- 1Frontier Science Center for Synthetic Biology and Key Laboratory of Systems Bioengineering (Ministry of Education), Tianjin University, Tianjin, China
- 2School of Chemical Engineering and Technology, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin, China
- 3Graduate School of Health Science, Suzuka University of Medical Science, Suzuka, Japan
Purpose: The purpose of our meta-analysis and systematic review was to compare the diagnostic performance of [18F]FDG PET/CT and [18F]FDG PET/MRI in colorectal liver metastasis.
Methods: We searched PubMed, Embase, and Web of Science for eligible articles until November 2022. Studies focusing on the diagnostic value of [18F]FDG PET/CT or PET/MRI for colorectal liver metastasis were included. Using a bivariate random-effect model, the pooled sensitivity and specificity for [18F]FDG PET/CT and [18F]FDG PET/MRI were reported as estimates with 95% confidence intervals (CIs). Heterogeneity among pooled studies was assessed using the I2 statistic. The Quality Assessment of Diagnostic Performance Studies (QUADAS-2) method was used to evaluate the quality of the studies that were included.
Results: There were a total of 2743 publications identified in the initial search, finally, a total of 21 studies comprising 1036 patients were included. The pooled sensitivity, specificity, and AUC of [18F]FDG PET/CT in were 0.86 (95% CI: 0.76-0.92), 0.89 (95% CI: 0.83-0.94), and 0.92(95% CI: 0.90-0.94). [18F]FDG PET/MRI were 0.84 (95% CI: 0.77-0.89), 1.00 (95% CI: 0.32–1.00), and 0.89(95% CI: 0.86-0.92), respectively.
Conclusion: [18F]FDG PET/CT shows similar performance compared to [18F]FDG PET/MRI in detecting colorectal liver metastasis. However, pathological results were not obtained for all patients in the included studies and PET/MRI results were derived from studies with small sample sizes. There is a need for additional, larger prospective studies on this issue.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier (CRD42023390949).
1. Introduction
With a 50-60% prevalence, the liver is the most significant metastatic location from colorectal cancer (CRC) (1, 2). Metastases are restricted to the liver in about one-third of these individuals at the time of detection, increasing curative therapy options (3, 4). Complete resection is a common treatment option for liver metastases, which is beneficial to the prognosis (5). This observation highlights the importance of correct staging or restaging of CRC for a personalized therapy decision.
Several diagnostic imaging modalities, including contrast-enhanced CT, MRI, and contrast-enhanced ultrasonography (US), are currently available for CRC staging or restaging. Contrast-enhanced CT is still considered the standard imaging modality for CRC staging and restaging, however, recent studies have revealed that MRI offers superior sensitivity for detecting liver metastases (6) contrast-enhanced US is a technique that depends a lot on the operator, which may explain the lower reported liver metastasis detection sensitivity (7). There have been reports of improved sensitivity with intraoperative or laparoscopic ultrasound, but this method cannot be performed without invasive procedures (8). As a result, the most effective imaging approach has not yet been defined.
Positron emission tomography/computed tomography (PET/CT) is an established modality for evaluating local recurrence or distant metastasis of colorectal cancer, which provides specific molecular and metabolic information (9–11). In terms of tumor staging, hybrid PET/CT system improves lesion localization and interpretation of colorectal cancer compared to PET or CT alone (12). Numerous studies have shown that PET/CT has unique advantages over conventional methods for colorectal liver metastasis (13–16). However, during the past decade, radionuclide imaging techniques such as hybrid positron emission tomography/magnetic resonance imaging (PET/MRI) have attracted attention as they allow well detection of cancer metastasis. Head-to-head comparison by Brendle et al. showed that PET/CT had lower sensitivity but better specificity than PET/MRI, however, the diagnostic performance of PET/CT was lower than previous studies due to the high percentage of mucinous tumors and limited spatial resolution (17). Nowadays, although many studies have reported the good performance of hybrid [18F]FDG PET/CT in colorectal liver metastasis, few have quantitatively assessed its relative performance compared to hybrid [18F]FDG PET/MRI.
Therefore, in the current study, we aimed to perform a meta-analysis by searching all available literature to obtain the diagnostic performance of hybrid [18F]FDG PET/CT and hybrid [18F]FDG PET/MRI modality in the diagnosis of colorectal liver metastasis.
2. Manuscript formatting
2.1. Material and methods
Our study protocol was registered on PROSPERO (CRD42023390949). This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplementary Table 1) (18).
2.1.1. Search strategy
A comprehensive search was conducted of the PubMed, Embase, and Web of Science databases for all available literatures through November, 2022 based on the following combination of terms (1): colon OR colorectal OR rectal (2); PET-MRI OR PET-MR OR Positron Emission Tomography Magnetic Resonance Imaging OR Positron Emission Tomography Computed Tomography OR PET/CT (3); liver metastasis OR liver metastases. Studies that might have been relevant were also included from the reference lists.
2.1.2. Inclusion and exclusion criteria
Only studies that met all of the following criteria were included (1): articles evaluating the diagnostic performance of [18F]FDG PET/CT or [18F]FDG PET/MRI for colorectal liver metastasis (2); number of patients or lesions ≥ 10 (3); histological pathology or follow-up imaging as gold standard. The exclusion criteria were (1): Irrelevant topic (2); duplicated articles (3); case reports, abstract, letters, review, or meta-analysis (4); true positive (TP), false positive (FP), true negative (TN), false negative (FN) data could not be extracted. After evaluating the titles and abstracts of the articles according to the inclusion and exclusion criteria, the full-text versions of the selected articles were examined to determine if they met the inclusion criteria. Disagreements among the researchers were settled through consensus.
2.1.3. Quality assessment and data extraction
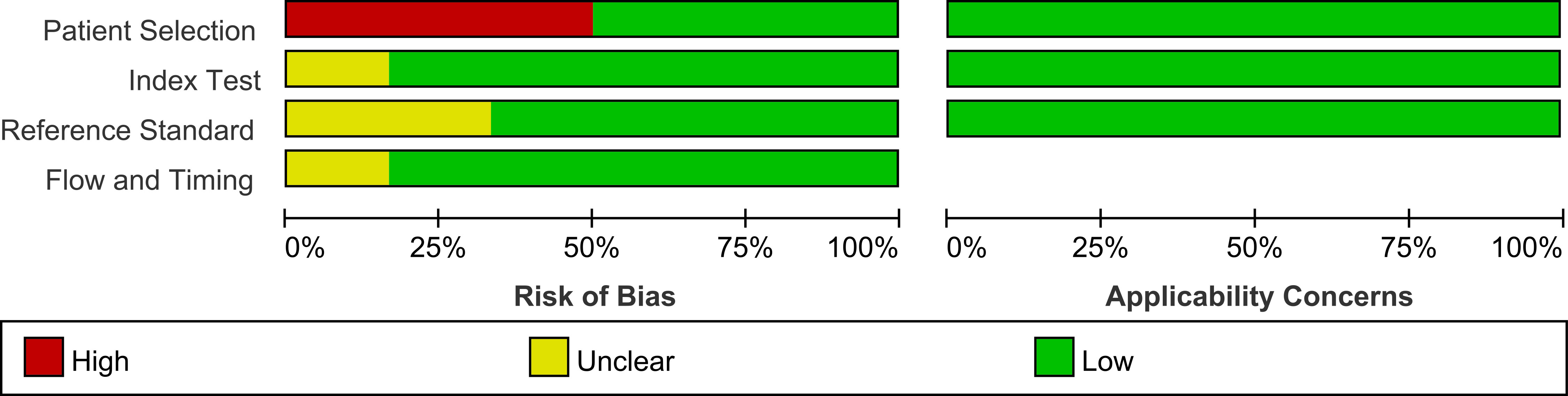
Using the Quality Assessment of Diagnostic Performance Studies (QUADAS-2) technique, two researchers independently assessed the quality of the included studies. Each study’s risk of bias and applicability were evaluated. It includes four important domains, including (1) patient selection (2); index test (3); reference standard; and (4) the flow and timing (19). For risk of bias, the question for patient selection was if consecutive patients enrolled; The question for the index test was if the index test results were interpreted without knowledge of the results of the reference standard; The question for reference standard was if the reference standard results interpreted without knowledge of the results of the index test; The question for the flow and timing was if there an appropriate interval between index tests and the reference(3 months). For application concern, the question for patient selection was if concerns that the included patients do match the review question; The question for index test was if there were concerns that the index test, its conduct, or its interpretation differ from the review question; The question for reference standard was if there concerns that the target condition as defined by the reference standard does not match the review question. The evaluation of each study was rated as high, low, or unclear in terms of risk of bias and applicability. To settle any potential disagreements, a third reviewer was engaged in. RevMan (version 5.3) was used for the analysis.
