- 1Department of Urology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Republic of Korea
- 2Department of Urology, School of Medicine, Keimyung University, Daegu, Republic of Korea
- 3Department of Urology, Ewha Womans Medical Center, Seoul, Republic of Korea
Purpose: This study aims to evaluate the association of serum lipid profile on prostate cancer (PC) risk and aggressiveness.
Methods: Men who underwent prostate biopsy between January 2005 and December 2015 were retrospectively analyzed. The association between lipid profile and the risk, stage, and Gleason grade group (GG) of the PC were investigated. Sensitivity analysis was conducted using univariate and multivariate quantile analysis for lipide profile on the risk and stage of PC.
Results: Of the 1740 study populations, 720 men (41.4%) were diagnosed as PC. From multivariate logistic regression analysis, age, prostate specific antigen, triglyceride (odds ratio (OR):1.05, confidence interval (CI):1.03-1.07, p-value<0.001) significantly increased PC risk, while total cholesterol (OR:0.96, CI:0.92-0.99, p-value=0.011) significantly decreased the PC risk. The increase of serum triglyceride increased the risk of both of locally advanced (OR:1.03, CI:1.00-1.07, p-value=0.025) and metastatic PC (OR:1.14, CI:1.04-1.25, p-value=0.004). The increase of serum triglyceride increased the risk of GG2-3 (OR:1.03, CI:1.00-1.06, p-value=0.027) and GG4-5 (OR:1.04, CI:1.01-1.08, p-value=0.027). Univariate quartile analysis founded serum triglyceride increasing risk of locally advanced disease than organ confined disease. (OR: 1.00, 1.25, 2.04, 4.57 for 1st, 2nd, 3rd and 4th quartile, p-value<0.001). Adjusted multivariate quartile analysis confirmed statistically significant increasing PC risk of triglyceride (OR: 1.00, 1.25, 2.04, 4.57 for 1st, 2nd, 3rd and 4th quartile, p-value<0.001).
Conclusions: This study findings suggested increased in triglyceride level increased the risk PC. Increased in triglyceride level also associated with aggressive presentation of PC, with higher stage and GG.
1 Introduction
Prostate cancer (PC) is the second most common malignancy in men and the second leading cause of cancer death worldwide (1). The age-standardised incidence of PC can differ 25-fold depending on geographic location; generally higher in Western compared with in Asian countries (2). There is an increased incidence of PC in Asian immigrants to Western countries (3) and temporal changes after adaptation to a westernized lifestyle in the northeast Asian population over the last decades (4, 5) suggest that an environmental factor highly influences the PC risk.
Dyslipidaemia is part of metabolic syndrome, characterized by an alteration of the plasma lipid profile, including LDL, HDL, and triglyceride levels. Both of genetic and environmental factors influence serum lipid levels (6, 7). Metabolic syndrome is another most common medical problem and is continuously increase in world-wide (8). The increasing incidence of both of PC and the metabolic syndrome suggest potential linkage between two diseases (8). While dyslipidaemia has been shown to be associated with PC risk in some in vivo and in vitro studies (9–12), epidemiologic and clinical studies have yielded conflicting results (13–16). Understanding the association between the lipid profile and prostate cancer risk is important because the lipid profile is considered to be a potentially modifiable risk factor.
Recent evidence suggests that metabolic syndrome might be associated with prostate cancer risk, aggressive features, and recurrence after treatment (15, 17). However, there is still debate surrounding the association between specific lipid profile and PC risk. This study aims to evaluate the association between the serum lipid profile and PC risk, aggressiveness, and staging.
2 Materials and methods
2.1 Study design and population
This study was approved by the Institutional Review Board (IRB No. 2022-1479) of the Asan Medical Center. The medical records of men who underwent a transrectal ultrasound (TRUS)-guided prostate biopsy at our institution between January 2005 and December 2015 were retrospectively reviewed. Prostate biopsies were conducted on men with a serum PSA level ≥ 4.0 ng/mL before 2009 and ≥ 3.0 ng/mL after that or if prostate cancer was clinically suspected. Among those who underwent a prostate biopsy, patients with a measured serum glucose and lipid profile, including the levels of serum total cholesterol, low-density level cholesterol (LDL, mg/dL), high-density level cholesterol (HDL, mg/dL), and triglyceride (mg/dL) in a fasting state within one year before prostate biopsy were enrolled. Quantification of each component of lipid profile measured by Beckman Coulter AU 5800 chemistry analyzer (Beckman Coulter, Brea, CA) (18). Age, body mass index (BMI), hypertension and diabetes were measured at the time of the biopsy. HbA1c was included in the values measured within three months before the biopsy, and statin co-medication was included in the analyses if the statin was taken for more than one month before the biopsy.
