
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 12 May 2023
Sec. Cancer Genetics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1109330
This article is part of the Research TopicCurrent Understanding of Genomic and Chromosomal Instabilities in Solid MalignanciesView all 9 articles
Background: Insulinoma is a rare type of pancreatic neuroendocrine tumor with low incidence and low-malignant features. While very few insulinomas present with malignant behaviours, such as lymph node and liver metastasis, only a few studies have focused on this field owing to the limitation of samples. Existing evidence suggests that metastatic insulinoma largely derive from non-functional pancreatic neuroendocrine tumor. However, we found a portion of metastatic insulinomas may derive from non-metastatic insulinomas and explored their clinicopathological signatures and genetic characteristics.
Methods: Four metastatic insulinoma patients with synchronous liver metastasis or lymph node metastasis at the Peking Union Medical College Hospital between October 2016 and December 2018 were enrolled, and whole exon and genome sequencing were performed on fresh frozen tissues and peripheral blood samples. Clinicopathological information and genomic sequencing results were collected and matched to explore the characteristics of the metastatic insulinomas.
Results: These four metastatic insulinoma patients underwent surgery or interventional therapy, and their blood glucose levels immediately increased and maintained within standard range after treatment. For these four patients, the proinsulin/insulin molar ratio <1 and primary tumors were all present as PDX1+, ARX-, and insulin+, which were similar to non-metastatic insulinomas. However, the liver metastasis showed PDX1+ and ARX+, insulin+. Meanwhile, genomic sequencing data showed no recurrently mutations and typical CNV patterns. However, one patient harboured the YY1 T372R mutation, a recurrently mutated gene in non-metastatic insulinomas.
Conclusions: A portion of metastatic insulinomas were largely derived from non-metastatic insulinomas in hormone secretion and ARX/PDX1 expression patterns. Meanwhile, the accumulation of ARX expression may be involved in the progression of metastatic insulinomas.
Insulinoma is a rare type of pancreatic neuroendocrine tumors (PanNETs), with a reported incidence of 1–4 cases per million persons per year (1–4). Insulinoma patients suffer from hypoglycaemia and neuropsychiatric symptoms, including sweating, confusion, amaurosis, and fainting (5, 6). Surgery is a common treatment for relieving symptoms (7), but partial pancreatectomy or enucleation of insulinoma may fail to relieve hypoglycaemia symptoms (8). The 5-year survival rate of insulinomas is approximately 97% because most insulinomas present with relatively benign biological behaviour (9). Nevertheless, very few insulinomas present with malignant behaviours, such as lymph node and liver metastasis. These patients could rely on second-line treatment (such as radiofrequency ablation and octreotide therapy for liver metastasis) to control tumor progression and hypoglycaemia symptoms. Several studies have discussed the clinical treatment of metastatic insulinomas. Enucleation is not recommended because of the potential risk of tumor metastasis; thus, thorough dissection of the primary focus and resectable liver metastasis is a better recommendation (10). In addition, some studies have suggested that tumor size may be related to malignancy, indicating that the larger tumor may have higher potential to metastasize (11).
The molecular mechanisms of metastatic insulinomas remain unclear because of their low incidence. Previous studies have suggested that metastatic insulinoma is mostly derived from NF-PanNETs, with ARX being the specific transcription factor (12). However, non-metastatic insulinomas tend to express PDX1, a specific transcription factor of islet β cells. Interestingly, a large proportion of patients have no history of non-metastatic insulinomas, while a portion of metastatic insulinoma patients have a history of NF-PanNET (8, 13). Existing evidence suggests that metastatic insulinoma may largely derive from NF-PanNETs, but this needs further validation.
PanNETs is heterogenous, and the primary tumor and metastatic foci may embrace different characteristics, such as genetic and hormone expression pattern (14). Therefore, genetic sequencing and immunohistochemical staining should be performed for both the primary tumor and metastasis. In this study, four patients with metastatic insulinoma at the Peking Union Medical College Hospital (PUMCH) were enrolled. The purpose of this study was to investigate the clinicopathological features, cell origin, and genetic characteristics of metastatic insulinomas.
Four metastatic insulinoma patients with synchronous lymph node or liver metastasis at the Puking Union Medical College Hospital (PUMCH) between October 2016 and December 2018 were enrolled. The inclusion criteria for metastatic insulinoma were as follows: 1) clinical diagnoses of insulinomas according to published criteria (15), 2) pathological diagnoses of PanNETs, and 3) PanNET metastasis to lymph nodes or distant organs, such as liver. We recorded detailed clinicopathological information, including 1) general information, such as sex, age at disease onset, and diagnosis; 2) qualitative diagnostic information, such as blood glucose and insulin levels; 3) imaging workups, including computed tomography (CT) and magnetic resonance imaging (MRI); 4) pathological information, such as tumor size, tumor staging, and immunohistochemical staining; and 5) treatments, including surgery, chemotherapy, arterial embolization, and microwave ablation. Follow-up visits were performed by phone calls, during which the patients stated their conditions.
