- Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, Heilongjiang, China
Objective: Although the survival rate of patients who undergo surgery for gastric cancer has greatly improved, still many patients have a poor prognosis. This retrospective study aimed to investigate the predictive ability of the PNI-IgM score, a combined prognostic nutritional index (PNI), and immunoglobulin M (IgM), on the prognosis of patients undergoing surgery for gastric cancer.
Methods: 340 patients with gastric cancer who underwent surgery from January 2016 to December 2017 were selected. The PNI-IgM score ranged from 1 to 3: score of 1, low PNI (< 48.45) and low IgM (< 0.87); score of 2, low PNI and high IgM, or high PNI and low IgM; score of 3, high PNI and high IgM. We compared the differences in disease-free survival (DFS) and overall survival (OS) among the three groups, while univariate and multivariate analyses calculated prognostic factors for DFS and OS. In addition, the nomograms were constructed based on the results of multivariate analysis to estimate the 1-, 3- and 5-year survival probability.
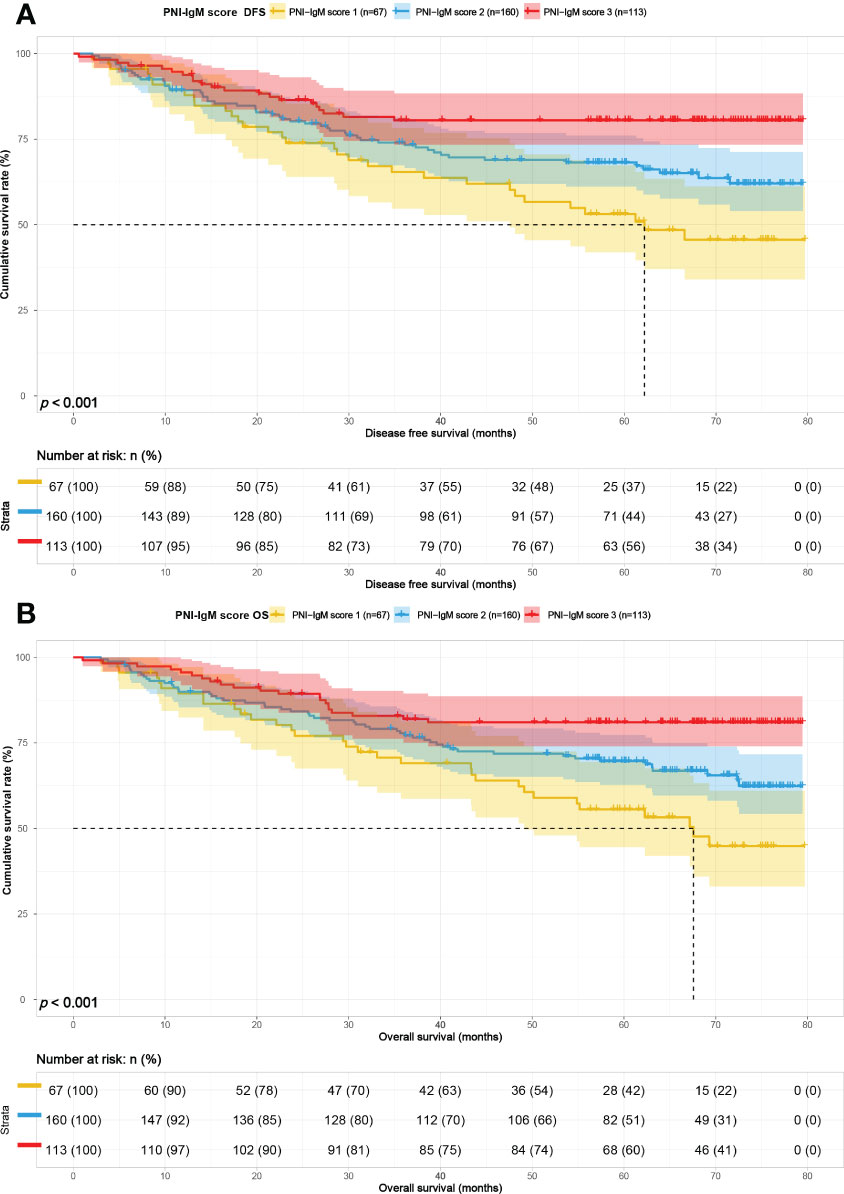
Results: There were 67 cases in the PNI-IgM score 1 group, 160 cases in the PNI-IgM score 2 group, and 113 cases in the PNI-IgM score 3 group. The median survival times of DFS in the PNI-IgM score group 1, the PNI-IgM score group 2, and the PNI-IgM score group 3 were 62.20 months, not reached, and not reached, and 67.57 months vs. not reached vs. not reached in three groups for OS. Patients in the PNI-IgM score group 1 had a lower DFS than the PNI-IgM score group 2 (HR = 0.648, 95% CI: 0.418-1.006, P = 0.053) and the PNI-IgM score group 3 (HR = 0.337, 95% CI: 0.194-0.585, P < 0.001). In stratified analysis, PNI-IgM score 1 had a worse prognosis in the age < 60 years group and CA724 < 2.11 U/m group.
Conclusion: PNI-IgM score is a novel combination of nutritional and immunological markers that can be used as a sensitive biological marker for patients with gastric cancer who undergo surgery. The lower the PNI-IgM score, the worse the prognosis.
Introduction
According to the World Health Organization, gastric cancer was the fifth leading cancer in the world, with nearly 1.09 million new cases of gastric cancer worldwide in 2020. Gastric cancer is the fourth leading cause of cancer deaths, with almost 770,000 deaths worldwide in 2020 (1). In 2022, China is expected to have 509,000 new cases and 400,000 estimated deaths per year (2). Currently, radical gastrectomy is the preferred and primary treatment modality for patients with gastric cancer, while other treatments include endoscopic intervention followed by gastrectomy, adjuvant chemotherapy (ACT), or neoadjuvant chemotherapy (NACT) (3–5). In recent years, immunotherapy has also been applied to the treatment (6). Unfortunately, outcomes remain unsatisfactory as a significant number of patients develop local recurrence or distant metastases after resection (7). Therefore, it appears essential to identify potential biomarkers that can accurately select appropriate treatment strategies for patients and predict patient prognosis.
The nutritional status of patients with gastric cancer, which could predict the progression of the treated cancer, has been identified an important factor (8–11). It was common for patients with gastric cancer to suffer from malnutrition and cachexia due to reduced food intake and increased energy consumption (12). Cachexia was reported to affect approximately 50%-80% of cancer patients and was associated with 20%-40% of cancer deaths (13–15). Nutritional markers such as albumin (ALB), prealbumin (PALB), and body mass index (BMI) have been found to be independent prognostic factors for gastric cancer (16). The prognostic nutritional index (PNI), as a simple and readily available nutritional indicator, has been shown to be related to the prognosis of many malignancies, such as gastric cancer, lymphoma, pancreatic head cancer, head and neck tumors, etc. al (17–21). Additionally, the body’s Inflammatory response could also affect to tumor recurrence and metastasis. Some studies have reported that lymphocytes, neutrophils, and C-reactive protein (CRP) are associated with disease progression in patients with gastric cancer (22). Immunoglobulin M (IgM) was primarily responsible for the primary humoral immune response following initial antigen stimulation and has been shown to be closely associated with the prognosis of many malignancies (23–25). Taken together, the prognosis of gastric cancer patients with malnutrition and inflammation was worse.
A large number of previous researches have pointed out that composite indicators of nutrition and immunity, such as Glasgow Prognostic Score (GPS), platelet-to-lymphocyte (PLR), and Controlling Nutritional Status (CONUT) score could predict the prognosis of gastric cancer patients (26, 27). However, no study has investigated the validity of a mixed index of PNI and IgM (PNI-IgM score) to predict the prognosis of patients with gastric cancer who underwent surgery.