Data extraction for all included papers was conducted separately by two researchers. The data that were extracted included (1): the author, year of publication (2); study characteristics including country, study design, analysis, reference standard (3); patient characteristics including number of patients, clinical indication, mean/median age, chemotherapy before PET (4); technical characteristics including types of imaging tests, scanner modality, ligand dose, time from injection to acquisition, image analysis, TP, FP, FN, TN. Data were manually retrieved from the literature, tables, and figures when not clearly stated. When the paper lacked sufficient information, we contacted the corresponding authors via email to request further data or clarification. Two researchers addressed their disagreements through consensus.
2.1.4. Data synthesis and statistical analysis
The Spearman rank correlation coefficient were used to evaluate threshold effect performance and a P value < 0.05 indicates that the threshold effect may contribute to the heterogeneity. Using a bivariate random-effect model, the pooled sensitivity and specificity for [18F]FDG PET/CT and [18F]FDG PET/MRI were reported as estimates with 95% confidence intervals (CIs). The summary receiver operating characteristic curve and area under the curve (AUC) were generated by using the summary receiver operating characteristic (SROC) model. If the 95% confidence intervals of the two modalities did not overlap, it was considered that there was a statistically significant difference in performance. If the 95% confidence intervals of the AUC of the two modalities did not overlap, it was considered that there was a statistically significant difference in performance. Multilevel mixed-effects logistic regression was used to compare the summary paired sensitivity or specificity data, adding test type (PET/CT or PET/MRI) as covariate. Likelihood ratio tests were used to obtain the statistical differences between the sensitivities and specificities of the two tests type by fitting alternative models, adding or removing the covariate term from the model (20).
Heterogeneity among pooled studies was assessed using the I2 statistic. Homogeneity among the studies was considered to be low, moderate, or high when the I2 value was 25%, 50%, or 75%. For PET/CT, the meta-regression analysis and leave-one-out sensitivity analysis were conducted in the case of substantial heterogeneity (I2≥ 50%) to explore possible sources of heterogeneity. For PET/MRI, we did not conduct meta-regression due to the small number of included studies (less than 10), but we perform leave-one-out sensitivity analysis to find the source of heterogeneity. Deeks’ funnel plot tests were used to evaluate publication bias. All analyses were conducted with Stata 15.1 and Meta-DiSc 1.4. Statistical significance was defined as a P value less than 0.05.
2.2. Results
2.2.1. Literature search and study selection
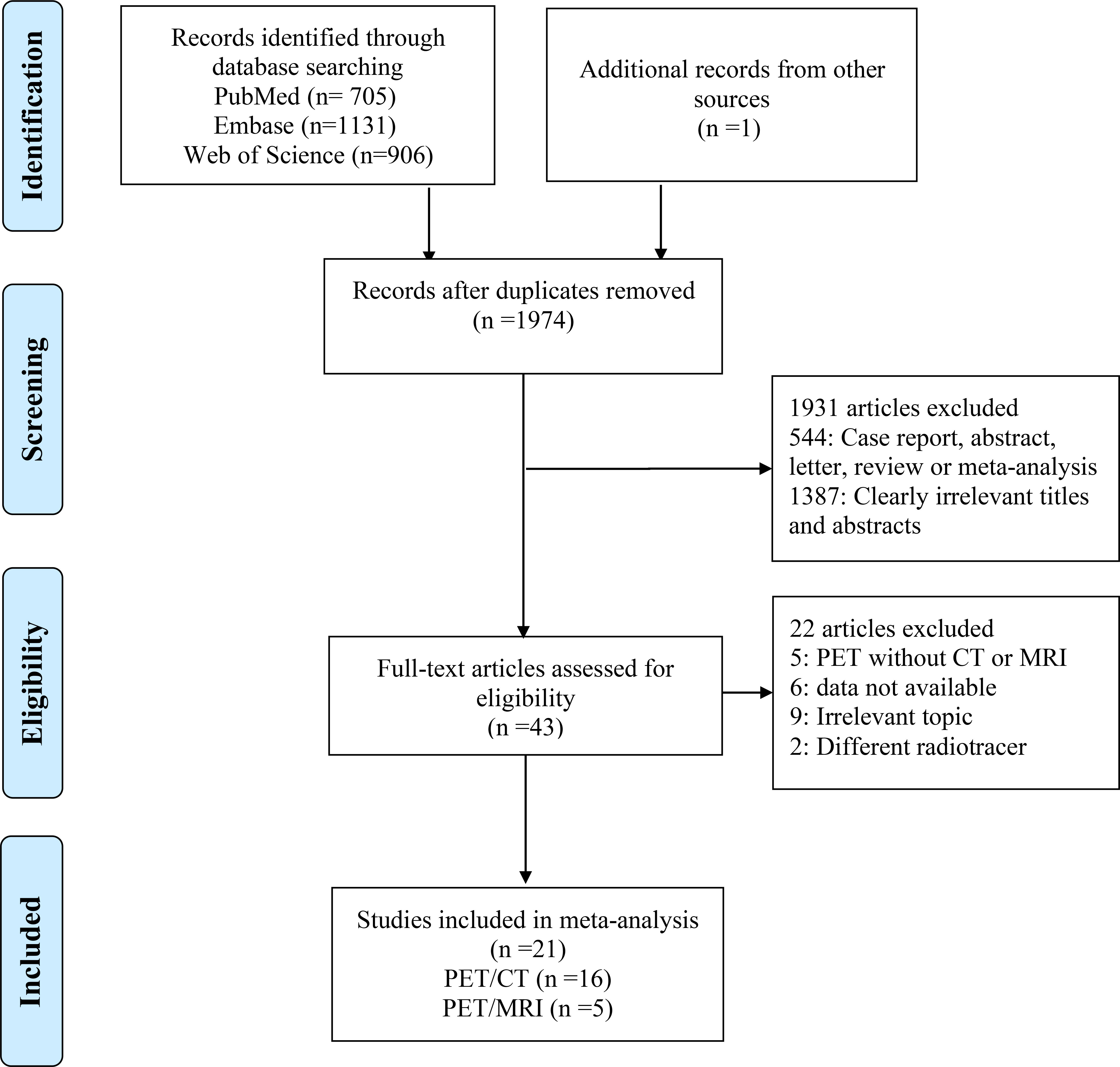
There were a total of 2743 publications identified in the initial search, 1974 studies were identified after excluding 769 duplicated studies. Based on the title or abstract,1931 studies were excluded. In the remaining results, 9 articles were irrelevant, 2 used different radiotracers, 6 data not available, and 9 PET without CT or MRI. Finally, a total of 21 articles evaluating the diagnostic performance for colorectal liver metastasis including 16 articles for PET/CT (9, 10, 13–17, 21–29), and 5 articles for PET/MRI were included (17, 29–32). The PRISMA flow diagram of the study selection process is shown in Figure 1. A full list of all reviewed full-text articles was shown in Supplementary Table 2.