Tissue samples were fixed in 4% paraformaldehyde (Sigma-Aldrich, Cat# 158127) overnight, dehydrated through a serial alcohol gradient, and embedded in paraffin. Before immunostaining, tissue sections were dewaxed in xylene, rehydrated using decreasing concentrations (100%, 95%, and 75%) of ethanol, and washed in phosphate buffered saline (PBS). The sections were then stained with haematoxylin and eosin (H&E).
Immunohistochemistry (IHC) for ARX (Novus, AF7068-SP), PDX1 (Abcam, ab134150), and insulin (Cell Signalling Technology, 4590S) was performed on insulinoma tissue sections. First, the antigens were unmasked by microwaving in 10 mmol/L citrate buffer, pH 9.0, then endogenous peroxide activity was blocked with 3% peroxidase, and the slides were incubated with 10% fetal bovine serum (FBS) to prevent nonspecific binding. The slides were then incubated with primary antibodies at an optimal dilution (ARX 1:20, PDX1 1:400, and insulin 1:100) and appropriate secondary antibodies. The 3,3’-diaminobenzidine (DAB) was used to detect antibody staining. After H&E and immunohistochemical staining, the tissue sections were dehydrated using increasing concentrations (95% and 100%) of ethanol and xylene.
For ARX and PDX1, intermediate/strong nuclear staining > 10% or weak nuclear staining >50% of cells was considered positive staining. For insulin, positive staining was defined as cytoplasmic staining >10% of cells. Meanwhile, pancreatic normal islets were used as both positive and negative controls (12, 16).
Whole genome sequencing (WGS) and whole exon sequencing (WES) libraries were constructed for fresh frozen tissues and peripheral blood samples for patients MI1 and MI2. The constructed libraries were sequenced based on BGISEQ-500 sequencing platforms, the following process including alignment to the human reference 19 genome (hg19), sorting, and duplicate marking of high-quality sequencing reads were analysed through DRAGEN software as previously described (17). The genomic sequencing data of two patients (MI3 and MI4) were sequenced and analysed in our previous study, and we downloaded the data from the China National GeneBank Nucleotide Sequence Archive with accession number CNP0000383 (https://db.cngb.org/cnsa/) (18). Then, the downloaded files and PUMCH datasets were combined for subsequent analyses.
Somatic mutations were identified for single nucleotide variants (SNVs) by MuTect (http://www.broadinstitute.org/cancer/cga/mutect) (19) and short insertions and deletions by Platypus (http://www.well.ox.ac.uk/platypus) (20), and annotated with Oncotator (http://www.broadinstitute.org/oncotator/) (21). The integer copy number variations (CNVs) were estimated using FACETS (22), and CNV status was determined by the threshold of >2 copies for amplification and <2 copies for deletion.
The four metastatic insulinoma patients all suffered from unconsciousness, and were diagnosed with insulinoma by imaging workups and typical hypoglycemia symptoms. Two patients with synchronous liver metastasis were detected by dynamic contrast-enhanced MRI, while other two patients with lymph node metastasis were verified by pathological reports (Figure 1B). All patients received surgery and chemotherapy (such as octreotide and everolimus) after surgery, while one patient lost follow-up. Meanwhile, patients with liver metastasis also received microwave ablation and arterial embolization for metastatic foci. The baseline characteristics and clinical evolution of each patient are summarised (Figure 1A and Table 1).
