- 1Case Western Reserve University School of Medicine, Case Western Reserve University, Cleveland, OH, United States
- 2Faculdade Israelita de Ciências da Saúde Albert Einstein, Hospital Israelita Albert Einstein, São Paulo, Brazil
- 3Division of Hematology and Oncology, Department of Medicine, University Hospitals Seidman Cancer Center, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 4Division of Thoracic and Esophageal Surgery, Department of Surgery, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 5University Hospitals Research in Surgical Outcomes and Effectiveness (UH-RISES), University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 6Division of Surgical Oncology, Department of Surgery, University Hospitals Seidman Cancer Center, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Background: The RxPONDER trial found that among breast cancer patients with estrogen receptor positive (ER+) breast cancer, 1-3 positive axillary nodes, and a recurrence score of ≤25, only pre-menopausal women benefitted from adjuvant chemoendocrine therapy; postmenopausal women with similar characteristic did not benefit from adjuvant chemotherapy. We aimed to replicate the RxPonder trial using a larger patient cohort with real world data to determine whether a RS threshold existed where adjuvant chemotherapy was beneficial regardless of age.
Methods: The National Cancer Database (NCDB) was queried for women with ER+, human epidermal growth factor receptor 2 (HER2) negative breast cancer, 1-3 positive axillary nodes, and RS ≤25 who received endocrine (ET) only or chemo-endocrine therapy (CET). Cox regression interaction was explored between CET and age as a surrogate for menopausal status.
Results: The final analytic cohort included 28,427 eligible women: 7,487 (26.3%) received adjuvant CET and 20,940 (73.7%) ET. In the entire cohort, RS had a normal distribution, with a median score of 14. After correcting for demographic and clinical variables, a threshold effect was observed with RS >20 being associated with a significantly inferior overall survival (OS) (P value range: < 0.001-0.019). In women with RS of 20-25, CET was associated with a significant improvement in OS compared to ET alone, regardless of age (age <=50: HR = 0.334, P=0.002; age>50: HR=0.521, P=0.019).
Conclusion: Among women with ER+/HER2- breast cancer with 1–3 positive nodes, and a RS of 20-25—in contrast to the RxPONDER trial—we observed that CET was associated with an OS benefit in women regardless of age.
1 Introduction
The 21-gene assay Oncotype DX® Breast Recurrence Score (RS), has been used widely to guide adjuvant chemotherapy utilizations in patients with estrogen receptor (ER)+/human epidermal growth factor receptor 2 (HER2) negative node-negative breast cancer (BC) (1–4), and are part of international consensus guidelines. In patients with axillary nodal metastasis, RS has also been demonstrated to identify which patients can safely forgo adjuvant chemotherapy when they are post-menopausal. Evaluation from RxPONDER (SWOG S1007) trial comparing endocrine therapy alone (ET) vs. chemotherapy in addition to endocrine therapy (CET) in patients with 1–3 positive axillary lymph nodes and RS ≤ 25 found that CET did not improve distant recurrence free survival compared to ET in postmenopausal women with RS 0-25, regardless of clinical features. By contrast, CET was found to be beneficial in premenopausal women in this trial regardless of RS (5).
Our aim in this study was to replicate the RxPONDER trial using real world data and larger sample size from National Cancer Database (NCDB) to determine whether a RS threshold could be identified where CET was beneficial regardless of age.
2 Methods
2.1 Data collection and data elements
A retrospective cohort study of the NCDB was performed. Jointly sponsored by the American College of Surgeons and the American Society, NCDB is a clinical oncology database sourced from hospital registry data representing more than 70% of newly diagnosed BC cases nationwide. The database covers more than 1,500 Commission on Cancer (CoC)-accredited facilities. Definition of the database variables are available from the dictionary of NCDB Participant Use Data File (http://ncdbpuf.facs.org). The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
2.2 Patient cohort and data analysis
The NCDB was queried to identify HR+HER2- BC patients who underwent definitive breast surgery and had 1 to 3 positive axillary lymph nodes from 2004-2018. Clinical staging data for the cohort was based on TNM classification in American Joint Committee on Cancer (AJCC) 7th edition. Patients were excluded if they were stage 0 or stage IV, male, had RS > 25, or if they were missing critical study information (e.g. follow-up data or RS).
