- 1Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, China
- 2Key Laboratory of High-Incidence-Tumor Prevention & Treatment, Ministry of Education, Nanning, China
Purpose: To evaluate the outcomes and prognostic factors for patients using conversion therapy with lenvatinib combined with transcatheter arterial chemoembolization (TACE) plus programmed cell death protein-1 (PD-1) inhibitors (LTP) for initially unresectable hepatocellular carcinoma (iuHCC).
Methods: Data on 94 consecutive patients with iuHCC who received LTP conversion therapy from November 2019 to September 2022 were retrospectively analyzed. Early tumor response was reported when patients showed complete or partial response at the time of their first follow-up (4–6 weeks) after initial treatment, in accordance with mRECIST. The endpoints consisted of conversion surgery rate, overall survival (OS), and progression-free survival (PFS).
Results: Early tumor response was found in 68 patients (72.3%) and not in the remaining 26 patients (27.7%) in the entire cohort. Early responders had a significantly higher conversion surgery rate than non-early responders (44.1% vs. 7.7%, p=0.001). Early tumor response was the only factor independently associated with successful conversion resection, as indicated by multivariate analysis (OR=10.296; 95% CI: 2.076–51.063; p=0.004). Survival analysis showed that early responders had longer PFS (15.4 vs. 7.8 months, p=0.005) and OS (23.1 vs. 12.5 months, p=0.004) than non-early responders. Early responders who underwent conversion surgery also had significantly longer median PFS and OS (not reached, not reached) than those who did not (11.2 months, p=0.004; 19.4 months, p<0.001). In multivariate analyses, early tumor response was identified as an independent prognostic factor for longer OS (HR=0.404, 95% CI: 0.171–0.954; p=0.039). Successful conversion surgery was also an independent predictive factor for longer PFS (HR=0.248, 95% CI: 0.099–0.622; p=0.003) and OS (HR=0.147, 95% CI: 0.039–0.554; p=0.005).
Conclusions: Early tumor response is an important predictive marker for successful conversion surgery and prolonged survival in patients with iuHCC treated using LTP conversion therapy. Conversion surgery is necessary to improve survival during conversion therapy, particularly for early responders.
Introduction
Liver cancer is the fourth leading cause of cancer-related mortality (1). Surgical resection can provide a good prognosis for resectable hepatocellular carcinoma (HCC). However, many patients are ineligible for resection because of excessive tumor burden, large vascular invasion, intrahepatic metastases, or external metastases (2). Conversion therapy aims to provide an improved prognosis by converting unresectable hepatocellular carcinoma for a chance to receive curative surgery. In clinical practice, multidrug combination therapy has been explored in intermediate to advanced HCC (3–5). Lenvatinib combined with transcatheter arterial chemoembolization (TACE) plus programmed cell death protein-1 (PD-1) inhibitors (LTP) has shown improved prognosis in initially unresectable hepatocellular carcinoma(iuHCC) (5–7). The objective response rate of LTP are higher than those of TACE alone and TACE combined with lenvatinib (6, 7). Thus, triple-combination therapy can potentially serve as a conversion therapy.
Achieving tumor shrinkage downstaging is an important goal in conversion therapy for the treatment of iuHCC. It provides patients with the opportunity for potential radical resection (2, 8). About 50% of patients have been found to exhibit obvious tumor shrinkage after triple-combination therapy and offered curative conversion resection (9, 10). In this context, early prediction of successful conversion surgery may guide surgical treatment strategies. Moreover, early identification of patients not benefitting from conversion therapy and timely switching to other second-line treatments may improve the prognosis.
Early tumor response could respond to the reduction of tumor burden from radiological evaluation earlier. It has been associated with good prognosis in numerous malignancies (11–17). In HCC patients receiving sorafenib, lenvatinib, and combined therapy, early tumor shrinkage can lead to improved outcomes and extended survival (18–20). However, early tumor response based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) does not prolong survival in HCC patients receiving PD-1 inhibitors plus bevacizumab (21). Thus, although early tumor response represents a rapid reduction in tumor burden, whether it has a predictive role in patients treated with immune combination therapy, particularly LTP conversion therapy, remains inconclusive.
This retrospective study was aimed at evaluating outcomes and prognostic factors for patients who underwent LTP conversion therapy for iuHCC.
Methods
Study population
Consecutive patients who received LTP conversion therapy from November 2019 to September 2022 for iuHCC were retrospectively analyzed. HCC was diagnosed by clinical assessment or histological examination (22). Tumors were considered unresectable either because of technical unresectability or oncologic unresectability, or both. Technical unresectability is defined in the presence of insufficient future remnant liver volume and lesions assessed by the surgeon as unsuitable for R0 resection. Oncological unresectability is defined as failure to obtain a better prognosis after surgical resection of intermediate-to-advanced HCC, such as major vascular invasion, intrahepatic metastases, and extrahepatic metastases. Other inclusion criteria were as follows: age between 18 and 80 years; Eastern Tumor Collaborative Group physical status (ECOG-PS) score of 0 to 1; Child–Pugh class A or B; adequate organ function; the absence of previous systemic and local treatment history for HCC, and at least one measurable target lesion in accordance with mRECIST. Those who received no radiologic evaluation at the first follow-up 4–6 weeks after the initial treatment for any reason were excluded. The ethics committee at our institution examined and approved the study protocol (LW2022147), and written informed consent was obtained from each patient.
Treatment strategies
Superselective TACE was performed by specialists with extensive surgical experience in the interventional unit (details in supplementary material). Repeat TACE was conducted when the active lesion area exceeded 50% of the baseline while the liver function was reasonable. Lenvatinib (LENVIMA®, Merck Sharp & Dohme, H20180052) and PD-1 inhibitors were given within 1 week after TACE, depending on the general condition and recovery of liver function. Patients were given either 8 mg (weight <60 kg) or 12 mg (weight ≥60 kg) of lenvatinib daily via the oral route. PD-1 inhibitors included either 200 mg of camrelizumab (AiRuiKa®, Jiangsu Hengrui Medicine Co. Ltd, S20190027) or 200 mg of sintilimab (Tyvyt®, Innovent Biologics and Eli Lilly and Company, S20180016) via intravenous injection every 3 weeks. All patients were treated until disease progression, intolerable toxicity, death, or withdrawal for any reason.
Hepatectomy was performed if patients met the criteria for resection and informed consent was obtained. The criteria for tumor resectability had to be satisfied, as follows: (1) adequate residual liver volume; (2) adequate cutting edge to achieve R0 resection; (3) tumor response assessment of complete response (CR) or partial response (PR) in accordance with mRECIST criteria, or patients with efficacy assessment of stable disease (SD) and maintenance for more than 8 weeks were considered to have controlled tumor biological behavior; (4) Child–Pugh grade A or B, and no other contraindications to hepatectomy.
Follow-up
The first follow-up was scheduled 4–6 weeks after the initial treatment and then every 2–4 months until October 20, 2022. Each follow-up evaluation includes tumor response and laboratory tests, such as blood tests, liver and kidney function, urine routine, tumor markers, myocardial enzymology examination, and thyroid function. Tumor response was evaluated on contrast-enhanced computed tomography (CE-CT) using mRECIST and RECIST1.1 by two senior hepatobiliary surgeons at our hospital.
Outcome assessments
Early tumor response was defined as patients with CR or PR at the time of the first follow-up (4–6 weeks) after initial treatment using mRECIST, whereas late tumor response was documented after the first follow-up. The non-early response included late tumor response, SD, or progressive disease (PD). Early α-fetoprotein (AFP) response was defined as a reduction of more than 75% in AFP levels following the initial treatment at the first follow-up (23).