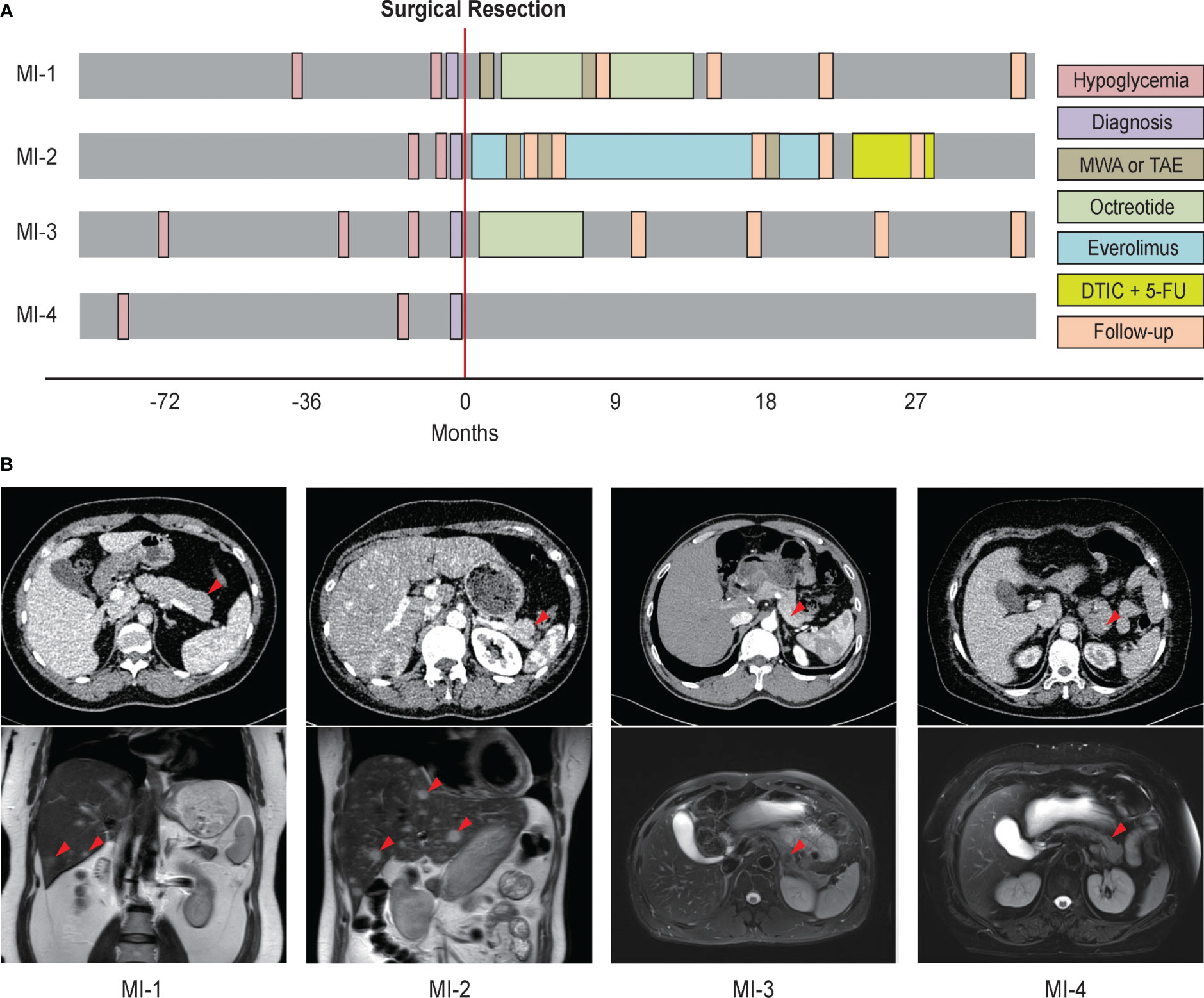
Figure 1 The imaging workups, diagnosis, and treatment of four patients with metastatic insulinoma. (A) Clinical evolution of four metastatic insulinoma patients. (B) The imaging report for primary tumors, lymph node metastasis, and liver metastasis of four metastatic insulinoma patients. MWA, microwave ablation. TAE, transcatheter arterial embolization. DTIC, dacarbazine. 5-FU, fluorouracil.
After surgical resection of pancreatic lesion, blood glucose levels of patients MI1, MI3, MI4 immediately increased and basically maintained within standard range. However, the blood glucose level of patient MI2 with synchronous liver metastasis was still lower than standard range, which immediately increased and maintained within standard range after treatment of liver metastasis, implying the insulin secretion capability of liver metastasis (Figure 2). Besides, all patients have been clinically excluded from the diagnosis of MEN-1 syndrome.
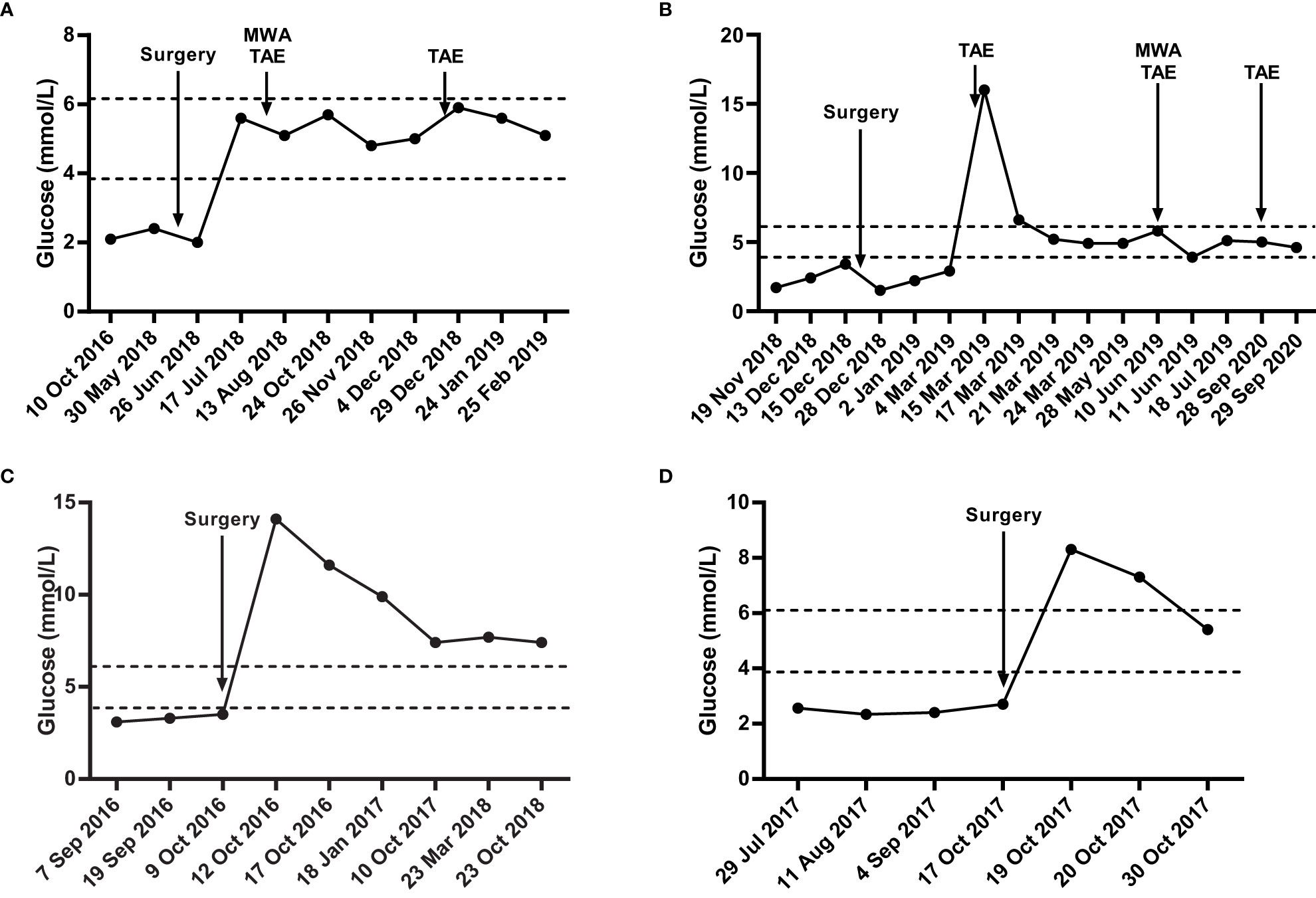
Figure 2 The blood glucose levels of four metastatic insulinoma patients. Blood glucose levels of MI1 (A), MI2 (B), MI3 (C), MI4 (D) during surgical and chemotherapy treatment. The area among the dotted lines indicates the standard range of blood glucose level (3.9 mmol/L – 6.1 mmol/L). MWA, microwave ablation. TAE, transcatheter arterial embolization.