2.2 Statistical analysis
Continuous variables were described by mean ± standard deviation, and discrete variables were described by number and frequency. Differences in demographic, clinical, and pathological factors between men with non-prostate cancer versus prostate cancer were examined using t-tests and Chi-squared (χ2) tests for continuous and categorical variables, respectively. Differences in the variables between Gleason grade (GG) and clinical stage were analysed with a one-way ANOVA test. Multivariate logistic regression models were used to assess the association between the PC risk and collected variables, including lipid profile. In addition, we analysed the association between PC aggressiveness and collected variables, by sub-group analysis of the Gleason grade group (GG; 1, 2-3 and 4-5) (19) and clinical stage (organ confined, locally advanced and metastatic prostate cancer). Locally advanced disease was defined as clinical stage is above T3 or suspected regional lymph node metastasis. Metastatic disease was defined as presence of distant or nonregional lymph node metastasis (20). The variables that showed p-value < 0.05 in the univariate analysis were selected for multivariate analysis. Finally, sensitivity analysis was conducted using adjusted univariate and multivariate quantile analysis for each subset of the lipid profile on the risk and stage of PC. All statistical analyses were done using SPSS version 21.0 (IBM, Armonk, NY, USA), and the significance level was 0.05 or less.
3 Results
3.1 Patient characteristics
Of the 1740 men who underwent prostate biopsy, 720 men (41.4%) were diagnosed with PC. Among the PC patients 34.3% (247/720), 36.4% (262/720), 29.3% (211/720) were GG 1, GG2-3 and GG 4-5. Most of patients (78.8%, 567/720) defined as localized disease and 15.5% (112/720) was locally advanced disease. Only 5.7% (41/720) were metastatic disease at the time of diagnosis. The patients who were diagnosed as PC had significantly higher age (66.3 vs. 60.1 years, p-value < 0.001), PSA level (32.9 vs. 4.7 ng/mL, p-value = 0.024) than non-PC population. The diabetes mellitus (23.2% vs. 12.4%, p-value < 0.001) and hypertension (50.0% vs. 35.5%, p-value < 0.001) were more frequent in PC patients. Statin comedication (12.0% vs. 9.7%) and BMI (24.5 vs 24.3, p-value = 0.174) was not significantly difference between PC and non-PC groups. For lipid profile, total cholesterol (178.0 vs 187.9 mg/dL, p-value < 0.001), LDL (111.7 vs. 119.6 mg/dL, p-value < 0.001) and HDL (49.8 vs. 52.0 mg/dL, p-value < 0.001) level were significantly lower and triglyceride level (141.0 vs. 119.1 mg/dL, p-value < 0.001) were significantly higher in PC patients than non-PC group. Detailed characteristics are described in Table 1.