We also evaluated the prognostic factors by using inflammatory indexes such as NLR, PLR, and systemic inflammation response index (SIRI). SIRI was calculated as neutrophil count × monocyte count/lymphocyte count (24). These inflammatory indices were split into two groups, based on their median value. Overall survival (OS) was measured from the initiation of the conversion therapy to death from any cause. Progression-free survival (PFS) was calculated from the initiation of the conversion therapy to progression, relapse, or death.
Statistical analysis
The data collected in this study were statistically analyzed using the software SPSS ver. 24.0 (IBM, Armonk, NY, United States) and R ver. 4.1.1 (http://www.R-project.org/). The Mann–Whitney U test or t-test was used to compare continuous variables, represented as median and quartiles or mean ± standard deviation. Categorical variables were presented as the number of cases and percentages by using the χ² test or Fisher’s exact probability method. Survival analysis was conducted using Kaplan–Meier methods and log-rank tests. The inverse Kaplan–Meier method was conducted to determine the median time of follow-up.
Potential predictive factors of successful conversion surgery were determined using binary logistic regression methods. In multivariate analysis, all factors with p<0.05 and clinically important variables in the univariate analyses were included via the enter method. Given the clinical correlation between early tumor response and AFP response, two models were used to include early tumor response and AFP response in separate multivariate logistic regression analyses to avoid collinearity. Considering that no patients with distant metastases had successful conversion surgery, we presented a sensitivity binary logistic regression analysis for patients without distant metastases.
Potential prognostic factors for PFS and OS were determined using the Cox proportional-hazards models. All factors with p<0.05 and clinically important variables for prognosis were included in the multivariate analysis via the enter method. Given the correlation between successful conversion surgery and early tumor response, we included two models in separate multivariate cox regression analyses to avoid collinearity. For all analyses, p<0.05 was considered statistically significant.
Results
Patient characteristics
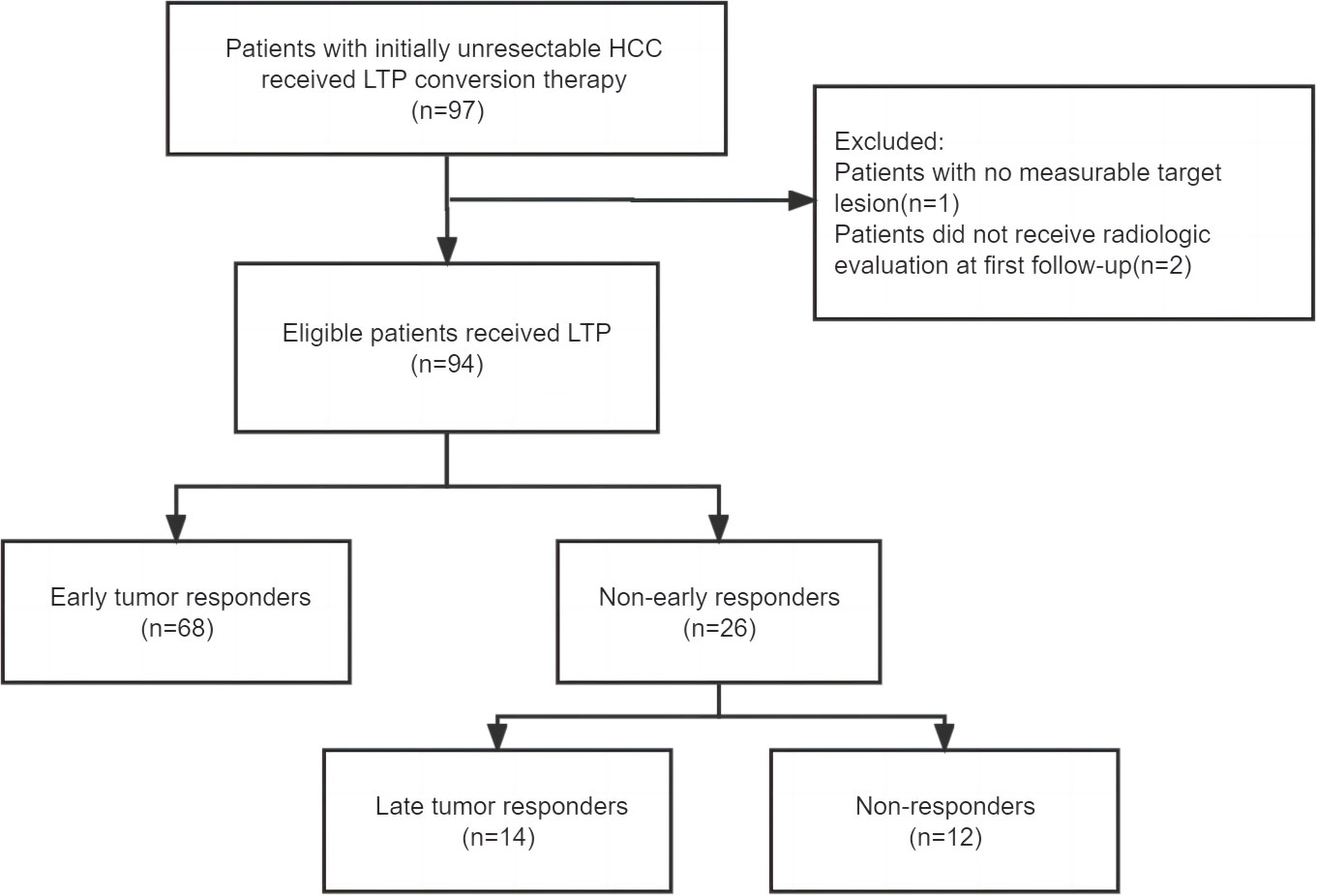
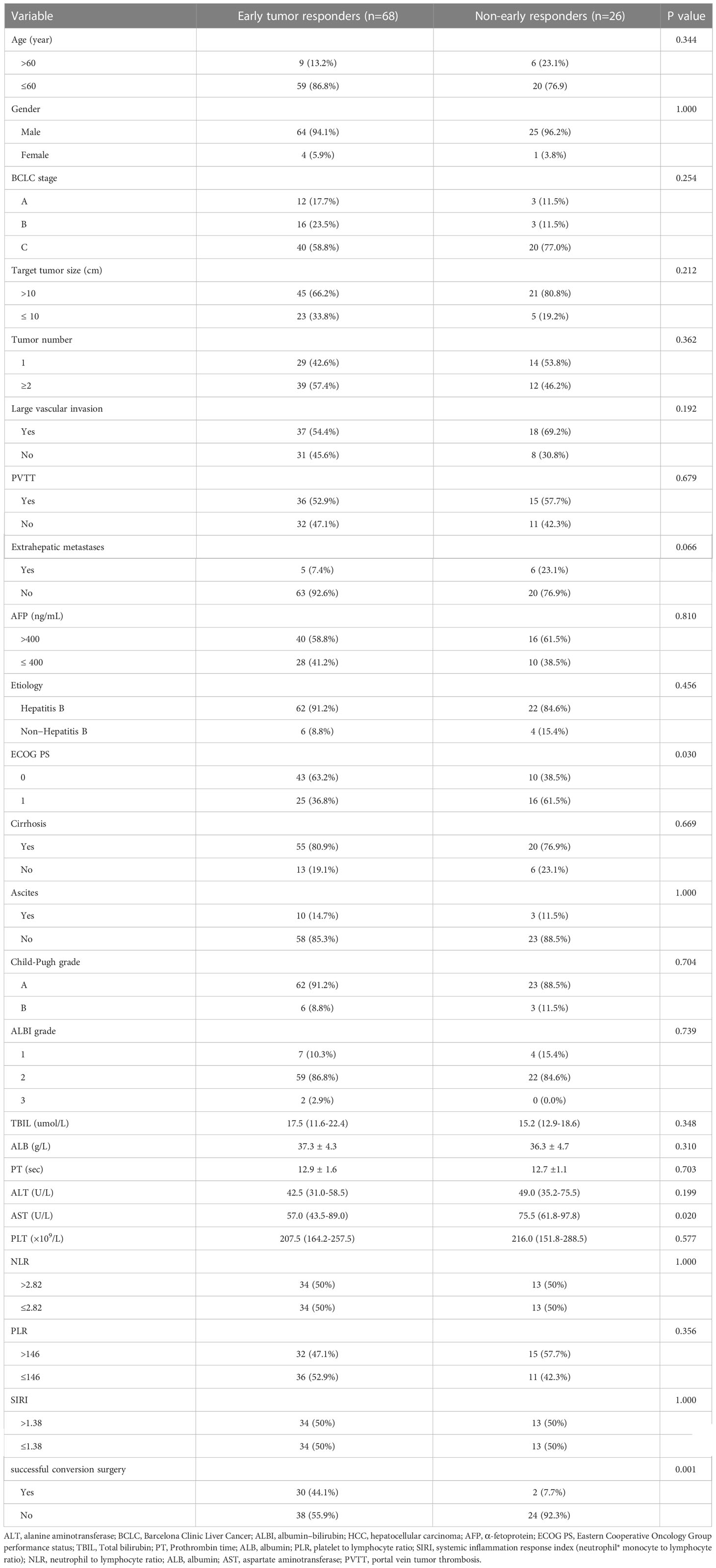