In this study, we evaluated the predictive effect of the PNI-IgM score on efficacy and prognosis in 340 patients with gastric cancer who underwent surgery. To further validate the PNI-IgM score, we performed a subgroup analysis and created nomograms.
Materials and methods
Patients
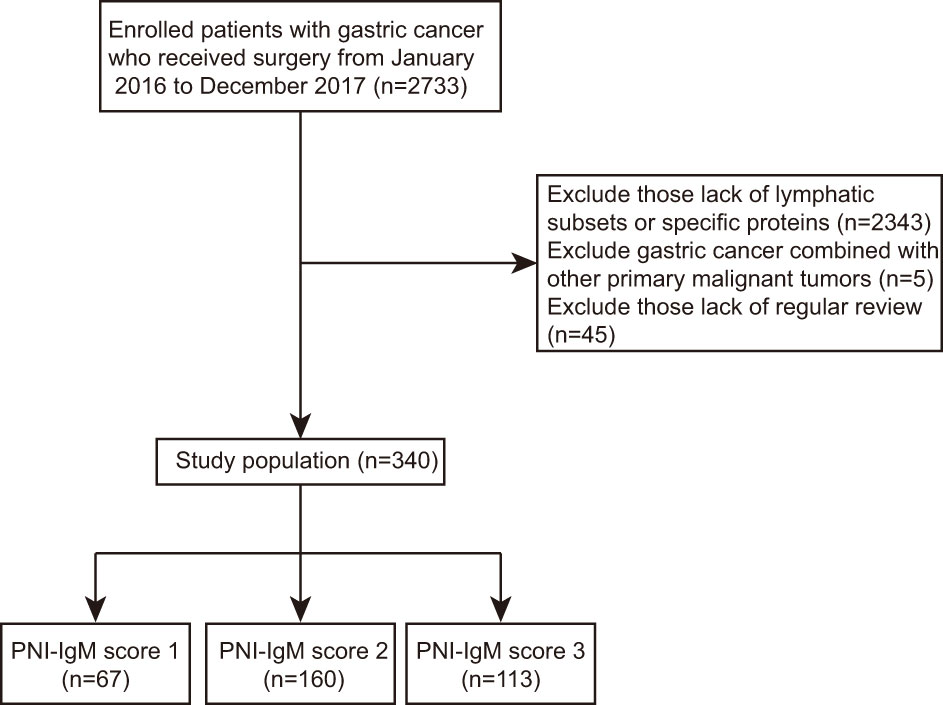
This is a retrospective study, so the Ethics Committee of Harbin Medical University Cancer Hospital waived informed consent. In total, we collected 340 consecutive patients with gastric cancer who received surgery at Harbin Medical University Cancer Hospital between January 2016 and December 2017 and were tested for lymphatic subsets and specific proteins. Statistical analysis of 340 patients and their clinical information was implemented according to the Helsinki Declaration and its amendments. All patients were included according to the following criteria: (1) all patients underwent surgical treatment; (2) all patients had no chronic disease; (3) all patients were tested for lymphatic subsets and specific proteins; (4) all patients did not display inflammatory response. (5) Patients with gastric cancer combined with other primary malignant tumors were excluded. Patients without complete clinical information and regular review after surgery were exclusion criteria. The flow chart of clinical case selection is shown in Figure 1. Electronic medical records system was used to collect clinical and pathological information.
Data collection
Patients were followed up by telephone or outpatient visit, every 3-6 months during the first 2 years, and every 6-12 months from the 3rd to 5th year, and annually thereafter. Disease-free survival (DFS) was comprehended as the period from the first day of surgery date to the date of disease progression. The evidence of progression was obtained by chest and abdomen X-ray or computed tomography. DFS was also defined as the date of death, death from any cause, or the date of withdrawal from the follow-up. Overall survival (OS) was described as the period from the first day of surgery date to the date of death, the date of withdrawal from the follow-up, or the time of the last follow-up. Electronic medical record system was used to acquire patients’ clinical and pathological information.
The peripheral venous blood was collected in fasting state after admission in all patients. The counts of peripheral lymphocytes (L) were measured and analyzed by an automatic blood analyzer (BACKMAN COULTER LH750), the levels of peripheral albumin were measured and analyzed by an automatic blood analyzer (ADVIA-2400), and the levels of peripheral IgM were measured and analyzed by a specific protein analyzer (IMMAGE800). PNI was calculated as follows: PNI = albumin (g/L) + 5 × total lymphocyte counts (109/L). The cut-off point was obtained by the receiver operating characteristic (ROC), which was based on OS for the prediction of patients’ death. The area under the ROC Curve (AUC) was used to evaluate the predictive ability of PNI and IgM. The optimal cut-off values of PNI and IgM with the highest Youden index were obtained.
Statistical analysis
Continuous variables are presented as means with standard deviations or as medians with interquartile ranges. Categorical variables were expressed as percentages. The comparison between continuous variables used the t-tests, one-way ANOVA, Kruskal-Wails rank sum test. We used the Chi-square test or Fisher’s exact test to compare the discrepancies between categorical variables. The Kaplan-Meier survival curve was used to compute the survival rate and the Log-rank test to compare the survival time difference. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Variables that achieved statistical significance at P < 0.05 were entered into the multivariate Cox regression analyses. Relative risks were assessed by the hazard ratio (HR) and 95% confidence interval (CI). The Cox proportional hazards regression model was constructed to analyze independent prognostic factors for DFS and OS. The nomograms were also constructed to predict the 1-, 3-, and 5-year survival probability for DFS and OS. The calibration curve analysis was used to assess the prognostic predictive ability of nomogram. All statistical analyses were completed through the R 4.1.3 (Vienna, Austria), IBM SPSS Statistics 25 (Chicago, IL, USA). Finally, we considered two-sided P values < 0.05 as statistical differences.