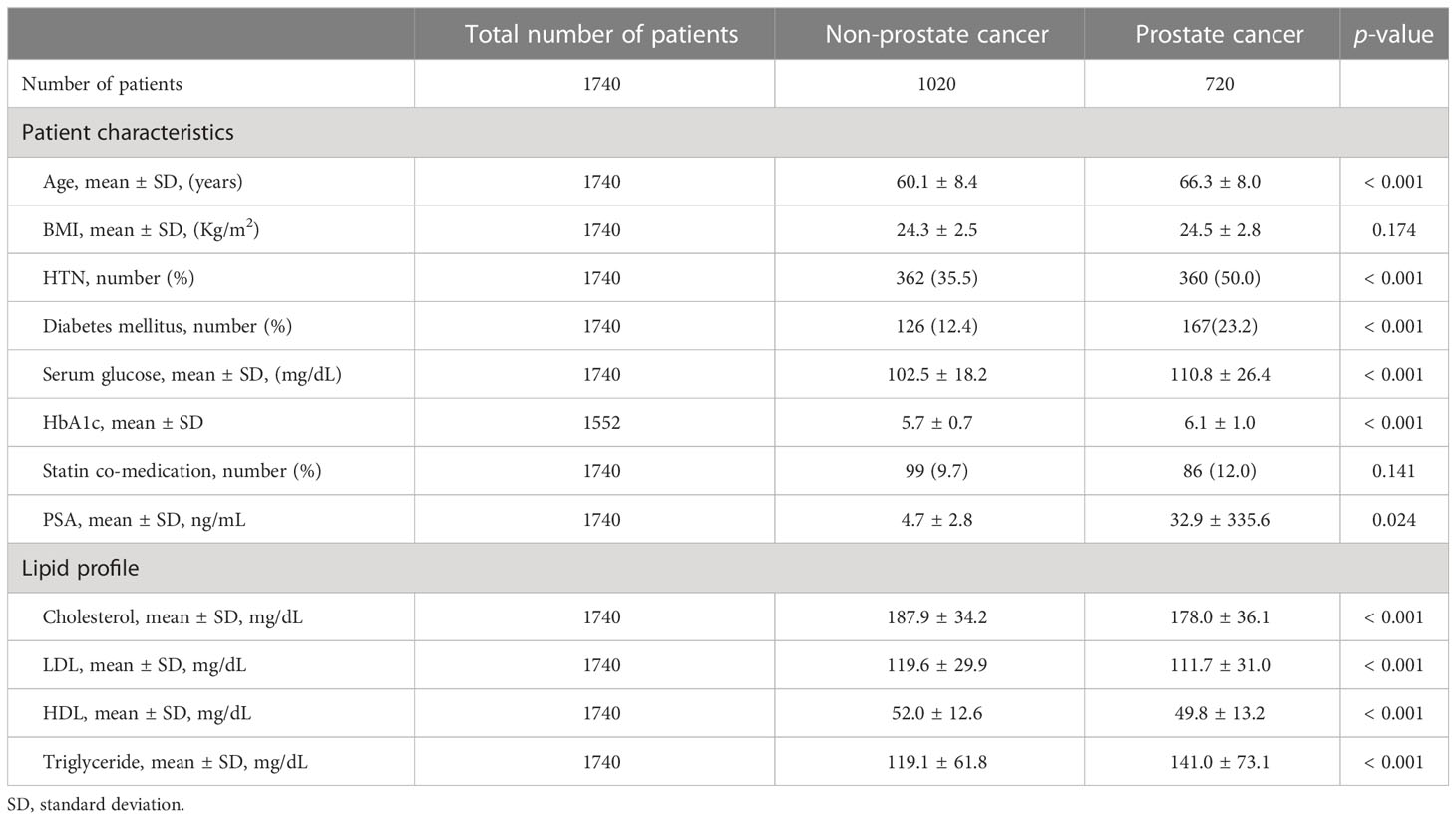
Table 1 General characteristics between prostate cancer patients and non-cancer patients before biopsy.
3.2 Lipid profile and prostate cancer risk
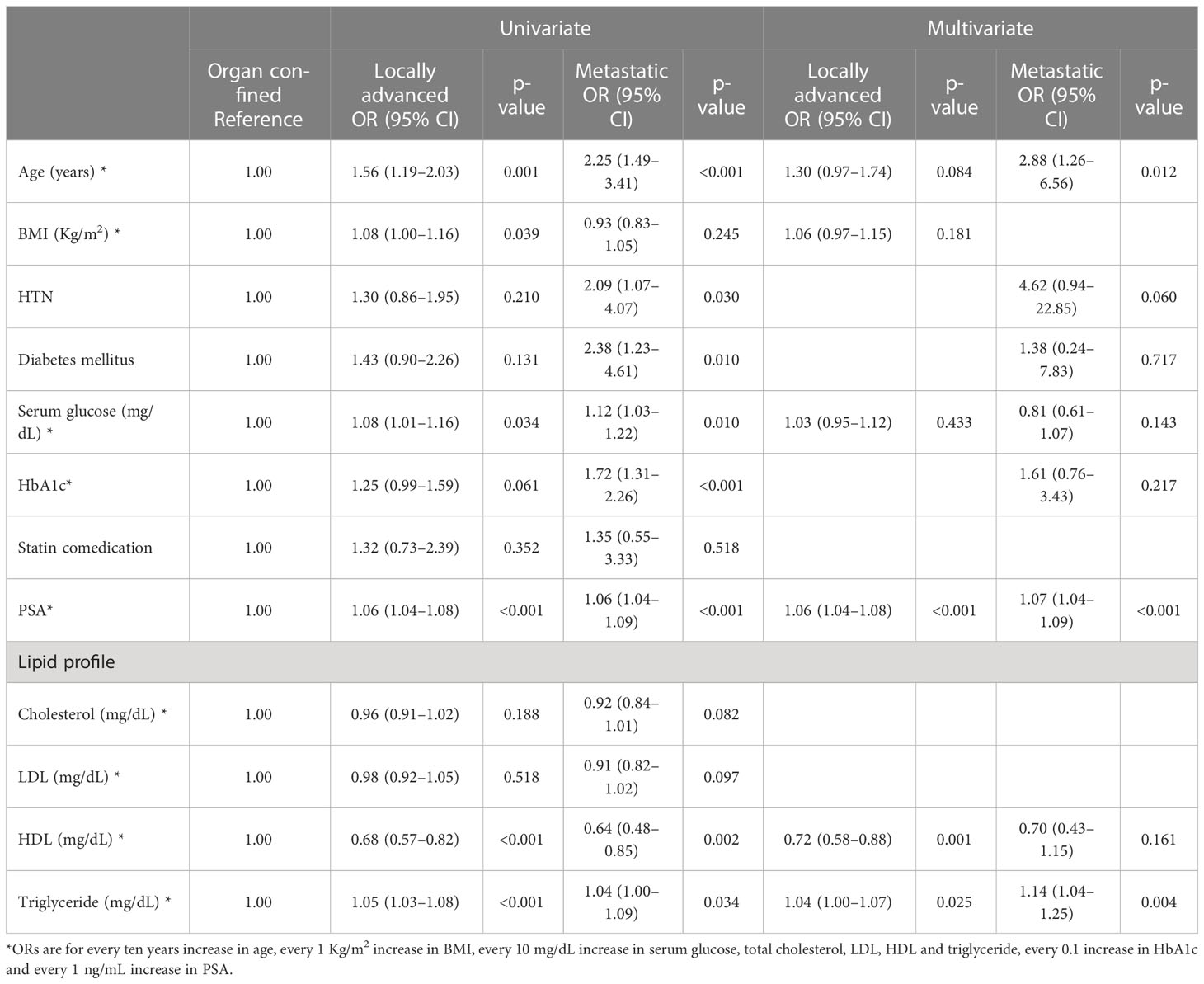
In univariate analysis, age, hypertension, diabetes mellitus, serum glucose level, HbA1c, PSA level, and all lipid profile components were significantly associated with PC risk. The multivariate analysis showed that age {Odds ratio (OR): 1.91, 95% confidence interval (CI): 1.64-2.22, p-value < 0.001}, PSA (OR: 1.08, 95% CI: 1.04-1.11, p-value < 0.001), total cholesterol (OR: 0.96, 95% CI: 0.92-0.99, p-value = 0.011) and triglyceride level (OR: 1.05, 95% CI: 1.03-1.07, p-value < 0.001) were associated with PC risk (Figure 1). In the adjusted multivariate quartile analysis of PC risk and the lipid profile, statistically significant trends of increasing odds following increasing triglyceride level (p-trend < 0.001) were found, however not in total cholesterol level (p-trend =0.243) (Supplementary Table 1).
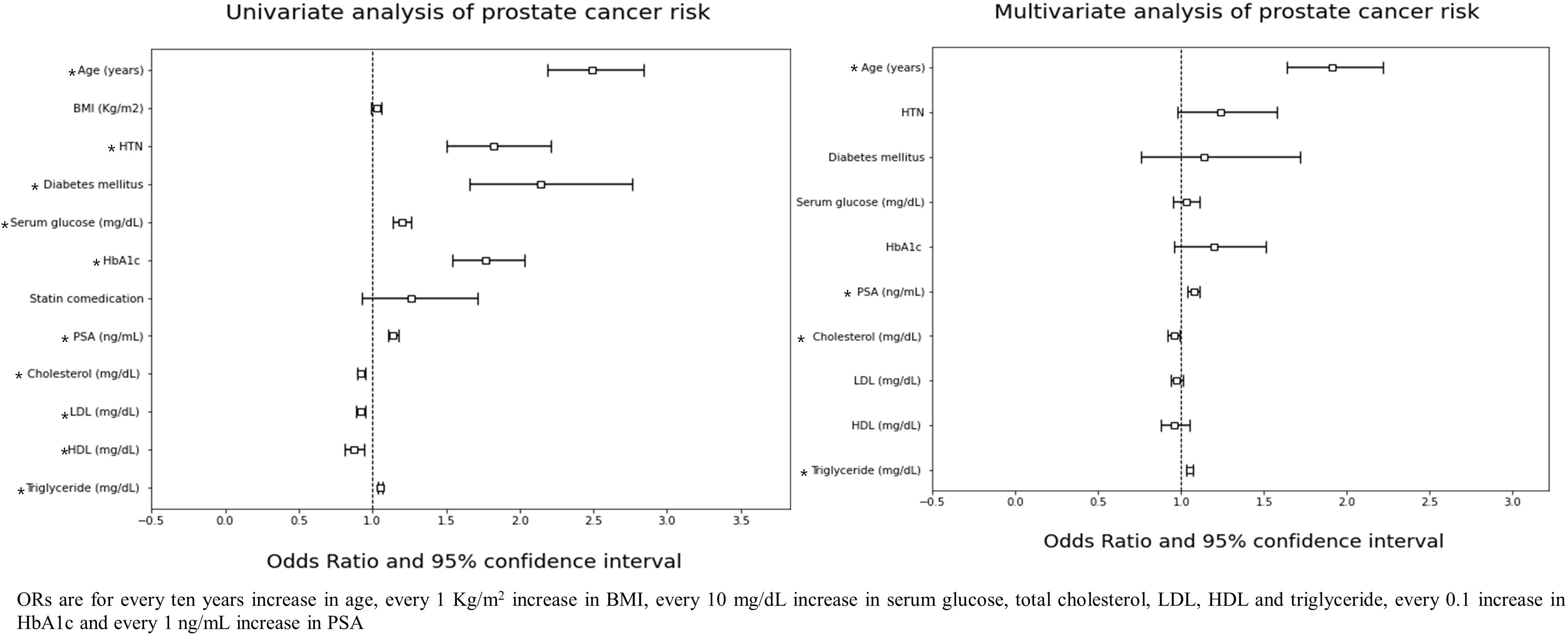
Figure 1 ORs are for every ten years increase in age, every 1 Kg/m2 increase in BMI, every 10 mg/dL increase in serum glucose, total cholesterol, LDL, HDL and triglyceride, every 0.1 increase in HbA1c and every 1 ng/mL increase in PSA.
