40, 41). This might indicate that, unlike the short-term influence of refractory mechanisms, metabolic disorders influence the longer-term development of MM.
Although few studies directly explore the association between metabolic disorders and obesity in MM, based on our findings, we speculate that the higher risk of readmission in metabolically unhealthy patients with MM is related, at least in part, to the above mechanisms. From our study, we have reason to believe that metabolic disorders have a greater impact on MM readmissions than obesity. We found that patients with metabolically unhealthy status had a higher readmission rate and readmission risk than those with metabolically healthy status. Meanwhile, patients with metabolic abnormalities and obesity may have a longer time in re-hospitalization stay. Through targeted interventions, such as diet, exercise and drug treatment, metabolic risk factors and quantity can be controlled to reduce the occurrence of adverse disease outcomes reduce the disease burden of MM. Readmission is considered to be a result of disease progression or unexpected complications on initial admission. Although our study may help to identify people who have a high risk of readmission, it is not clear whether the readmission of metabolically unhealthy patients is due to progression of the disease itself or related complications. Therefore, further investigations are necessary to explore the mechanisms of the effect of metabolic disorders on the development of MM.
Although a large sample size of patients was used for follow-up, our study had many other limitations. First, all diseases were diagnosed based on the ICD-10 codes, and we could not verify the accuracy of disease diagnoses in our study. Secondly, obesity defined by BMI did not consider the effect of fat distribution on the disease, which had implications for MM outcomes (15). Moreover, due to data limitations, the stage, karyotype, oncogenic mutations and treatment regimen information of MM patients were not available, which could have influenced the results. To reduce these effects, we corrected for patient antitumor chemotherapy and stem cell transplant status in the analysis, which were the primary treatment for MM. Although we did not perform a subgroup analysis based on MM stage and treatment type due to the limitations of the database, the results of this study have good representativeness and reliability among MM patients based on the large patient population in the NRD database. Further studies in more detailed cohorts are required to validate the relationship of obesity and metabolism to MM specific disease characteristics. In addition, the analyzed data were from NRD 2018, before the coronavirus disease 2019 (COVID-19) epidemic, and we were unable to assess the NRD data during the pandemic. Therefore, our analysis could not take into account the effect of COVID-19 on the results. Recent studies have shown that COVID-19 increased rehospitalizations and mortality rates in patients and had adverse effects on cancer patients, including MM (42–44). However, obesity and metabolic risk factors contribute to COVID-19 infection and adverse outcomes and increase the risk of hospitalization (45, 46). Thus, we speculate that during the pandemic, COVID-19, obesity and metabolic risk factors could increase the risk of readmission and adverse outcomes in MM patients jointly. Future clinical practice should focus on patients with obesity and metabolic abnormalities, control the occurrence of metabolic abnormalities and the number of metabolic risk factors, and prevent the adverse effects of obesity and metabolism on patients with cancer and COVID-19.
Taken together, we observed that metabolic disorders, not obesity, were independently associated with higher readmission risk in patients with MM. Moreover, as the number of metabolic risk factors increased, the risk of MM readmission elevated. In the management of MM, attention should be paid to advance directives and optimized nursing for patients with metabolic disorders to reduce the readmission rate and hospitalization burden.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Biomedical Research Ethic Committee of Shandong Provincial Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
We kindly acknowledge the participants and researchers who participated in this study. YZ, XF, KL and YFZ contributed to the design of the study protocol. YZ performed the data analyses and wrote the first draft of the manuscript. JH and ZSY contributed to the analysis plan. Chunhui Zhao contributed to the data analysis. ZNY, YC and YW revised the manuscript to create the final version. All authors proofread the manuscript and agreed with the submission and publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1116307/full#supplementary-material
aHR, adjusted hazard ratios; HR, unadjusted hazard ratio; BMI, body mass index; CVD, cardiovascular disease; ICD-10-CM, Diseases-Tenth Revision-Clinical Modification; LOS, length of stay; MHNO, metabolically healthy non-obese; MHO, metabolically healthy obese; MM, Multiple myeloma; MUNO, metabolically unhealthy non-obese; MUO, metabolically unhealthy obese; NRD, Nationwide Readmissions Database.
