- 1Warwick Clinical Trials Unit, University of Warwick, Coventry, United Kingdom
- 2School of Health Sciences, University of East Anglia, Norwich, United Kingdom
- 3Oxford Clinical Trials Research Unit, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom
- 4Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Medical Sciences Division, University of Oxford, Oxford, United Kingdom
- 5University of Exeter Medical School, University of Exeter, Exeter, United Kingdom
- 6Nuffield Department of Surgical Sciences, Medical Sciences Division, University of Oxford, Oxford, United Kingdom
- 7Oxford Cancer Centre, Churchill Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom
Objective: To determine the feasibility of a randomised controlled trial to estimate the effectiveness and cost-effectiveness of a rehabilitation intervention following neck dissection (ND) after head and neck cancer (HNC).
Design: Two-arm, open, pragmatic, parallel, multicentre, randomised controlled feasibility trial.
Setting: Two UK NHS hospitals.
Participants: People who had HNC in whom a ND was part of their care. We excluded those with a life expectancy of six months or less, pre-existing, long-term neurological disease affecting the shoulder and cognitive impairment.
Intervention: Usual care (standard care supplemented with a booklet on postoperative self-management) was received by all participants. The GRRAND intervention programme consisted of usual care plus up to six individual physiotherapy sessions including neck and shoulder range of motion and progressive resistance exercises, advice and education. Between sessions, participants were advised to complete a home exercise programme.
Randomisation: 1:1 randomisation. Allocation was based on minimisation, stratified by hospital site and spinal accessory nerve sacrifice. It was not possible to mask treatment received.
Main outcome measures: Primary: Participant recruitment, retention and fidelity to the study protocol and interventions from study participants and staff at six months post-randomisation (and 12 months for those reaching that time-point). Secondary: clinical measures of pain, function, physical performance, health-related quality of life, health utilisation and adverse events.
Results: 36 participants were recruited and enrolled. The study achieved five of its six feasibility targets. These included consent - 70% of eligible participants were consented; intervention fidelity - 78% participants discharged completed the intervention sessions; contamination - none - no participants in the control arm received the GRRAND-F intervention and retention - 8% of participants were lost to follow-up. The only feasibility target that was not achieved was the recruitment target where only 36 of the planned 60 participants were recruited over 18 months. This was principally due to the COVID-19 pandemic which caused all research activity to be paused or reduced, with a subsequent reduction in.
Conclusions: Based on the findings a full-trial can now be designed to better understand whether this proposed intervention is effective.
Clinical Trial Registration: https://www.isrctn.com/ISRCTN1197999, identifier ISRCTN11979997.
Introduction
Annually, head and neck cancer (HNC) is diagnosed in 700,000 people worldwide and over 11,000 in the UK (1, 2). Within the UK, tumours of the oropharynx are the most common and have seen a two-fold increase in incidence over the last 20 years, largely attributed to human papillomavirus (HPV) (3, 4). Over the last 20 years, there has also been a 30% increase in oral cancer; these increases are predicted to continue (1). In addition, there is a significant health burden from thyroid cancer as well as skin cancers which are all predicted to increase in prevalence (1). People affected by HNC are now younger, more active and more ethnically diverse than previous generations of HNC survivors (1).
The treatment pathway for HNC is complex. Surgery and/or radiotherapy and/or chemo-radiotherapy is used to treat the primary tumour. From a surgical perspective, a neck dissection (ND) can be performed. Historically, a ND involved removal of all the lymph nodes as well as potentially key structures such as the spinal accessory nerve, the internal jugular vein and the sternocleidomastoid muscle. While these radical procedures are now relatively uncommon, it is now more common to remove selected lymph node levels that have been defined and preserve key structures (5).
Side-effects from surgery can be substantial, including swallowing problems, neck and shoulder problems, difficulties sleeping, fatigue and anxiety (6, 7). Post-operative complications are common following ND, occurring in 50-100% of patients (8–10). Early complications can include shoulder pain and infection. Late complications may not appear until three months post-treatment and can continue to present over five years (11). These complications include shoulder movement dysfunction, speech, swallowing and musculoskeletal problems such as cervical contracture and muscle wastage (11). Shoulder dysfunction is particularly evident where injury to the accessory nerve occurs during surgery (12). Post-operative psychosocial complications are also common, predominantly being fatigue, anxiety, depression, sleep disturbance and social isolation. Sequelae of shoulder dysfunction and psychosocial complications are strongly associated with reduced return to work. Up to 50% of patients ceasing working due to shoulder disability alone (10, 13).
There is currently no national standard best practice for effective rehabilitation following HNC treatment which involved ND. The 2016 National Institute for Health and Care Excellence (NICE) Clinical Guideline on the management of HNC (8) recommended clinicians “consider progressive resistance training for people with impaired shoulder function, as soon as possible after ND”. The review noted that this evidence was from small trials with a high risk of bias. As such, physiotherapy practice varies across the UK. Rehabilitation in the form of physiotherapy is not routinely available to some patients with HNC, in either in-patient or outpatient settings and when it is offered, it is often not evidence-based (14). Furthermore there remains a gap in knowledge on how to rehabilitate patient’s wider side-effects following surgery for HNC such as fatigue, anxiety, poor sleep and return to work.
There is limited research on whether rehabilitation interventions such as physiotherapy may improve shoulder or neck function, quality of life or reduce complications neck dissection for HNC. Given the health challenge which people following neck cancer for HNC face post-operatively, testing rehabilitation interventions to improve clinical outcomes is therefore valuable. Understanding how feasible it would be to recruit and retain participants and whether a rehabilitation intervention is acceptable and can be delivered are key trail design uncertainties which require to be answered to determine whether a full-trial is appropriate. Given this uncertainty, the aim of this study was to evaluate whether it was feasible to conduct a randomised controlled trial (RCT) to assess the effectiveness of a rehabilitation intervention in improving pain, function and health-related quality of life following ND after HNC.
Methods
Study design
A full protocol has been published previously (15). The methods and results of the qualitative sub-study associated with this trial have been previously reported (16).
This study has been reported in accordance with the CONSORT extension for pilot and feasibility studies reporting checklist (17).
This was a two-arm, open, pragmatic, parallel, multicentre, randomised controlled feasibility trial. Participants were recruited from two UK National Health Service (NHS) hospitals by the clinical team once they had been listed for ND surgery for HNC. Recruitment occurred between January 2020 to June 2021. Interventions were delivered in physiotherapy departments within these hospitals.
Study objectives
We aimed to determine:
1. Recruitment and retention rates from study participants across sites.
2. Potential risks of intervention contamination.
3. Feasibility and acceptability of the intervention from patient and physiotherapist perspectives.
4. Sample size calculation for a definitive trial.
5. Wider experiences and perceptions of the study design from a patient and physiotherapist perspective.
Objective 5 has been previously reported in a qualitative sub-study paper (16).
