
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 16 February 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1109274
This article is part of the Research Topic Tumor Organoid Models and Epigenetic Regulation in Cancer Research View all 5 articles
Patients with non-small cell lung cancer (NSCLC) who carry epidermal growth factor receptor (EGFR) mutations can benefit significantly from EGFR tyrosine kinase inhibitors (EGFR TKIs). However, it is unclear whether patients without EGFR mutations cannot benefit from these drugs. Patient-derived tumor organoids (PDOs) are reliable in vitro tumor models that can be used in drug screening. In this paper, we report an Asian female NSCLC patient without EGFR mutation. Her tumor biopsy specimen was used to establish PDOs. The treatment effect was significantly improved by anti-tumor therapy guided by organoid drug screening.
The 5-year survival rate of lung cancer is only 18% (1). Non-small cell lung cancer (NSCLC) accounts for 75%–80% of all lung cancers. Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor that activates cell proliferation pathways on the cell surface. Approximately 50% of Asian NSCLC patients harbor EGFR mutations (2, 3). Therefore, EGFR mutation detection has become a mandatory part of the management of Asian NSCLC patients (4, 5). Deletion-Exon19 (Del19) and exon 21 L858R substitution (L858R) account for 90% of EGFR mutations in NSCLC (6). Patients with EGFR mutations can benefit greatly from EGFR tyrosine kinase inhibitors (EGFR TKIs). However, due to the limitations of detection methods and the complexity of lung cancer, there are still many factors that will affect the accuracy of EGFR mutation detection. It is important to develop patient-specific drug prediction models for personalized anti-tumor therapy.
Patient-derived tumor organoids (PDOs) are three-dimensional models produced from a patient’s cancer tissue, combining genetic analysis with drug screening (7). PDOs can be used to simulate the occurrence and development of lung cancer, study the mechanism of drug resistance, and trace mutated genes (8). PDO can also be used in vitro for lung cancer drug screening, biomarker validation, and personalized anti-tumor drug treatment response prediction (9).
Here, we report an Asian female NSCLC patient without EGFR mutation. Anti-tumor therapy was administered based on drug screening of PDOs. The outcome of the patient after treatment was significantly improved. This case report provides a case basis for PDOs to become a clinical prediction model, helping in the development of precision medicine.
This case involves a 66-year-old non-smoking Asian female NSCLC patient. She was admitted to the hospital for chest tightness, shortness of breath, intermittent cough for 1 year, and left chest pain for 1 month. The patient had no family history of lung tumors and had not previously received anti-tumor therapy. The patient’s vital signs were stable except for a few cardiac arrhythmias. Moreover, other hematological tests were in normal ranges except for tumor markers (carcinoembryonic antigen (CEA), 2.60 ng/ml; neuron-specific enolase (NSE), 18.80 ng/ml; CYFR21-1, 14.48 ng/ml). However, chest computed tomography (CT) showed a large space-occupying lesion in the upper lobe of the left lung with a maximum cross-sectional area of 7.3 cm × 5.5 cm. Moreover, there were multiple metastatic lymph nodes in the mediastinum and neck.
The patient could not receive surgical treatment due to the vast lesion and multiple lymph node metastases. Therefore, a tracheoscopic biopsy was performed immediately under flexible diagnostic bronchoscopy. The pathology report showed that the vast lesion was a hypofractionated adenocarcinoma classified as an epithelial tumor of NSCLC with high malignancy (No. 215758 from Hebei General Hospital Pathology Department).
Targeted therapy is a highly effective treatment option for Asian non-smoking female lung adenocarcinoma patients with an EGFR mutation. In this case, EGFR mutation detection was first performed at ShuWen Biology Laboratory. The pathology department provided formalin-fixed paraffin-embedded sections for the genetic testing. Regarding eight types of EGFR mutations, including Deletion-Exon19, L858R, and Insertion-Exon20, the test report suggested wild type with insensitivity to EGFR TKIs (such as gefitinib and erlotinib). The report (No. W0113008960) is shown in Table 1. The T790M mutation is the main reason for resistance to EGFR TKIs in patients with advanced NSCLC (8). Therefore, T790M mutation testing at Guangzhou KingMed Center was carried out. The center used the QX200 Droplet Digital PCR system with Human EGFR Gene T790M Mutation Detection Kit (S-ddPCR) to detect the T790M mutation. However, this report (No. QS20D-3953) also showed a lack of the T790M mutation. Because of the EGFR mutation negativity, the patient only received standard chemotherapy with a pemetrexed and carboplatin regimen. However, the patient experienced nausea and vomiting and could not eat during chemotherapy. Choosing an appropriate and effective treatment for her was a major dilemma.