The derivation of metastatic insulinomas is still unclear from existing studies. Previous studies have verified that metastatic insulinoma assembles NF-PanNETs from four aspects: natural history, circulating biochemical profile, gene expression, and treatment strategy (13). Therefore, we explored the clinicopathological characteristics mentioned above of the four metastatic insulinomas. In addition, we performed genomic sequencing to uncover the genetic signatures of metastatic insulinomas.
All four patients had no history of NF-PanNETs but a long-term duration of insulinomas before the final diagnosis, which could be concluded from the typical hypoglycaemia symptoms and multiple imaging examinations.
Previous studies have reported that patients with metastatic insulinomas have elevated proinsulin levels (>1000 pmol/L) and a high proinsulin/insulin molar ratio (>3), while non-metastatic insulinomas always present proinsulin levels <200 pmol/L and proinsulin/insulin molar ratio of nearly 1 (8, 23). In our study, the proinsulin and insulin levels were 347.60 pmol/L and 405.54 pmol/L for MI1, 195.85 pmol/L and 273.18 pmol/L for MI2, 160.49 pmol/L and 154.66 pmol/L for MI3, and 22.36 pmol/L and 130.28 pmol/L for MI4. The proinsulin/insulin molar ratios were 0.96, 0.89, 1.04, and 0.17, respectively.
Previous studies have shown that non-metastatic insulinomas exclusively expresses PDX1, whereas liver metastatic foci and metastatic insulinoma are more likely to express ARX (12, 16). In this study, all the primary tumors were PDX1+, ARX-, and insulin+, while liver metastasis foci of patients MI1 and MI2 both showed PDX1+, ARX+, and insulin+ (Figure 3).