Participant eligibility
Participants were eligible if they were adults who had HNC which involved ND as part of their care; were willing to attend the physiotherapy outpatient department (if randomised to the experimental arm), and provided they gave written informed consent. We excluded people whose treatment was palliative (expected survival six months or less), those with a pre-existing, long-term neurological disease affecting the shoulder, for example, hemiplegia and people with cognitive impairment (defined as an Abbreviated Mental Test Score of seven or less) (18). Consented participants were randomised post-surgery.
Study treatments
Usual care group
Usual care was received by both control and experimental intervention groups. This consisted of standard NHS recovery and rehabilitation following NC for HNC including of simple range of motion (ROM) exercises for face, neck and shoulder, respiratory care to promote sputum clearance, breathing control and exercise tolerance, education on body positioning, oral health to reduce food pocketing and pain management advice. All participants on discharge from the in-patient setting received a booklet providing advice on postoperative self-management strategies including exercise, pain management, return to work and activities of daily living. Reflecting usual care, those allocated to the usual care group, once discharged from hospital, were not routinely referred to physiotherapy. This reflects usual practice in the UK NHS service, allowing the design the compare how a different rehabilitation approach (experimental intervention) compares to current service delivery.
Experimental group
Participants randomised to this group received the same in-patient rehabilitation programme as participants in the usual care group PLUS an individualised rehabilitation programme. As described in full previously (19), this was delivered by a physiotherapist trained in the experimental intervention in an outpatient setting. This was delivered either face-to-face in hospital or virtually. In brief, the intervention permitted physiotherapists to prescribe treatments to address modifiable physical and psychosocial factors associated with poor recovery following HNC surgery. These could include: muscle weakness, limited ROM, reduced sensation, pain and fear avoidance beliefs. Programmes were individualised to contain one, several or all treatment options, dependent on participant’s needs. Participants were provided with a home exercise programme to supplement face-to-face sessions.
The experimental intervention could be delivered over a maximum of six sessions during a six-month period. The first session was aimed to occur within 14 days of surgery. The initial session was up-to 60 minutes in duration with subsequent sessions up to 45 minutes. The physiotherapist, in collaboration with the participant, agreed the spacing of sessions based on need depending on clinical presentation, participant preference and symptoms during adjunctive treatments which may impact on require or capacity to participate in the rehabilitation sessions.
Data collection
Baseline data were collected prior to randomisation, once consent had been obtained.
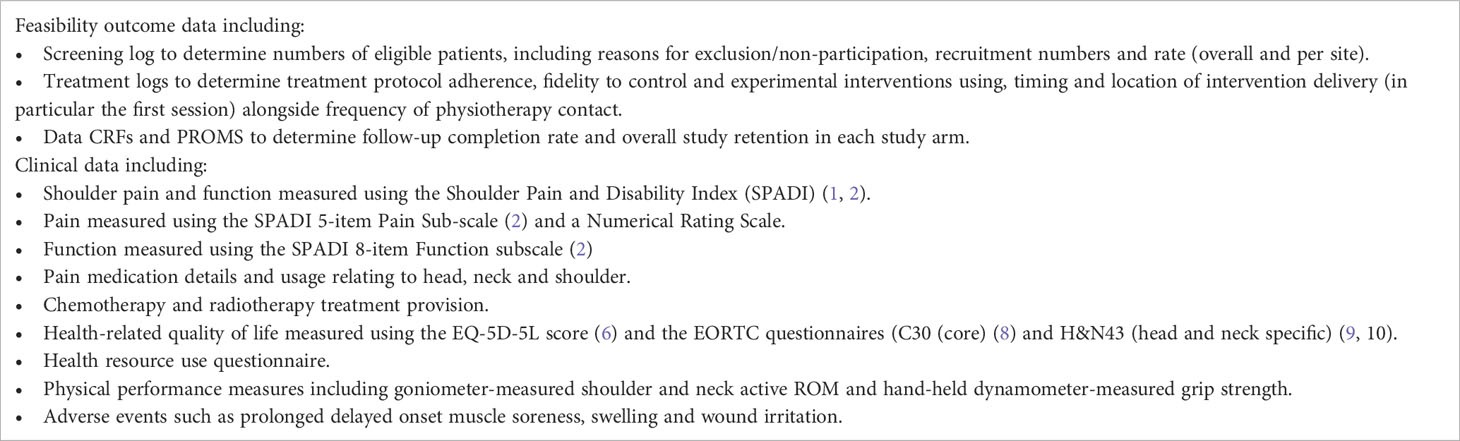
Data were clinical and participant-reported and collected using questionnaires at baseline and six months post-randomisation (primary end-point) during routine clinical appointments. Data were also collected for those participants who reach 12-month follow-up during the data collection phase. Data collected is summarised in Table 1. The clinical outcome data collected were included for three reasons: (1) to ensure that we were able to assess completion rate and overall study retention for the outcome measures used; (2) to provide the parameters to inform the sample size calculation for a definitive trial, and; (3) to offer a ‘signal’ of treatment efficacy which may infer promise the treatment may be beneficial, offering additional justification for the need for a definitive trial.
Randomisation, blinding and allocation concealment
Random allocation was 1:1. Randomisation was performed using a centralised computer randomisation programme provided by Oxford Clinical Trials Research Unit (OCTRU). Research nurses and physiotherapists at recruiting centres assigned participants by accessing the online randomisation programme to adopt a concealed allocation approach. Allocation was based on minimisation, stratified by hospital site and spinal accessory nerve sacrifice.
Due to the nature of the intervention, masking participants or the teams providing interventions was not possible. Investigators taking the clinical measurements were blinded to the intervention.
Sample size
We originally planned to recruit 60 participants, based on Whitehead et al. (20) and Teare et al’s recommendation (21). This assumed a 10% drop-out. Based on our 2017 data, this was considered realistic from two participating sites where approximately 160 potentially eligible participants were identified in that year. However, recruitment was significantly impacted by the COVID-19 pandemic where both clinical and research activity was halted at intervals during the study period.
Data analysis methods and progression criteria
To assess the trial feasibility, we calculated the rate of eligible participants who consented to be included in the trial, trial recruitment rate and retention to six months. The flow of participants through the study from identification to screening and then to follow-up was summarised using a CONSORT diagram (17). Availability of data at each follow-up time point were summarised. The baseline comparability of the two intervention groups in terms of minimisation factors and baseline characteristics are described as proportions for categorical variables, and as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables depending on the distribution. The number of withdrawals, protocol deviations, losses to follow-up, deaths, adverse events and details of treatment received were summarised by treatment group.
We analysed the clinical outcomes to explore whether there was a ‘signal’ of efficacy for the experimental intervention for the shoulder pain and function measured using the Shoulder Pain and Disability Index (SPADI) (22, 23) and EORTC questionnaires (C30 (core) and H&N43 (head and neck specific) (24, 25) since these assessed the key domains of pain, function and HRQoL. These were analysed on an intention-to-treat (ITT) basis. Treatment differences and 95% confidence intervals (CI) are reported throughout SPADI and EORTC QLQ C30 and H&N43 items were summarised using with mean and SDs presented by treatment arms. A linear model adjusting for baseline factors only was used to estimate the treatment differences between the two arms at the six month follow-up time-point. Due to only a small number of participants reached the 12-month follow-up time-point, the treatment differences calculated at 12 months were only exploratory. No p-values are reported as the study was not powered to test for differences between clinical or patient-reported outcomes.