2.2.2. Study description and quality assessment
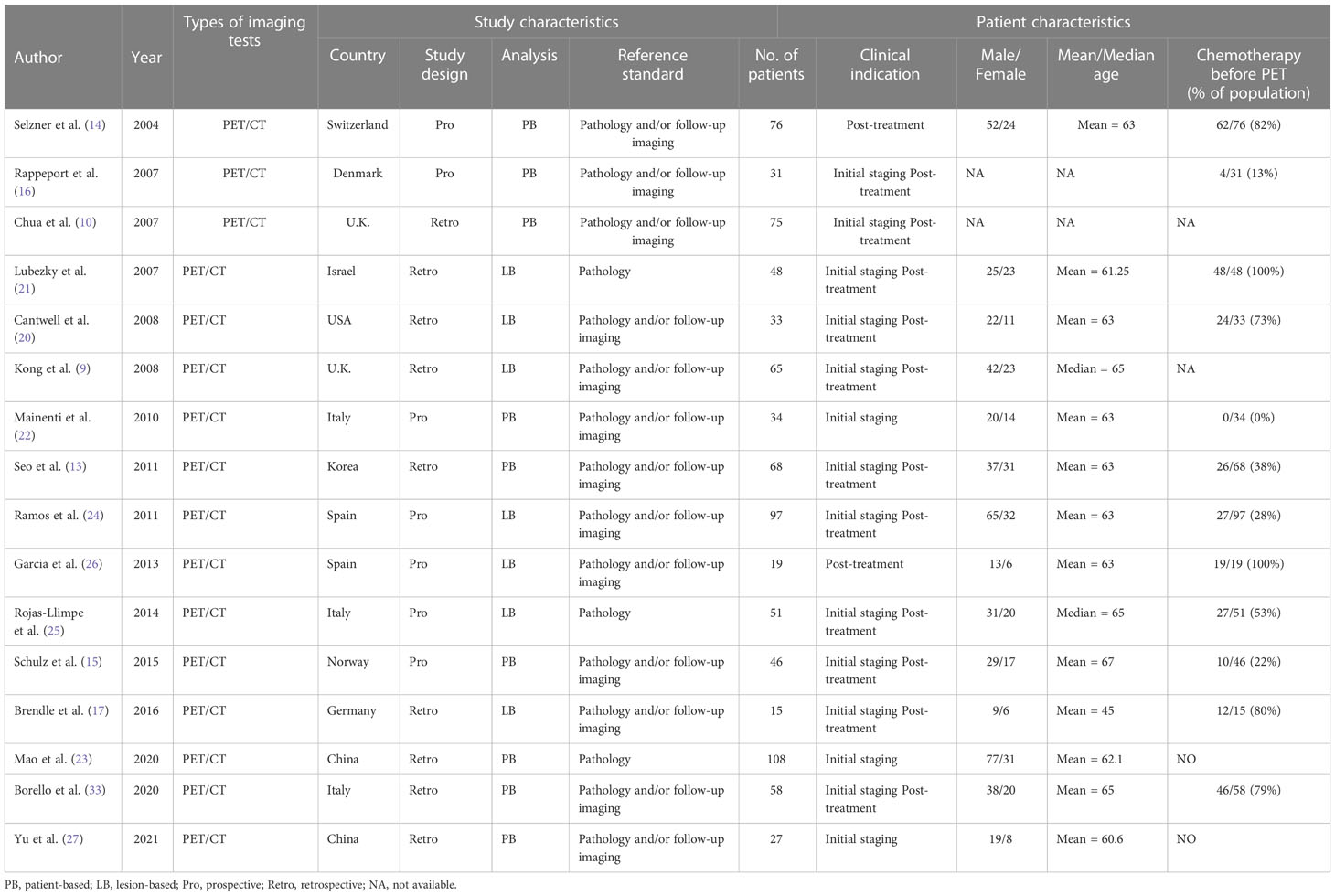
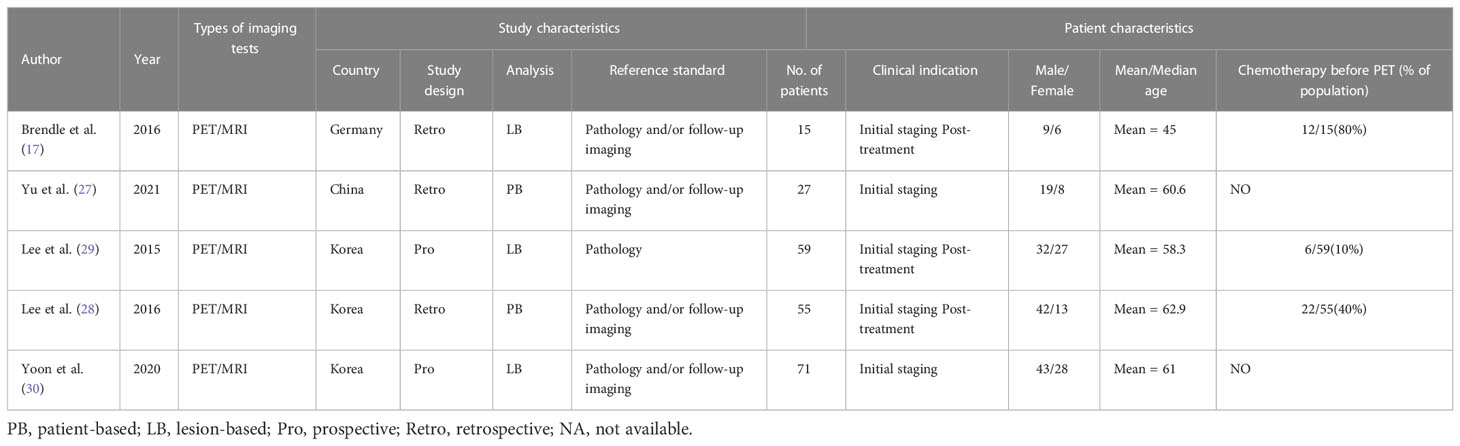
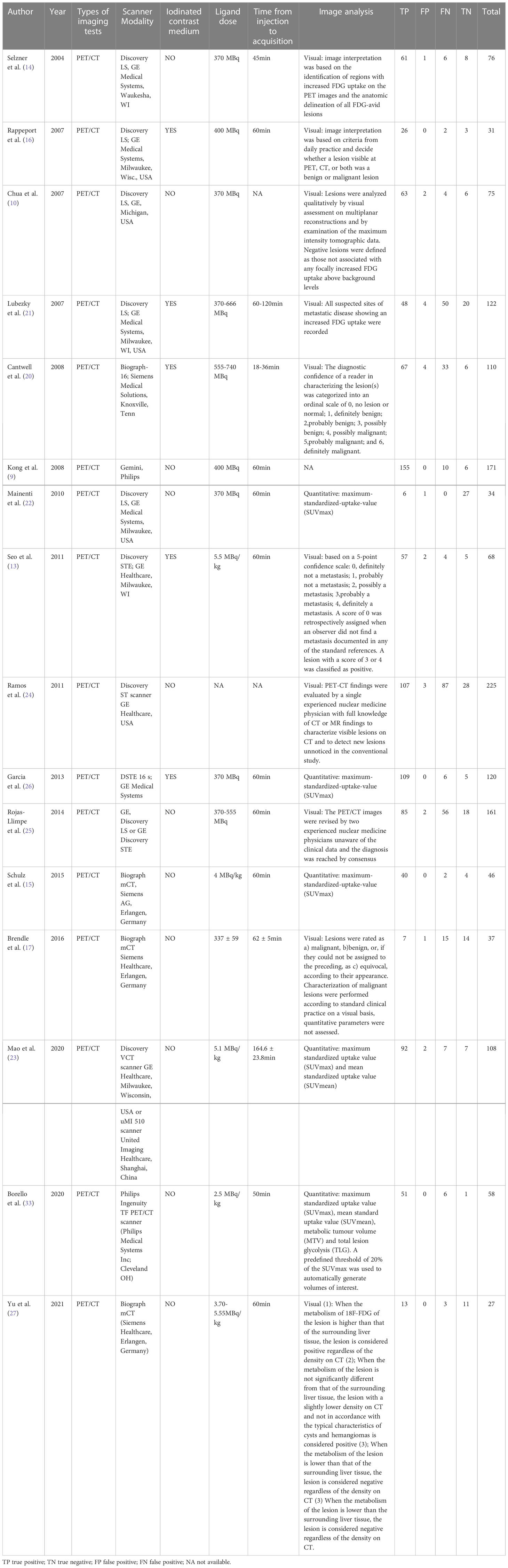
The study and patient characteristics from the 21 studies covering 1036 patients were listed in Table 1 and Table 2. Technical aspects were displayed in Table 3 and Table 4. What’ s more, an assessment on the quality of involved studies was carried out depending on the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. The quality assessment graph revealed high-risk bias concerns mainly concentrated on the field of patient selection (Figure 2), caused by that most of these studies did not include consecutive patients. Overall, the risk bias of the articles was considered satisfactory.
2.2.3. Diagnostic performance of [18F]FDG PET/CT and PET/MRI for colorectal liver metastasis
For [18F]FDG PET/CT, the results of the Spearman correlation coefficient demonstrated no threshold effect heterogeneity (Spearman correlation coefficient =0.074, P=0.786), similarly, [18F]FDG PET/MRI also showed no threshold effect heterogeneity (Spearman correlation coefficient =-0.500, P=0.391). The results of pooled sensitivity of [18F]FDG PET/CT for colorectal liver metastasis were 0.86 (95% CI, 0.76-0.92) and specificity were 0.89 (95% CI, 0.83-0.94) (Figure 3). The pooled sensitivity of [18F]FDG PET/MRI were 0.84 (95% CI, 0.77-0.89) and specificity were 1.00 (95%CI, 0.32-1.00) (Figure 4). The sensitivity of the two tests didn’t differ significantly (P= 0.58),and the specificity of the two tests also didn’t differ significantly (P= 0.27). Figure 5 illustrated the SROC curve for [18F]FDG PET/CT and [18F]FDG PET/MRI, which exhibited an AUC of 0.92 (95%CI: 0.90-0.94) and 0.89 (95% CI: 0.86-0.92).