A study involving 84 advanced lung cancer organoid models demonstrated that PDOs largely preserve somatic alterations in patients with advanced lung cancer, including the driving genes of tumors (10). Therefore, a CT-guided lung biopsy was performed, and we sent fresh tumor tissue samples to Beijing K2 Oncology Laboratory and successfully established a lung cancer PDO (OrganoidPro™ culture kit, K20-M-NSCLC). The PDO was treated with different concentrations and combinations of antineoplastic drugs. ATP quantification by CellTiter-Glo® 3D Assay was used to detect tumor cell viability (CellTiter-Glo® 3D Cell Viability Assay, Promega, Madison, WI, USA; G9681). Subsequently, a POLARstar Omega fully automated multifunctional device was used for the detection of enzyme markers. The results showed that carboplatin was ineffective at inhibiting the cancer cells, as was pemetrexed. However, gefitinib was 78% effective. Although carboplatin combined with pemetrexed was ineffective at inhibiting the cancer cells, gefitinib combined with carboplatin combined with pemetrexed was 78% effective. This report provides different information from the EGFR mutation report. The PDO drug screening report (No. KOLU-223) is shown in Table 2. Finally, standard chemotherapy was discontinued, and the patient was administered gefitinib for targeted therapy. The timeline of the diagnostic and therapeutic process is shown in Figure 1.
The treatment of this patient was encouraging. The patient was followed up several times over 2 years. First, her vital signs were stable. Other symptoms, such as chest tightness, shortness of breath, and chest pain, were significantly alleviated. Only a mild cough remained. In addition, her tumor markers gradually decreased to the normal range (CEA, 1.59 ng/ml; NSE, 9.98 ng/ml; CYFR21-1, 1.64 ng/ml). Chest CT also showed a gradual reduction of the tumor to 9 mm × 4 mm. The patient’s living conditions improved significantly. The anti-tumor effect of the patient was evaluated as complete remission (CR). The chest CT results before and after treatment are shown in Figure 2.
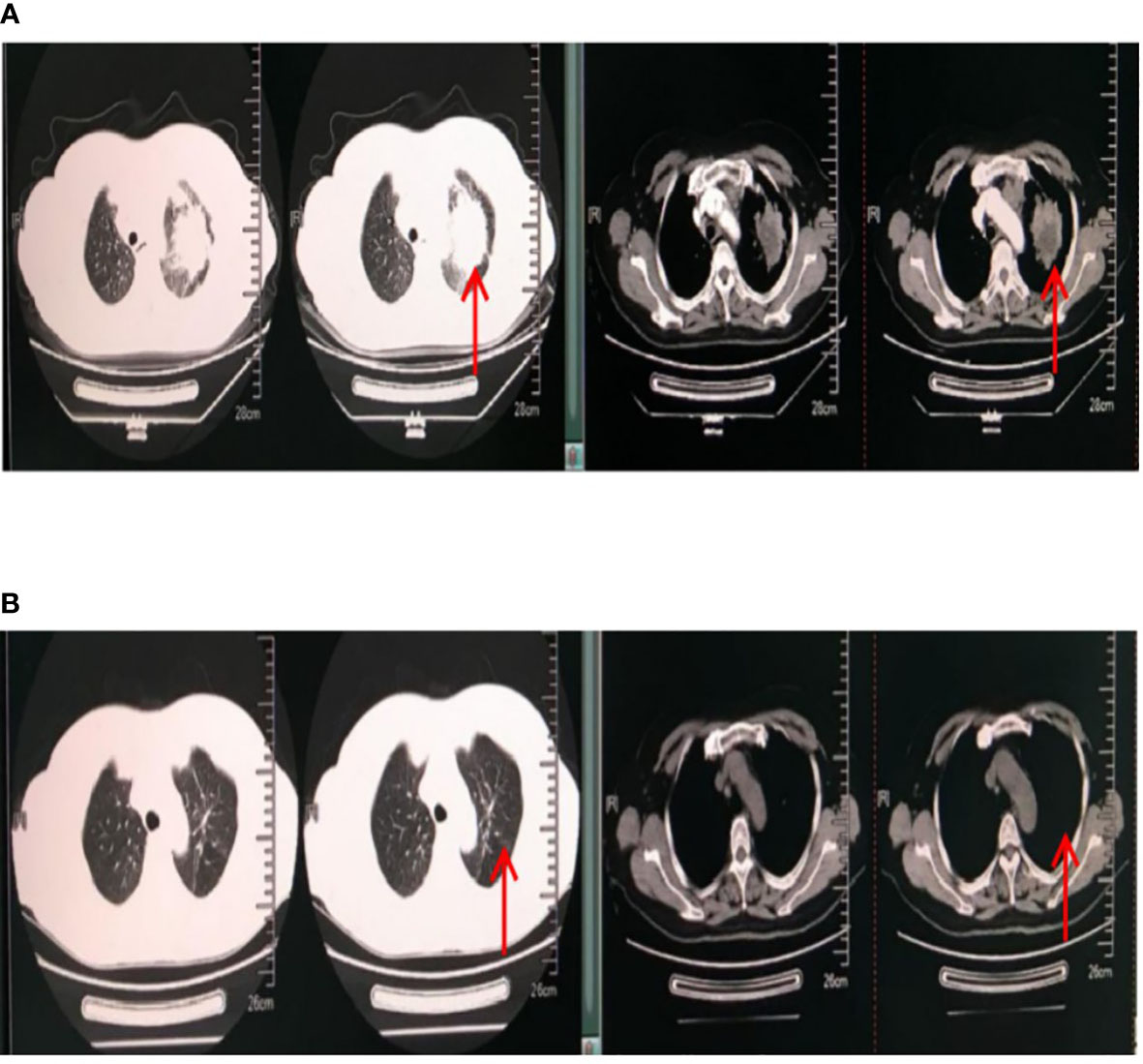
Figure 2 Chest CT results. The red arrow points to the lung tumor. (A) Chest CT results on 13 December 2017 (before treatment). The maximum cross-sectional area of the space-occupying lesion in the upper lobe of the left lung was 7.3 cm × 5.5 cm. Multiple metastatic lymph nodes were found in the mediastinum and neck. There was pleural effusion in the left chest. (B) Chest CT results on 27 May 2019 (after treatment). The maximum cross-sectional area of the space-occupying lesion was 9 mm × 4 mm. Multiple small lymph nodes were found in the mediastinum and neck.
With the advancement of molecular testing technologies, genetic testing has become a mandatory component in the management of NSCLC (4, 5). One study enrolling 1,482 patients from seven Asian countries and regions found that the overall EGFR mutation rate in Asian NSCLC patients was 51.4% (11). Therefore, EGFR has become the most common target for mutation testing.