Results
Patient characteristics
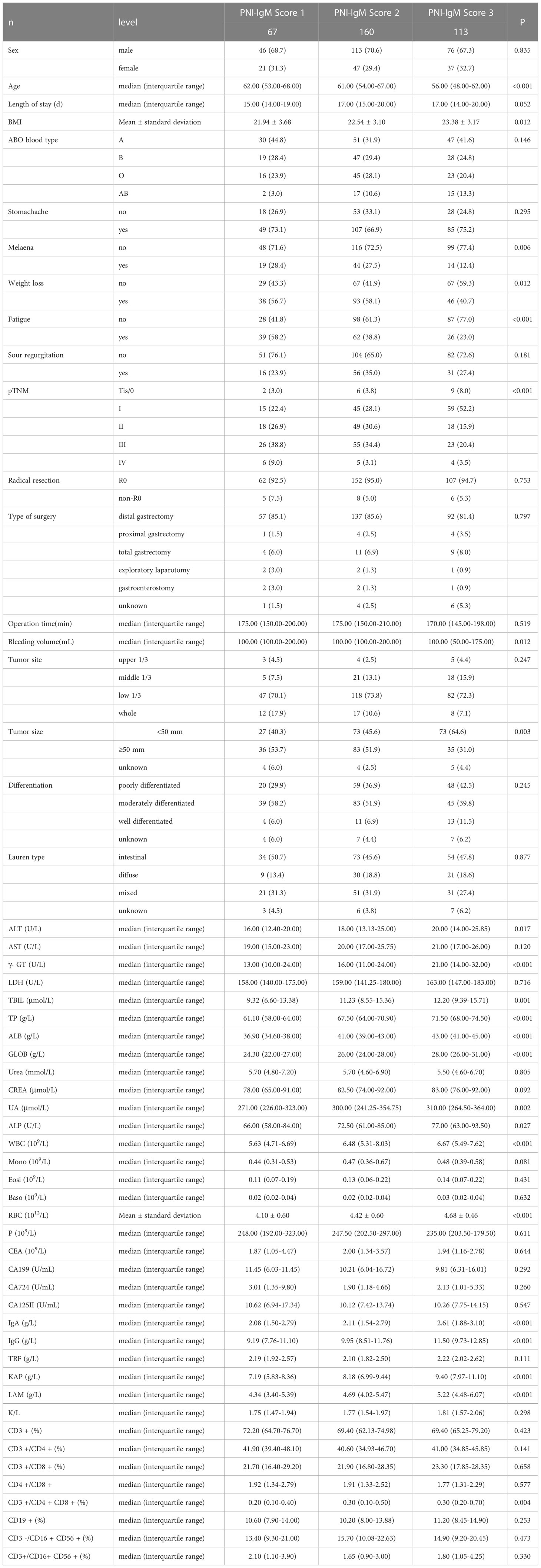
The median age of patients was 60 years, and there were 105 women (30.9%) and 235 men (69.1%) in all two groups’ cases. The optimal cut-off value of PNI was 48.45. The optimal cut-off value of IgM was 0.87 g/L. According to the optimal cut-off values of PNI and IgM, all patients were divided into three groups: PNI-IgM score of 3 (n = 113): high IgM (≥ 0.87) and high PNI (≥ 48.45); PNI-IgM score of 2 (n = 160): high IgM (≥ 0.87) and low PNI (< 48.45), or low IgM (< 0.87) and high PNI (≥ 48.45); PNI-IgM score of 1(n = 67): low IgM (< 0.87) and low PNI (< 48.45). The Chi-square test or Fisher’s exact test showed that PNI-IgM score was related to melaena (P = 0.006), weight loss (P = 0.012), fatigue (P < 0.001), pTNM stage (P < 0.001) and tumor size (P = 0.003). The one-way ANOVA Kruskal-Wails rank sum test showed that PNI-IgM score was related to age (P < 0.001), BMI (P = 0.012), bleeding volume (P = 0.012). The detailed clinical characteristics of all 340 cases grouped by PNI-IgM score are displayed in Table 1.
In this study, we also collected patients’ nutritional and hematological parameters before surgery, including total protein (TP), PALB, alanine aminotransferase (ALT), aspartate transaminase (AST), glutamyl transpeptidase (γ- GT), lactate dehydrogenase (LDH), total bilirubin (TBIL), globulin (GLOB), urea, creatinine (CREA), urate (UA), alkaline phosphatase (ALP), monocyte (Mono), eosinophils (Eosi), basophil (Baso), red blood cell (RBC), platelet (P), carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199), carbohydrate antigen 724 (CA724), carbohydrate antigen 125II (CA125II). We also collected lymphatic subsets and specific proteins, including immunoglobulin A (IgA), immunoglobulin G (IgG), transferrin (TRF), light-chain immunoglobulin (KAP), heavy-chain immunoglobulin (LAM), KAP/LAM, CD3 + cells (T cells), CD3 +/CD4 + cells (Th cells), CD3 +/CD8 + cells (CTL cells), CD4 +/CD8+ cells, CD3 +/CD4 + CD8 + cells, CD19 + cells (B cells), CD3 -/CD16 + CD56 + cells (NK cells), CD3 +/CD16 + CD56 + cells (NKT cells). We analyzed their relationship to the PNI-IgM score by Kruskal-Wails rank sum test (Table 1). We found that the PNI-IgM score was related to ALT (P = 0.017), γ- GT (P < 0.001), TBIL (P < 0.001), TP (P < 0.001), ALB (P < 0.001), GLOB (P < 0.001), UA (P = 0.002), ALP (P = 0.027), WBC (P < 0.001), RBC (P < 0.001), IgA (P < 0.001), IgG (P < 0.001), KAP (P < 0.001), LAM (P < 0.001) and CD3 +/CD4 + CD8 + cells (P = 0.004).
Univariate and multivariate Cox hazard analysis for DFS and OS
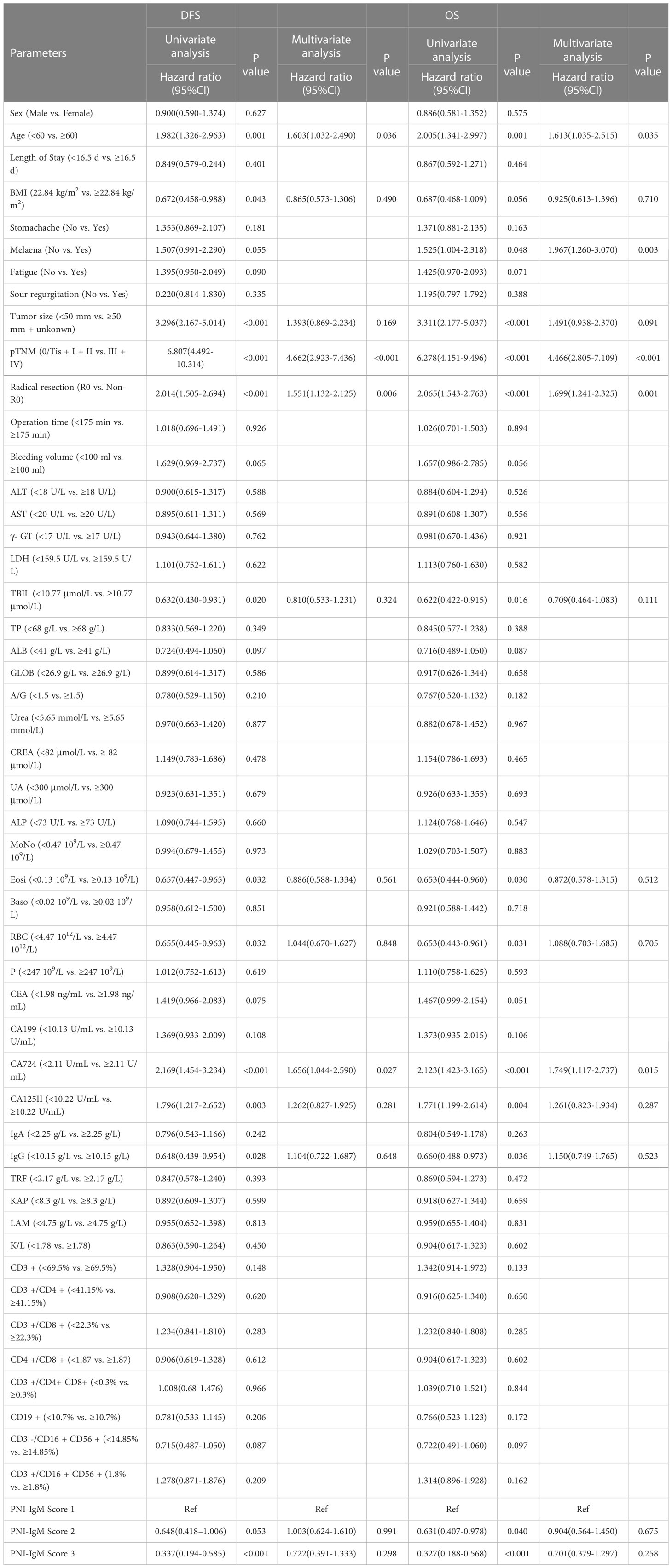
According to univariate analysis, the prognosis factors for patients’ DFS in this study were age (P = 0.001), BMI (P = 0.043), tumor size (P < 0.001), pTNM stage (P < 0.001), radical resection (P < 0.001), TBIL (P = 0.020), Eosi (P = 0.032), RBC (P = 0.032), CA724 (P < 0.001), CA125II (P = 0.003), IgG (P = 0.028), PNI-IgM score (P < 0.05). And the prognosis factors of patients in this study for OS were age (P = 0.001), melaena (P = 0.048), tumor size (P < 0.001), pTNM stage (P < 0.001), radical resection (P < 0.001), TBIL (P = 0.016), Eosi (P = 0.030), RBC (P = 0.031), CA724 (P = 0.004), CA125II (P = 0.004), IgG (P = 0.036), PNI-IgM score (P < 0.05). The multivariate analysis indicated that age (P = 0.036 vs. P = 0.035), pTNM stage (P < 0.001 vs. P < 0.001), radical resection (P = 0.006 vs. P = 0.001), and CA724 (P = 0.027 vs. P = 0.015) were both independent prognostic factors for DFS and OS. In addition, melaena (P = 0.003) was the independent prognostic factor for OS (Table 2).