To determine whether this trial could progress onto a phase III definitive trial, we used pre-specified traffic light stop-amend-go progression criteria (19) and interpretation from the qualitative aspects of the study (16). This was reviewed by the Trial Oversight Committee (TOC) to provide a recommendation on the outcome of feasibility principally based on the results of the progression criteria.
Study monitoring
A TOC was appointed to independently review data on safety, protocol adherence and trial recruitment.
Results
Patient characteristics and treatment
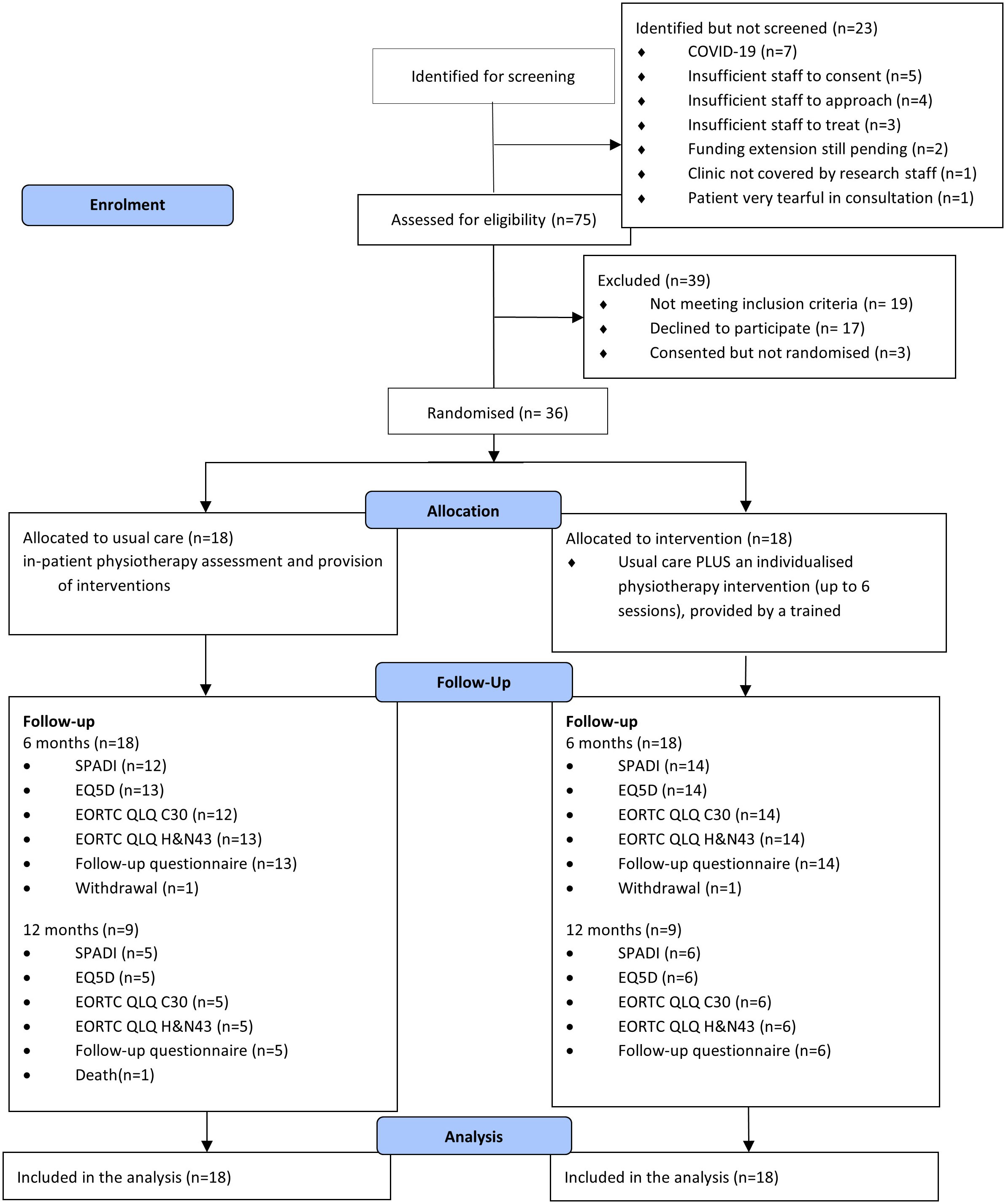
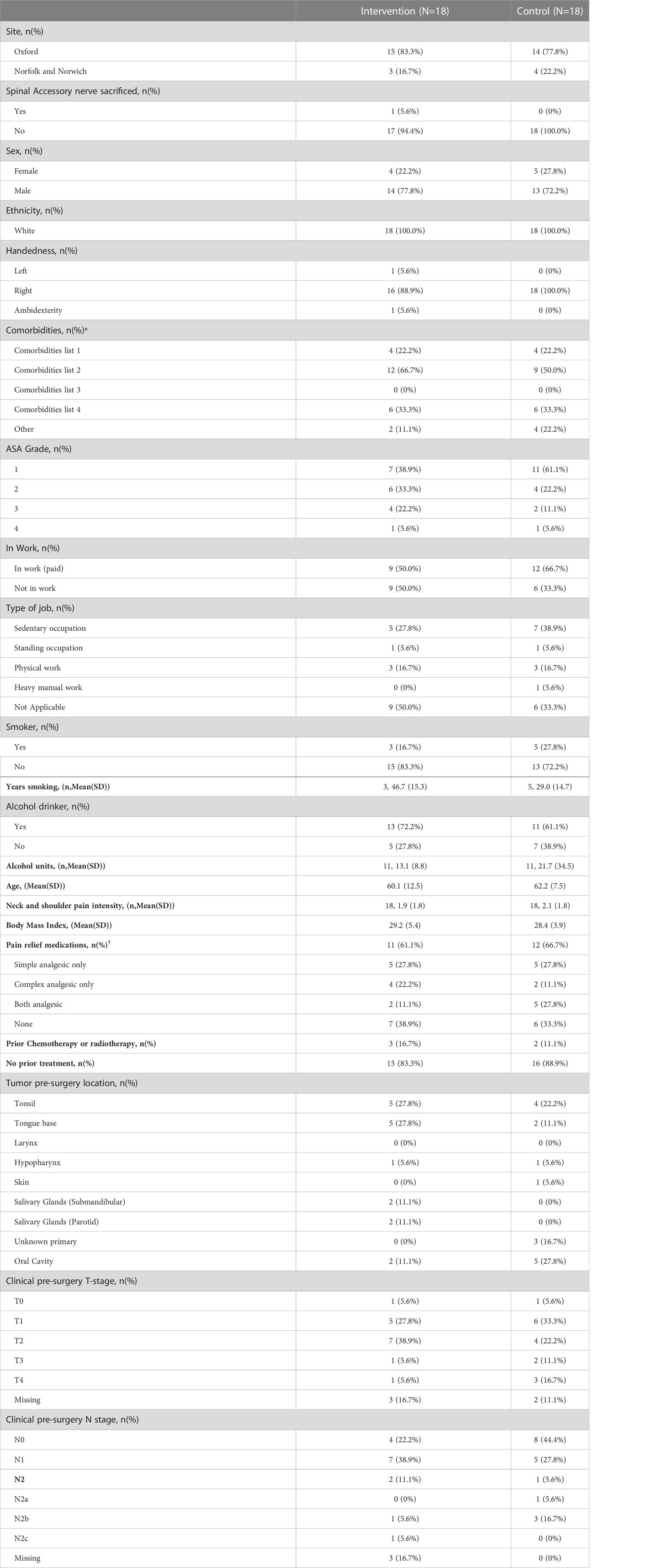
Thirty-six participants were recruited. This is summarised in Figure 1. The complete list of baseline characteristics in both experimental intervention and control groups is presented in Table 2.