1. van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. Lancet (2021) 397(10272):410–27. doi: 10.1016/S0140-6736(21)00135-5
2. Bazarbachi AH, Al Hamed R, Malard F, Harousseau JL, Mohty M. Relapsed refractory multiple myeloma: A comprehensive overview. Leukemia. (2019) 33(10):2343–57. doi: 10.1038/s41375-019-0561-2
3. Sonneveld P, Broijl A. Treatment of relapsed and refractory multiple myeloma. Haematologica. (2016) 101(4):396–406. doi: 10.3324/haematol.2015.129189
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med (2016) 374(16):1543–51. doi: 10.1056/NEJMsa1513024
5. Visade F, Babykina G, Puisieux F, Bloch F, Charpentier A, Delecluse C, et al. Risk factors for hospital readmission and death after discharge of older adults from acute geriatric units: Taking the rank of admission into account. Clin Interv Aging. (2021) 16:1931–41. doi: 10.2147/CIA.S327486
6. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med (2009) 360(14):1418–28. doi: 10.1056/NEJMsa0803563
7. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. (2006) 113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
8. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
9. Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: A meta-analysis of prospective studies. Eur J Cancer. (2011) 47(11):1606–15. doi: 10.1016/j.ejca.2011.01.020
10. Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, et al. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: A nationwide cohort study in Sweden. Cancer Res (2007) 67(12):5983–6. doi: 10.1158/0008-5472.CAN-07-0274
11. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X
12. Zheng X, Peng R, Xu H, Lin T, Qiu S, Wei Q, et al. The association between metabolic status and risk of cancer among patients with obesity: Metabolically healthy obesity vs. Metabolically Unhealthy Obes Front Nutr (2022) 9:783660. doi: 10.3389/fnut.2022.783660
13. Stefan N, Häring H-U, Hu FB, Schulze MB. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinology. (2013) 1(2):152–62. doi: 10.1016/S2213-8587(13)70062-7
14. Thyagaturu HS, Bolton AR, Li S, Kumar A, Shah KR, Katz D. Effect of diabetes mellitus on 30 and 90-day readmissions of patients with heart failure. Am J Cardiol (2021) 155:78–85. doi: 10.1016/j.amjcard.2021.06.016
15. Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: A pooled analysis of 20 prospective studies. Br J Haematol (2014) 166(5):667–76. doi: 10.1111/bjh.12935
16. Park SM, Lim MK, Jung KW, Shin SA, Yoo KY, Yun YH, et al. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National health insurance corporation study. J Clin Oncol (2007) 25(30):4835–43. doi: 10.1200/JCO.2006.10.3416
17. Ooi DSQ, Toh JY, Ng LYB, Peng Z, Yang S, Rashid N, et al. Dietary intakes and eating behavior between metabolically healthy and unhealthy obesity phenotypes in Asian children and adolescents. Nutrients (2022) 14(22):4796. doi: 10.3390/nu14224796
18. Zhang H, Tang X, Hu D, Li G, Song G. Transition patterns of metabolism-weight phenotypes over time: A longitudinal study using the multistate Markov model in China. Front Public Health (2022) 10:1026751. doi: 10.3389/fpubh.2022.1026751
19. Guerrero-Romero F, Rodríguez-Morán M. Concordance between the 2005 international diabetes federation definition for diagnosing metabolic syndrome with the national cholesterol education program adult treatment panel III and the world health organization definitions. Diabetes Care (2005) 28(10):2588–9. doi: 10.2337/diacare.28.10.2588a
20. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). JAMA (2001) 285(19):2486–97. doi: 10.1001/jama.285.19.2486
21. Ragbourne SC, Maghsoodi N, Streetly M, Crook MA. The association between metabolic syndrome and multiple myeloma. Acta Haematol (2021) 144(1):24–33. doi: 10.1159/000505992
22. Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: A meta-analysis of observational studies. Blood. (2012) 119(21):4845–50. doi: 10.1182/blood-2011-06-362830
23. Chari A, Mezzi K, Zhu S, Werther W, Felici D, Lyon AR. Incidence and risk of hypertension in patients newly treated for multiple myeloma: A retrospective cohort study. BMC Cancer. (2016) 16(1):912. doi: 10.1186/s12885-016-2955-0
24. Lim JSL, Chong PSY, Chng WJ. Metabolic vulnerabilities in multiple myeloma. Cancers (Basel). (2022) 14(8):1905. doi: 10.3390/cancers14081905
25. Gavriatopoulou M, Paschou SA, Ntanasis-Stathopoulos I, Dimopoulos MA. Metabolic disorders in multiple myeloma. Int J Mol Sci (2021) 22(21):11430. doi: 10.3390/ijms222111430
26. Parikh R, Tariq SM, Marinac CR, Shah UA. A comprehensive review of the impact of obesity on plasma cell disorders. Leukemia. (2022) 36(2):301–14. doi: 10.1038/s41375-021-01443-7
27. Reagan MR, Fairfield H, Rosen CJ. Bone marrow adipocytes: A link between obesity and bone cancer. Cancers (Basel). (2021) 13(3):364. doi: 10.3390/cancers13030364