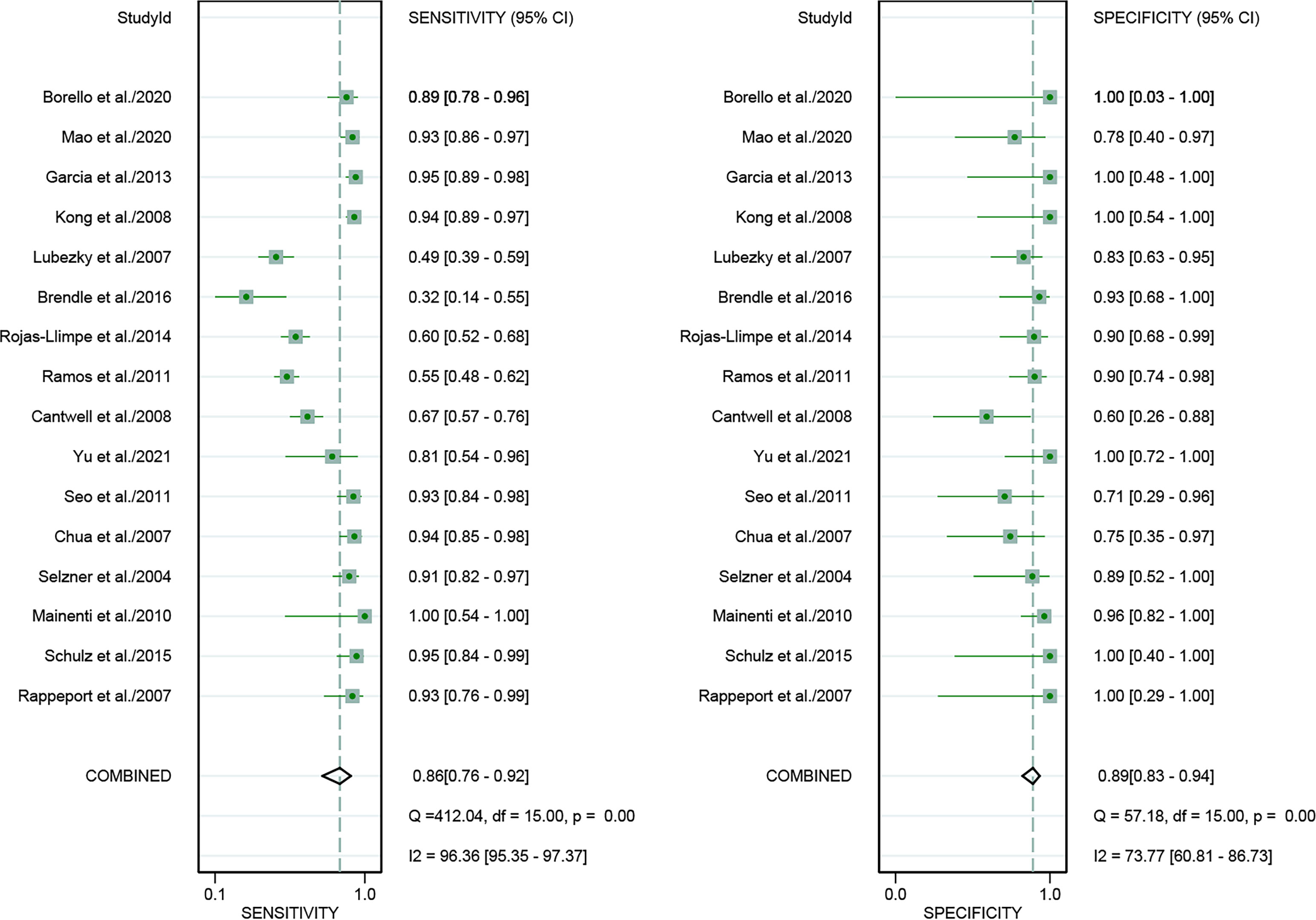
Figure 3 Forest plots of the combined [18F]FDG PET/CT sensitivity and specificity for colorectal liver metastasis. Squares denoted the sensitivity and specificity in each study, while horizontal bars indicated the 95% confidence interval.
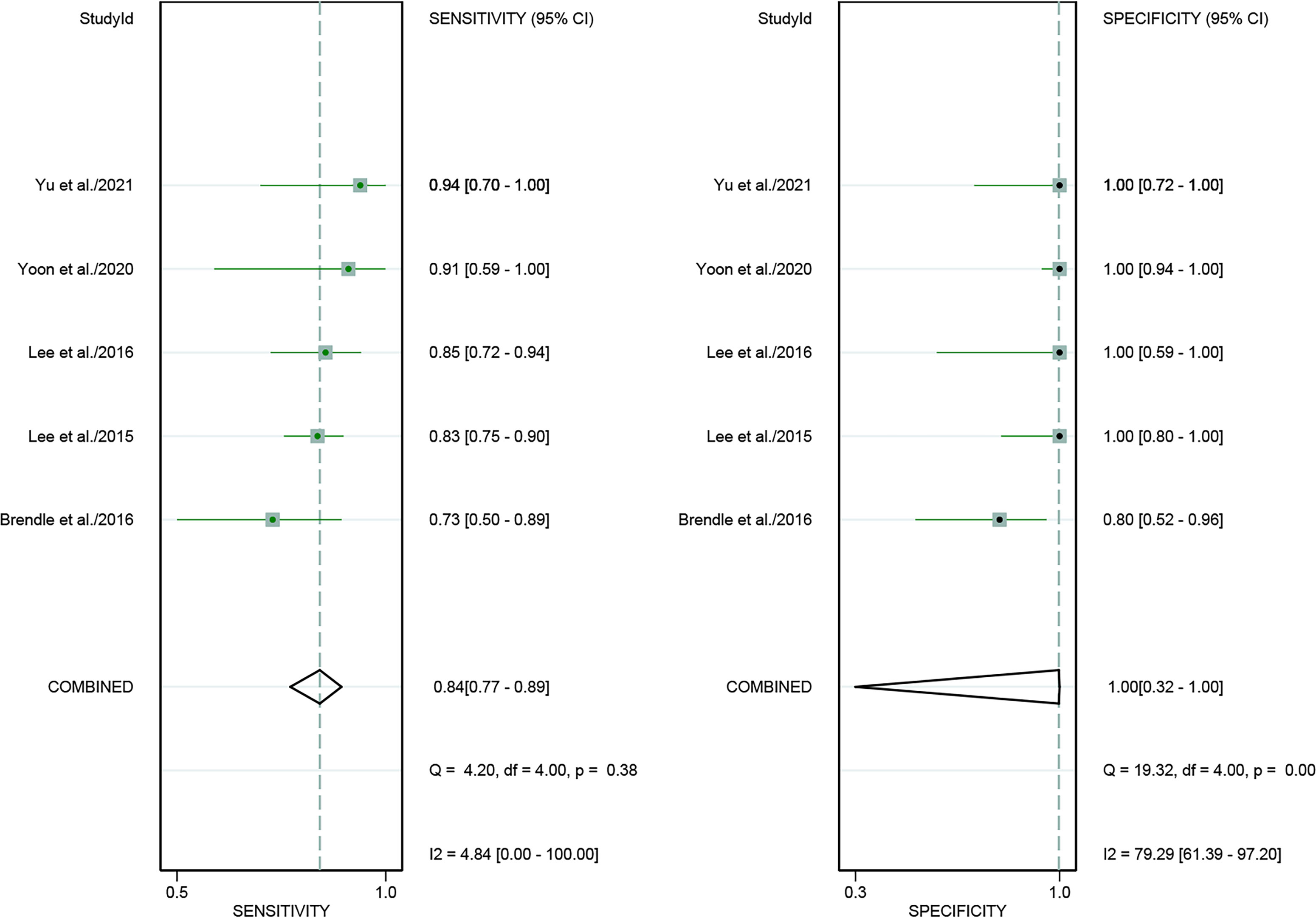
Figure 4 Forest plots of the combined [18F]FDG PET/MRI sensitivity and specificity for colorectal liver metastasis. Squares denoted the sensitivity and specificity in each study, while horizontal bars indicated the 95% confidence interval.