The cohort was divided by patients who received CET and ET. The primary outcome was overall survival (OS). Analysis included univariate comparison of patient factors associated with receipt of CET (vs. ET). Logistic regression analysis was performed to identify clinical factors that were predictive of CET. To compare the two groups, Wilcoxon rank-sum test was utilized for continuous variables and chi-square for categorical data. Difference in OS between the groups was analyzed using Kaplan-Meier survival estimates and compared through log-rank test. To control for confounding effects, multivariable Cox proportional hazard analysis was performed. The covariates included were: age, gender, race, insurance provider, facility, and patient clinical characteristics. Interaction between menopausal status (age <50 or above) and CET receipt was explored.
All statistical analysis was performed using STATA/MP, version 16.0 (Stata Corp LLC, College Station, TX). Institutional Review Board (IRB) approval was exempted by the University Hospitals Cleveland Medical Center IRB as all NCDB data is de-identified and does not contain any protected health information.
3 Results
The final analytic cohort included 28,427 women with a primary diagnosis of pathological stage I-III HR+HER2- BC with 1–3 positive axillary lymph nodes, i.e. pN1 (Table 1). The median follow-up time was 52.7 months (interquartile range [IQR] 35.3-74.3 months), 7,487 patients (26.3%) received CET and 20,940 (73.7%) received ET. Patients who received ET were more likely to be older (median age 61 vs. 54, P=0.001), White (87.1% vs. 86.5%, P=0.045), have non-private insurance (44.5% vs. 26.5%, P<0.001), have a greater number of comorbidities (1+ Charlson-Deyo Score 16.6% vs. 12.6% P<0.001), and a lower RS (<11, 37.9% vs 17.0%, P<0.001) compared to patients who received CET.

Table 1 Demographic, clinical and treatment characteristics of pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes, NCDB 2004-2018.
Multivariable logistic regression was performed to determine patient and clinical characteristics that were independently associated with CET vs. ET (Table 2). After accounting for available demographic and clinical-pathological factors, patients with the following factors were more likely to receive CET: higher RS (OR = 2.8, 95% confidence interval [CI] 2.6-3.0, P<0.001), grade 2-3 BC (OR = 1.5, 95% CI 1.3-1.6, P<0.001), lympho-vascular invasion (OR = 1.2, 95% CI 1.1-1.3, P<0.001), and private insurance (OR = 1.2, 95% CI 1.1-1.3, P<0.001). Conversely, age was inversely related to likelihood of receipt of CET (OR = 0.9, 95% CI 0.9-0.9, P<0.001).

Table 2 Multivariable logistic regressions for predictors of receipt of chemotherapy in pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes, NCDB 2004-2018.
Using the Kaplan-Meier estimate, OS was superior in CET compared to ET in the entire cohort (P<0.001, Figure 1). In the entire cohort, RS had a normal distribution (Figure 2), with a median RS of 14. To further explore the relationship of RS and OS benefit with receipt of CET, a multivariate Cox regression was performed with each individual RS of 11-25 (Table 3). After correcting for demographic and clinical features, we observed a threshold effect as patients with RS of >20 had a significantly inferior OS (P value ranged from <0.001-0.019). Patients were divided into two groups using a RS of 19 as a cut-off, and examined whether any interactions existed between CET and age as a surrogate for menopausal status. Among patients with RS of 0-19, CET was not associated with a significantly improved OS when compared to ET (Table 4) regardless of age (≤50, P=0.068; >50, P=0.770). By contrast, in women with RS of 20-25, the combination CET was associated with a significant improvement in OS compared to ET alone, regardless of age (HR = 0.334, P=0.002 for age ≤50, and HR=0.521, P=0.019 for age >50, Table 4 and Figure 3). In the subgroup of women over 50 and a RS of 20-25, CET was associated with a significant improvement in OS (HR = 0.84, 95% CI 0.5-0.9, p=0.038) compared to ET (Supplemental Table 1).