A total of 97 patients who received conversion therapy to treat iuHCC were assessed; 3 patients were subsequently excluded (Figure 1). Among the 94 patients included, 84 (89.4%) had hepatitis B-associated HCC. At baseline, 85 patients (90.4%) were classified as Child–Pugh grade A, and the majority were assigned with an ALBI grade >1 (88.3%). Further, 60 (63.8%) were of Barcelona clinical liver cancer (BCLC) stage C and 19 cases (20.2%) were of BCLC stage B; 56 (59.6%) patients had an initial AFP > 400 ng/mL. In 70.2% of patients, the tumors measured >10 cm in diameter. Multiple tumors were found in 51 patients (54.3%). The laboratory tests were also summarized, including the results for the hepatobiliary enzyme, total bilirubin, NLR, PLR, and SIRI (Table 1). Although adverse events in varying degrees affected all patients, they were within controllable levels (Table S1).
Treatment response and successful conversion surgery
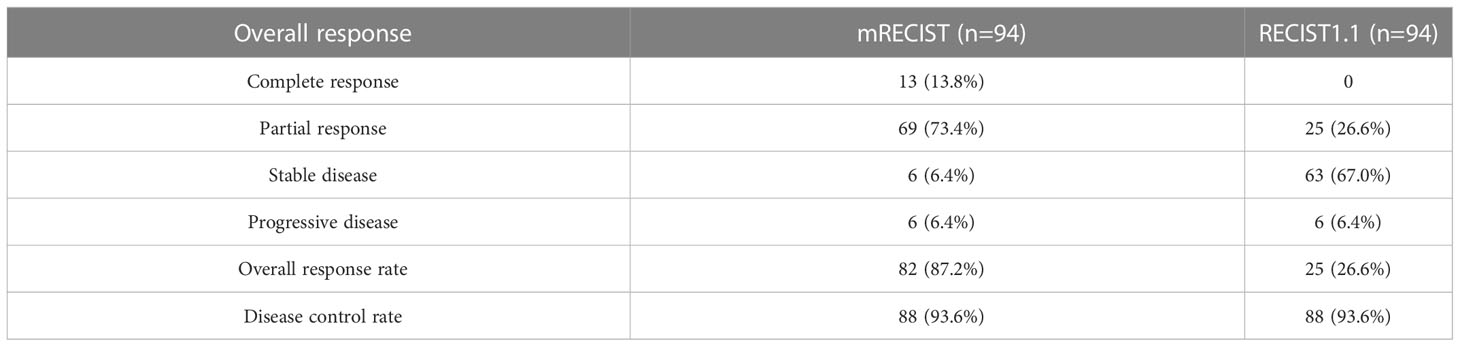
The median follow-up period was 14.4 (10.7–18.2) months. For all patients, the overall response rate was 87.2%, and the disease control rate was 93.6% based on mRECIST (Table 2). Among the patients, 32 (34.0%) underwent conversion surgery. The median time to surgery was 3.8 (3.1–5.4) months. All patients who were successfully converted had no distant metastases. Of the entire cohort, 68 (72.3%) patients showed an early tumor response, and 26 (27.7%) had no early tumor response. The patients who showed early tumor response had a significantly higher conversion surgery rate than those who showed no such response (44.1% vs. 7.7%, p=0.001). The patients with early tumor response had similar baseline characteristics to those with no early tumor response, in addition to the ECOG-PS score (p=0.030) and AST (p=0.020) (Table 1). Representative cases are presented in Supplementary Figure 1.
Relationship between early tumor response and conversion resection rate
The first multivariate model incorporated early tumor response in the 94 patients included in the study. The result indicates that the only independent predictive factor for conversion surgery was early tumor response (OR=10.296; 95% CI: 2.076–51.063; p=0.004). Early AFP response was included in the second multivariate model; however, early AFP response was not a predictor of conversion resection (Table 3). The result was confirmed in 83 patients with non-distant metastases (OR=9.659; 95% CI: 1.899–49.125; p=0.006)(Table S2).
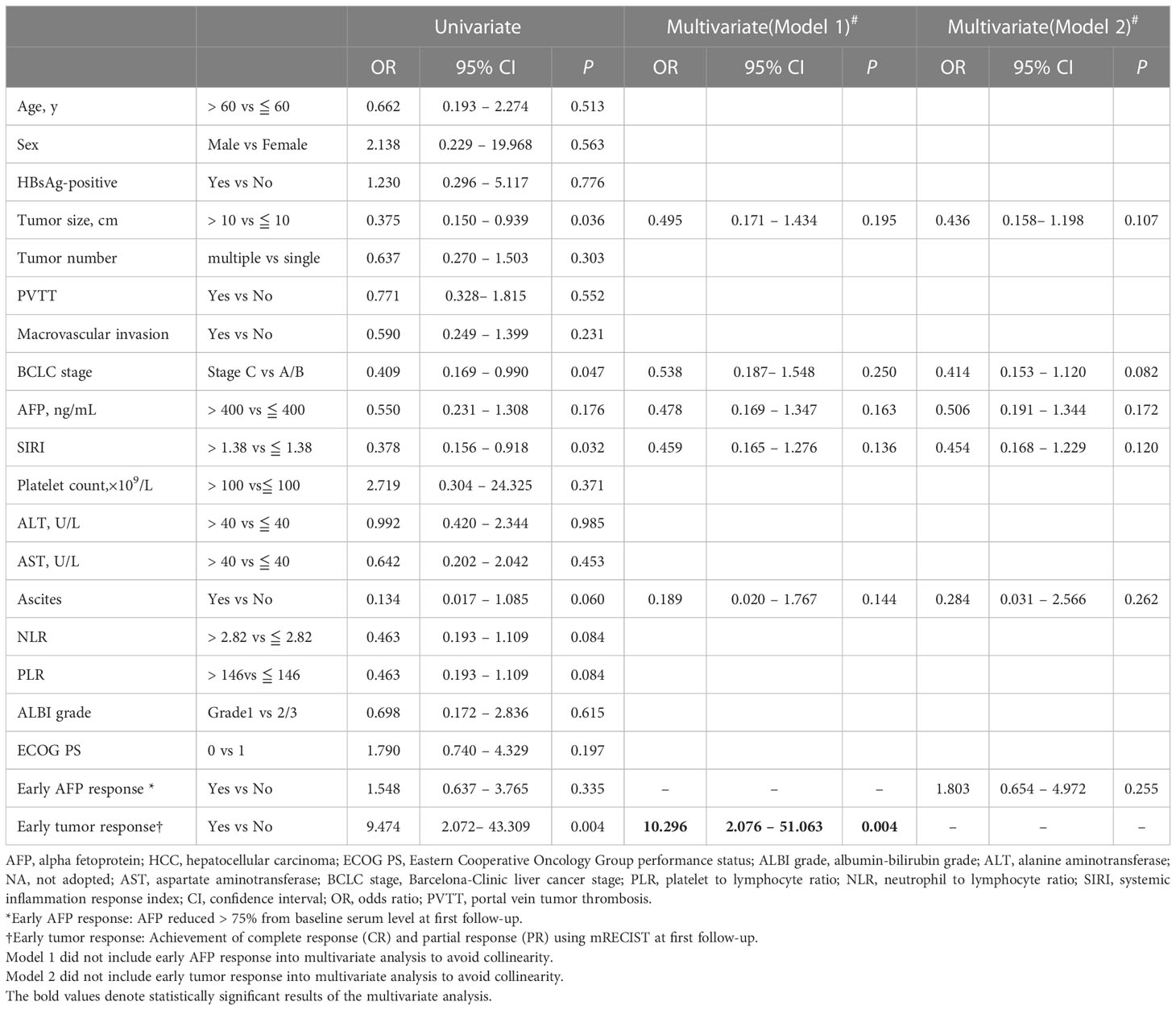
Table 3 Factors associated with successful conversion surgery in 94 patients who received conversion therapy for initially unresectable HCC.