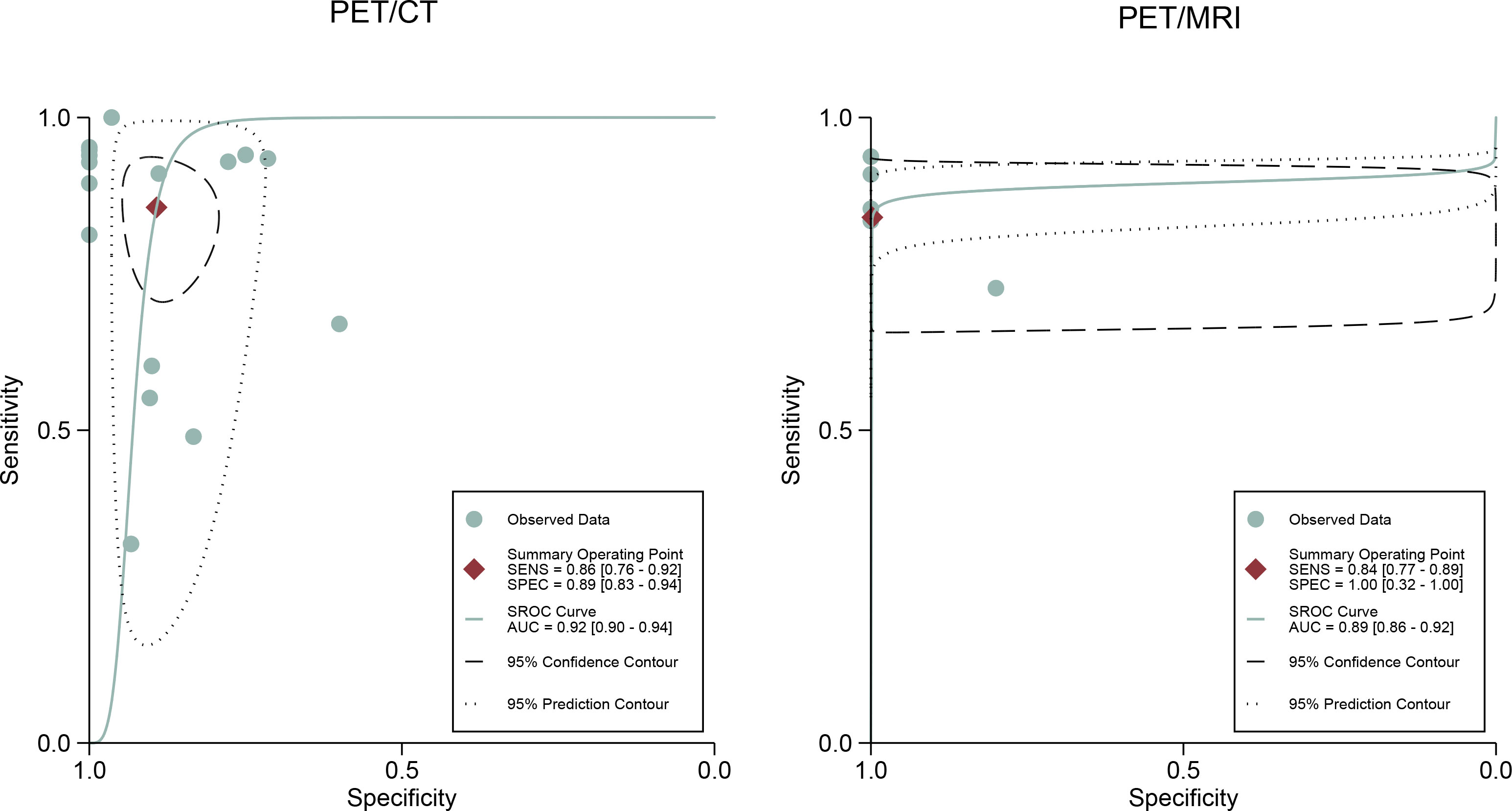
Figure 5 [18F]FDG PET/CT and [18F]FDG PET/MRI summary receiver operating characteristic (SROC) curves. The summary point is the optimal combination of sensitivity and specificity. The black dotted lines surrounding each summary point indicates the 95% confidence interval.
In addition, we also performed a subgroup forest plot for PET/CT in patient-based analysis (Figure 6), the results of pooled sensitivity of [18F]FDG PET/CT in patient-based analysis for colorectal liver metastasis were 0.92 (95% CI, 0.82-0.97) and specificity were 0.89 (95% CI, 0.52-1.00).
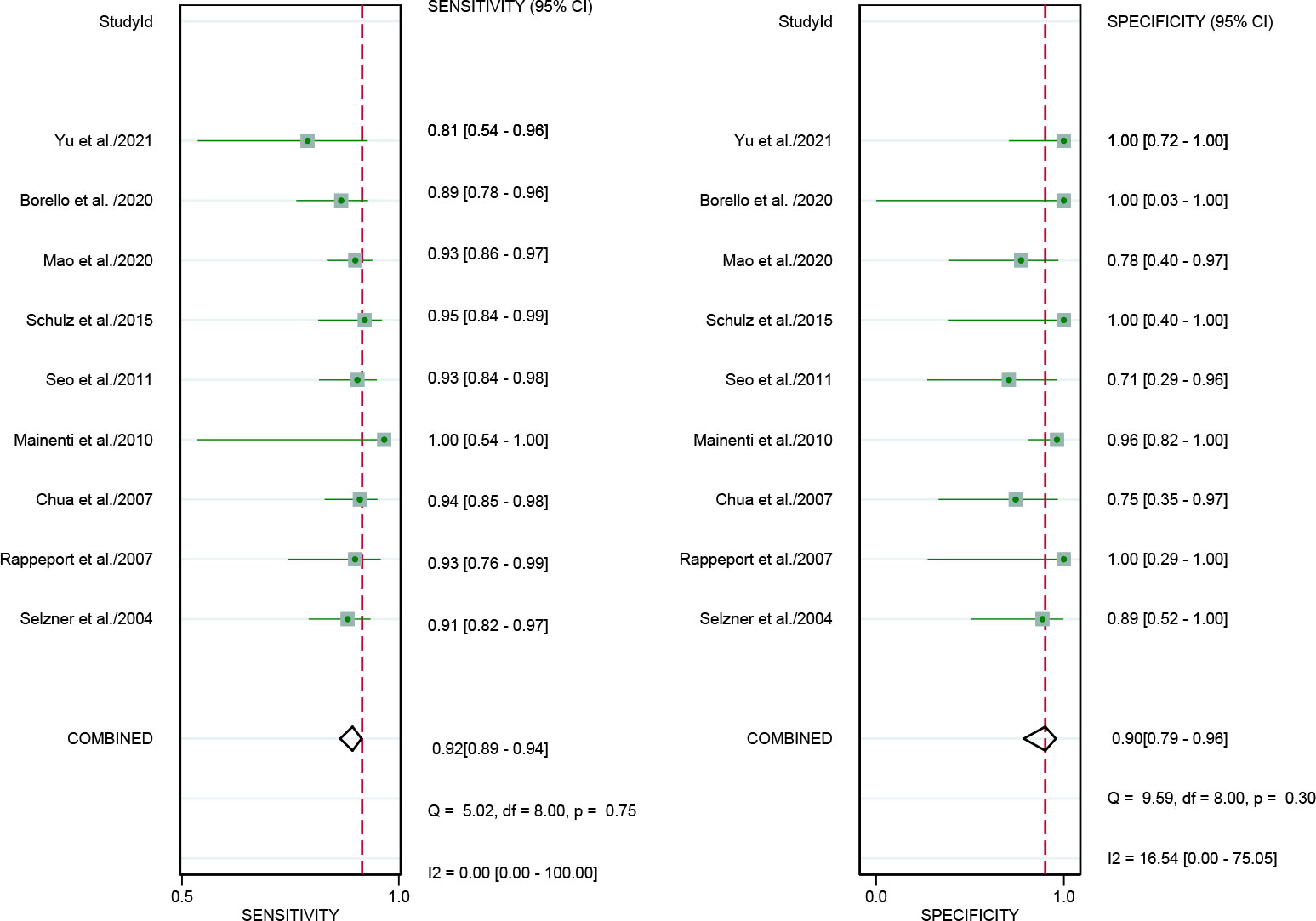
Figure 6 Forest plots of the combined [18F]FDG PET/CT sensitivity and specificity in patient-based analysis for colorectal liver metastasis. Squares denoted the sensitivity and specificity in each study, while horizontal bars indicated the 95% confidence interval.