28. Kilgore LL, Patterson BW, Parenti DM, Fisher WR. Immune complex hyperlipidemia induced by an apolipoprotein-reactive immunoglobulin a paraprotein from a patient with multiple myeloma. Characterization this immunoglobulin. J Clin Invest. (1985) 76(1):225–32. doi: 10.1172/JCI111951
29. Tirado-Velez JM, Benitez-Rondan A, Cozar-Castellano I, Medina F, Perdomo G. Low-density lipoprotein cholesterol suppresses apoptosis in human multiple myeloma cells. Ann Hematol (2012) 91(1):83–8. doi: 10.1007/s00277-011-1246-8
30. Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: Facts and fantasies. J Clin Invest. (2019) 129(10):3978–89. doi: 10.1172/JCI129186
31. Stefan N. Metabolically healthy and unhealthy normal weight and obesity. Endocrinol Metab (Seoul). (2020) 35(3):487–93. doi: 10.3803/EnM.2020.301
32. Echouffo-Tcheugui JB, Short MI, Xanthakis V, Field P, Sponholtz TR, Larson MG, et al. Natural history of obesity subphenotypes: Dynamic changes over two decades and prognosis in the framingham heart study. J Clin Endocrinol Metab (2019) 104(3):738–52. doi: 10.1210/jc.2018-01321
33. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the nurses' health study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinology. (2018) 6(9):714–24. doi: 10.1016/S2213-8587(18)30137-2
34. Wu W, Merriman K, Nabaah A, Seval N, Seval D, Lin H, et al. The association of diabetes and anti-diabetic medications with clinical outcomes in multiple myeloma. Br J Cancer. (2014) 111(3):628–36. doi: 10.1038/bjc.2014.307
35. Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol (2019) 16(2):83–99. doi: 10.1038/s41569-018-0097-6
36. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
37. Morris EV, Edwards CM. Adipokines, adiposity, and bone marrow adipocytes: Dangerous accomplices in multiple myeloma. J Cell Physiol (2018) 233(12):9159–66. doi: 10.1002/jcp.26884
38. Kanda J, Matsuo K, Inoue M, Iwasaki M, Sawada N, Shimazu T, et al. Association of anthropometric characteristics with the risk of malignant lymphoma and plasma cell myeloma in a Japanese population: a population-based cohort study. Cancer Epidemiol Biomarkers Prev (2010) 19(6):1623–31. doi: 10.1158/1055-9965.EPI-10-0171
39. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3. 5 Million Men Women. J Am Coll Cardiol (2017) 70(12):1429–37. doi: 10.1016/j.jacc.2017.07.763
40. Dimopoulos MA, Terpos E, Niesvizky R, Palumbo A. Clinical characteristics of patients with relapsed multiple myeloma. Cancer Treat Rev (2015) 41(10):827–35. doi: 10.1016/j.ctrv.2015.07.005
41. Mohan M, Weinhold N, Schinke C, Thanedrarajan S, Rasche L, Sawyer JR, et al. Daratumumab in high-risk relapsed/refractory multiple myeloma patients: Adverse effect of chromosome 1q21 gain/amplification and GEP70 status on outcome. Br J Haematol (2020) 189(1):67–71. doi: 10.1111/bjh.16292
42. Yousufuddin M, Yamani MH, Kashani KB, Zhu Y, Wang Z, Seshadri A, et al. Characteristics, treatment patterns, and clinical outcomes after heart failure hospitalizations during the COVID-19 pandemic, march to October 2020. Mayo Clin Proc (2023) 98(1):31–47. doi: 10.1016/j.mayocp.2022.09.005
43. O'Neil JC, Geisler BP, Rusinak D, Bassett IV, Triant VA, McKenzie R, et al. Discharge to post-acute care and other predictors of prolonged length of stay during the initial COVID-19 surge: A single site analysis. Int J Qual Health Care (2023) 35(1):mzac098. doi: 10.1093/intqhc/mzac098
44. Wang B, Van Oekelen O, Mouhieddine TH, Del Valle DM, Richter J, Cho HJ, et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol (2020) 13(1):94. doi: 10.1186/s13045-020-00934-x
45. Westheim AJF, Bitorina AV, Theys J, Shiri-Sverdlov R. COVID-19 infection, progression, and vaccination: Focus on obesity and related metabolic disturbances. Obes Rev (2021) 22(10):e13313. doi: 10.1111/obr.13313
Keywords: MM, multiple myeloma, metabolic disorders, obesity phenotypes, hospitalization burden, readmission
Citation: Zhang Y, Fan X, Zhao C, Yuan Z, Cheng Y, Wu Y, Han J, Yuan Z, Zhao Y and Lu K (2023) Association between metabolic obesity phenotypes and multiple myeloma hospitalization burden: A national retrospective study. Front. Oncol. 13:1116307. doi: 10.3389/fonc.2023.1116307
Received: 07 December 2022; Accepted: 06 February 2023;
Published: 23 February 2023.
Edited by:
Ioannis Ntanasis-Stathopoulos, National and Kapodistrian University of Athens Medical School, GreeceReviewed by:
Maria Gavriatopoulou, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Zhang, Fan, Zhao, Yuan, Cheng, Wu, Han, Yuan, Zhao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanfei Zhao, eXpoYTkxMjVAdW5pLnN5ZG5leS5lZHUuYXU=; Keke Lu, bHVrZWtlQHNkZm11LmVkdS5jbg==
†ORCID: Yue Zhang, orcid.org/0000-0003-4070-3619
Yuanfei Zhao, orcid.org/0000-0003-3964-0454
Keke Lu, orcid.org/0000-0002-2063-8250
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.