Effect of early tumor response on PFS and OS
The median PFS for the patients with early tumor response was 15.4 months, whereas the median PFS with no early tumor response was 7.8 months (p=0.005; Figure 2A). However, early tumor response was not an independent predictive factor of PFS (HR= 0.576, 95% CI: 0.302–1.097; p=0.093) (Table 4, Multivariate model 1). The median OS of the patients with early tumor response was 23.1 months (p=0.004; Figure 2B), whereas that of patients with no early tumor response was 12.5 months. In multivariate analysis, early tumor response was independently correlated with OS (HR= 0.404, 95% CI: 0.171–0.954; p=0.039) and jointly with the following conditions: baseline AFP>400 ng/mL (p=0.020), portal vein tumor thrombosis (PVTT) (p=0.011), and extrahepatic metastasis (p=0.001)(Table 5, Multivariate model 1). The effects of early tumor response on PFS (HR= 0.569, 95% CI: 0.278 – 1.164; p=0.123) and OS (HR= 0.329, 95% CI: 0.119 – 0.910; p=0.032) were confirmed in 83 patients with non-distant metastases (Tables S3, S4, Multivariate model 1).
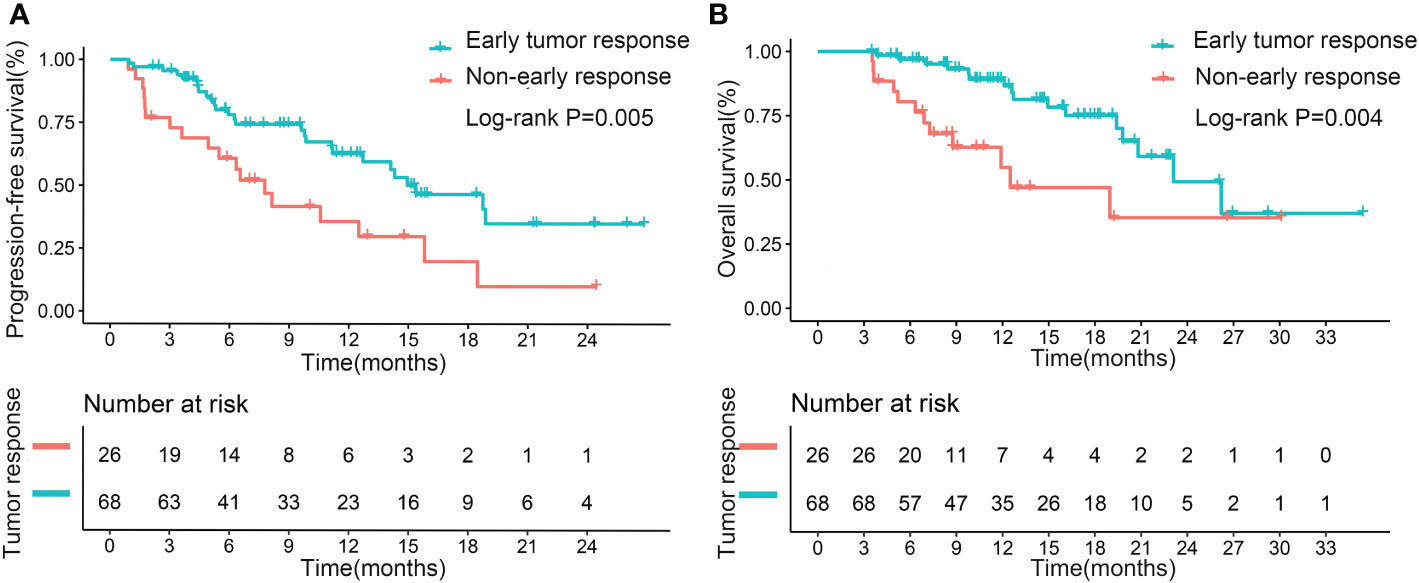
Figure 2 Kaplan–Meier curves for progression-free survival (A) and overall survival (B), based on early tumor response in the entire cohort.
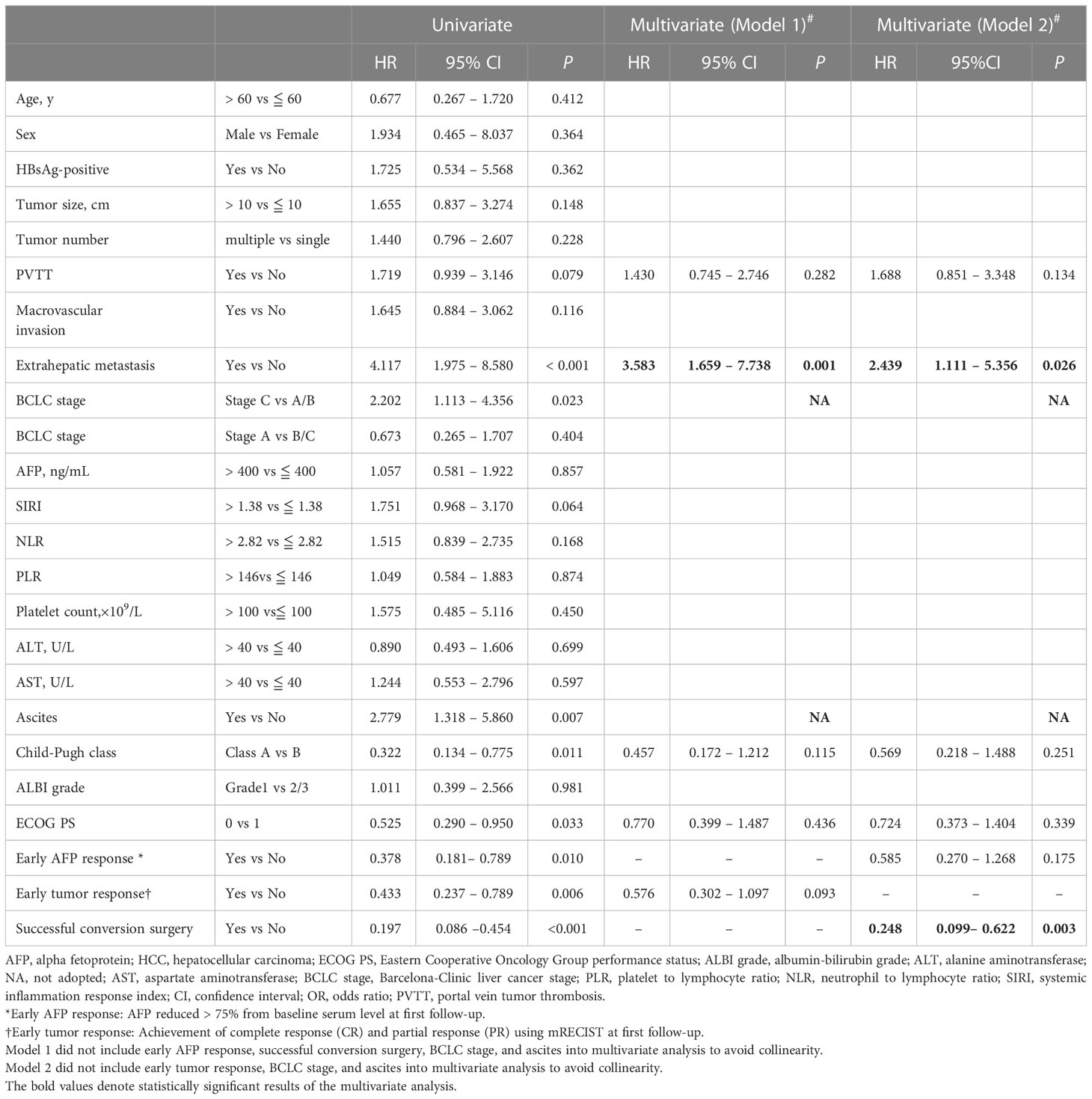
Table 4 Factors associated with progression-free survival in 94 patients who received conversion therapy for initially unresectable HCC.