2.2.4. Heterogeneity analysis
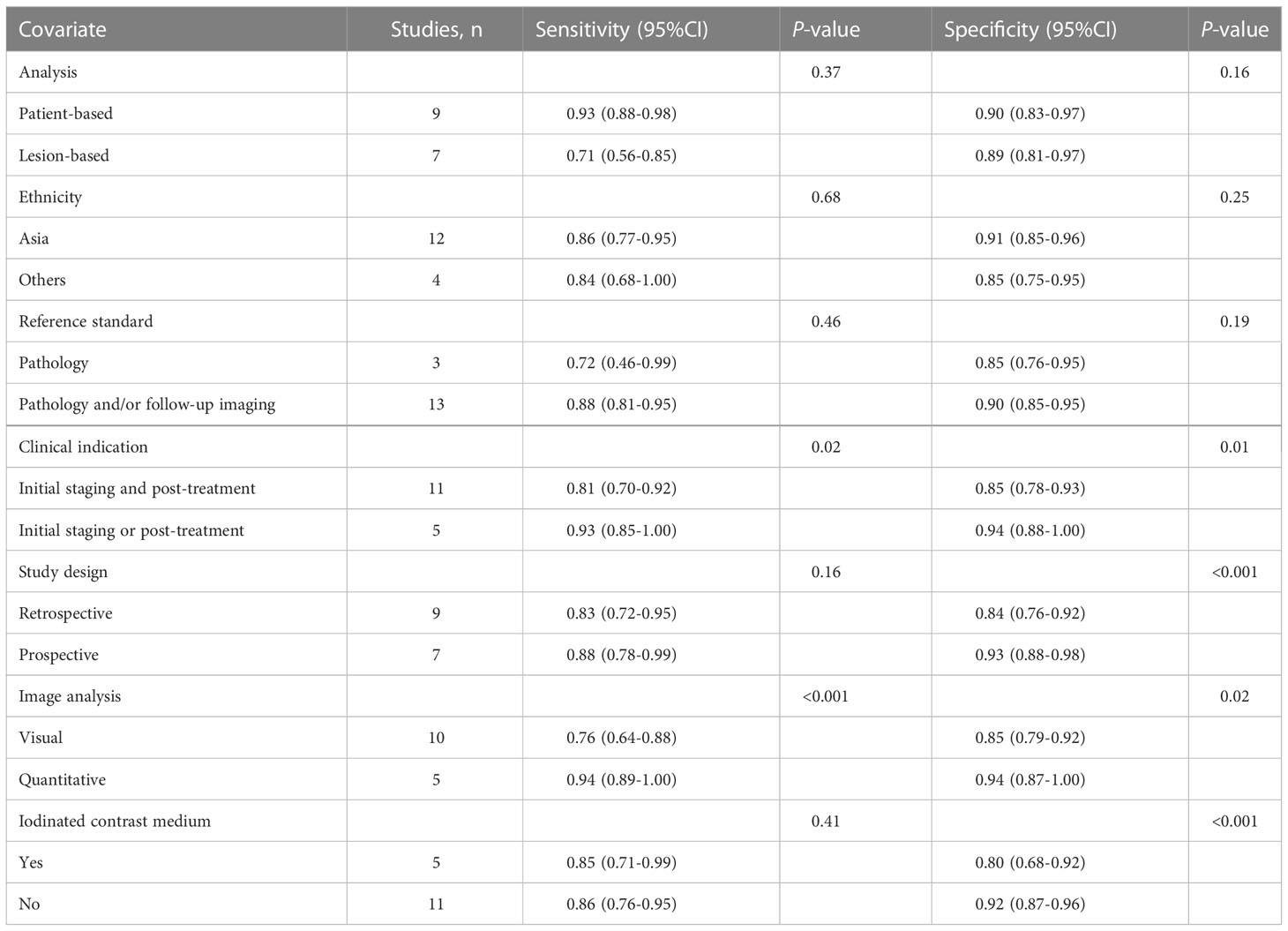
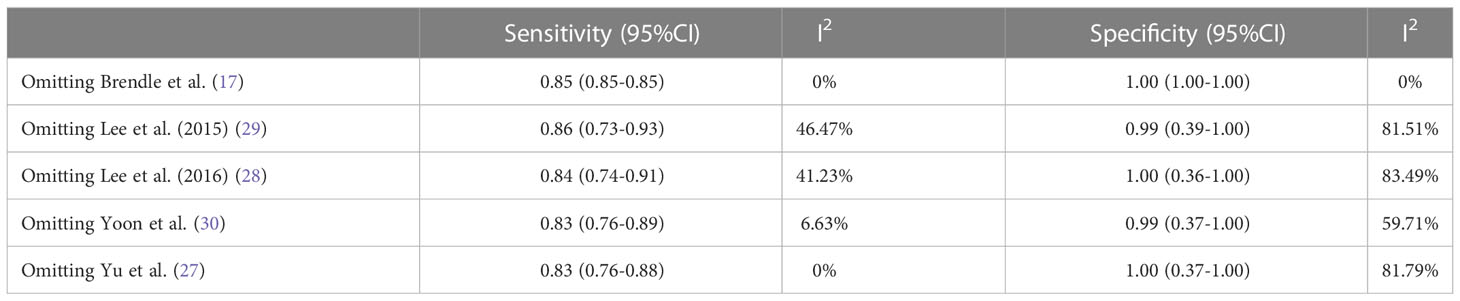
Regarding the pooled sensitivity and specificity of [18F]FDG PET/CT for colorectal liver metastasis, the I2 was 96.36%, 73.77%, respectively. In terms of the heterogeneity of [18F]FDG PET/MRI, the I2 were 79.29% and 4.84%. For [18F]FDG PET/CT, meta-regression analysis showed that image analysis(P<0.001 for sensitivity, P=0.02 for specificity), study design (P<0.001 for specificity), clinical indication (P=0.02 for sensitivity, P=0.01 for specificity), and iodinated contrast medium (P<0.001 for specificity) were the possible cause of heterogeneity (Table 5 and Figure 7). For [18F]FDG PET/MRI, sensitivity analysis by excluding data from Brendle et al. demonstrated a combined specificity of 1.00(95% CI: 1.00–1.00), with no heterogeneity (I2 = 0%) (Table 6).
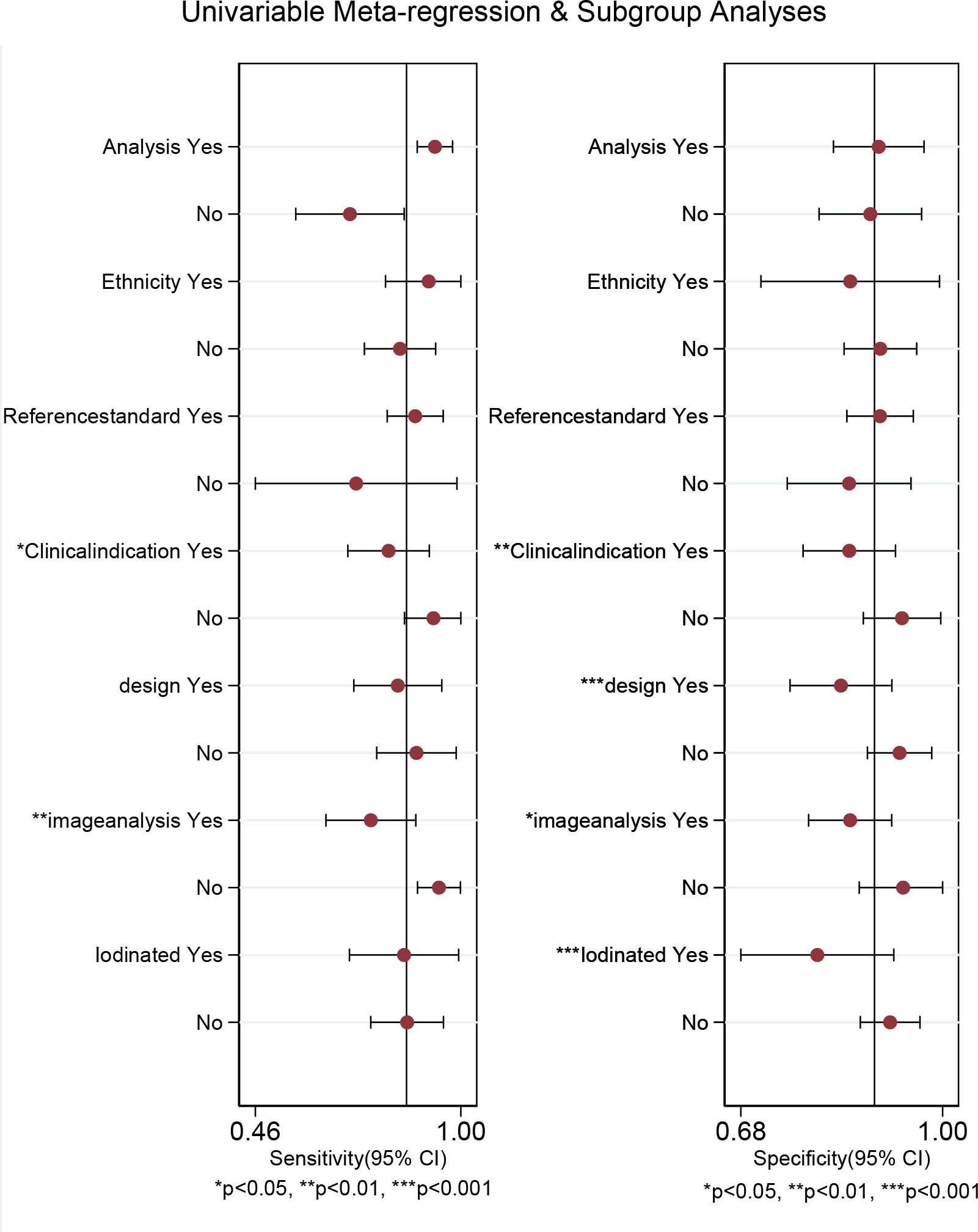
Figure 7 Multiple univariable meta-regression forest plot of [18F]FDG PET/CT for colorectal liver metastasis.