Twenty-seven (75%) males and nine (25%) female participants were recruited with a mean age of 61 years (SD: 10.2). Twenty-one (58%) participants were in employment at the time of recruitment. Sixteen participants (44%) had tumours involving the oropharynx, tonsil and tongue base. Twenty-two (61%) participants had T1/2 tumours. Eight (22%) had undergone neck treatment in the preceding six months (surgery or radiotherapy). Five participants had prior chemotherapy or radiotherapy, (three intervention group; two control group).
The characteristics of surgical intervention are summarised in Table 3. The time to randomisation following ND was similar in both groups: experimental group mean 1.6 days (SD: 0.6) and the control group mean 1.9 days (SD: 1.5).
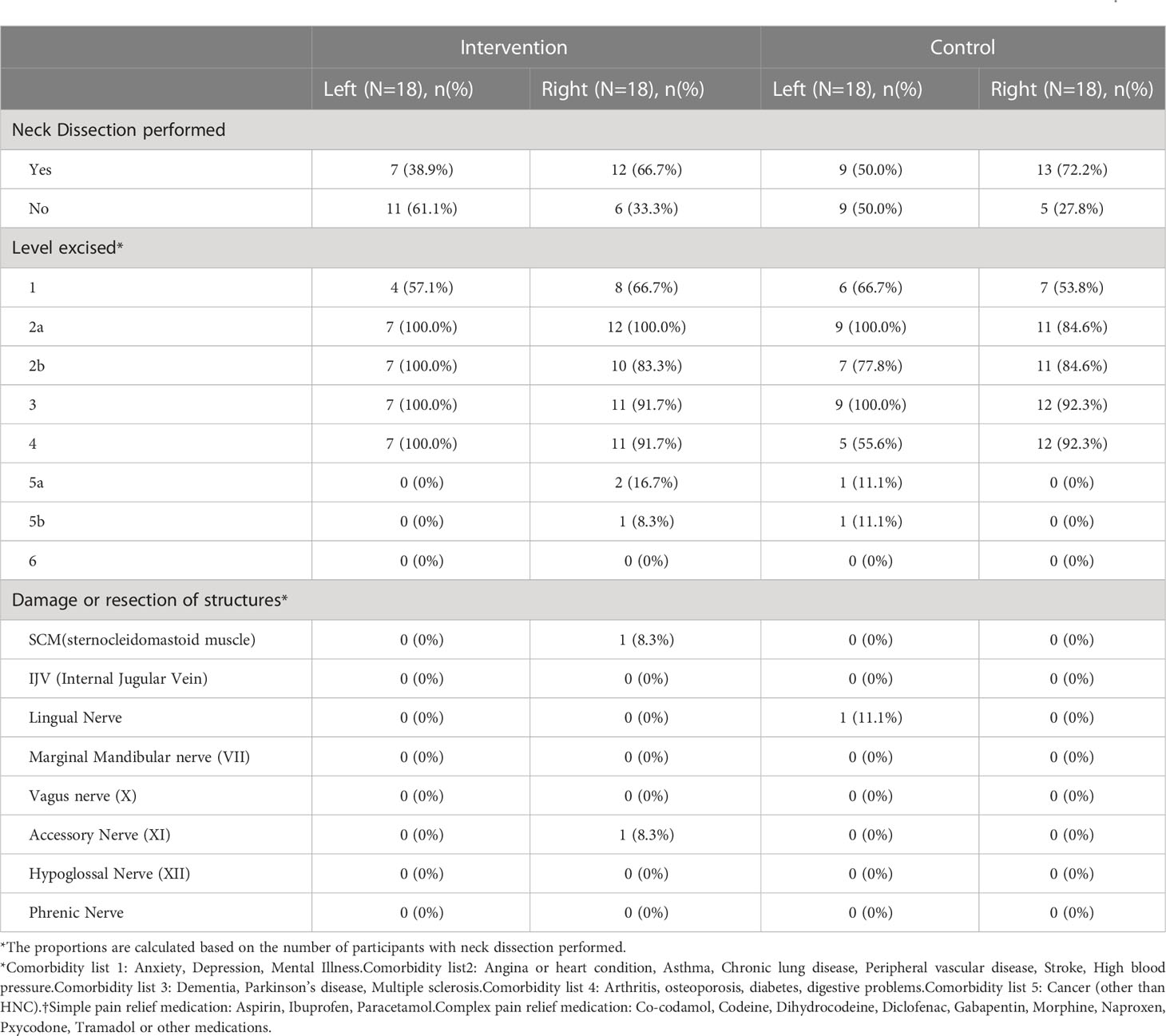
Table 3 Surgical characteristics of the cohort, illustrating neck dissections performed, levels dissected and damage/resection of key structures.
In both the experimental intervention and control groups, pathological lymph nodes were predominantly in level 2a/b. The Supplementary Table 1 illustrates the levels involved in both groups. Patients in both groups were in hospital for a similar number of days; experimental group median 4.0 days and the control group median 5.0 days.
Feasibility outcomes
Recruitment and retention
The trial identified 98 potential participants. However, due to COVID-19 pandemic, 23 (23.4%) identified potential participants did not complete the screening process. Out of the 75 screened participants, 56 (75%) were eligible, of which 39 (70%) consented to the trial. Due to COVID-19 or participants having died between consent and randomisation, three consented participants were excluded. Therefore 36 participants were successfully recruited. The trial was able to actively recruit for 18 months across two sites. The recruitment rate was one recruitment per site, per actively recruiting month, accounting for study pauses due to the COVID-19 pandemic.