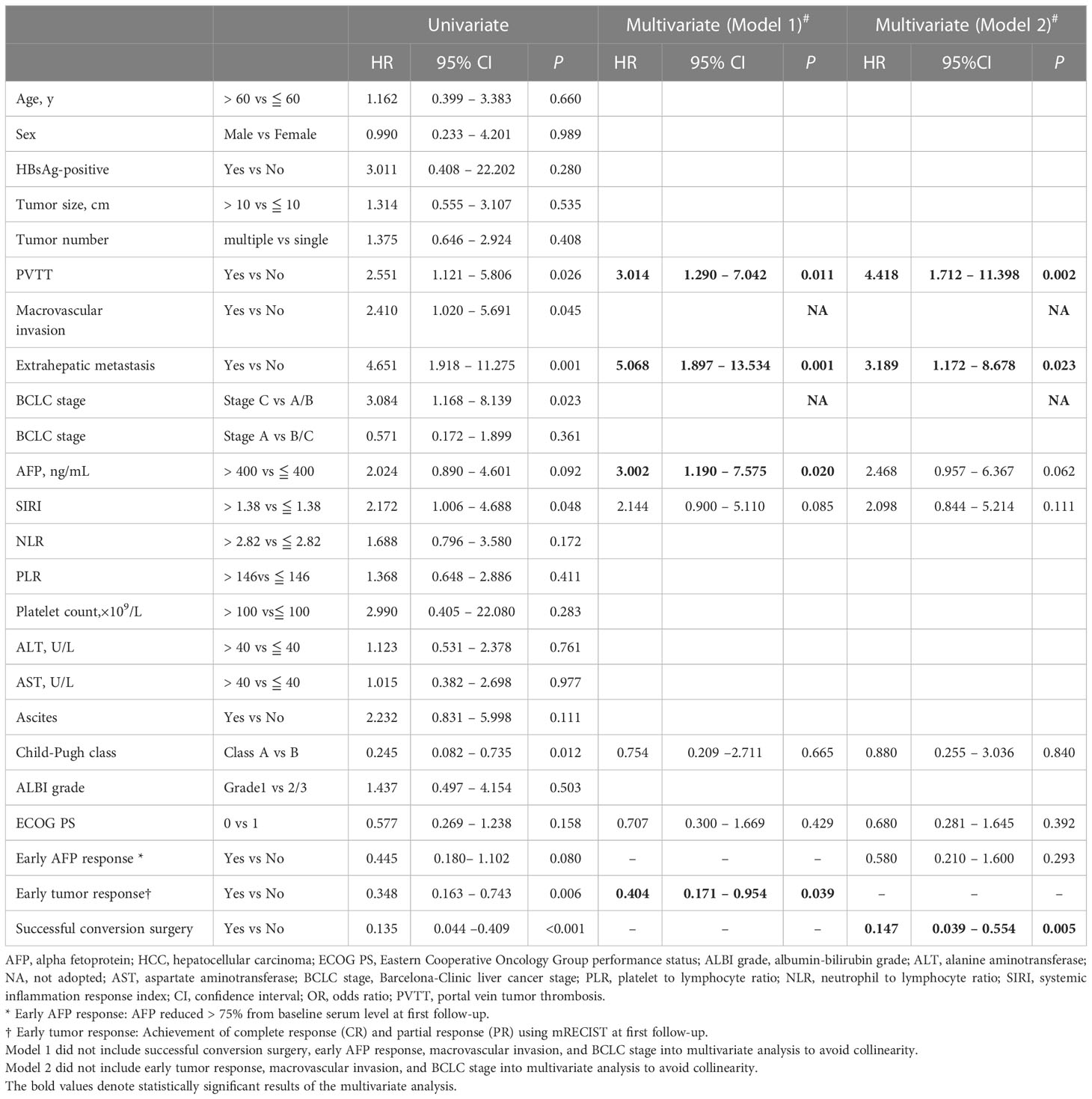
Table 5 Factors associated with overall survival in 94 patients who received conversion therapy for initially unresectable HCC.
Given that non-early tumor response was a risk factor for OS, we assigned different scores to each risk factor on the basis of the beta coefficient score in multivariate analysis. We further divided the patients into 4 groups, based on the risk factor. Survival curves showed that the best median OS was in the score 0 group, which declined as the risk factor scores increased (Figure 3).