2.2.5. Publication bias
Deeks’ funnel plot asymmetry test showed that there was a significant publication bias for [18F]FDG PET/CT (P=0.01), and no significant publication bias was observed for [18F]FDG PET/MRI (P=0.76) (Figure 8).
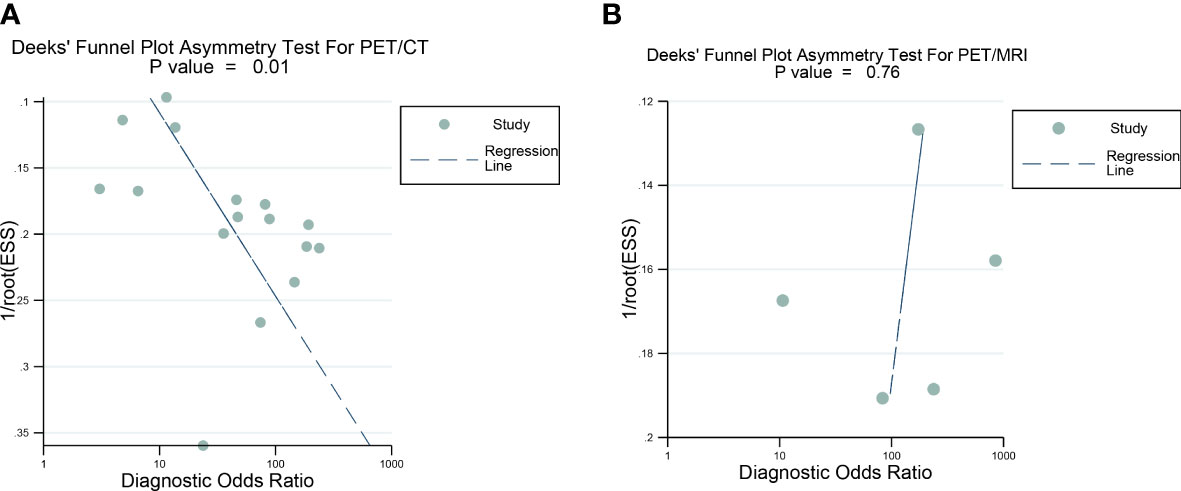
Figure 8 Deek’s funnel plot was used to evaluate the publication bias of [18F]FDG PET/CT and [18F]FDG PET/MRI. (A) Deek's funnel plot for PET/CT; (B) Deek's funnel plot for PET/CT. P<0.05 was considered significant.
2.3. Discussion
This is, to our knowledge, the first systematic review and meta-analysis comparing the diagnostic performance of [18F]FDG PET/CT and [18F]FDG PET/MRI for colorectal liver metastasis. The pooled sensitivity, specificity, and AUC of [18F]FDG PET/CT in were 0.86, 0.89, and 0.92. [18F]FDG PET/MRI were 0.84 and 1.00, and 0.89. PET/CT and PET/MRI seemed to have similar performance in detecting colorectal liver metastases because their 95% confidence intervals for AUC values were highly overlapping.
The staging or restaging of colorectal cancer’s local and distant metastases is crucial in assessing a patient’s survival and risk of recurrence, which was valuable for choosing the appropriate treatment strategy. Previously, three meta-analyses on PET or PET/CT for colorectal liver metastasis have been published. Niekel et al. conducted a meta-analysis that included only prospective trials (34). The authors reported that PET had a higher sensitivity on a per-patient basis (0.94), in comparison to both MRI (0.88) and CT (0.75). According to another meta-analysis conducted by Floriani et al., PET showed the highest sensitivity (0.94), followed by MRI (0.81), CT (0.75), and ultrasound for the diagnosis of liver metastases (6). A meta-analysis conducted by Maffione et al. indicated that PET is less sensitive (0.93) but more specific (0.93) than MRI and has an impact on the therapy of roughly one-fourth of patients (35). However, all of the previous meta-analyses didn’t mention hybrid PET/MRI system, which was an effective modality in detecting colorectal liver metastasis proven by recent studies. Thus, there was an urgent need to assess the diagnostic performance of PET/CT versus PET/MRI.
Our pooled sensitivity for PET/CT was inferior compared with the findings of both previously published meta-analyses (0.86 vs. 0.94 and 0.94). The specificity of PET/CT in our results were also inferior compared with the results of previous meta-analysis (0.89 vs. 0.94 and 0.93). This was due to our inclusion of studies that analyzed data from both patients and lesions. When we pooled sensitivity with only patient-based studies, the sensitivity (0.93) and specificity (0.90) were in line with the previous meta-analyses.
Even while [18F]FDG PET/CT and PET/MRI were increasingly being utilized to detect distant metastases in suspected cases of colorectal cancer, they were not the first-choice modality in the routine evaluation of CRC. However, Ruers et al. demonstrated the addition of PET over conventional imaging modalities, which indicated that the number of inefficient surgical procedures was dramatically decreased by include PET in the presurgical work-up (36). While [18F]FDG PET/CT has proven to be successful in a variety of clinical settings, [18F]FDG PET/MRI has not met with the same level of success. There were several reasons accounting for it, including expenses, logistical difficulties, and the original perception that PET/MRI was not superior to PET/CT in staging diagnostic ability (37), which was further confirmed in our study. Our results based on patient and lesion levels showed similar diagnostic performance between PET/CT and PET/MRI. When considering the high cost and diagnostic performance of PET/MRI, it was determined that the diagnostic value offered by PET/CT was effective enough to serve as a suitable alternative. In terms of the diagnostic performance, our findings may be applicable and timely for clinical decision-making. However, due to the small sample size of hybrid PET/MRI, further larger prospective studies focusing in the diagnostic performance of PET/MRI in colorectal liver metastasis are still needed to obtain a more robust result.
In terms of heterogeneity, there was high heterogeneity in [18F]FDG PET/CT (sensitivity and specificity) and [18F]FDG PET/MRI (specificity). We examined the sources of heterogeneity among the studies by performing meta-regression and sensitivity analysis. For [18F]FDG PET/CT, meta-regression analysis showed that image analysis, study design,clinical indication, and iodinated contrast medium were the possible cause of heterogeneity. For [18F]FDG PET/MRI, we got an acceptable heterogeneity (I2 = 0%) by excluding data from Brendle et al., which could be explained by different previous treatment and cut-off thresholds. Nevertheless, there may be further causes, such as differences in patients, technique, and study design. It yielded a same specificity (1.00) when we omitting the study by Brendle et al., which further proved the robustness of the results.
The limitations of our meta-analysis should also be mentioned. First, only five studies offered sufficient information that evaluated the diagnostic performance of the hybrid PET/MRI system for the staging or restaging of liver metastases in CRC patients, which leads to small sample size. This was because hybrid PET/MRI were introduced in the recent years and still lack of well-designed trials. Second, Due to the limited number of research that satisfied the inclusion criteria, we included either patient-based or lesion-based analysis studies, which could increase potential bias. Third, most of the reference standard for diagnosis colorectal liver metastasis was pathology and follow-up imaging, pathological results are not obtained for all patients in the included studies. These results therefore need to be interpreted with caution.
2.4. Conclusion
[18F]FDG PET/CT shows similar performance compared to [18F]FDG PET/MRI in detecting colorectal liver metastasis. However, pathological results were not obtained for all patients in the included studies and PET/MRI results were derived from studies with small sample sizes. There is a need for additional, larger prospective studies on this issue.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ZM and XZ conceived and designed the study, which were proofed by ZM. XZ and XL collected and analyzed the data. ZM wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1114059/full#supplementary-material
References
1. Artigas Martín JM, Alonso Orduña V, Serrablo Requejo A, Larrosa López R, Martín Cuartero J. Epidemiology and diagnosis of liver metastases. Revisiones en Cancer (2008) 22(1):1–13.
2. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg (2006) 244(2):254–9. doi: 10.1097/01.sla.0000217629.94941.cf
3. Belli G, D'Agostino A, Ciciliano F, Fantini C, Russolillo N, Belli A. Liver resection for hepatic metastases: 15 years of experience. J Hepatobiliary Pancreat Surg (2002) 9(5):607–13. doi: 10.1007/s005340200082
4. Smith MD, McCall JL. Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg (2009) 96(10):1101–13. doi: 10.1002/bjs.6735
5. Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer (2006) 42(14):2212–21. doi: 10.1016/j.ejca.2006.04.012
6. Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: A systematic review and meta-analysis. J OF MAGNETIC RESONANCE Imaging (2010) 31(1):19–31. doi: 10.1002/jmri.22010
7. Kekelidze M, D'Errico L, Pansini M, Tyndall A, Hohmann J. Colorectal cancer: Current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J OF Gastroenterol (2013) 19(46):8502–14. doi: 10.3748/wjg.v19.i46.8502
8. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg (2002) 235(6):759–66. doi: 10.1097/00000658-200206000-00002
9. Kong G, Jackson C, Koh DM, Lewington V, Sharma B, Brown G, et al. The use of 18F-FDG PET/CT in colorectal liver metastases–comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging (2008) 35(7):1323–9. doi: 10.1007/s00259-008-0743-z
10. Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, et al. The impact of (18)F-FDG PET/CT in patients with liver metastases. Eur J OF Nucl Med AND Mol Imaging (2007) 34(12):1906–14. doi: 10.1007/s00259-007-0518-y
11. Georgakopoulos A, Pianou N, Kelekis N, Chatziioannou S. Impact of 18F-FDG PET/CT on therapeutic decisions in patients with colorectal cancer and liver metastases. Clin Imaging (2013) 37(3):536–41. doi: 10.1016/j.clinimag.2012.09.011
12. Mottaghy FM, Sunderkötter C, Schubert R, Wohlfart P, Blumstein NM, Neumaier B, et al. Direct comparison of [18F]FDG PET/CT with PET alone and with side-by-side PET and CT in patients with malignant melanoma. Eur J Nucl Med Mol Imaging (2007) 34(9):1355–64. doi: 10.1007/s00259-006-0358-1
13. Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced f-18-Fluorodeoxyglucose positron emission Tomography/Computed tomography for the detection of colorectal liver metastases. Invest Radiol (2011) 46(9):548–55. doi: 10.1097/RLI.0b013e31821a2163
14. Selzner MK, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann OF Surg (2004) 240(6):1027–36. doi: 10.1097/01.sla.0000146145.69835.c5
15. Schulz A, Viktil E, Godt JC, Johansen CK, Dormagen JB, Holtedahl JE, et al. Diagnostic performance of CT, MRI and PET/CT in patients with suspected colorectal liver metastases: the superiority of MRI. Acta RADIOLOGICA (2016) 57(9):1040–8. doi: 10.1177/0284185115617349
16. Rappeport ED, Loft A, Berthelsen AK, von der Recke P, Larsen PN, Mogensen AM, et al. Contrast-enhanced FDG-PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients with liver metastases from colorectal cancer: A prospective study with intraoperative confirmation. Acta RADIOLOGICA (2007) 48(4):369–78. doi: 10.1080/02841850701294560
17. Brendle C, Schwenzer NF, Rempp H, Schmidt H, Pfannenberg C, la Fougere C, et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J OF Nucl Med AND Mol Imaging (2016) 43(1):123–32. doi: 10.1007/s00259-015-3137-z
18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med (2009) 3(3):e123–30.
19. Cantoni V, Green R, Acampa W, Zampella E, Assante R, Nappi C, et al. Diagnostic performance of myocardial perfusion imaging with conventional and CZT single-photon emission computed tomography in detecting coronary artery disease: A meta-analysis. J Nucl Cardiol (2021) 28(2):698–715. doi: 10.1007/s12350-019-01747-3
20. Van Houwelingen HC, Zwinderman KH, Stijnen T. A bivariate approach to meta-analysis. Stat Med (1993) 12(24):2273–84. doi: 10.1002/sim.4780122405
21. Borello A, Russolillo N, Lo Tesoriere R, Langella S, Guerra M, Ferrero A. Diagnostic performance of the FDG-PET/CT in patients with resected mucinous colorectal liver metastases. SURGEON-JOURNAL OF THE R COLLEGES OF SURGEONS OF EDINBURGH AND IRELAND (2021) 19(5):E140–E5. doi: 10.1016/j.surge.2020.09.004
22. Cantwell CP, Setty BN, Holalkere N, Sahani DV, Fischman AJ, Blake MA. Liver lesion detection and characterization in patients with colorectal cancer: A comparison of low radiation dose non-enhanced PET/CT, contrast-enhanced PET/CT, and liver MRI. J OF Comput ASSISTED TOMOGRAPHY (2008) 32(5):738–44. doi: 10.1097/RCT.0b013e3181591d33
23. Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Klausner JM, et al. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: Comparison with operative and pathological findings. J OF GASTROINTESTINAL Surg (2007) 11(4):472–8. doi: 10.1007/s11605-006-0032-8
24. Mainenti PP, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, et al. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. ABDOMINAL Imaging (2010) 35(5):511–21. doi: 10.1007/s00261-009-9555-2
25. Mao W, Zhou J, Qiu L, Yin H, Tan H, Shi H. The added value of dual-time-point 18F-FDG PET/CT imaging in the diagnosis of colorectal cancer liver metastases. Abdom Radiol (NY) (2020) 45(4):1075–81. doi: 10.1007/s00261-019-02396-3
26. Ramos E, Valls C, Martinez L, Llado L, Torras J, Ruiz S, et al. Preoperative staging of patients with liver metastases of colorectal carcinoma. does PET/CT really add something to multidetector CT? Ann OF Surg Oncol (2011) 18(9):2654–61. doi: 10.1245/s10434-011-1670-y
27. Rojas Llimpe FL, Di Fabio F, Ercolani G, Giampalma E, Cappelli A, Serra C, et al. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: results of the PROMETEO-01 study. Br J Cancer (2014) 111(4):667–73. doi: 10.1038/bjc.2014.351
28. Vicente AMG, Ferreras ED, Perez VS, Garcia VMP, Guzman JCV, Aragon FJ, et al. Response assessment of colorectal liver metastases with contrast enhanced CT/18F-FDG PET. Eur J OF Radiol (2013) 82(6):E255–E61. doi: 10.1016/j.ejrad.2012.12.029
29. Yu Jun ZY, Dongdong R, Cong C, Yang Li, Chunling R, Jun C. Comparative analysis of 18F-FDG PET/CT and PET/MRI in the diagnosis of colorectal cancer liver metastases. Int J Radiat Med Nucl Med (2021) 45(2):75–82. doi: 10.3760/cma.j.cn121381–202003009–00013
30. Lee DH, Lee JM, Hur BY, Joo I, Yi NJ, Suh KS, et al. Colorectal cancer liver metastases: Diagnostic performance and prognostic value of PET/MR imaging. RADIOLOGY (2016) 280(3):782–92. doi: 10.1148/radiol.2016151975
31. Lee SJ, Seo HJ, Kang KW, Jeong SY, Yi NJ, Lee JM, et al. Clinical performance of whole-body 18F-FDG PET/Dixon-VIBE, T1-weighted, and T2-weighted MRI protocol in colorectal cancer. Clin Nucl Med (2015) 40(8):E392–E8. doi: 10.1097/RLU.0000000000000812
32. Yoon JH, Lee JM, Chang W, Kang HJ, Bandos A, Lim HJ, et al. Initial m staging of rectal cancer: FDG PET/MRI with a hepatocyte-specific contrast agent versus contrast-enhanced CT. RADIOLOGY (2020) 294(2):310–9. doi: 10.1148/radiol.2019190794
33. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology (1983) 148(3):839–43. doi: 10.1148/radiology.148.3.6878708
34. Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: A meta-analysis of prospective studies including patients who have not previously undergone treatment. RADIOLOGY (2010) 257(3):674–84. doi: 10.1148/radiol.10100729
35. Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic accuracy and impact on management of f-18-FDG PET and PET/CT in colorectal liver metastasis: A meta-analysis and systematic review. Eur J OF Nucl Med AND Mol Imaging (2015) 42(1):152–63. doi: 10.1007/s00259-014-2930-4
36. Ruers TJ, Wiering B, van der Sijp JR, Roumen RM, de Jong KP, Comans EF, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. J Nucl Med (2009) 50(7):1036–41. doi: 10.2967/jnumed.109.063040
Keywords: PET/CT, PET/MRI, colorectal neoplasms, liver metastasis, meta-analysis
Citation: Miao Z, Zhao X and Li X (2023) [18F]FDG PET/CT versus [18F]FDG PET/MRI for the diagnosis of colorectal liver metastasis: A systematic review and meta-analysis. Front. Oncol. 13:1114059. doi: 10.3389/fonc.2023.1114059
Received: 13 December 2022; Accepted: 31 January 2023;
Published: 13 February 2023.
Edited by:
Mario Petretta, IRCCS SYNLAB SDN, ItalyReviewed by:
Roberta Green, Federico II University Hospital, ItalyTeresa Mannarino, University of Naples Federico II, Italy
Copyright © 2023 Miao, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Miao, MTAxOTIwNzE2N0B0anUuZWR1LmNu
†These authors have contributed equally to this work
 Zhi Miao
Zhi Miao Xiaomeng Zhao1,2†
Xiaomeng Zhao1,2†