Figure 1 Overall survival compared between endocrine therapy alone versus endocrine therapy plus chemotherapy in pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes and RS 0-25. (OS at 3 and 5 years were 98.5% and 96.4% for the endocrine therapy alone cohort compared to 99.3% and 97.6%, respectively for the endocrine plus chemotherapy group (P<0.001).
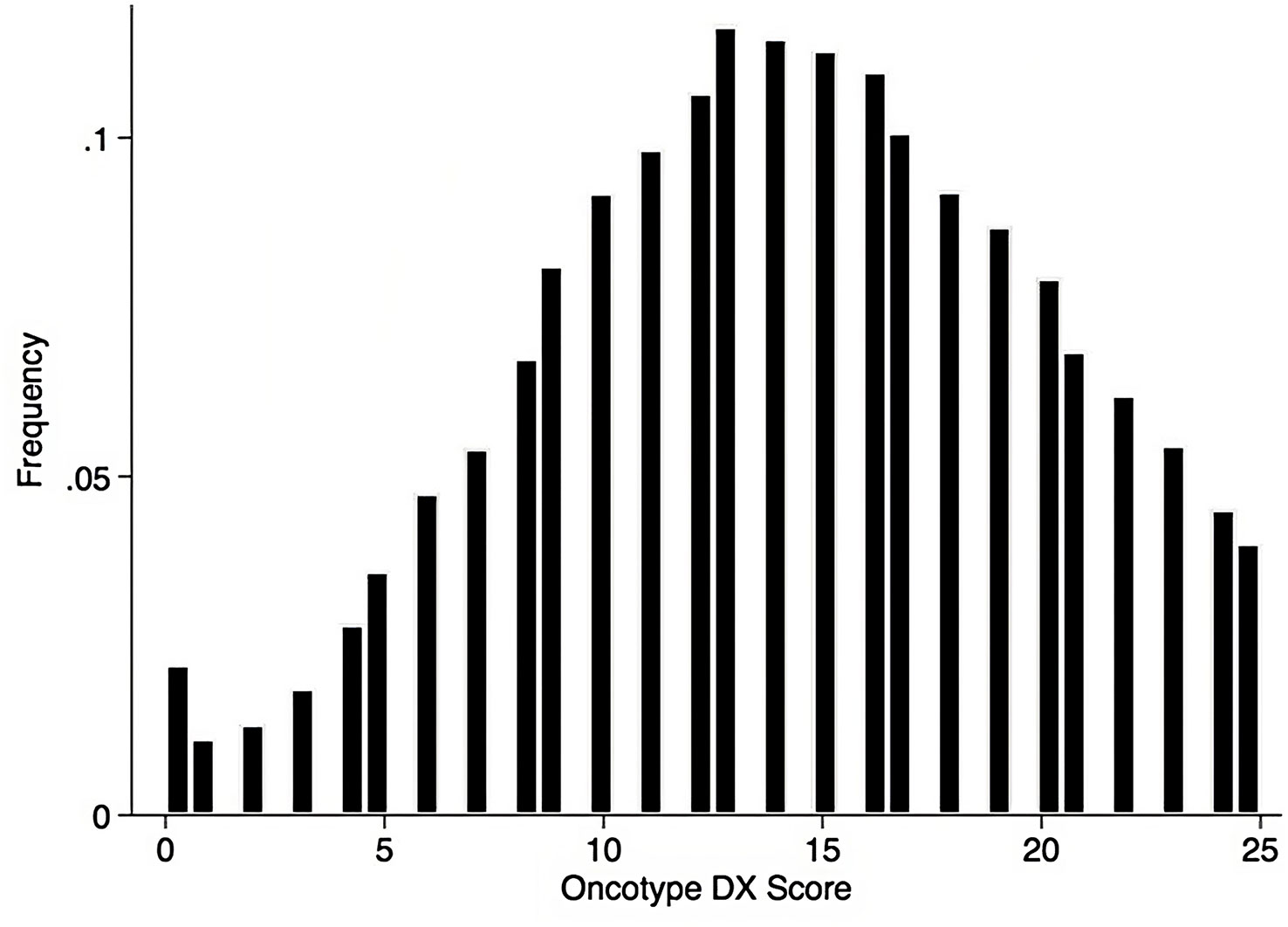
Figure 2 Histogram of RS among the pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes, data shown as percent of patients.