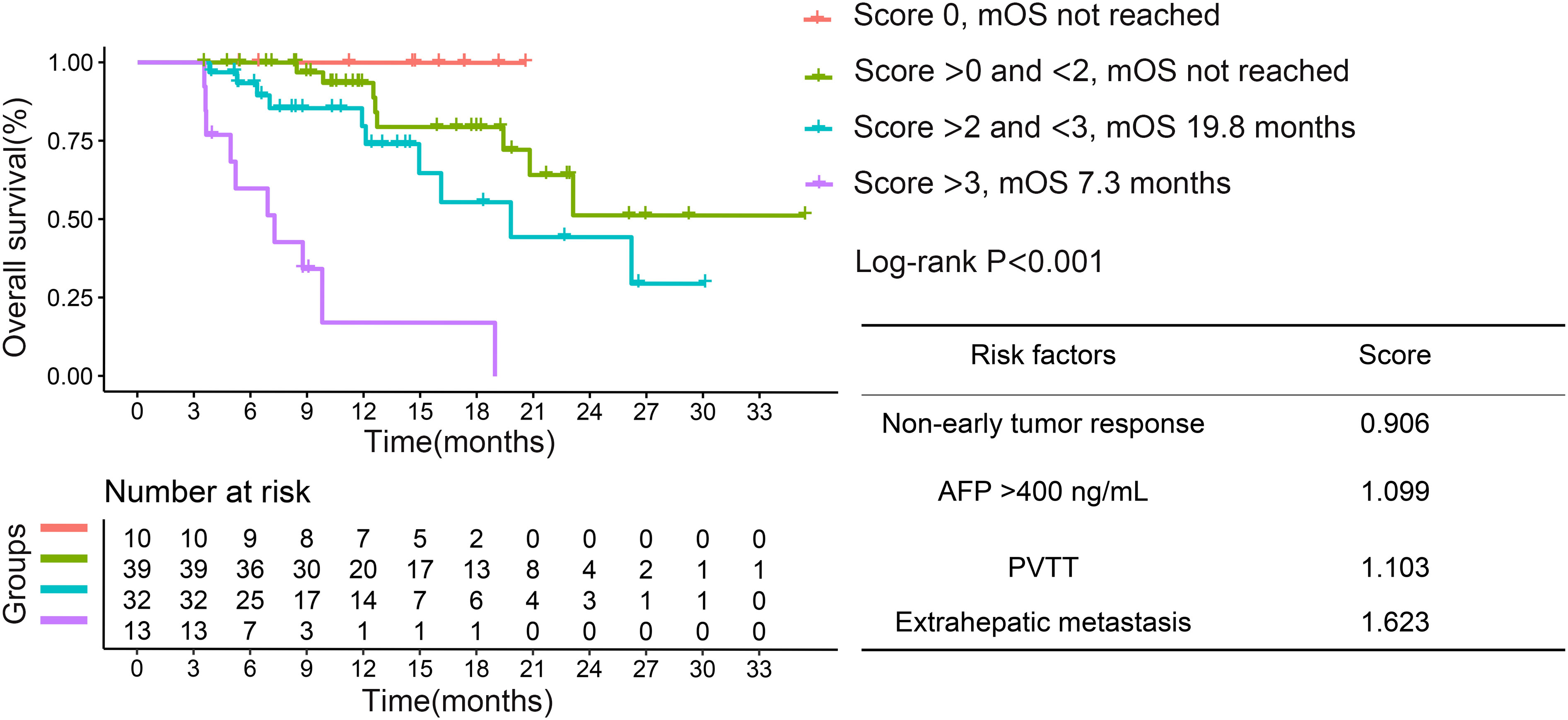
Figure 3 Kaplan–Meier curves for overall survival based on different risk factor scores in the entire cohort. PVTT, portal vein tumor thrombosis; AFP, α-fetoprotein; OS, overall survival.
Relevance of successful conversion surgery for PFS and OS
Median PFS and OS (not reached, not reached) were significantly longer in patients who underwent successful conversion surgery than in those patients who received no such surgery (9.8 months, p<0.001; 14.9 months, p<0.001; Figures 4A, B). We further assessed the role of conversion surgery in patients with early tumor response. Results showed that for patients with early tumor response, those who underwent conversion surgery also had significantly longer median PFS and OS (not reached, not reached) than those who did not undergo conversion resection (11.2 months, p=0.004; 19.4 months, p<0.001; Figures 5A, B). Furthermore, early responders combined with late responders had a similar median OS to that of early responders (p=1.000). Both had significantly longer OS than non-responders (p<0.001, p<0.001; respectively; Supplementary Figure 2A). We further assessed the role of conversion surgery in late tumor responders. The results showed that late responders who underwent conversion surgery had no significantly longer median OS than those who did not (p=0.3; Supplementary Figure 2B).
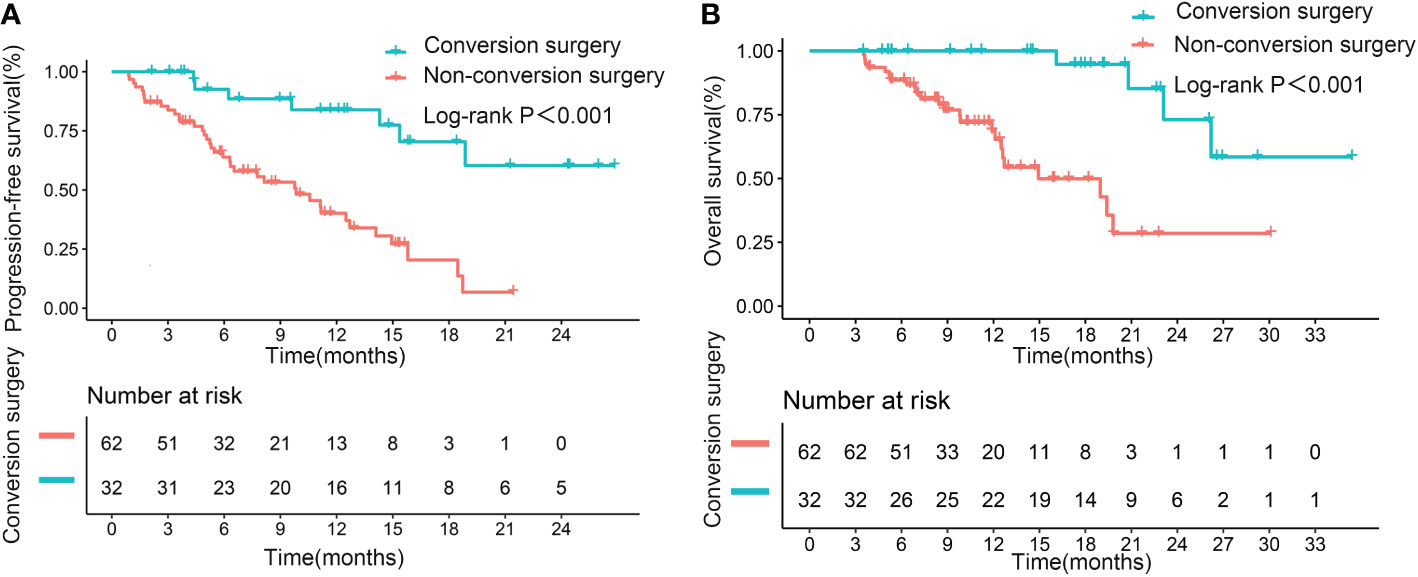
Figure 4 Kaplan–Meier curves for progression-free survival (A) and overall survival (B), based on successful conversion surgery, in the entire cohort.
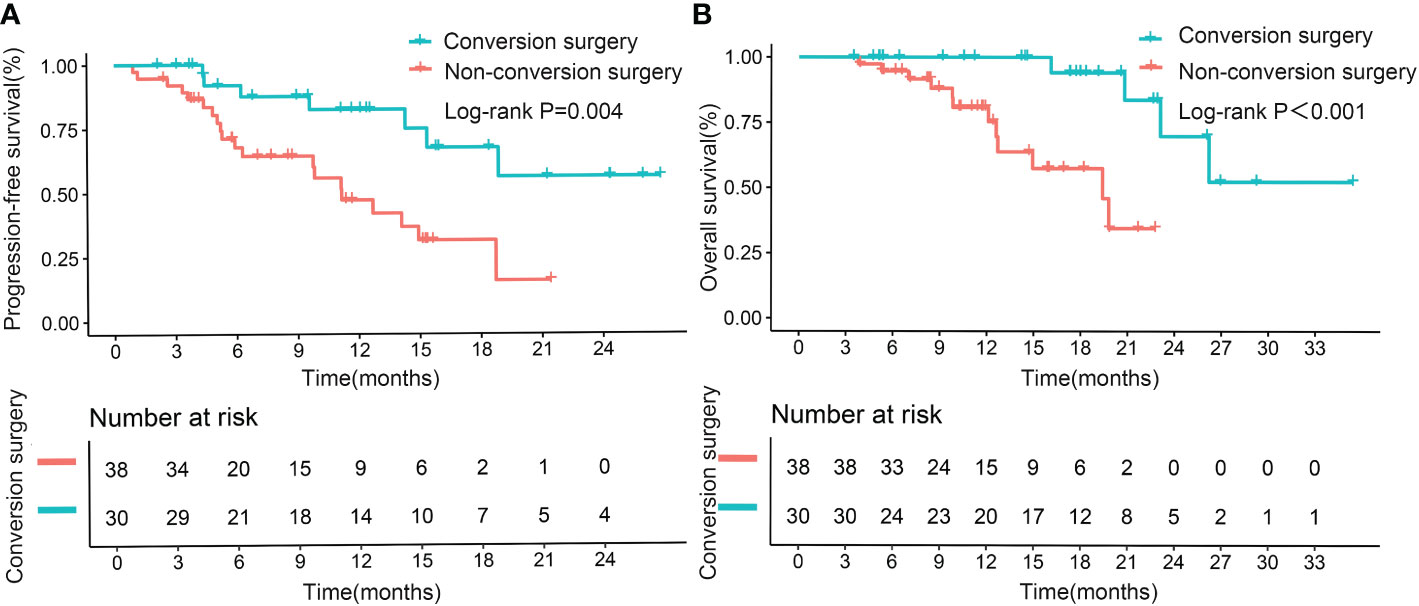
Figure 5 Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in patients with early tumor response, based on successful conversion surgery.