3.3 Lipid profile and prostate cancer aggressiveness: gleason grade group
Age, hypertension, diabetes mellitus, serum glucose level, HbA1c, PSA level, total cholesterol, HDL, and triglycerides were significantly different concerning GG (Supplementary Table 2). From univariate and multivariable analysis, age, PSA level, and triglyceride level were significantly associated with a higher GG. (Table 2) However, in the univariate quartile analysis of GG and the lipid profile, a statistically significant trend of odds following increasing triglyceride levels in both GG 2-3 (p-trend = 0.646) and GG 4-5 (p-trend = 0.136) (Supplementary Table 3) could not be found.
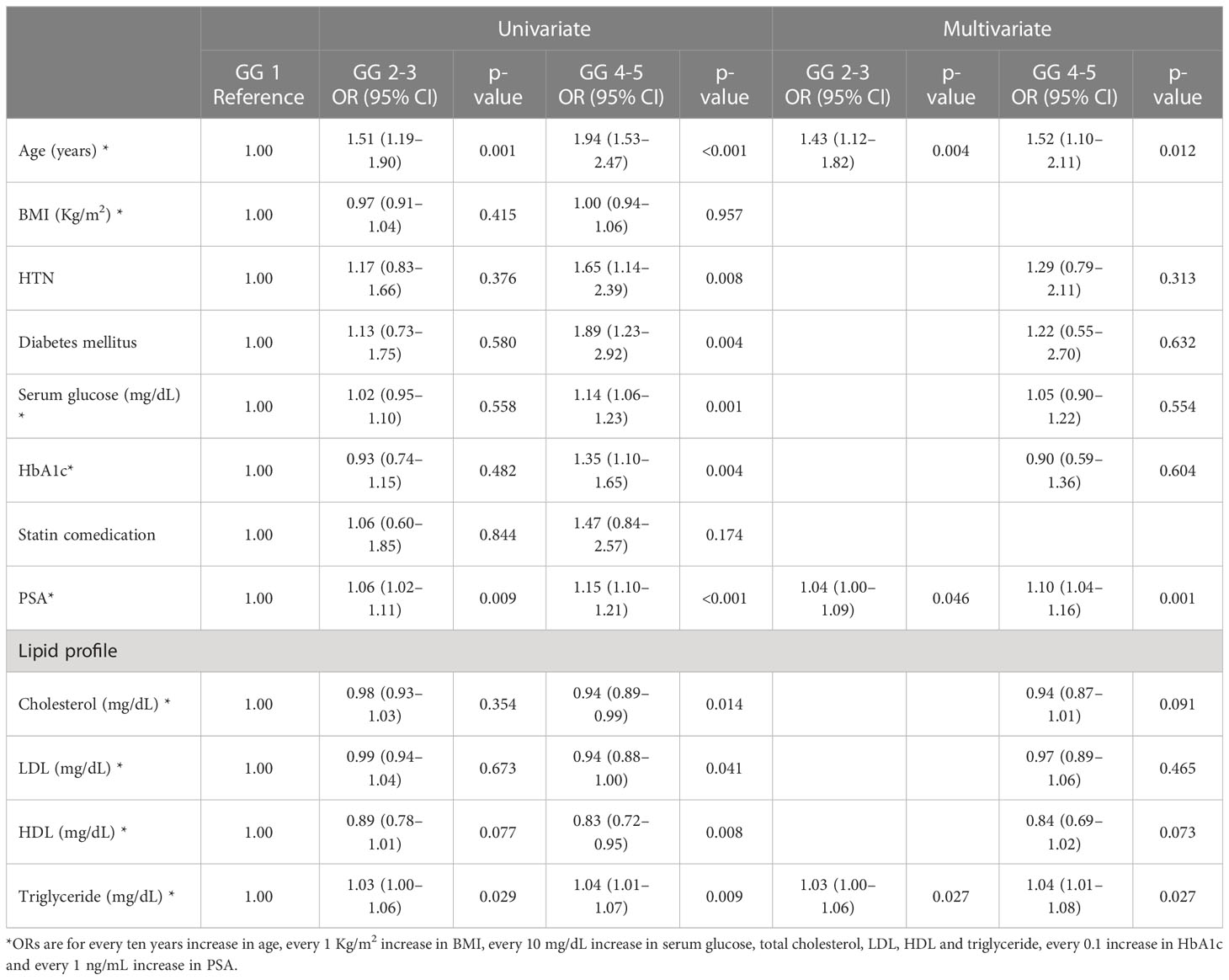
Table 2 Logistic regression analysis for pathologic Gleason grade group (GG) in prostate cancer patients.