Stratified analyses by potential effect modifiers
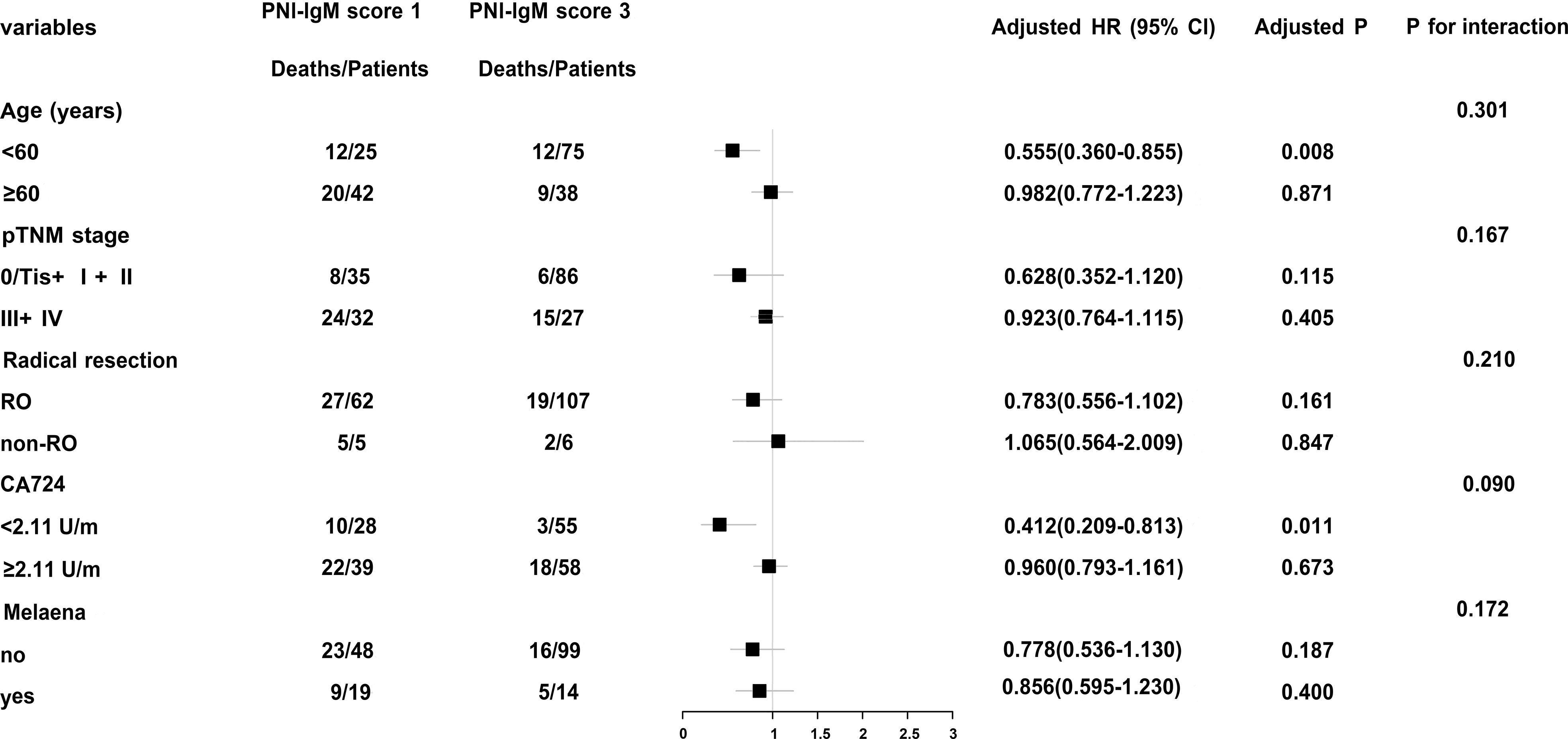
In order to further study PNI-IgM score, we conducted a stratified analysis and found that patients with PNI-IgM score 1 had shorter OS in age < 60 years group (HR = 0.555, 95% CI: 0.360-0.855, P = 0.008) and in CA724 < 2.11U/m group (HR = 0.412, 95% CI: 0.209-0.813, P = 0.011) (Figure 2).
PNI-IgM score and prognosis
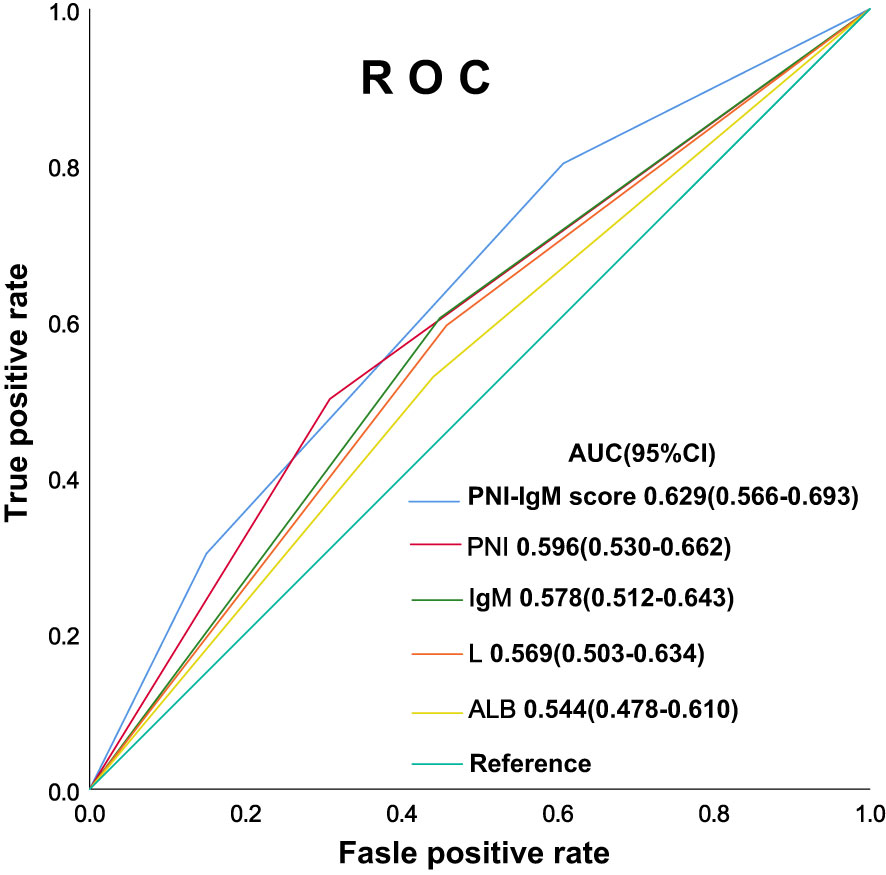
The PNI-IgM score 1 group’s median survival time for DFS was 62.20 months, the 1-, 3-, and 5-year DFS probability was 89.4% (95% CI: 82.3%-97.1%), 65.4% (95% CI: 54.7%-78.3%), 53.1% (95% CI: 41.9%-67.3%). The PNI-IgM score 1 group’s median survival time for OS was 67.57 months, the 1-, 3-, and 5-year OS probability was 89.5% (95% CI: 82.4%-97.2%), 69.0% (95% CI: 58.6%-81.3%), 55.6% (95% CI: 44.5%-69.4%). The PNI-IgM score 2 group’s median survival time for DFS and OS were both not achieved. The 1-, 3-, and 5-year DFS and OS probability were 89.3% (95% CI: 84.6%-94.2%), 74.0% (95% CI: 67.4%-81.3%), 68.2% (95% CI: 61.1%-76.1%); 89.9% (95% CI: 85.4%-94.7%), 77.8% (95% CI: 71.6%-84.6%), 69.7% (95% CI: 62.8%-77.3%), respectively. The PNI-IgM score 3 group’s median survival time for DFS and OS were both not achieved. The 1-, 3-, and 5-year DFS and OS probability were 93.8% (95% CI: 89.4%-98.4%), 80.5% (95% CI: 73.4%-88.4%), 80.5% (95% CI: 73.4%-88.4%); 95.6% (95% CI: 91.9%-99.4%), 82.0% (95% CI: 75.1%-89.4%), 81.0% (95% CI: 74.0%-88.7%), respectively. To further determine whether the PNI-IgM score could predict the prognosis of gastric cancer patients. The ROC curves were based on OS for the prediction of patients’ death. For the traditional clinicopathologic factors, including ALB, L, PNI, and IgM, each feature and the combined PNI-IgM score were plotted, and the point with the highest AUC was illustrated on the ROC curve. The PNI-IgM score exhibited a higher prognostic accuracy for DFS and OS than PNI and other clinicopathological risk factors (Figure 3). Patients with the PNI-IgM score 1 have a worse DFS than patients with the PNI-IgM score 2 (HR = 0.648, 95% CI: 0.418-1.006, P = 0.053) or the PNI-IgM score 3 (HR = 0.337, 95% CI: 0.194-0.585, P < 0.001). Patients with the PNI-IgM score 1 have a shorter OS than patients with the PNI-IgM score 2 (HR = 0.631, 95% CI: 0.407-0.978, P = 0.040) or the PNI-IgM score 3 (HR = 0.327, 95% CI: 0.188-0.568, P < 0.001) (Figures 4A, B).