Multivariate results confirmed that successful conversion surgery (HR=0.248, 95% CI: 0.099–0.622; p=0.003) was independently correlated with PFS (Table 4, Multivariate model 2). In addition, multivariate analysis confirmed that successful conversion surgery was also independently correlated with OS (HR = 0.147, 95% CI: 0.039 – 0.554; p=0.005), in addition to PVTT (p=0.002) and extrahepatic metastasis (p=0.023)(Table 5, Multivariate model 2). The results on the relevance of successful conversion surgery for PFS (HR= 0.265, 95% CI: 0.105– 0.669; p=0.005) and OS (HR= 0.088, 95% CI: 0.021 – 0.363; p=0.001) were confirmed in 83 patients with non-distant metastases (Table S3, S4, Multivariate model 2).
Discussion
The triple-combination LTP is a trend in conversion therapy for patients with iuHCC (5). However, LTP provides improved treatment outcomes while challenging the prediction of successful conversion surgery and prognosis. We found that early tumor response was independently associated with successful conversion surgery and better survival. Moreover, successful conversion surgery after LTP is essential for a better prognosis, especially early responders.
Some markers might help predict the prognosis of people with liver cancer. NLR is a prognostic factor in iuHCC for patients receiving triple-combination therapy (20). Baseline PLR and SIRI are also associated with the prognosis for HCC (9, 25, 26). In addition, the combination of C-reactive protein (CRP) and AFP showed a prognostic role in HCC patients receiving tyrosine kinase inhibitors (TKIs) combined with immunotherapy (27, 28). However, CRP is not a mandatory test for every patient in our hospital, hence its limited application in current clinical practice. To explore their value in conversion therapy, we chose inflammatory indexes in blood routine, such as PLR, NLR, and SIRI substituted for CRP. Further, we used the baseline AFP and early AFP response to explore the potential predictive factor. However, among the aforementioned indicators, only baseline AFP was independently associated with OS. Therefore, the above indicators have a limited role in LTP conversion therapy.
Several studies have been conducted on the prognostic role of early tumor response in iuHCC, but the results have been inconclusive. Earlier studies by Hashi et al. and Öcal et al. evaluated the relationship between survival and early tumor response in patients treated with TKI, in accordance with RECIST and mRECIST, respectively (18, 19). They found that early tumor response was independently associated with prognosis. Current studies recommend mRECIST-based tumor response assessment, given that RECIST is not considered applicable for viable tumors (18, 29, 30). However, a real-world study indicated that early tumor response based on mRECIST was not correlated with prognosis in patients receiving PD-1 inhibitors combined with bevacizumab (21). The study found that the Choi criteria and revised Choi criteria, which consider tumor density on CE-CT, might provide a more suitable evaluation of early tumor response and its correlation with prognosis (21). We considered that applying the Choi criteria and revised Chio criteria is not preferable because of the use of TACE in triple-combination therapy. Therefore, we still chose mRECIST to evaluate early tumor response. We determined that early tumor response was the only factor independently correlated with successful conversion resection. The result is also applicable to patients without distant metastases. A significant association was also found between early tumor response and OS but not between early tumor response and PFS, a finding that is inconsistent with previous results (20). The main reason is that in the previous study, only two patients underwent conversion surgery, whereas in the current study, 34.0% of the patients underwent conversion resection. Therefore, early tumor response is an important impact factor in conversion surgery and the prognosis of patients undergoing LTP conversion therapy. We further included early tumor response for risk stratification and found that OS decreased with increasing risk factor scores. It demonstrates the potential clinical value of this stratification that takes into account early tumor response and provides evidence to support the importance of future larger-scale studies.
Conversion resection may be the only means to obtain a cure for patients with iuHCC (2). No more evidence of whether surgical resection should be performed after conversion therapy for the treatment of iuHCC meets the criteria for resectability. In our multivariable prognosis analysis, conversion surgery was an independent predictor of prognosis. This finding suggests that conversion surgery is important and necessary. To further identify the significance of conversion surgery, we compared the survival of patients with early tumor response who received conversion surgery with the survival of patients who were yet to meet the criteria for resectability despite a favorable early tumor response. We also found a better prognosis for patients who had undergone conversion surgery. Therefore, hepatectomy should be performed if patients meet the criteria for resection in early responders. However, late responders only demonstrated a trend toward prolonged OS after conversion surgery, with no significant difference in OS. In addition, late responders had low conversion surgery rate and may be no less effective than early responders. Further sample size expansion is required to confirm this result.Therefore, conversion surgery significantly improved the prognosis in the overall population, but its efficacy in late responders requires further testing.
Several limitations should be noted. First, the small sample size in one hospital prevented us from further exploring the factors influencing the prognosis of patients who received successful conversion surgery. Second, potential selection bias is inevitable because of the retrospective nature of the study. A large sample of prospective studies is necessary to further explore the subject. Third, we used two PD-1 inhibitors. Although no differences in the prognostic impact of these immunotherapeutic drugs were found (data not shown), further studies on the effects of different PD-1 inhibitors on conversion therapy are still needed.