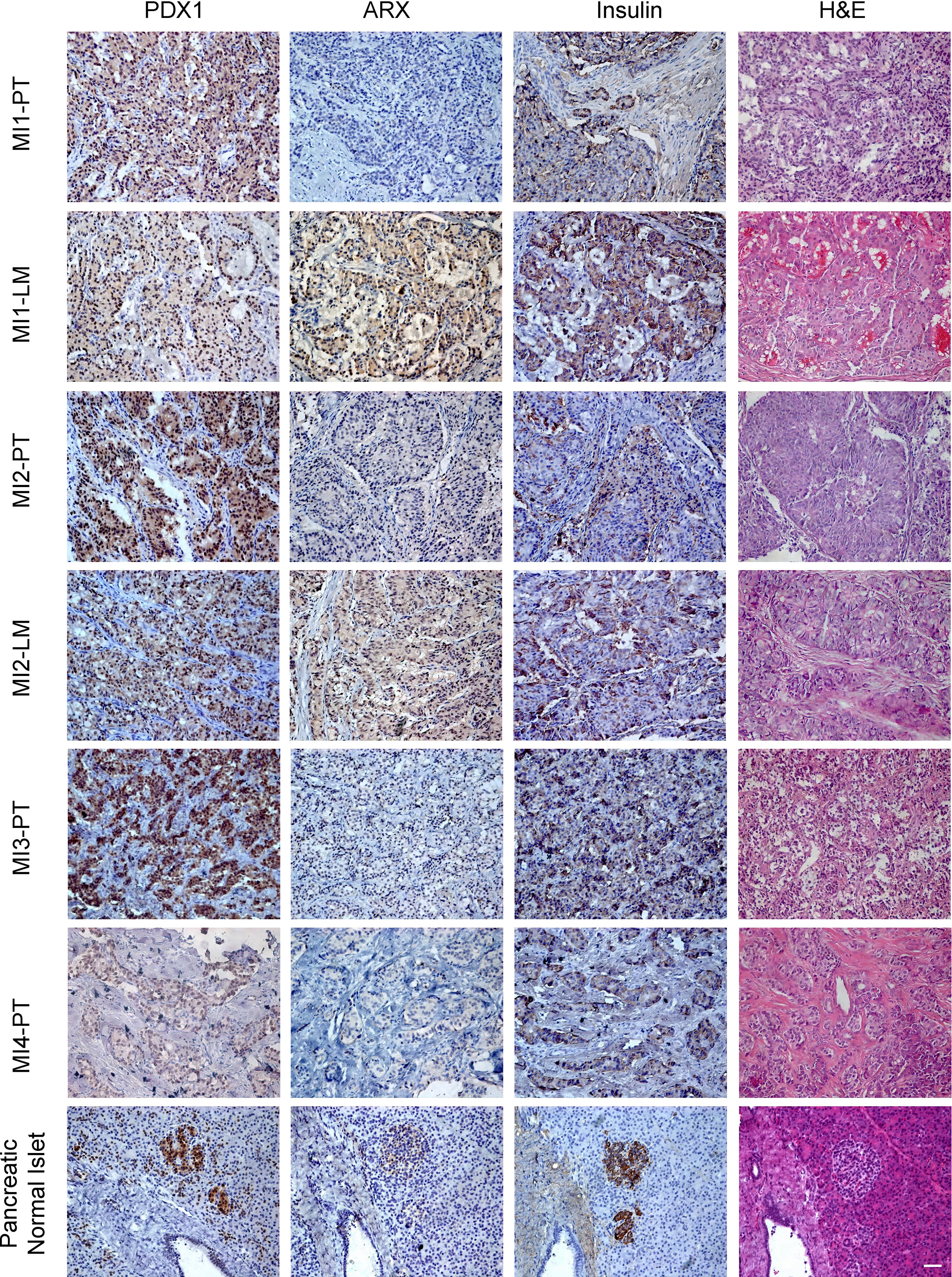
Figure 3 The H&E and immunohistochemical staining of ARX, PDX1, and Insulin of primary tumors and liver metastasis. PT, primary tumor. LM, liver metastasis. Scale bar, 100μm. H&E, haematoxylin and eosin staining.
We performed WES and WGS on the primary tumors of the four patients and liver metastasis of MI2 to explore somatic gene mutations and CNVs. Genomic sequencing data showed that gene mutations previously detected in NF-PanNETs (such as MEN1, DAXX, and ATRX, etc) were not found in these four patients. However, MI4 harboured the YY1 T372R mutation, which is a recurrently mutated gene in non-metastatic insulinomas. The non-silent mutations of these four patients were summarized in Table 2. However, it seems that there were no typical CNV pattern for these four patients. The typical CNV patterns of NF-PanNETs [chromosome loss of 1, 2, 3, 6, 8, 10, 11, 15, 16, 21, and 22, and chromosome gain of 4, 5, 7, 9, 12, 13, 14, 17, 18, 19, and 20 (18, 24)] were not found in these metastatic insulinomas (Figure 4).