Survival for pTNM stage
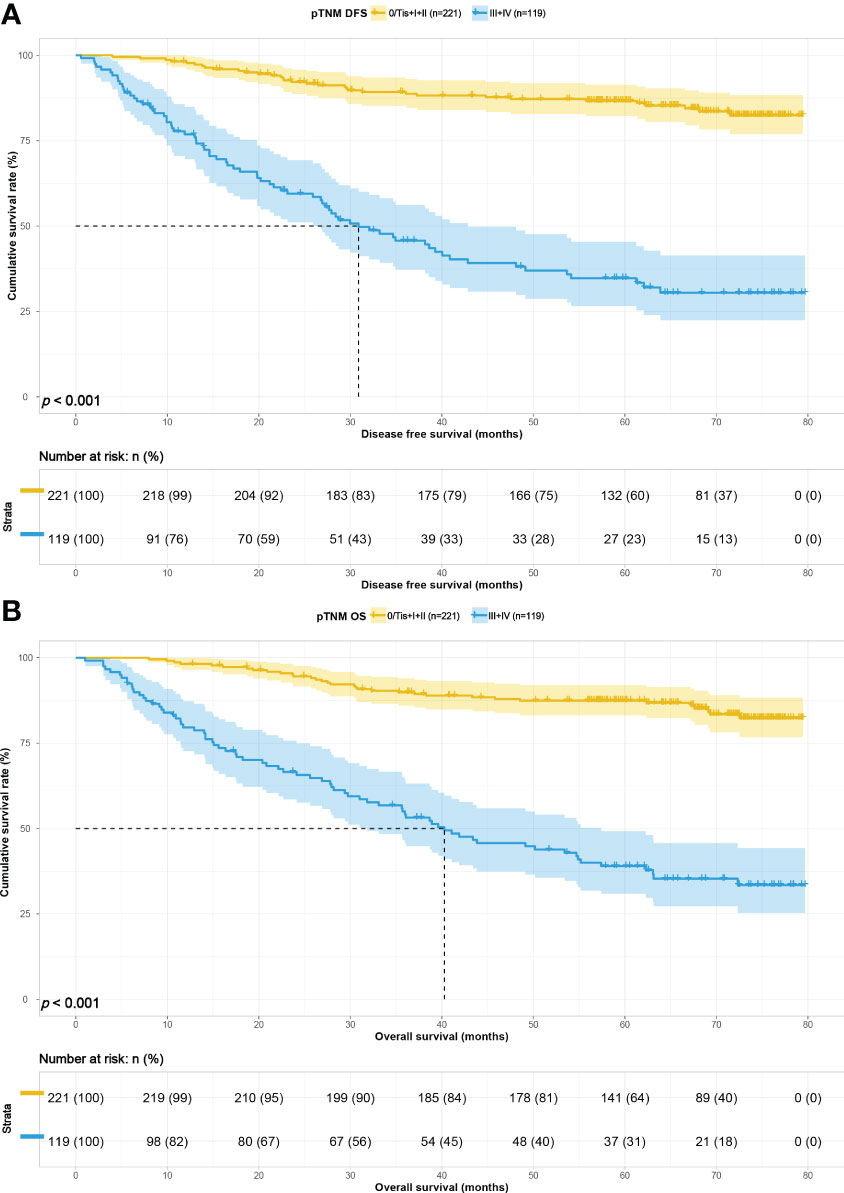
To study the predictive ability of PNI-IgM score for prognosis of gastric cancer patients in correlation with pTNM stage, we divided the 340 patients into early pTNM stage (0/Tis + I + II) group (221 patients) and advanced pTNM stage (III + IV) group (119 patients). The median survival time for DFS and OS in the early pTNM stage group were both not reached. The 1-, 3-, and 5-year DFS and OS probability were 98.2% (95% CI: 96.4%-100.0%) vs. 98.2% (95% CI: 96.4%-100.0%), 89.2% (95% CI: 85.2%-93.5%) vs. 89.9% (95% CI: 86.0%-94.0%), 86.7% (95% CI: 82.2%-91.4%) vs. 87.4% (95% CI: 83.1%-92.0%). The median survival time for DFS and OS in the advanced pTNM stage group were 30.90 months and 40.27 months. The 1-, 3-, and 5-year DFS and OS probability were 76.9% (95% CI: 69.6%-84.9%) vs. 79.6% (95% CI: 72.7%-87.2%); 45.7% (95% CI: 37.2%-56.2%) vs. 54.1% (95% CI: 45.7%-64.0%); 34.7% (95% CI: 26.6%-45.4%) vs. 39.0% (95% CI: 30.9%-49.3%). Patients with the PNI-IgM score 1 have a shorter DFS than patients with the PNI-IgM score 2 (HR = 0.814, 95% CI: 0.356-1.860, P = 0.626) or the PNI-IgM score 3 (HR = 0.278, 95% CI: 0.096-0.800, P = 0.018). Patients with the PNI-IgM score 1 also have a shorter OS than patients with the PNI-IgM score 2 group (HR = 0.821, 95% CI: 0.359-1.876, P = 0.640) or the PNI-IgM score 3 group (HR = 0.280, 95% CI: 0.097-0.808, P = 0.019). Patients in the advanced pTNM stage had lower DFS (HR = 4.394, 95% CI: 2.737-7.055, P < 0.001) and OS (HR = 4.466, 95% CI: 2.805-7.109, P < 0.001) than those early pTNM stage patients (Figures 5A, B).