In summary, early tumor response is an important predictive marker for successful conversion surgery and prolonged survival in patients with iuHCC treated using LTP conversion therapy. Conversion surgery is necessary to improve survival during conversion therapy, particularly for early responders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Guangxi Medical University Cancer Hospital(approval number: LW2022147). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL, JC, LL and FW contributed for study concept, design, and finished manuscript writing. XL, JC, XW, TB, and SL performed data analysis and critically revised the article. XL, JC, TW, ZT, CH, BZ, and BL were involved in data collection and verification. All authors contributed to the article and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81860502), Key Laboratory of High-Incidence-Tumor Prevention & Treatment, Ministry of Education(GKE2019-06), Key Laboratory of High-Incidence-Tumor Prevention & Treatment, Ministry of Education(GKE-ZZ202129), Key Laboratory of High-Incidence-Tumor Prevention & Treatment, Ministry of Education(GKE-ZZ202004), and Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education (GKE-ZZ202216).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1110689/full#supplementary-material
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
2. Arita J, Ichida A, Nagata R, Mihara Y, Kawaguchi Y, Ishizawa T, et al. Conversion surgery after preoperative therapy for advanced hepatocellular carcinoma in the era of molecular targeted therapy and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci (2022) 29:732–40. doi: 10.1002/jhbp.1135
3. Yang F, Xu GL, Huang JT, Yin Y, Xiang W, Zhong BY, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: Efficacy and systemic immune response. Front Immunol (2022) 13:847601. doi: 10.3389/fimmu.2022.847601
4. He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol (2021) 13:17588359211002720. doi: 10.1177/17588359211002720
5. Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The significance of transarterial Chemo(Embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: A systematic review. Front Immunol (2022) 13:913464. doi: 10.3389/fimmu.2022.913464
6. Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, et al. Efficacy and safety of tace combined with lenvatinib plus pd-1 inhibitors compared with tace alone for unresectable hepatocellular carcinoma patients: A prospective cohort study. Front Oncol (2022) 12:874473. doi: 10.3389/fonc.2022.874473
7. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus tace with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring pd-L1 expression: A retrospective study. J Cancer Res Clin Oncol (2022) 148(8):2115–25. doi: 10.1007/s00432-021-03767-4
8. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-Pd-1 antibody combinations. Liver Cancer (2021) 10(4):320–9. doi: 10.1159/000514313
9. Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu XT, et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus tace: Real-world study. BJS Open (2022) 6(5):zrac114. doi: 10.1093/bjsopen/zrac114
10. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-Pd-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/jhc.S332420
11. Bouchahda M, Boige V, Smith D, Karaboué A, Ducreux M, Hebbar M, et al. Early tumour response as a survival predictor in previously- treated patients receiving triplet hepatic artery infusion and intravenous cetuximab for unresectable liver metastases from wild-type kras colorectal cancer. Eur J Cancer (2016) 68:163–72. doi: 10.1016/j.ejca.2016.09.011
12. Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: Results from phase iii tribe trial by the gruppo oncologico del nord ovest. Ann Oncol (2015) 26(6):1188–94. doi: 10.1093/annonc/mdv112
13. Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol (2013) 31(30):3764–75. doi: 10.1200/jco.2012.42.8532
14. Fucà G, Corti F, Ambrosini M, Intini R, Salati M, Fenocchio E, et al. Prognostic impact of early tumor shrinkage and depth of response in patients with microsatellite instability-high metastatic colorectal cancer receiving immune checkpoint inhibitors. J Immunother Cancer (2021) 9(4):e002501. doi: 10.1136/jitc-2021-002501
15. Vivaldi C, Fornaro L, Cappelli C, Pecora I, Catanese S, Salani F, et al. Early tumor shrinkage and depth of response evaluation in metastatic pancreatic cancer treated with first line chemotherapy: An observational retrospective cohort study. Cancers (Basel) (2019) 11(7):939. doi: 10.3390/cancers11070939
16. Kawachi H, Fujimoto D, Morimoto T, Hosoya K, Sato Y, Kogo M, et al. Early depth of tumor shrinkage and treatment outcomes in non-small cell lung cancer treated using nivolumab. Invest New Drugs (2019) 37(6):1257–65. doi: 10.1007/s10637-019-00770-y
17. Miyake H, Miyazaki A, Imai S, Harada K, Fujisawa M. Early tumor shrinkage under treatment with first-line tyrosine kinase inhibitors as a predictor of overall survival in patients with metastatic renal cell carcinoma: A retrospective multi-institutional study in Japan. Target Oncol (2016) 11(2):175–82. doi: 10.1007/s11523-015-0385-6
18. Öcal O, Schinner R, Schütte K, de Toni EN, Loewe C, van Delden O, et al. Early tumor shrinkage and response assessment according to mrecist predict overall survival in hepatocellular carcinoma patients under sorafenib. Cancer Imaging (2022) 22(1):1. doi: 10.1186/s40644-021-00439-x
19. Takahashi A, Moriguchi M, Seko Y, Shima T, Mitsumoto Y, Takashima H, et al. Early tumor shrinkage as a predictive factor for outcomes in hepatocellular carcinoma patients treated with lenvatinib: A multicenter analysis. Cancers (Basel) (2020) 12(3):754. doi: 10.3390/cancers12030754
20. Li X, Fu Z, Chen X, Cao K, Zhong J, Liu L, et al. Efficacy and safety of lenvatinib combined with pd-1 inhibitors plus tace for unresectable hepatocellular carcinoma patients in China real-world. Front Oncol (2022) 12:950266. doi: 10.3389/fonc.2022.950266
21. Xu Y, Yang Y, Li L, Zhou A, Zhang H, Ye F, et al. Different radiological criteria for early tumor response evaluation in patients with unresectable hepatocellular carcinoma treated with anti-Pd-1 antibody plus bevacizumab. Front Oncol (2022) 12:848129. doi: 10.3389/fonc.2022.848129
22. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
23. Zhu AX, Dayyani F, Yen CJ, Ren Z, Bai Y, Meng Z, et al. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin Cancer Res (2022) 28:3537–45. doi: 10.1158/1078-0432.Ccr-21-3275
24. Eissa M, Shaarawy S, Abdellateif MS. The role of different inflammatory indices in the diagnosis of covid-19. Int J Gen Med (2021) 14:7843–53. doi: 10.2147/ijgm.S337488
25. Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Neutrophil-lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: A multicenter analysis. Eur J Gastroenterol Hepatol (2022) 34(6):698–706. doi: 10.1097/meg.0000000000002356
26. Hong YM, Yoon KT, Cho M. Systemic immune-inflammation index predicts prognosis of sequential therapy with sorafenib and regorafenib in hepatocellular carcinoma. BMC Cancer (2021) 21(1):569. doi: 10.1186/s12885-021-08124-9
27. Yang Y, Ouyang J, Zhou Y, Zhou J, Zhao H. The crafity score: A promising prognostic predictor for patients with hepatocellular carcinoma treated with tyrosine kinase inhibitor and immunotherapy combinations. J Hepatol (2022) 77:574–6. doi: 10.1016/j.jhep.2022.03.018
28. Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the crafity score. J Hepatol (2022) 76(2):353–63. doi: 10.1016/j.jhep.2021.09.035
29. Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. Objective response by mrecist as a predictor and potential surrogate end-point of overall survival in advanced hcc. J Hepatol (2017) 66(6):1166–72. doi: 10.1016/j.jhep.2017.01.012
30. Vincenzi B, Di Maio M, Silletta M, D’Onofrio L, Spoto C, Piccirillo MC, et al. Prognostic relevance of objective response according to easl criteria and mrecist criteria in hepatocellular carcinoma patients treated with loco-regional therapies: A literature-based meta-analysis. PloS One (2015) 10(7):e0133488. doi: 10.1371/journal.pone.0133488
Keywords: conversion therapy, PD-1 inhibitors, initially unresectable hepatocellular carcinoma, transcatheter arterial chemoembolization, lenvatinib
Citation: Li X, Chen J, Wang X, Bai T, Lu S, Wei T, Tang Z, Huang C, Zhang B, Liu B, Li L and Wu F (2023) Outcomes and prognostic factors in initially unresectable hepatocellular carcinoma treated using conversion therapy with lenvatinib and TACE plus PD-1 inhibitors. Front. Oncol. 13:1110689. doi: 10.3389/fonc.2023.1110689
Received: 29 November 2022; Accepted: 17 January 2023;
Published: 30 January 2023.
Edited by:
Xiong Chen, Nanjing General Hospital of Nanjing Military Command, ChinaReviewed by:
Chi-Leung Chiang, The University of Hong Kong, Hong Kong SAR, ChinaJaejun Lee, Catholic University of Korea, Republic of Korea
Copyright © 2023 Li, Chen, Wang, Bai, Lu, Wei, Tang, Huang, Zhang, Liu, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lequn Li, bGlfbGVxdW5AMjYzLm5ldA==; Feixiang Wu, d3VmZWl4aWFuZ0BneG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xingzhi Li
Xingzhi Li Jie Chen1†
Jie Chen1† Zhihong Tang
Zhihong Tang Lequn Li
Lequn Li Feixiang Wu
Feixiang Wu