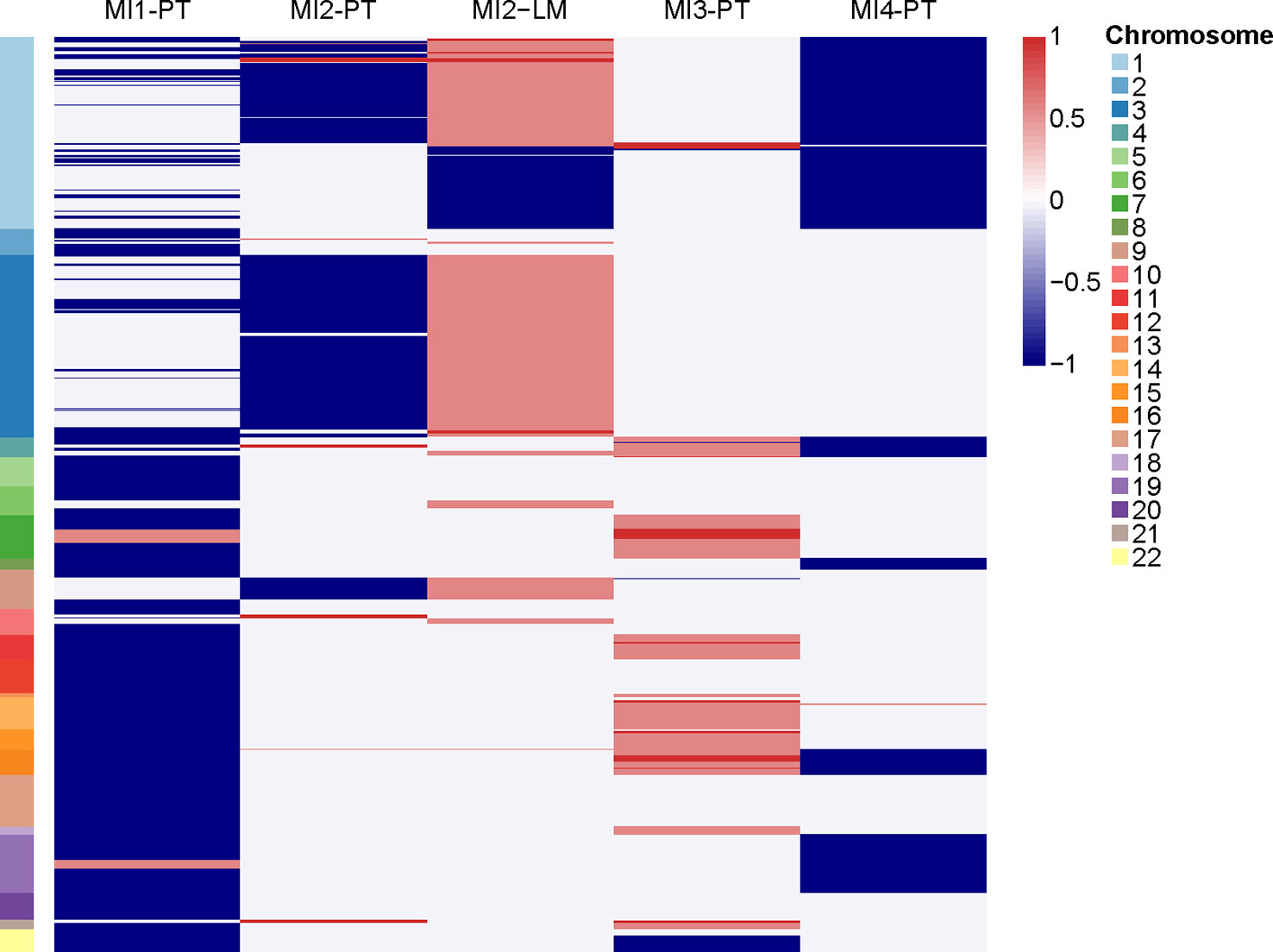
Figure 4 The copy number variations of primary tumors and liver metastasis. PT, primary tumor. LM, liver metastasis.
The natural history, circulating hormone levels, and IHC staining results indicate that primary tumors of these four metastatic insulinomas remained clinicopathological features of non-metastatic insulinomas, indicating it may largely derive from non-metastatic insulinomas other than NF-PanNETs. Nevertheless,
the liver metastasis showed ARX+ features resembling NF-PanNETs, indicating the accumulated ARX expression may play an important role in the progression of metastatic insulinomas.
Metastatic insulinoma is a rare type of PanNET that exhibits both malignant biological behaviours and excess hormone secretion patterns. Previous studies have shown that metastatic insulinoma has a longer duration of symptoms, higher insulin and C-peptide levels, and a larger tumor size. However, reliable indicators for the detection of metastatic insulinomas are still lacking. Some researchers have found that metastatic insulinomas may largely be derived from NF-PanNETs (8, 13), but it needs further exploration.
The preoperative elevated proinsulin levels and proinsulin/insulin molar ratio could predict the malignancy of insulinoma as well as ARX expression by IHC staining (23, 25). However, the hormone levels and IHC staining of primary tumors for these four metastatic insulinomas were similar to non-metastatic insulinomas, while liver metastasis showed signature of both α and β cell, indicating the complexity of metastatic insulinoma. The ARX and PDX1 expression is necessary for α and β cell differentiation, respectively. PDX1 staining positive was commonly found in non-metastatic insulinomas, while ARX staining positive was preferred in a portion of NF-PanNETs. The primary tumors of these four patients all showed PDX1+ and ARX-, while the liver metastasis of patient MI1 and MI2 both showed PDX1+ and ARX+. It seems that the accumulated ARX expression was important for malignant signatures of metastatic insulinomas, but it needs further exploration.