Construction of nomograms to predict DFS and OS
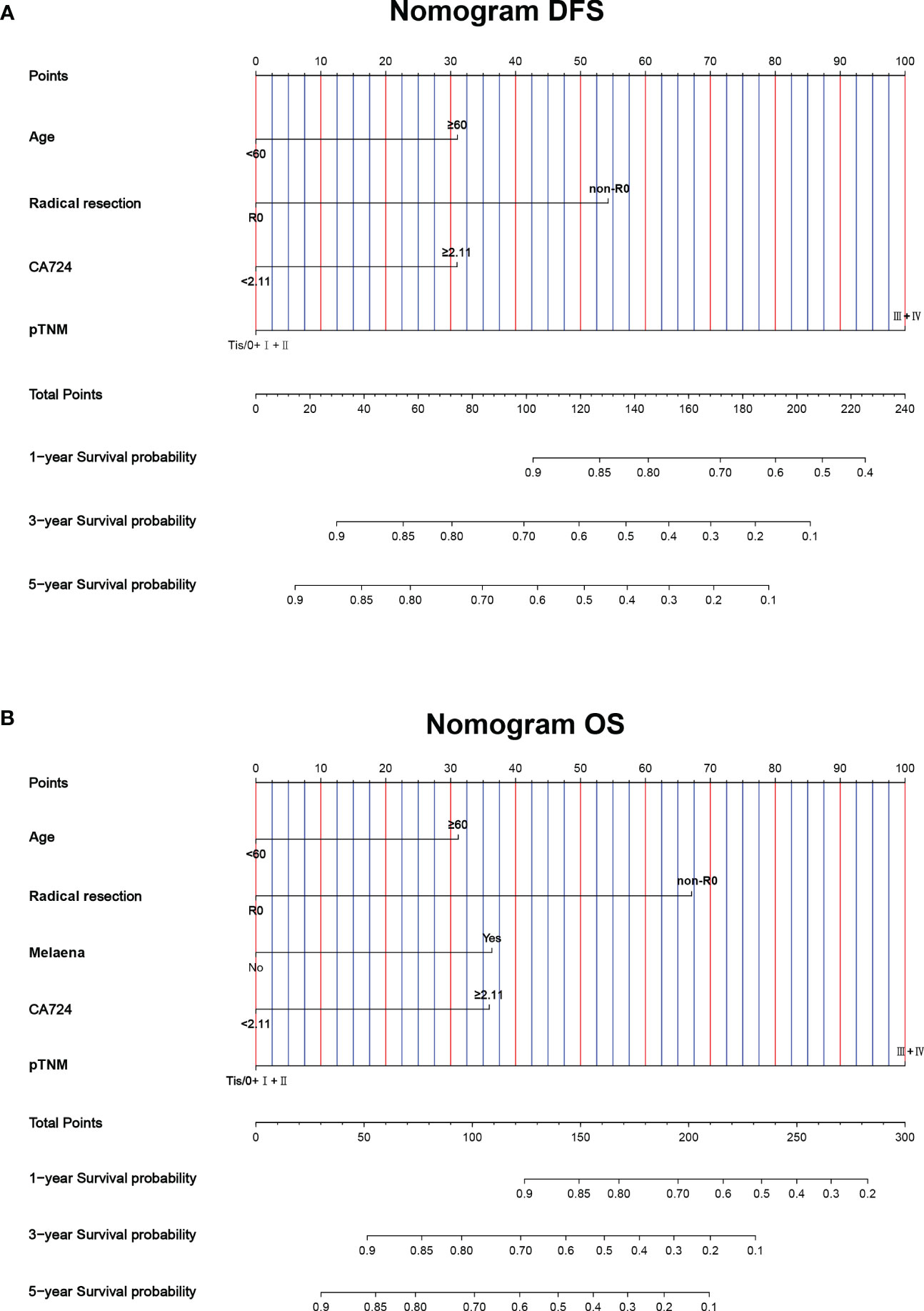
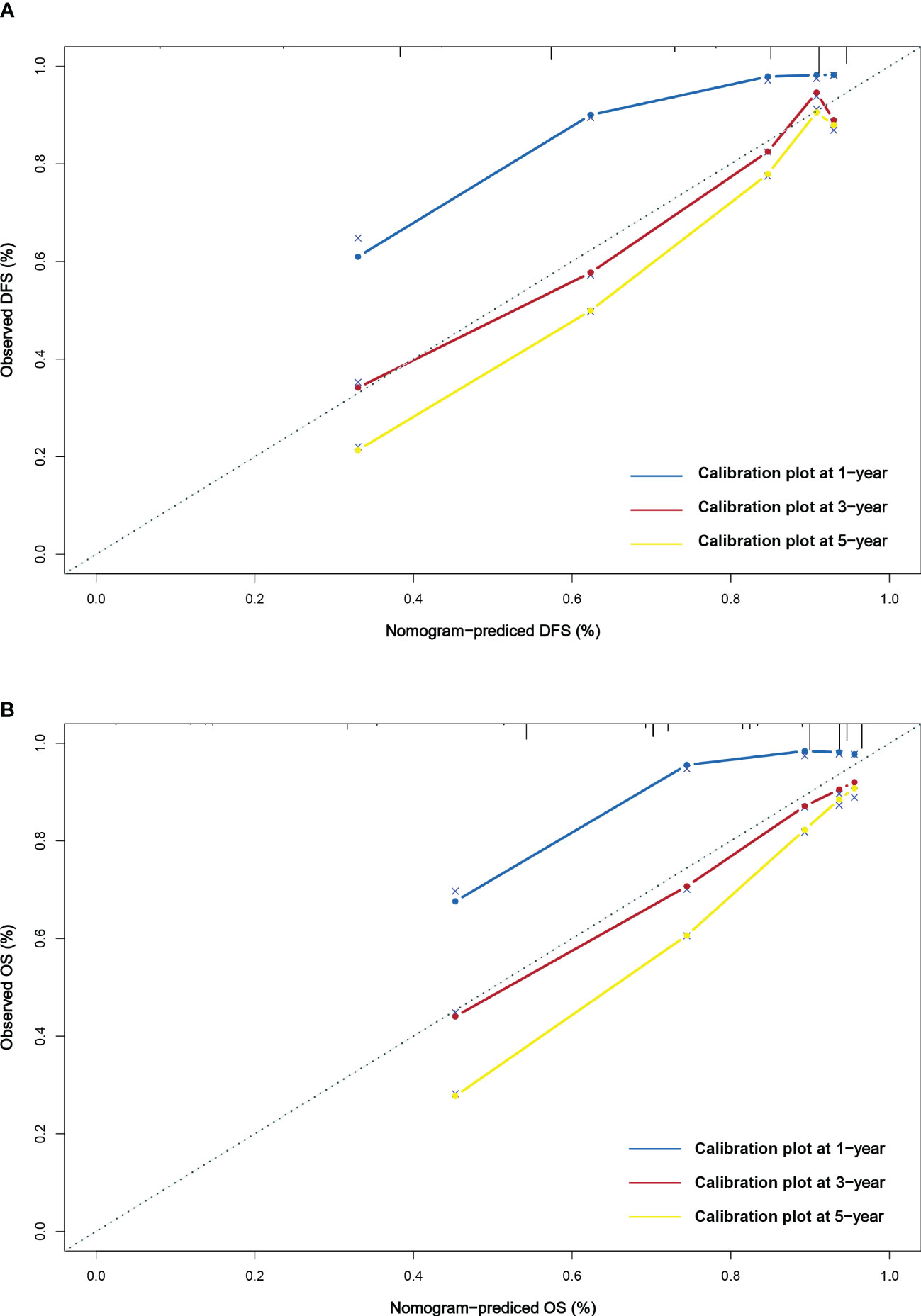
This study found that age, radical resection, CA724, and pTNM stage were the independent prognostic factors for DFS. By constructing Cox proportional hazard regression model, age, melaena, radical resection, CA724, and pTNM stage were the independent prognostic factors for OS. Based on the results of multivariate analysis, the nomograms to predict the 1-, 3-, and 5-year survival probability for DFS and OS were established (Figures 6A, B). The C-index and 95% CI for predicting the survival probability of DFS and OS were 0.770 (0.724-0.817) and 0.772 (0.724-0.819). Calibration curves for the DFS probability at 3-, 5-years in the demonstrated satisfactory consistency between the nomogram-predicted and actual survival. The calibration curves for the probability of OS at 3-, 5-years also suggested correlation between the observed and nomogram-predicted survival (Figures 7A, B).