3.4 Lipid profile and prostate cancer aggressiveness: clinical stage
Age, hypertension, diabetes mellitus, serum glucose level, HbA1c, PSA level, HDL, and triglycerides differed significantly between organ-confined, locally advanced, and metastatic stages (Supplementary Table 4). From univariate and multivariable analysis, PSA, HDL, and triglyceride levels were significantly associated with locally advanced disease and age. PSA and triglyceride levels were significantly associated with metastatic disease (Table 3). In the univariate quartile analysis of the clinical stage and the lipid profile, a statistically significant odds trend following increasing triglyceride levels was found in locally advanced disease (p-trend = 0.003) but not in metastatic disease (p-trend = 0.119) (Supplementary Table 3).
4 Discussion
In the present study, we found that the lipid profile components were associated with PC risk and aggressiveness (both in higher GG and advanced clinical stage). In the multivariate analysis, PC risk was significantly associated with age, PSA, and lipid profile (total cholesterol and triglyceride level). The higher GG was significantly associated with age, PSA, and triglyceride levels. Locally advanced disease was significantly associated with a higher PSA, triglyceride level, and lower HDL level. Metastatic disease was significantly associated with older age, higher PSA, and triglyceride levels. From univariate and multivariate quartile analysis, only statistically significant trends of increasing odds were found in PC risk and locally advanced disease when assessed with regard to triglyceride level.
Prostate cancer tumorigenesis and proliferation directly or indirectly affect lipid metabolism (10). The increased intake of fatty acids is required for the rapid proliferation of prostate cancer; thus, de novo and alternative lipogenesis pathways are enhanced (9). Accumulation of intra tumoral lipid droplets due to PC cells via de novo fatty acid synthesis, gave higher survival change during nutrition depletion conditions, such as progression or metastasis (21). Moreover, prostate cancer highly depends on cholesterol-derived steroid hormones, including androgens (11). In some in vitro studies, hypercholesterolaemia has led to prostate tumour growth, volume increase, and metastasis, and cholesterol is an important factor controlling the signal transduction of PC cells (12, 22). Some In vivo studies also supported correlation of lipid profile and PC progression. Inhibition of fatty acid synthesis in metastatic castration resistance PC xenograft and organoid model, reduced tumor growth by downregulate androgen receptor pathways (23). David et al. (24) reported high fat diet reprogramed PC metabolism and accelerate disease progression, through mimicking MYC overexpression in the mouse model. Thus, alteration of the lipid profile might be associated with an increased PC risk.
Despite in vivo and in vitro evidence of a positive correlation between dyslipidaemia and PC risk, epidemiologic and clinical studies show conflicting results (13–16). For example, the Swedish Apolipoprotein MOrtality RISk (AMORIS) (14) and Austrian cohort studies (13) found evidence of lipid metabolism being associated with PC risk. However, Liu et al. reviewed a large prospective cohorts and found that total blood cholesterol, HDL, and LDL levels were not associated with PC risk and high-grade PC risk (16). Unfortunately, this meta-analysis does not assess the association between triglyceride level and PC. Prostate cancer association group to investigate cancer-associated alterations in the genome (PRACTICAL) consortium underwent mendelian randomization study to reveal causal influence of lipid profile to PC risk (25). PRACTICAL consortium reported total cholesterol level does not alter PC risk, however they founded weak association of high LDL and triglyceride on PC. Owing to the complex manifestation of LDL, triglyceride, and HDL among the lipid profiles and the effect of statin comedication on prostate cancer appear complicated, it is necessary to analyze the separation of each component of lipid profile.