To determine the molecular characteristics of metastatic insulinoma, we attempted to elucidate them through genomic sequencing. Only one patient harboured YY1 T372R mutation, which is a recurrently mutated gene in non-metastatic insulinomas. We previously described somatic mutations of NF-PanNETs (MEN1, DAXX, ATRX, and mTOR pathway-related genes), but there was no such genetic mutation in these four metastatic insulinomas. Meanwhile, no typical signature of CNV patterns was found in these four metastatic insulinomas, which may be due to the limited cases. The typical CNV patterns of NF-PanNETs were also not shown in all NF-PanNET patients, the absence of the typical patterns in these four patients also make sense. Previous studies have indicated that chromosome 6q loss and 12q, 14q, and 17pq gains are strongly associated with metastatic insulinoma, whereas such CNV patterns was not found in our study.
For metastatic insulinoma, primary tumor should be resected to improve the life quality, and metastasis also need to be removed because the tumors could secrete excess insulin resulting in hypoglycemia symptoms. For instance, MI2 patient underwent resection of the pancreatic body and tail, but the metastasis could not be completely resected because of the extensive spread of the liver metastatic lesions. After surgery, the patient still suffered from hypoglycaemia, but the symptoms were immediately controlled when the interventional therapy for metastasis was performed.
At present, there is still a lot of debate regarding whether to enucleate insulinomas. Previous studies indicated that patients with liver metastasis before surgery generally choose pancreatectomy over enucleation. For insulinoma patients without metastasis before surgery, the proportion of metachronous metastasis after enucleation is approximately 2% (10). Currently, guidelines recommend active surgical treatment for insulinoma patients. Enucleation is only suitable for insulinoma with a diameter ≤ 2 cm and the distance > 2–3 mm from the main pancreatic duct, and rapid frozen pathological examination indicates that it is benign. However, predicting the occurrence of early metastasis before surgery remains difficult.
The limitation of this study is the small sample size. We only found four cases which met the criteria for metastatic insulinoma with clear Whipple symptoms, however, we basically collected all clinicopathological information and genomic sequencing data. More cases should be collected and analysed in the future. Moreover, the molecular mechanisms of metastatic insulinoma remain unclear because of its rarity, and transcriptomic and epigenetic changes need to be further elucidated.
According to national legislation/guidelines, specifically the Administrative Regulations of the People’s Republic of China on Human Genetic Resources (http://www.gov.cn/zhengce/content/2019-06/10/content_5398829.htm, http://english.www.gov.cn/policies/latest_releases/2019/06/10/content_281476708945462.htm), no additional raw data is available at this time. Data of this project can be accessed after an approval application to the China National Genebank (CNGB, https://db.cngb.org/cnsa/). Please refer to https://db.cngb.org/, or email: CNGBdb@cngb.org for detailed application guidance. The accession code CNP0000383 should be included in the application.
The studies involving human participants were reviewed and approved by Peking Union Medical College Hospital Ethical Review Committee. The patients/participants provided their written informed consent to participate in this study.
JZ is responsible for article drafting and clinical data collection. RJ is responsible for immunohistochemical staining and sequencing data analysis. XHo was responsible for whole genome sequencing and whole exome sequencing of four tumors. HW is responsible for pathology examination. XHa and WW are responsible for coordinating the whole article, guiding and reviewing the article. XHa and WW are corresponding authors for this article. All authors contributed to the article and approved the submitted version.