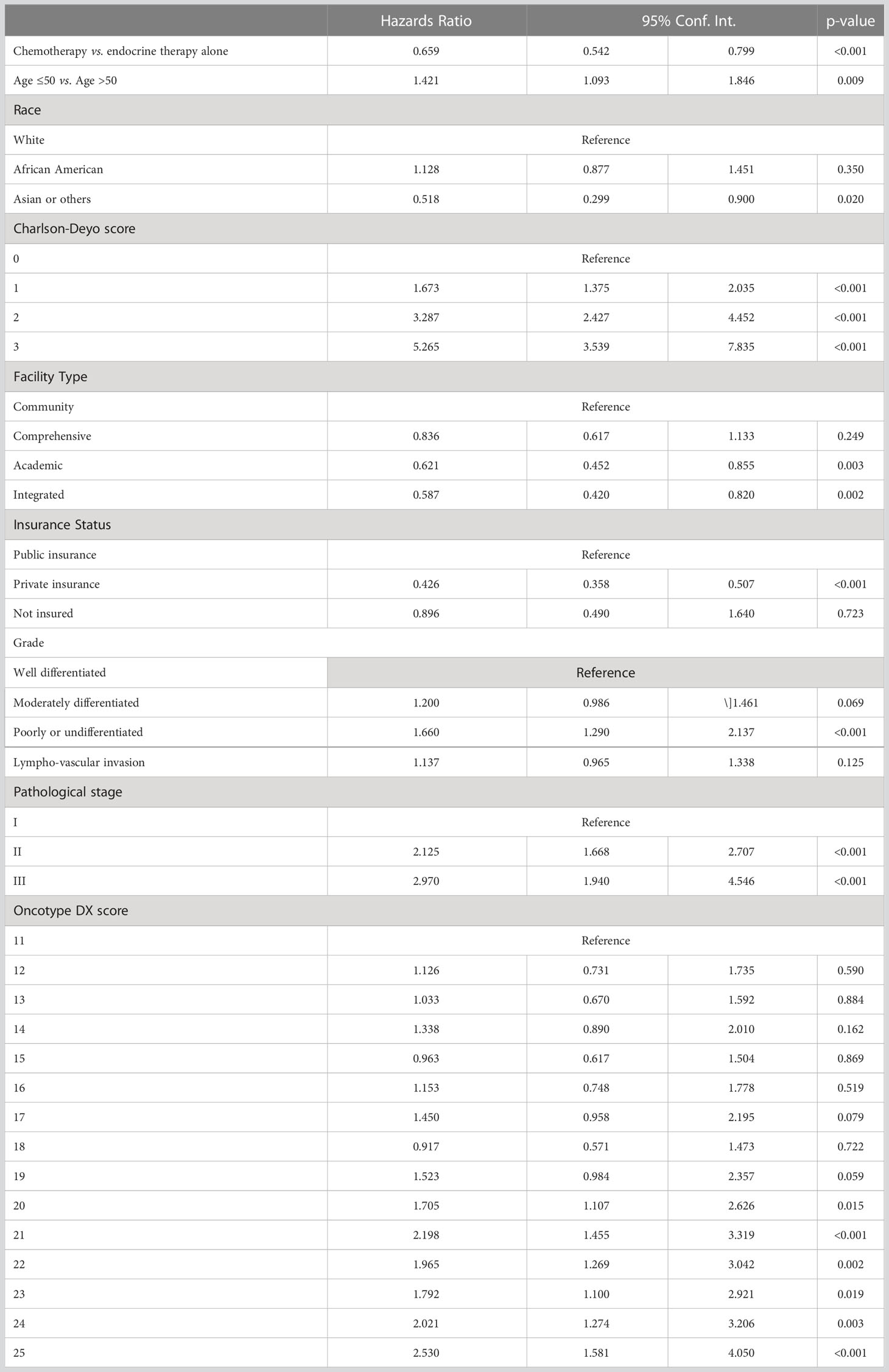
Table 3 Cox proportional hazard regression for overall survival pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes and individual RS 11-25.