The patient reported herein was diagnosed with pulmonary adenocarcinoma and was a non-smoking Asian woman. Her characteristics were fully consistent with those of EGFR mutation (12, 13). However, her EGFR genetic test results showed negativity for eight EGFR mutation types (including the classic mutation type Del19 in Asian patients), with EGFR TKI insensitivity. It is worth mentioning that the patient was only tested for eight types of EGFR mutation. Other mutational tests were not performed. We suggested that the patient proceeds with KRAS or ALK testing. Additionally, we suggested that the entire EGFR gene and the whole exon be sequenced to find rare mutations that activate the EGFR receptor. Unfortunately, the patient rejected these recommendations due to the high cost and long testing period. However, Deletion-Exon19, L858R, T790M, and Insertion-Exon20, the four most common mutation types in Asian women, were all negative (14). Thus, finding a more reliable drug sensitivity assay and providing patients with accurate treatment have become our greatest challenges.
PDOs have the advantage of saving time and cost. These models can be successfully established in 14 days (15). We successfully established a PDO for this patient with fresh tumor tissues from CT-guided lung biopsy and obtained a very interesting result from PDO drug screening assays. The combination of carboplatin, pemetrexed, and gefitinib did not increase cancer cell inhibition over gefitinib alone. This result indicated that the patient had undergone heavy physical burden of standard chemotherapy with little benefit. Based on the PDO drug screening result, the patient received gefitinib monotherapy.
With the help of the PDO drug screening result, patients can be treated appropriately and effectively. First, the physical and financial burden caused by chemotherapy can be avoided. Compared with chemotherapy, gefitinib only needs to be taken orally once daily, which greatly improves patient compliance and quality of life. Second, the treatment effect was very good in our case. The tumor was significantly reduced. The concentration of the detected tumor markers also decreased to the normal range. Her discomfort symptoms were also significantly relieved. Third, the whole treatment period was shorter. The accurate prediction of PDO greatly shortened the exploration period of effective treatment. In this regard, tumor patients will benefit greatly, especially those with advanced tumors (16). Finally, PDO drug screening can accurately predict multiple drugs rather than assess tumor response in the context of genomic profiling for mutations (17). For example, gefitinib is a first-generation targeted drug, whereas dasatinib is a second-generation targeted drug. Gefitinib’s cancer cell inhibition rate in PDO was 78%, significantly higher than that of dasatinib. This result shows that the advantage of accurate drug prediction by PDO over single-gene mutation prediction by genetic testing is clear.