The most common reason for potential participants being identified but not screened were: no further approach due to COVID-19 (30%), not followed-up due to insufficient staffing to approach (22%) or consent (17%). For those screened but ineligible, reasons for ineligibility were due to ND not planned as part of participant care (63%), follow-up by the participant being difficult due to not having access to the internet (21%). For those eligible but declined, the most common reason was participants not interested in taking part of research (41%) and distance of travel for follow-up (24%). The full list of reasons is included in Supplementary Table 2.
Of the 36 randomised participants, 26 completed the compulsory six months follow-up case report forms (CRFs). The retention rate to six months was 72% (95% CI: 58% to 87%).
Study intervention fidelity
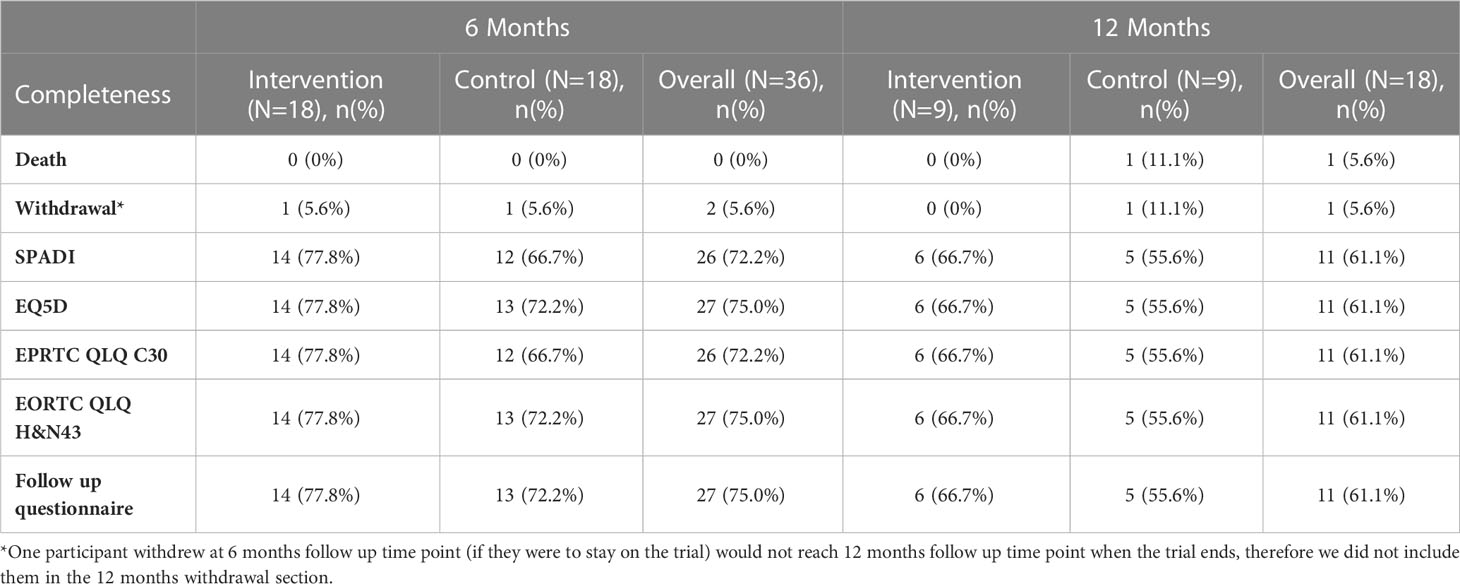
Table 4 shows the completion rates at six- and 12-month follow-up for key secondary outcomes. Participants in both treatment arms attended in-patient assessment and treatment programme before they were discharged from hospital. Participants in the intervention group attended a median of two sessions (IQR: 1.0, 3.8), while those in the control group attended a median of 2.5 sessions (IQR: 1.0, 4.8). Figure 2 illustrates the range of rehabilitation interventions prescribed during these sessions.
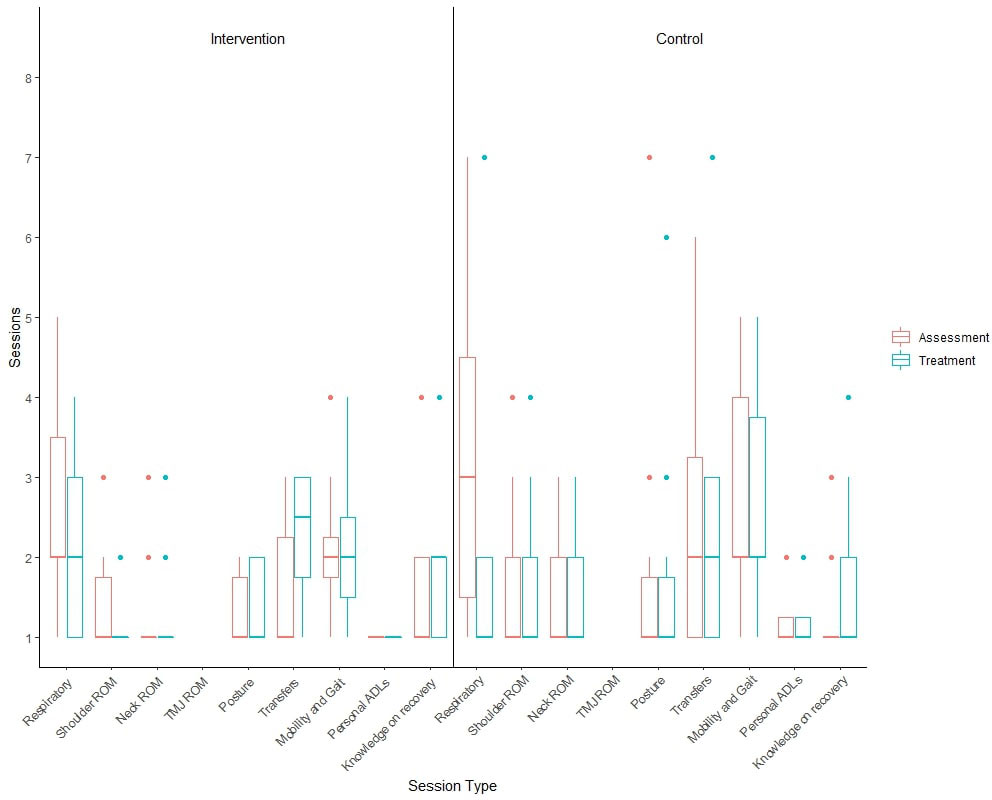
Figure 2 Boxplot illustrating the number of GRRAND sessions attended by participants for each of the in-patient programme.
Participants randomised to the intervention arm received their first post-discharge physiotherapy session a mean of 9.6 days (SD: 4.9) from in-patient discharge. The number of sessions received by participants is presented in Supplementary Table 3. Due to the COVID-19 pandemic, participants could receive these intervention sessions either in hospital or virtually. The location and range of interventions is presented in Supplementary Table 4.