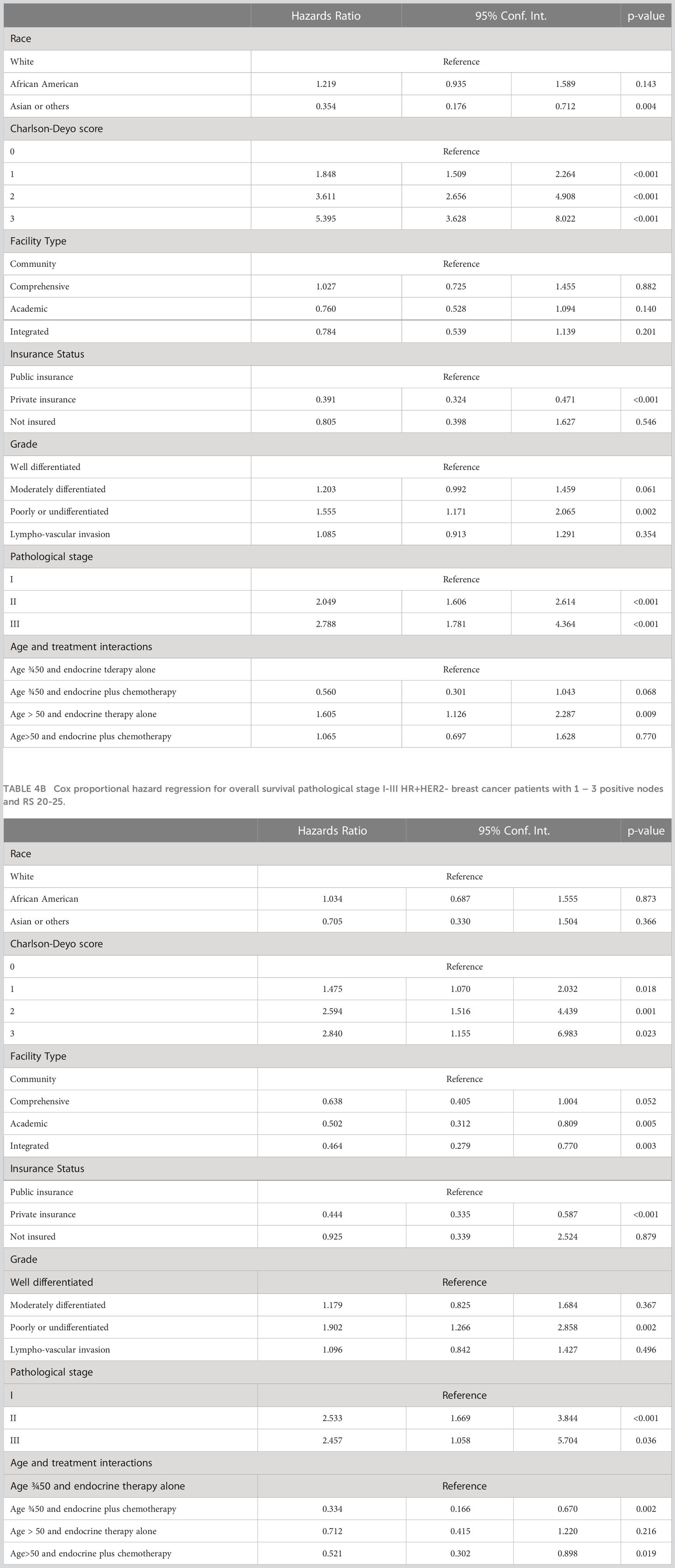
Table 4A Cox proportional hazard regression for overall survival pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes and RS 0-19.