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-002, National Natural Science Foundation of China (82141102 and 82072954), National Key Research and Development Program of China (2019YFC1315901), and Beijing Natural Science Foundation (Z190022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology (2008) 135(5):1469–92. doi: 10.1053/j.gastro.2008.05.047
2. Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas (2010) 39(6):735–52. doi: 10.1097/MPA.0b013e3181ebb168
3. de Herder WW, Niederle B, Scoazec J-Y, Pauwels S, Klöppel Gü, Falconi M, et al. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology (2006) 84(3):183–8. doi: 10.1159/000098010
4. Jiang R, Hong X, Zhao Y, Wu W. Application of multiomics sequencing and advances in the molecular mechanisms of pancreatic neuroendocrine neoplasms. Cancer Lett (2021) 499:39–48. doi: 10.1016/j.canlet.2020.11.012
5. Dai H, Chen H, Hong X, Han X, Xu Q, Pang H, et al. Early detection of cognitive impairment in patients with insulinoma. Endocrine (2019) 65(3):524–30. doi: 10.1007/s12020-019-01994-x
6. Ding Y, Wang S, Liu J, Yang Y, Liu Z, Li J, et al. Neuropsychiatric profiles of patients with insulinomas. Eur Neurol (2010) 63(1):48–51. doi: 10.1159/000268166
7. Mathur A, Gorden P, Libutti SK. Insulinoma. Surg Clin North Am (2009) 89(5):1105–21. doi: 10.1016/j.suc.2009.06.009
8. Yu R, Nissen NN, Hendifar A, Tang L, Song YL, Chen YJ, et al. A clinicopathological study of malignant insulinoma in a contemporary series. Pancreas (2017) 46(1):48–56. doi: 10.1097/MPA.0000000000000718
9. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am (2011) 40(1):1–18, vii. doi: 10.1016/j.ecl.2010.12.005
10. Crippa S, Zerbi A, Boninsegna L, Capitanio V, Capitanio V, Balzano G, et al. Surgical management of insulinomas: short- and long-term outcomes after enucleations and pancreatic resections. Arch Surg (2012) 147(3):261–6. doi: 10.1001/archsurg.2011.1843
11. Sada A, Glasgow AE, Vella A, Thompson GB, McKenzie TJ, Habermann EB, et al. Malignant insulinoma: a rare form of neuroendocrine tumor. World J Surg (2020) 44(7):2288–94. doi: 10.1007/s00268-020-05445-x
12. Hackeng WM, Schelhaas W, Morsink FHM, Heidsma CM, Eeden Sv, Valk GD, et al. Alternative lengthening of telomeres and differential expression of endocrine transcription factors distinguish metastatic and non-metastatic insulinomas. Endocr Pathol (2020) 31(2):108–18. doi: 10.1007/s12022-020-09611-8
13. Yu R. Malignant insulinoma is largely derived from nonfunctioning pancreatic neuroendocrine tumors: a contemporary view. Pancreas (2020) 49(6):733–6. doi: 10.1097/MPA.0000000000001562
14. Kimura H, Ohtsuka T, Fujimoto T, Date K, Matsunaga T, Cases AI, et al. Different hormonal expression patterns between primary pancreatic neuroendocrine tumors and metastatic sites. Pancreas (2016) 45(7):947–52. doi: 10.1097/MPA.0000000000000570
15. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology (2016) 103(2):153–71. doi: 10.1159/000443171
16. Cejas P, Drier Y, Dreijerink KMA, Brosens LAA, Deshpande V, Epstein CB, et al. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat Med (2019) 25(8):1260–5. doi: 10.1038/s41591-019-0493-4
17. Wu K, Zhang X, Li F, Xiao D, Hou Y, Zhu S, et al. Frequent alterations in cytoskeleton remodelling genes in primary and metastatic lung adenocarcinomas. Nat Commun (2015) 6:10131. doi: 10.1038/ncomms10131
18. Hong X, Qiao S, Li F, Wang W, Jiang R, Wu H, et al. Whole-genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: leading to a new classification system. Gut (2020) 69(5):877–87. doi: 10.1136/gutjnl-2018-317233
19. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol (2013) 31(3):213–9. doi: 10.1038/nbt.2514
20. Rimmer A, Phan H, Mathieson I, Iqbal Z, Twigg SRF, WGS500 Consortium, et al. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat Genet (2014) 46(8):912–8. doi: 10.1038/ng.3036
21. Ramos AH, Lichtenstein L, Gupta M, Lawrence MS, Pugh TJ, Saksena G, et al. Oncotator: cancer variant annotation tool. Hum Mutat (2015) 36(4):E2423–9. doi: 10.1002/humu.22771
22. Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res (2016) 44(16):e131. doi: 10.1093/nar/gkw520
23. Camara-de-Souza AB, Toyoshima MTK, Giannella ML, Camacho CP, Lourenço DM Jr., Rocha MS, et al. Insulinoma: a retrospective study analyzing the differences between benign and malignant tumors. Pancreatology (2018) 18(3):298–303. doi: 10.1016/j.pan.2018.01.009
24. Scarpa A, Chang DK, Nones K, Corbo V, Patch A-M, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature (2017) 543(7643):65–71. doi: 10.1038/nature21063
Keywords: genomic sequencing, insulinoma, malignant, metastasis, pancreatic neuroendocrine tumour
Citation: Zhang J, Jiang R, Hong X, Wu H, Han X and Wu W (2023) Metastatic insulinoma: exploration from clinicopathological signatures and genetic characteristics. Front. Oncol. 13:1109330. doi: 10.3389/fonc.2023.1109330
Received: 27 November 2022; Accepted: 27 April 2023;
Published: 12 May 2023.
Edited by:
Jin He, Johns Hopkins University, United StatesReviewed by:
Christopher M. Heaphy, Boston University, United StatesCopyright © 2023 Zhang, Jiang, Hong, Wu, Han and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianlin Han, aGFueGlhbmxpbkBwdW1jaC5jbg==; Wenming Wu, ZG9jdG9yd3V1QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.