There were two withdrawals, one from the control arm and one from the experimental intervention arm. Both participants who withdrew did not provide reasons of their withdrawal. The participant in the experimental intervention arm withdrew before receiving any intervention. Accordingly, overall retention in the trial to six months was 92%.
Data response rate
Due to COVID-19, many participants were not able to attend clinic and therefore did not have their physical performance measured at baseline and during follow-up visits. In the experimental intervention group, 14 had baseline measurements recorded, but at six and 12 months this had reduced to four and one participant respectively. In the control arm, 13 had baseline measurements. This reduced to seven and three participants at six and 12 months. The return rate is shown in Table 4. Of note, 16 participants reached the 12-month time-point. In summary, 72% to 75% of six-month PROMs data, dependent on questionnaire, were completed and returned.
Clinical and patient-reported outcomes
The SPADI score ranges from 0 to 100, where 0 indicating the best and 100 indicating the worst shoulder function (22, 23). The summary statistics of SPADI scores at different time-points for the two treatment groups is displayed in Supplementary Table 5. There was a trend for the observed treatment differences in SPADI at six months to be in-favour of the intervention arm. The total SPADI score was a mean of -9.37 (95% CI: -20.66 to 1.93), whilst sub-scores were a mean of -0.09 (95% CI:-13.4 to 13.2) for pain and -13.56 (95%CI: -25.52, -1.60) for disability.
The results of the EORTC QLQ C30 and H&N43 (24, 25) are presented in Supplementary Table 6. In summary, those allocated to the experimental intervention demonstrated higher HRQoL scores across the domains compared to control group participants. This did not reach a statistically significant threshold as expected for this underpowered analysis.
Progression criteria
Table 5 presents the findings of the progression criteria analysis. As this indicates, the study design reached thresholds for feasibility for five of the six criteria. The study reached ‘green’ thresholds for consent where 70% of eligibility participants consented, intervention fidelity, where 78% of participants discharged completed the intervention sessions, contamination there no participants in the control group received the GRRAND intervention and retention where only 8% of participants were lost to follow-up. One criterion was categorised as ‘amber’ where 28% of participants demonstrated missing data in the questionnaire. Recruitment was the single criteria which was not met, with a ‘red’ outcome where only 36 from the originally 50 participants were recorded within 18 months. However, as stated earlier, this study was conducted throughout the COVID-19 pandemic where site activity was interrupted. Accordingly interpreting this criterion is challenging. The TOC on 20th June 2022 were presented with the findings. They recommended, with modification, the trial was feasible.
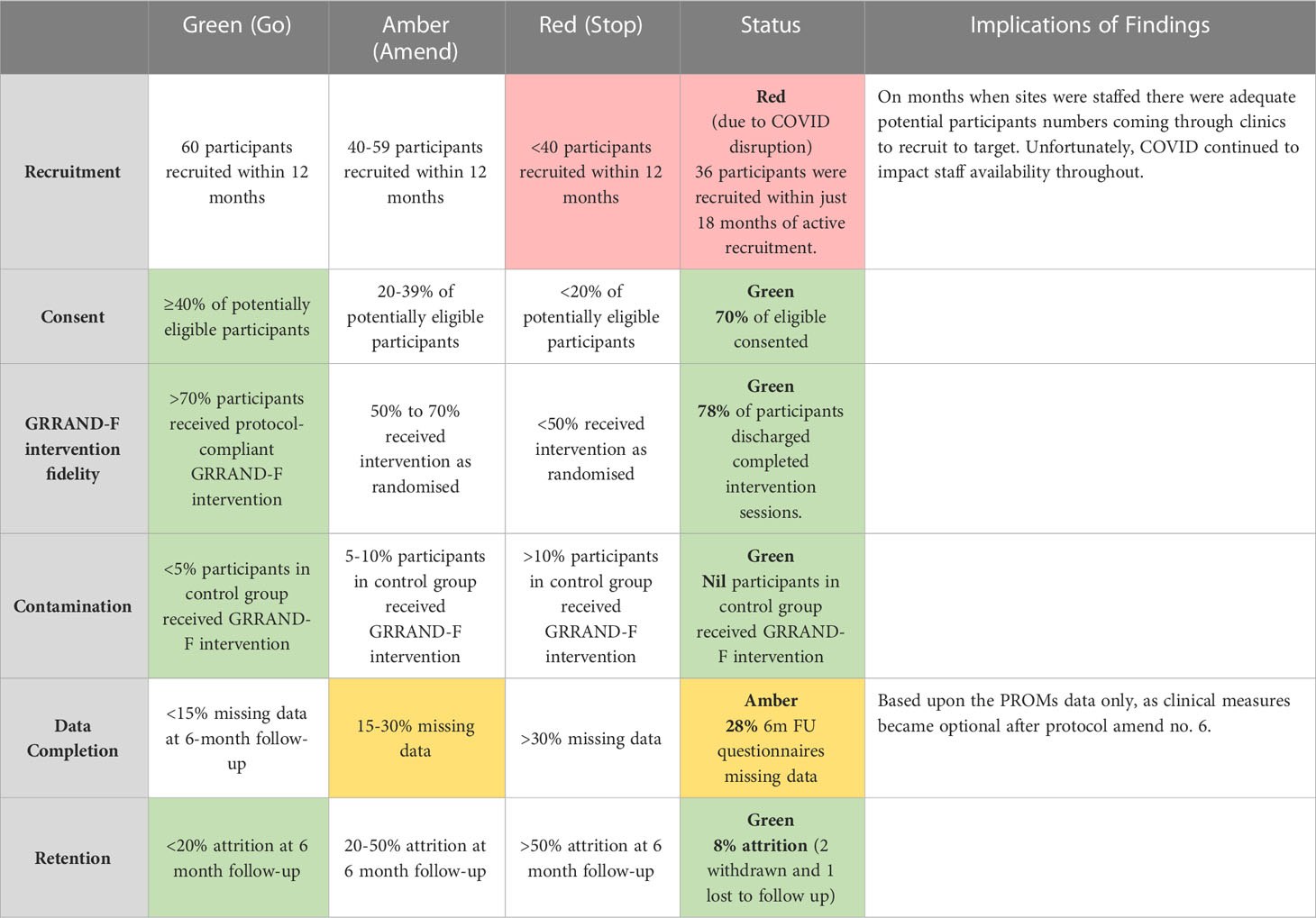
Table 5 A summary of the results of the progression criteria based on the traffic-light stop-go criteria approach.
Discussion
The findings of this study indicate that, whilst modifications may improve its efficiency, this proposed trial design for a pragmatic, multicentre RCT investigating the effectiveness and cost-effectiveness of a rehabilitation intervention on pain, function and HRQoL following ND for HNC is feasible. The findings also provide a signal that this intervention is potentially efficacious, certainly over a short-term, and at a level which is clinically significant. The impact of the COVID-19 pandemic on NHS site activities reduced the ability to test the design across the intended number of sites. Nonetheless, where tested, there were consistent data to indicate that both the approaches proposed to screen and consent participants in addition to collecting outcome data are suitable. Furthermore physiotherapists delivering the interventions demonstrated fidelity to the GRRAND intervention. As highlighted by the a priori progression criteria, there is strong evidence that this trial design would be feasible for the intervention to be tested in a full trial.