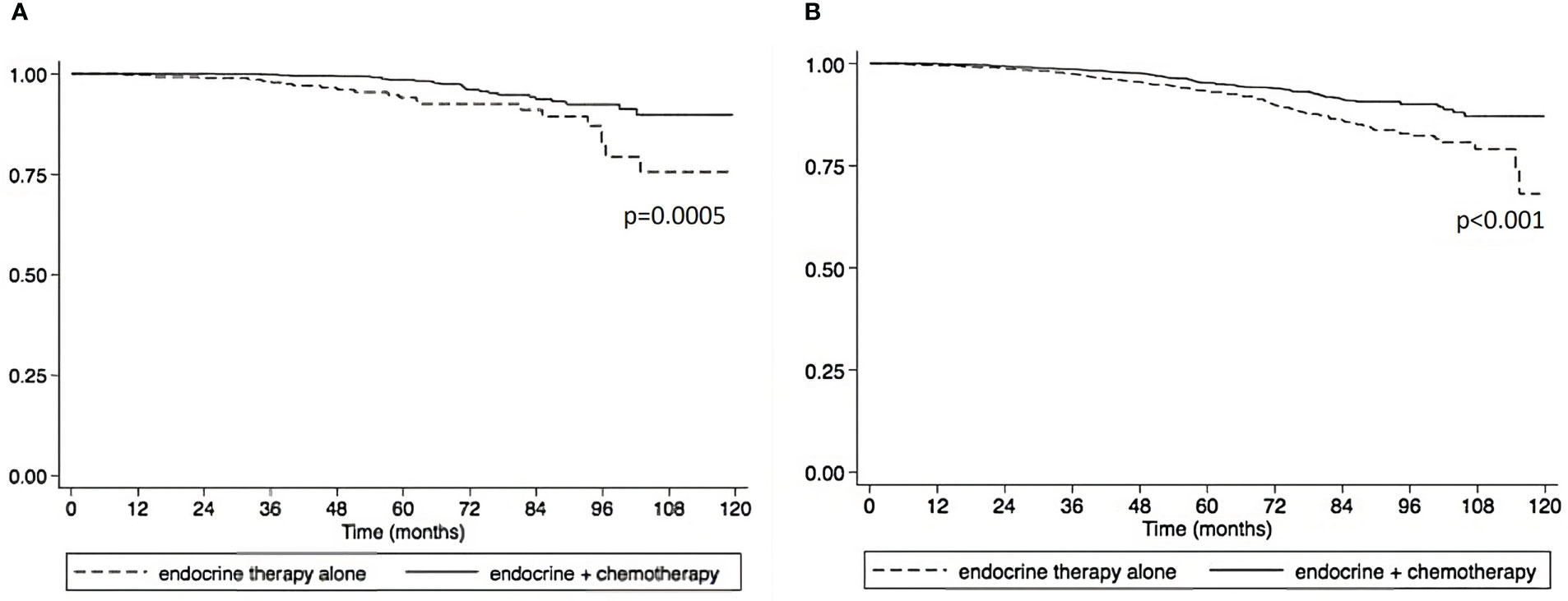
Figure 3 Overall survival compared between endocrine therapy alone versus endocrine therapy plus chemotherapy in pathological stage I-III HR+HER2- breast cancer patients with 1 – 3 positive nodes and RS 20-25. (A) premenopausal patients. (B) postmenopausal patients).