Discussion
Gastric cancer is a common malignant tumor in China, with the third highest incidence and mortality rate (1). Although there are many treatments for gastric cancer, it is still a very challenging disease (28). With the development of medical technology, the 5-year survival rate of gastric cancer patients undergoing surgery has steadily increased, but there is still a large proportion of patients with poor prognoses (29). Numerous researches have shown that gastric cancer patients’ prognoses were related to disease tumor markers, body nutrition and immune status (30–32). Therefore, there is a need to develop a more accurate prognostic risk stratification system to stratify patients and help individualize the choice of treatment.
Our study is the first to assess the association between the PNI-IgM score, which a composite indicator of immunity and nutrition, clinicopathological factors and survival. It also demonstrated that the PNI-IgM score predicted prognosis among gastric cancer patients who underwent resection. We found that low PNI and IgM were associated with poor patient prognosis. This study found that age, pTNM stage, radical resection, and CA724 were independent prognostic factors for DFS and OS. In addition, an independent prognostic factor for OS was melaena. In the Stratified analyses, we found age <60 years and CA724 < 2.11 U/m had shorter OS in PNI-IgM score 1.
Although many studies have confirmed the prognostic association of PNI and IgM with gastric cancer (33–35) and other solid tumors (36–38). In this study, PNI and IgM were also demonstrated to predict the prognosis of gastric cancer patients. There are several possible mechanisms explaining the association of PNI-IgM score with the prognosis of patients with gastric cancer. PNI is a composite indicator consisting of albumin and lymphocytes, which mainly reflects the nutritional status of the body, but also the immune status of the body (39, 40). Albumin is a well-known indicator of the nutritional status of the body, and many studies have concluded that low albumin is a sign of malnutrition in the body (41, 42). Lymphocytes are an important component of the body’s immune system and reflect the overall immune status of the body (43). Low serum lymphocytes have been reported to be associated with tumor progression and metastasis in patients with gastric cancer (44–46). IgM accounts for about 10% of the total serum immunoglobulins and mainly reflects the recent immune response. Tumor-reactive IgM had been shown to eliminate malignant cells through complement fixation (47), induction of apoptosis (48), and induction of secondary immune responses against neoantigens (49).
This study also has some limitations. First, this was a single-region, single-center retrospective study with limited sample size and potential selection bias. Second, we only selected patients with gastric cancer who underwent surgery. Third, the cut-off value of PNI and IgM is usually derived from the ROC curve, and the optimal cut-off value is uncertain. Therefore, multi-regional, multi-center, and larger sample size studies are needed to validate our findings.
Conclusion
In conclusion, our study found that the PNI-IgM score is a valid scoring tool for patients with gastric cancer who received surgery. Patients in the PNI-IgM score 1 group had a worse prognosis than those in the PNI-IgM score 2 group, and the PNI-IgM score 3 group. Therefore, the PNI-IgM score could be used as a biomarker to develop better treatment strategies for patients undergoing surgery for gastric cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the ethics committee of Harbin Medical University Cancer Hospital. All patients provided written informed consent before the study.
Author contributions
Writing-original draft and Writing-review & editing: ZD, HaS; Data curation and Investigation: RZ, GD, and HP; Methodology and Supervision: YZ, RH; Resources, Funding acquisition and Project administration: YX, and HoS. All authors contributed to the article and approved the submitted version.
Funding
Clinical Research Foundation of Wu Jieping Medical Foundation (No: 320.6750.2022-07-13).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin Med J-Peking (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
3. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet (2012) 379(9813):315–21. doi: 10.1016/S0140-6736(11)61873-4
4. Yoshikawa T, Morita S, Tanabe K, Nishikawa K, Ito Y, Matsui T, et al. Survival results of a randomised two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of s-1 plus cisplatin (SC) and paclitaxel plus cisplatin (PC) followed by D2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer (2016) 62:103–11. doi: 10.1016/j.ejca.2016.04.012
5. Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol (2012) 30(3):268–73. doi: 10.1200/JCO.2011.39.1953
6. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discovery (2015) 14(8):561–84. doi: 10.1038/nrd4591
7. Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev (2020) 85:101980. doi: 10.1016/j.ctrv.2020.101980
8. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr (2021) 40(5):2898–913. doi: 10.1016/j.clnu.2021.02.005
9. Langius JA, Zandbergen MC, Eerenstein SE, van Tulder MW, Leemans CR, Kramer MH, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr (2013) 32(5):671–8. doi: 10.1016/j.clnu.2013.06.012
10. Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr (2018) 37(4):1202–9. doi: 10.1016/j.clnu.2017.05.027
11. Viana ECRM, Oliveira IDS, Rechinelli AB, Marques IL, Souza VF, Spexoto MCB, et al. Malnutrition and nutrition impact symptoms (NIS) in surgical patients with cancer. PloS One (2020) 15(12):e0241305. doi: 10.1371/journal.pone.0241305
12. Loman BR, Luo M, Baggs GE, Mitchell DC, Nelson JL, Ziegler TR, et al. Specialized high-protein oral nutrition supplement improves home nutrient intake of malnourished older adults without decreasing usual food intake. JPEN-Parenter Enter (2019) 43(6):794–802. doi: 10.1002/jpen.1467
13. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer (2006) 14(10):999–1011. doi: 10.1007/s00520-006-0079-9
14. Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer (2008) 44(8):1124–32. doi: 10.1016/j.ejca.2008.02.033
15. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer (2002) 2(11):862–71. doi: 10.1038/nrc927
16. Liu ZJ, Ge XL, Ai SC, Wang HK, Sun F, Chen L, et al. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroentero (2017) 23(27):4978–85. doi: 10.3748/wjg.v23.i27.4978
17. Nogueiro J, Santos-Sousa H, Pereira A, Devezas V, Fernandes C, Sousa F, et al. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbeck Arch Surg (2022) 407(7):2703–14. doi: 10.1007/s00423-022-02627-0
18. Shen Z, Wang F, He C, Li D, Nie S, Bian Z, et al. The value of prognostic nutritional index (PNI) on newly diagnosed diffuse Large b-cell lymphoma patients: a multicenter retrospective study of HHLWG based on propensity score matched analysis. J Inflammation Res (2021) 14:5513–22. doi: 10.2147/JIR.S340822
19. Ma S, Zhang B, Lu T, Li D, Li T, Shen Z, et al. Value of the prognostic nutritional index (PNI) in patients with newly diagnosed, CD5-positive diffuse large b-cell lymphoma: a multicenter retrospective study of the huaihai lymphoma working group. Cancer-Am Cancer Soc (2022) 128(19):3487–94. doi: 10.1002/cncr.34405