In the present study, the higher triglyceride level shows a robust correlation with PC risk, advanced stage, and grades. The first (14) and extended (26) results of the AMORIS cohort are similar to our study. The AMORIS study’s strength in the extensive review of the glucose and lipid profile (14). A primitive analysis shows no relation between the triglyceride level and the PC risk. But when the glucose level was controlled for, the positive correlation between high triglyceride and PC risk in high glycaemic populations was revealed. Similar to our result, the follow-up study to AMORIS showed a positive correlation between high triglyceride levels and PC aggressiveness and severity (26). AMORIS study result has limitation on lack of information of patients diabetes status and history of statin or metformin co-medication, which can provide misclassification of patients lipid profile status (26). However, our study has the strength of complete patient data, as we collected information on all patients’ diabetes status and statin-medication status. Although metformin co-medication status was not collected in this study, we collected 87.2% (1552/1780) of patients’ HbA1c status, which reflects their recent several months of diabetes control status. This allowed us to evaluate the potential impact of diabetes on the association between lipid profiles and PC risk and aggressiveness.
Several pieces of background evidence were established supporting the positive correlation between triglycerides and PC risk and aggressiveness. Several studies have reported that triglyceride-rich residues cause carcinogenesis by cell signalling pathways such as MEK/ERK and Akt pathways associated with cell growth, cell proliferation, apoptosis and lipid biosynthesis (27, 28). High triglycerides are also associated with the development of insulin resistance and an increase in insulin-like growth factor-1, as well as increased reactive oxygen species and oxidative stress, all of which are associated with PC (27, 29) The in vitro studies support our results, and we emphasize an intense relationship between triglycerides and PC risk and aggressiveness.
Our study had some limitations. First, due to the retrospective nature of this study, there are inevitable risk of selection bias. The staging and GG in this study based on prostate biopsy result and clinical images, thus there are disparity between clinical and pathologic stage. As a clinical observational study, the background evidence of the association of triglyceride and PC risk is still, and additional translation research is needed to confirm these findings. Although our results show association between lipid profiles and PC risk and aggressiveness, this data does not collect survival outcomes, thus further investigation should be needed. Despite these limitations, our study was a large study of 1,740 men from a single institution and is valuable as the only study that has examined the relationship between the lipid profile and risk, grade, and clinical stage of PC. Additionally, our data contained information on each patient’s diabetes status, statin-medication status, and HbA1c status, which are potential confounding factors that could affect patient misclassification if not evaluated.
5 Conclusions
Our findings suggest that high triglycerides are the most important component of the lipid profile to influence PC risk. Therefore, for patients who want lifetime monitoring of PC risk, it might be beneficial to focus on the triglyceride level. However, the protective effect of lowering the triglyceride level needs to be investigated through randomized-controlled trials in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Asan Medical Center Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Jungyo Suh: Writing - Original Draft, Formal analysis, Teak Jun Shin: Writing - Original Draft Dalsan You: Data Curation, Writing - Review & Editing In Gab Jeong: Data Curation, Writing - Review & Editing Jun Hyuk Hong: Data Curation, Writing - Review & Editing Choung-Soo Kim: Data Curation, Writing - Review & Editing Hanjong Ahn: Conceptualization, Methodology, Writing - Review & Editing, Supervision
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1113226/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Chen R, Ren S, Yiu MK, Fai NC, Cheng WS, Ian LH, et al. Prostate cancer in Asia: a collaborative report. Asian J Urol (2014) 1:15–29. doi: 10.1016/j.ajur.2014.08.007
3. Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the united states and native koreans in south Korea. Cancer Control (2007) 14:78–85. doi: 10.1177/107327480701400111
4. Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol (2018) 25:524–31. doi: 10.1111/iju.13593