Using a RS of 11 as a cutoff to examine the interaction between CET and menopausal status, we found that CET was associated with a significant improvement in OS —using age as a surrogate for premenopausal status — in women 50 and under with an RS of 12-25 (Supplemental Tables 2A, B).
4 Discussion
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) have consistently shown in multiple meta-analyses that adjuvant chemotherapy significantly reduces cancer related mortality—a benefit that is independent of age (6, 7). In the 2005 meta-analysis the EBCTCG reported that in women with ER+ BC anthracycline-based adjuvant poly-chemotherapy reduced annual BC death rates by 38% in women younger than 50 years of age at time of initial diagnosis, and by about 20% for women 50–69 years when diagnosed. This benefit of adjuvant chemotherapy was observed largely irrespective of the use of tamoxifen and of ER status, nodal status, or other tumor characteristics.
This differential benefit of adjuvant chemotherapy in younger pre-menopausal vs. older post-menopausal women is possibly related to an indirect anti-estrogen effect, by the ability of chemotherapy to induce premature ovarian failure. The Zebra trial demonstrated in premenopausal women with ER-positive and node-positive early stage BC, that ovarian ablation with goserelin provided a benefit which was similar to that of adjuvant CMF chemotherapy (8).
Therefore, it was somewhat unexpected that in RxPONDER in post-menopausal women with pN1 disease no significant benefit in OS of CET was observed. Our study, by contrast was more consistent with results of EBCTCG, in that among women >50 with ER+/HER2- BC with 1–3 positive nodes, and a RS of 20-25, CET was associated with an OS benefit. These results using a real world cohort of patients from NCDB suggests that women >50, many of whom are presumably post-menopausal, with a RS of 20-25 appear to still derive an OS benefit from CET compared to patients who received ET.
Similarly Pagani et al. demonstrated that CET was associated with a significantly improved disease-free survival among postmenopausal women with ER-positive, node-positive breast cancer— although the magnitude of the benefit was less in patients highly ER+ tumors, with only 1 axillary lymph node, or in older women (9). Similarly, in a retrospective analysis of SWOG-8814 a significant benefit from adjuvant anthracycline based CET was reported by Albain et al. (10) in postmenopausal women with node-positive, ER+ BC, and RS >31. Interestingly, in the 103 women with intermediate RS (18–30), although the number of events was small, there was a trend towards an improved DFS with CET vs. ET (HR=0.72; 95% CI 0.39−1.31) which improved over time. In our study, we did observe a threshold effect with RS of 20 and above as associated with an inferior OS which was statistically significant (P value ranged from < 0.001 to 0.019).
Our study does have numerous limitations, despite the advantages of the large sample size and long follow-up times. Firstly, because the NCDB does not include local regional recurrence and disease-free survival, one major limitation of our study is that our analysis of long-term outcomes was limited to OS. Another limitation in our study is that we used age >50 as a surrogate for menopause status since NCDB does not specifically define menopausal status. However, age is not a therapeutic target and many women in their fifties maintain ovarian function. Therefore, a persistent endocrine effect of cytotoxics of chemotherapy may produce a larger impact of chemotherapy in younger postmenopausal women (less than 60 years) (9). Similarly, we do not have access to detailed granular data on the precise steroid hormone receptor concentrations (ER or PR) in the primary tumor or specific chemotherapeutic or ET treatment regimens which therefore cannot be factored into analyses. Finally, the NCDB only receives data from Commission on Cancer (CoC) accredited hospitals, and therefore excludes patients treated in many non-CoC accredited centers in the United States. Despite these limitations, these data suggest that there is a sub-population of postmenopausal women with RS 20-25 who appear to benefit from CET.