The trial design indicated modification to two key aspects. Firstly, whilst the return of CRFs was within intended expectations, the return of intervention exercises diaries was low (50%). The use of exercise diaries in this trial design was to ascertain adherence levels between rehabilitation sessions. Whilst the embedded qualitative study indicated adherence was good from study participants (16), the numerical data which we anticipated could accompany this, was lacking. Exercise diaries and assessment of rehabilitation adherence has been acknowledged as a major challenges in other trials (26). Given this, and the pragmatic nature of the trial design, we propose a ‘light-touch’ assessment to exercise diary data where participants in the full trial may indicate adherence and compliance to the intervention through attendance in physiotherapy, which was well-collected in the health-resource use questionnaire. Secondly, as a result of the COVID-19 pandemic, a number of participants attended follow-up appointments remotely. It is anticipated that this may continue longer-term with online consultations in the future (27). The change from face-to-face to remote consultations resulted in an inability to collect physical function measures, notably joint range of motion and handheld dynamometry assessments. As the SPADI includes elements of physical performance and capability within its subsections, it is proposed that assessing physical function through such a PROM rather than physical function may not only improve the flexibility of collecting this domain, as not reliant on face-to-face consultations, but may be more time and cost-effective by collecting via post or online rather than requiring transport and associated costs. Furthermore, as a number of patients receive their surgery within tertiary centres in the NHS for HNC ND, the travel to these specialist centres can be considerable. Accordingly, collecting such data remotely may reduce the burden on these patients, particularly during a potentially stressful healthcare episode following ND.
This study presented with a number of major successes. Firstly, there was clear support from participants for the design and conduct of this study. This is evidenced with our high conversion rate between eligible participant approach to consent (70%). Furthermore, the participant attitudes towards the GRRAND intervention, as reported in (16), further augment this notion. Secondly, given the challenges in managing site opening and research conduct during the COVID-19 pandemic, the ability to undertake this across two NHS hospitals was a major success. However, as a weakness, due to this reason we were unable to open the planned further two sites and recruited 24 fewer participants than originally planned (19). These sites would have offered further learning on the study design to supplementary the findings reported in this study. Finally, as a result of the pandemic, the planned face-to-face data collection processes were not implemented throughout. However, this opportunity meant we were agile and able to make protocol amendments to ensure not only the intervention delivery could be delivered virtually, but also data collection could be modified for this eventually. The benefit of this meant we now have the knowledge on how to adopt both face-to-face or virtual approaches for intervention delivery and data collection mechanisms to the benefit of designing a full trial. Finally, the result indicate heterogeneity in pre-operative status and adjunctive treatments across the cohort. Whilst there is a risk that this differed between the groups for this small sample, it is anticipated that equivalence would be achieved with a larger cohort. Consideration should be made on whether these are important prognostic factors to warrant inclusion as part of the minimisation randomisation procedure for a full-trial.
During the design of this feasibility study, we did not stipulate a proposed primary outcome measure for a full trial. Armed with the evidence from this feasibility study, based on high data returns and the ability to collect multiple domains from the same PROM, the SPADI would appear to be an appropriate instrument. This has been endorsed by our patient and public involvement in research members. Based on this, the estimated sample size for a future trial with SPADI as primary outcome is 416, assuming participants are randomised 1:1 using a clinically meaningful difference of eight points in the SPADI from the GRASP trial (26), SD of 21.7 scores, 90% power, 5% significance level and 20% attrition rate. Alternatively the estimated sample sizes for a future trial with EORTC C-30 global health status as primary outcome are 588 or 376, based on the estimated minimal difference from a group study which investigated six EORTC trials (28). This information will form the basis of our plans for the full-trial to test the GRRAND intervention.
Conclusions
The findings from this feasibility study indicate the proposed trial design for a pragmatic, multicentre RCT of testing a rehabilitation intervention following ND for HNC was assessed as feasible with modifications. A full-trial, based on these findings, can now be designed to better understand whether this proposed rehabilitation interventions is clinically and cost-effective for this population. This remains important as given the increase in HNC prevalence in younger patients, these individuals increasingly require an evidence-based rehabilitation programme to reduce morbidly and improve functional performance after this surgical procedure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by South Central - Oxford B Research Ethics Committee. IRAS project ID: 268845. The patients/participants provided their written informed consent to participate in this study.
Author contributions
The author list comprises the study group responsible for administration and execution of the trial. TS, SD, SL, and SW were responsible for conception, design and execution of the study; AG, AM, and VG, were responsible for administrative management of the study; BF was responsible for qualitative research; SD, TL and MCJ were responsible for statistics. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Institute for Health Research (NIHR) Research for Patient Benefit grant (PB-PG-1217- 20031). The study was supported by the Oxford Clinical Trials Research Unit, University of Oxford and the National Institute for Health and Care Research Oxford Biomedical Research Centre. SEL was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. Trial Sponsor: Oxford University Hospitals NHS Foundation Trust (OUH Research & Development, Joint Research Office, 2nd Floor, OUH Cowley, Unipart Business Centre, Garsington Road, Oxford, OX4 2PG. Email:T1VILlNwb25zb3JzaGlwQG94bmV0Lm5ocy51aw==
Acknowledgments
GRRAND-F Collaborators: Miss Harriet Finze (Oxford University Hospital NHS Foundation Trust), Mr Ray Derkacz (Patient and Public Member), Sites: Principal Investigators: Mr Richard Sisson, Norfolk and Norwich University Hospitals NHS Foundation Trust; Mr Stuart Winter, Oxford University Hospital NHS Foundation Trust; Ms Emma King, Poole Hospital NHS Foundation Trust; Professor Hisham Mehanna, University Hospitals Birmingham NHS Foundation Trust. Trial Steering Committee: Professor Matthew Maddocks (Kings College London), Professor Vinidh Paleri (The Royal Marsden Hospital NHS Foundation Trust, London).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1110500/full#supplementary-material
References
1. (IARC). WHOIAfRoC. GLOBOCAN 2022: estimated cancer incidence, mortality and prevalence worldwide. (2022). Available at: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates.
2. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers (2020) 6(1):92. doi: 10.1038/s41572-020-00224-3
3. Schache AG, Powell NG, Cuschieri KS, Robinson M, Leary S, Mehanna H, et al. HPV–related oropharyngeal cancer in the united kingdom: An evolution in understanding of disease etiology. Cancer Res (2016) 76(22):6598–606. doi: 10.1158/0008-5472.CAN-16-0633
4. NCIU. Profile of head and neck cancers in England: Incidence, mortality and survival. National Cancer Inteligence Unit (2010). Available at: www.ncin.org.uk.
5. Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg (2008) 134(5):536–8. doi: 10.1001/archotol.134.5.536
6. Roerink SH, Coolen L, Schenning ME, Husson O, Smit JW, Marres HA, et al. High prevalence of self–reported shoulder complaints after thyroid carcinoma surgery. Head Neck (2017) 39(2):260–8. doi: 10.1002/hed.24579
7. Rogers SN, Ferlito A, Pellitteri PK, Shaha AR, Rinaldo A. Quality of life following neck dissections. Acta Otolaryngol (2004) 124(3):231–6. doi: 10.1080/00016480310015317
8. NICE. National Institute for Healthcare and Excellence (NICE). Cancer of the upper aerodigestive tract: Assessment and management in people aged 16 and over. National Institute for Health and Care Excellence (2016). Available at: https://www.nice.org.uk/guidance/ng36.
9. Cappiello J, Piazza C, Giudice M, De Maria G, Nicolai P. Shoulder disability after different selective neck dissections (levels II–IV versus levels II–v): A comparative study. Laryngoscope (2005) 115(2):259–63. doi: 10.1097/01.mlg.0000154729.31281.da
10. Shone GR, Yardley MP. An audit into the incidence of handicap after unilateral radical neck dissection. J Laryngol Otol (1991) 105(9):760–2. doi: 10.1017/S0022215100117232
11. Chan JY, Wong ST, Chan RC, Wei WI. Shoulder dysfunction after selective neck dissection in recurrent nasopharyngeal carcinoma. Otolaryngol Head Neck Surg (2015) 153(3):379–84. doi: 10.1177/0194599815590589
12. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Scapular muscle exercises following neck dissection surgery for head and neck cancer: A comparative electromyographic study. Phys Ther (2013) 93(6):786–97. doi: 10.2522/ptj.20120385
13. Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck (1999) 21(6):531–7. doi: 10.1002/(SICI)1097-0347(199909)21:6<531::AID-HED6>3.0.CO;2-M
14. Robinson M, Ward L, Mehanna H, Paleri V, Winter SC. Provision of physiotherapy rehabilitation following neck dissection in the UK. J Laryngol Otol (2018) 132(7):624–7. doi: 10.1017/S0022215118000671
15. Gallagher KK, Sacco AG, Lee JS, Taylor R, Chanowski EJ, Bradford CR, et al. Association between multimodality neck treatment and work and leisure impairment: A disease–specific measure to assess both impairment and rehabilitation after neck dissection. JAMA Otolaryngol Head Neck Surg (2015) 141(10):888–93. doi: 10.1001/jamaoto.2015.2049
16. Fordham B, Smith TO, Lamb S, Morris A, Winter SC. Patient and physiotherapist perceptions of the getting recovery right after neck dissection (GRRAND) rehabilitation intervention: A qualitative interview study embedded within a feasibility trial. BMJ Open (2022) 12(11):e064269. doi: 10.1136/bmjopen-2022-064269
17. Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ (2016), 355:i5239. doi: 10.1136/bmj.i5239
18. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing (1972) 1(4):233–8. doi: 10.1093/ageing/1.4.233
19. Gallyer V, Smith TO, Fordham B, Dutton S, Chester–Jones M, Lamb SE, et al. Getting recovery right after neck dissection (GRRAND–f): mixed–methods feasibility study to design a pragmatic randomised controlled trial protocol. BMJ Open (2021) 11(6):e045741. doi: 10.1136/bmjopen-2020-045741
20. Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res (2016) 25(3):1057–73. doi: 10.1177/0962280215588241
21. Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: A simulation study. Trials (2014) 15:264. doi: 10.1186/1745-6215-15-264
22. Roach KE, Budiman–Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res (1991) 4(4):143–9. doi: 10.1002/art.1790040403
23. Eden MM, Flores AM, Galantino ML, Spinelli BA. Recommendations for patient–reported outcome measures for head and neck cancer–related shoulder dysfunction: A systematic review. Rehabil Oncol (2014) 32(3):6–19. doi: 10.1097/01893697-201432030-00003
24. Bjordal K, Hammerlid E, Ahlner–Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: Validation of the European organization for research and treatment of cancer quality of life questionnaire–H&N35. J Clin Oncol (1999) 17(3):1008–19. doi: 10.1200/JCO.1999.17.3.1008
25. Singer S, Amdal CD, Hammerlid E, Tomaszewska IM, Castro Silva J, Mehanna H, et al. International validation of the revised European organisation for research and treatment of cancer head and neck cancer module, the EORTC QLQ–HN43: Phase IV. Head Neck (2019) 41(6):1725–37. doi: 10.1002/hed.25609
26. Hopewell S, Keene DJ, Marian IR, Dritsaki M, Heine P, Cureton L, et al. Progressive exercise compared with best practice advice, with or without corticosteroid injection, for the treatment of patients with rotator cuff disorders (GRASP): A multicentre, pragmatic, 2 × 2 factorial, randomised controlled trial. Lancet (2021) 398(10298):416–28. doi: 10.1016/S0140-6736(21)00846-1
27. Gilbert AW, Jones J, Stokes M, May CR. Factors that influence patient preferences for virtual consultations in an orthopaedic rehabilitation setting: A qualitative study. BMJ Open (2021) 11(2):e041038. doi: 10.1136/bmjopen-2020-041038
28. Musoro ZJ, Coens C, Fiteni F, Pogoda K, Cardoso F, Tribius S, et al. PCN378 – minimally important difference for interpreting EORTC QLQ–C30 scores in melanoma, breast cance and head and neck cancer patients on behalf of the EORTC breast, head and neck, melanoma and quality of life groups. Value Health (2018) 21:S78. doi: 10.1093/jncics/pkz037
Keywords: head and neck, cancer, shoulder, rehabilitation, surgery
Citation: Smith TO, Garrett A, Liu T, Morris A, Gallyer V, Fordham BA, Dutton SJ, Chester-Jones M, Lamb SE and Winter SC (2023) Getting Recovery Right After Neck Dissection (GRRAND-F): Mixed-methods feasibility study to design a pragmatic randomised controlled trial. Front. Oncol. 13:1110500. doi: 10.3389/fonc.2023.1110500
Received: 28 November 2022; Accepted: 24 February 2023;
Published: 16 March 2023.
Edited by:
Gyorgy B. Halmos, University Medical Center Groningen, NetherlandsReviewed by:
Bruce Ashford, Illawarra Shoalhaven Local Health District (ISLHD), AustraliaLisette Vd Molen, Antoni van Leeuwenhoek Hospital, Netherlands
Copyright © 2023 Smith, Garrett, Liu, Morris, Gallyer, Fordham, Dutton, Chester-Jones, Lamb and Winter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stuart Charles Winter, c3R1YXJ0LndpbnRlckBvdWgubmhzLnVr
 Toby O. Smith
Toby O. Smith Angela Garrett3
Angela Garrett3 Tianshu Liu
Tianshu Liu Bethany A. Fordham
Bethany A. Fordham Stuart Charles Winter
Stuart Charles Winter