20. Jiang P, Li X, Wang S, Liu Y. Prognostic significance of PNI in patients with pancreatic head cancer undergoing laparoscopic pancreaticoduodenectomy. Front Surg (2022) 9:897033. doi: 10.3389/fsurg.2022.897033
21. Shi Y, Zhang Y, Niu Y, Chen Y, Kou C. Prognostic role of the prognostic nutritional index (PNI) in patients with head and neck neoplasms undergoing radiotherapy: a meta-analysis. PloS One (2021) 16(9):e0257425. doi: 10.1371/journal.pone.0257425
22. Lu J, Xu BB, Zheng ZF, Xie JW, Wang JB, Lin JX, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer (2019) 22(3):536–45. doi: 10.1007/s10120-018-0892-0
23. Roney MSI, Lanagan C, Sheng YH, Lawler K, Schmidt C, Nguyen NT, et al. IgM and IgA augmented autoantibody signatures improve early-stage detection of colorectal cancer prior to nodal and distant spread. Clin Transl Immunol (2021) 10(9):e1330. doi: 10.1002/cti2.1330
24. Fitzgerald S, O’Reilly JA, Wilson E, Joyce A, Farrell R, Kenny D, et al. Measurement of the IgM and IgG autoantibody immune responses in human serum has high predictive value for the presence of colorectal cancer. Clin Colorectal Canc (2019) 18(1):e53–60. doi: 10.1016/j.clcc.2018.09.009
25. Yasuda M, Take N, Shinohara S, Chikaishi Y. Serum immunogloblins might be useful predictors of immune-related adverse events after immune checkpoint inhibitor usage in lung cancer. Thorac Cancer (2022) 13(17):2536–8. doi: 10.1111/1759-7714.14573
26. Wang W, Tong Y, Sun S, Tan Y, Shan Z, Sun F, et al. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol (2022) 12:936206. doi: 10.3389/fonc.2022.936206
27. Chen L, Sun H, Zhao R, Huang R, Pan H, Zuo Y, et al. Controlling nutritional status (CONUT) predicts survival in gastric cancer patients with immune checkpoint inhibitor (PD-1/PD-L1) outcomes. Front Pharmacol (2022) 13:836958. doi: 10.3389/fphar.2022.836958
28. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
29. Arnold M, Morgan E, Bardot A, Rutherford MJ, Ferlay J, Little A, et al. International variation in oesophageal and gastric cancer survival 2012-2014: differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study). Gut (2022) 71(8):1532–43. doi: 10.1136/gutjnl-2021-325266
30. Vidra N, Kontogianni MD, Schina E, Gioulbasanis I. Detailed dietary assessment in patients with inoperable tumors: potential deficits for nutrition care plans. Nutr Cancer (2016) 68(7):1131–9. doi: 10.1080/01635581.2016.1213867
31. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
32. Garla P, Waitzberg DL, Tesser A. Nutritional therapy in gastrointestinal cancers. Gastroenterol Clin N (2018) 47(1):231–42. doi: 10.1016/j.gtc.2017.09.009
33. Takagi A, Hawke P, Tokuda S, Toda T, Higashizono K, Nagai E, et al. Serum carnitine as a biomarker of sarcopenia and nutritional status in preoperative gastrointestinal cancer patients. J Cachexia Sarcopeni (2022) 13(1):287–95. doi: 10.1002/jcsm.12906
34. Waki K, Shichijo S, Uedo N, Takeuchi Y, Maekawa A, Kanesaka T, et al. Long-term outcomes after endoscopic resection for late-elderly patients with early gastric cancer. Gastrointest Endosc (2022) 95(5):873–83. doi: 10.1016/j.gie.2021.12.028
35. Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao R, et al. Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front Nutr (2022) 9:1038118. doi: 10.3389/fnut.2022.1038118
36. Cui Z, Zhang J, Zhang J, Xu L. Evaluation of IgG, IgM, CD4+ and CD8+ T cells during neoadjuvant chemotherapy with tezio and apatinib in gastric cancer patients. Cell Mol Biol (2020) 66(3):113–8. doi: 10.14715/cmb/2020.66.3.17
37. Chen L, Bai P, Kong X, Huang S, Wang Z, Wang X, et al. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Dev Biol (2021) 9:656741. doi: 10.3389/fcell.2021.656741
38. Monroy-Iglesias MJ, Crescioli S, Beckmann K, Le N, Karagiannis SN, Van Hemelrijck M, et al. Antibodies as biomarkers for cancer risk: a systematic review. Clin Exp Immunol (2022) 209(1):46–63. doi: 10.1093/cei/uxac030
39. Zhang X, Zhao W, Chen X, Zhao M, Qi X, Li G, et al. Combining the fibrinogen-to-Pre-Albumin ratio and prognostic nutritional index (FPR-PNI) predicts the survival in elderly gastric cancer patients after gastrectomy. Onco Targets Ther (2020) 13:8845–59. doi: 10.2147/OTT.S264199
40. Yan D, Shen Z, Zhang S, Hu L, Sun Q, Xu K, et al. Prognostic values of geriatric nutritional risk index (GNRI) and prognostic nutritional index (PNI) in elderly patients with diffuse Large b-cell lymphoma. J Cancer (2021) 12(23):7010–7. doi: 10.7150/jca.62340
41. Guo Y, Wei L, Patel SH, Lopez G, Grogan M, Li M, et al. Serum albumin: early prognostic marker of benefit for immune checkpoint inhibitor monotherapy but not chemoimmunotherapy. Clin Lung Cancer (2022) 23(4):345–55. doi: 10.1016/j.cllc.2021.12.010
42. Yang Z, Zheng Y, Wu Z, Wen Y, Wang G, Chen S, et al. Association between pre-diagnostic serum albumin and cancer risk: results from a prospective population-based study. Cancer Med (2021) 10(12):4054–65. doi: 10.1002/cam4.3937
43. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer (2019) 19(1):672. doi: 10.1186/s12885-019-5903-y
44. Eo WK, Jeong DW, Chang HJ, Won KY, Choi SI, Kim SH, et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroentero (2015) 21(9):2668–76. doi: 10.3748/wjg.v21.i9.2668
45. Shen Q, Liu W, Quan H, Pan S, Li S, Zhou T, et al. Prealbumin and lymphocyte-based prognostic score, a new tool for predicting long-term survival after curative resection of stage II/III gastric cancer. Brit J Nutr (2018) 120(12):1359–69. doi: 10.1017/S0007114518002854
46. Park SJ, Lee J, Kim H, Shin K, Lee M, Park JM, et al. Association between absolute lymphocyte count and overall mortality in patients with surgically resected gastric cancer. Korean J Intern Med (2021) 36(3):679–88. doi: 10.3904/kjim.2019.358
47. Díaz-Zaragoza M, Hernández-Ávila R, Viedma-Rodríguez R, Arenas-Aranda D, Ostoa-Saloma P. Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (Review). Oncol Rep (2015) 34(3):1106–14. doi: 10.3892/or.2015.4095
48. Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol (2012) 188(3):939–45. doi: 10.4049/jimmunol.1102107
Keywords: prognostic nutritional index, IgM, gastric cancer, surgery, prognosis
Citation: Du Z, Sun H, Zhao R, Deng G, Pan H, Zuo Y, Huang R, Xue Y and Song H (2023) Combined with prognostic nutritional index and IgM for predicting the clinical outcomes of gastric cancer patients who received surgery. Front. Oncol. 13:1113428. doi: 10.3389/fonc.2023.1113428
Received: 01 December 2022; Accepted: 25 May 2023;
Published: 09 June 2023.
Edited by:
Paola Parente, IRCCS Casa Sollievo Della Sofferenza Hospital, ItalyReviewed by:
Weihua Fu, Tianjin Medical University General Hospital, ChinaVasileios I Lagopoulos, Aristotle University of Thessaloniki, Greece
Copyright © 2023 Du, Sun, Zhao, Deng, Pan, Zuo, Huang, Xue and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjiang Song, c29uZ2hvbmdqaWFuZzE5NzJAMTYzLmNvbQ==
†These authors have contributed equally to this article
‡ORCID: Zhongze Du, orcid.org/0000-0001-5434-6072
 Zhongze Du
Zhongze Du Hao Sun
Hao Sun Ruihu Zhao
Ruihu Zhao Guiming Deng
Guiming Deng Yanjiao Zuo
Yanjiao Zuo