In summary, among women with ER+/HER2- BC with 1–3 positive axillary lymph nodes, and a RS of 20-25—in contrast to the RxPONDER—we observed that CET was associated with an OS benefit in women regardless of age underscoring that there could be hormone independent anti-tumor effects of chemotherapy in ER+ breast cancer.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/.
Author contributions
LC and AM contributed to the conception or design of the work. LC, NS, XL and CT contributed to the acquisition, analysis, or interpretation of data for the work. LC drafted the work. LC, NS, AA, AM and CT revised it critically for important intellectual content. LC, NS, CT, XL, AA and AM approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
NS is supported through funding from the Sociedade Beneficente Israelita Brasileira Albert Einstein on the program “Marcos Lottenberg and Marcos Wolosker International Fellowship for Physicians Scientist - Case Western”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1115208/full#supplementary-material
References
1. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies–improving the management of early breast cancer: St gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol (2015) 26(8):1533–46. doi: 10.1093/annonc/mdv221
2. Harris LN, Ismaila N, McShane LM, Hayes DF. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American society of clinical oncology clinical practice guideline summary. J Oncol Pract (2016) 12(4):384–9. doi: 10.1200/JOP.2016.010868
3. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26 Suppl 5:v8–30. doi: 10.1093/annonc/mdv298
4. Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, Peretz T, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer (2017) 3:32. doi: 10.1038/s41523-017-0033-7
5. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med (2021) 385(25):2336–47. doi: 10.1056/NEJMoa2108873
6. Early Breast Cancer Trialists' Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet (2012) 379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5
7. Group EBCTC. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (2005) 365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0
8. Jonat W, Kaufmann M, Sauerbrei W, Blamey R, Cuzick J, Namer M, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the zoladex early breast cancer research association study. J Clin Oncol (2002) 20(24):4628–35. doi: 10.1200/JCO.2002.05.042
9. Pagani O, Gelber S, Simoncini E, Castiglione-Gertsch M, Price KN, Gelber RD, et al. Is adjuvant chemotherapy of benefit for postmenopausal women who receive endocrine treatment for highly endocrine-responsive, node-positive breast cancer? international breast cancer study group trials VII and 12-93. Breast Cancer Res Treat (2009) 116(3):491–500. doi: 10.1007/s10549-008-0225-9
10. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol (2010) 11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6
Keywords: adjuvant (chemo)radiotherapy, chemotherapy, breast cancer, ER+ breast cancer, HER2- breast cancer, survival, oncotype
Citation: Stabellini N, Cao L, Towe CW, Luo X, Amin AL and Montero AJ (2023) Adjuvant chemotherapy is associated with an overall survival benefit regardless of age in ER+/HER2- breast cancer pts with 1-3 positive nodes and oncotype DX recurrence score 20 to 25: an NCDB analysis. Front. Oncol. 13:1115208. doi: 10.3389/fonc.2023.1115208
Received: 03 December 2022; Accepted: 11 April 2023;
Published: 24 April 2023.
Edited by:
José Bines, National Cancer Institute (INCA), BrazilReviewed by:
Makiko Ono, Cancer Institute Hospital of Japanese Foundation for Cancer Research, JapanNapa Parinyanitikul, King Chulalongkorn Memorial Hospital, Thailand
Copyright © 2023 Stabellini, Cao, Towe, Luo, Amin and Montero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto J. Montero, QWxiZXJ0by5Nb250ZXJvQFVIaG9zcGl0YWxzLm9yZw==
†These authors have contributed equally to this work
 Nickolas Stabellini
Nickolas Stabellini Lifen Cao
Lifen Cao Christopher W. Towe1,4,5
Christopher W. Towe1,4,5 Xun Luo
Xun Luo Alberto J. Montero
Alberto J. Montero