5. Han HH, Park JW, Na JC, Chung BH, Kim CS, Ko WJ. Epidemiology of prostate cancer in south Korea. Prostate Int (2015) 3:99–102. doi: 10.1016/j.prnil.2015.06.003
6. De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol (2012) 61:560–70. doi: 10.1016/j.eururo.2011.11.013
7. Heller DA, de Faire U, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med (1993) 328:1150–6. doi: 10.1056/NEJM199304223281603
8. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
9. Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell (2004) 5:253–61. doi: 10.1016/S1535-6108(04)00055-8
10. Suburu J, Chen YQ. Lipids and prostate cancer. Prostaglandins Other Lipid Mediators (2012) 98:1–10. doi: 10.1016/j.prostaglandins.2012.03.003
11. Butler LM, Centenera MM, Swinnen JV. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocrine-Related Cancer (2016) 23:R219–27. doi: 10.1530/ERC-15-0556
12. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest (2005) 115:959–68. doi: 10.1172/JCI200519935
13. Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, Diem G, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer (2009) 101:1202–6. doi: 10.1038/sj.bjc.6605264
14. Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, et al. Prostate cancer risk in the Swedish AMORIS study. Cancer (2011) 117:2086–95. doi: 10.1002/cncr.25758
15. Gacci M, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, Tubaro A, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis (2017) 20:146–55. doi: 10.1038/pcan.2017.1
16. Yu Peng L, Yu Xue Z, Fei LP, Cheng C, Ya Shuang Z, Da Peng L, et al. Cholesterol levels in blood and the risk of prostate cancer: a meta-analysis of 14 prospective studies. Cancer Epidemiol Biomarkers Prev (2015) 24:1086–93. doi: 10.1158/1055-9965.EPI-14-1329
17. Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev (2014) 23:2349–56. doi: 10.1158/1055-9965.EPI-14-0458
18. Pih GY, Gong EJ, Choi JY, Kim M-J, Ahn JY, Choe J, et al. Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Res Treat (2021) 53:445–56. doi: 10.4143/crt.2020.599
19. Pow-Sang JM. Management of patients with nonmetastatic prostate cancer. J Natl Compr Cancer Network (2022) 20:566–9. doi: 10.6004/jnccn.2022.5012
20. Hager B, Kraywinkel K, Keck B, Katalinic A, Meyer M, Zeissig SR, et al. Increasing use of radical prostatectomy for locally advanced prostate cancer in the USA and Germany: a comparative population-based study. Prostate Cancer Prostatic Dis (2017) 20:61–6. doi: 10.1038/pcan.2016.43
21. Scaglia N, Frontini-López YR, Zadra G. Prostate cancer progression: as a matter of fats. Front Oncol (2021) 11:719865. doi: 10.3389/fonc.2021.719865
22. Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27(Kip1) expression. J Biol Chem (2000) 275:24500–5. doi: 10.1074/jbc.M003145200
23. Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci USA (2019) 116:631–40. doi: 10.1073/pnas.1808834116
24. Labbé DP, Zadra G, Yang M, Reyes JM, Lin CY, Cacciatore S, et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat Commun (2019) 10:4358. doi: 10.1038/s41467-019-12298-z
25. Bull CJ, Bonilla C, Holly JMP, Perks CM, Davies N, Haycock P, et al. Blood lipids and prostate cancer: a mendelian randomization analysis. Cancer Med (2016) 5:1125–36. doi: 10.1002/cam4.695
26. Arthur R, Møller H, Garmo H, Holmberg L, Stattin P, Malmstrom H, et al. Association between baseline serum glucose, triglycerides and total cholesterol, and prostate cancer risk categories. Cancer Med (2016) 5:1307–18. doi: 10.1002/cam4.665
27. Sekine Y, Koike H, Nakano T, Nakajima K, Takahashi S, Suzuki K. Remnant lipoproteins induced proliferation of human prostate cancer cell, PC-3 but not LNCaP, via low density lipoprotein receptor. Cancer Epidemiol (2009) 33:16–23. doi: 10.1016/j.canep.2009.04.004
28. Steelman LS, Chappell WH, Abrams SL, Kempf CR, Long J, Laidler P, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mtor pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (2011) 3:192–222. doi: 10.18632/aging.100296
Keywords: cancer risk, lipid profile, metabolic syndrome, prostate cancer, triglycerides
Citation: Suh J, Shin TJ, You D, Jeong IG, Hong JH, Kim C-S and Ahn H (2023) The association between serum lipid profile and the prostate cancer risk and aggressiveness. Front. Oncol. 13:1113226. doi: 10.3389/fonc.2023.1113226
Received: 01 December 2022; Accepted: 18 April 2023;
Published: 15 May 2023.
Edited by:
Valeria Naponelli, University of Parma, ItalyReviewed by:
Stefano Cacciatore, International Centre for Genetic Engineering and Biotechnology (ICGEB), South AfricaChui Yan Mah, University of Adelaide, Australia
Copyright © 2023 Suh, Shin, You, Jeong, Hong, Kim and Ahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanjong Ahn, aGphaG5AYW1jLnNlb3VsLmty
 Jungyo Suh
Jungyo Suh Teak Jun Shin2
Teak Jun Shin2 Dalsan You
Dalsan You