However, there are still limitations in this case report. First, the patient was tested only for common EGFR mutations and did not receive genetic testing for other rare or different combinations of mutations. Performing all of these tests is difficult in the clinic. However, more comprehensive genetic testing data, or even high-throughput sequencing (HTS), is still necessary to explain negative EGFR mutation but effective TKI therapy. In 2015, Baik et al. reported a patient with a negative EGFR common mutation but effective EGFR TKI treatment (18). With next-generation sequencing (NGS), Baik found that EGFR exon 18–25 kinase domain duplication (EGFR-KDD) was the reason for the effective treatment of this patient. EGFR gene mutation is the most common mutation type in NSCLC patients, of which nearly 90% are Del19 and exon 21 L858R. However, there are still some mutation types with a small proportion that respond to EGFR TKI treatment. EGFR-KDD is a rare potential carcinogenic mutation, and its incidence in lung cancer is approximately 0.2% (19). Therefore, NGS can help patients obtain more accurate individualized treatment.
Second, Del19 and L858R were not detected in this case, but treatment with gefitinib was effective. In general, EGFR mutations are EGFR gene mutations that result in changes in the EGFR receptor on the cell membrane. Gefitinib is a small molecule receptor TKI that binds to the intracellular segment of the EGFR receptor to exert anti-tumor effects. There were no EGFR mutations detected in this case. However, gefitinib also inhibits insulin-like growth factor (IGF) and platelet-derived growth factor (PDGF), which are members of the tyrosine kinase receptor subfamily. It is unclear whether other tyrosine kinases and their signaling pathways can influence EGFR receptor mutations, affecting the efficacy of gefitinib therapy (20, 21).
In the literature, PDOs greatly preserve the histological and genetic characteristics of a primary tumor. First, Chen et al. (16) found >80% concordance between tumor and PDO samples for the top 20 NSCLC-related gene mutations. Second, PDOs were highly consistent with clinical treatment in terms of predicting the sensitivity of chemotherapeutic drugs (22). Similarly, in targeted therapy, PDO responses to targeted drugs correlated partially with the mutation profile, revealing similarities and differences between tumors and their corresponding PDOs. For example, Chen et al. (16) reported that PDOs show resistance to gefitinib but a therapeutic response to osimertinib. Matched clinical tumor tissues showed abnormal amplification of cMET, indicating the mechanism of gefitinib resistance. In addition, another PDO, with an insertion-exon 20 mutation, was resistant to gefitinib but exhibited a significant response to osimertinib and chemotherapy. Current genetic tests use genomic analysis to assess tumor response and can only predict the effect of drugs for this class of genetic spectrum mutations. It is difficult to predict the effectiveness of specific drugs (23). However, a drug screening assay was performed using the PDO for this patient, and the results showed that the PDO was resistant to gefitinib and sensitive to axitinib. Overall, there is a great advantage of PDO over genetic testing.
Based on early experience with PDO in precision medicine, the advantages of PDO over genetic testing are clear. We recommend combining genetic testing with PDO drug screening assays, especially for patients with negative genetic testing results. Moreover, PDO can be used as an adjunctive approach to help clinicians identify the most beneficial drugs for their patients. Overall, we report an interesting case. Although NGS sequencing data are lacking, this case highlights the power of personalized treatment decisions guided by PDO drug screening and the value of combining different diagnostic tools (such as genetic testing and PDO drug screening). The finding will accelerate the development of precision medicine for lung cancer.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This study was approved by the Medical Ethics Committee of Hebei Provincial People’s Hospital, No. (2018) Research Ethics No. (01). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YP, HC and YS performed the material preparation and data analysis. YP wrote the first draft of the manuscript. Also, YP and YS treated the patient. All authors contributed to the article and approved the submitted version.
We want to thank the patient who voluntarily participated in the study and the ShuWen Biology Laboratory and the Guangzhou KingMed Center for support of genetic testing support. We would like to thank the K2 Oncology Laboratory (Beijing) for the technical support in organoid culture.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1109274/full#supplementary-material
1. Zappa C, Mousa SA. Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res (2016) 5(3):288–300. doi: 10.21037/tlcr.2016.06.07
2. Liu H, Li Y, Chen G, Wang J, Li Y, Wang Y, et al. Detection and its clinical significance of EGFR gene mutation and gene amplification in 187 patients with non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2009) 12(12):1219–28.
3. Salto-Tellez M, Tsao MS, Shih JY, Thongprasert S, Lu S, Chang GC, et al. Clinical and testing protocols for the analysis of epidermal growth factor receptor mutations in East Asian patients with non-small cell lung cancer: A combined clinical-molecular pathological approach. J Thorac Oncol (2011) 6(10):1663–9. doi: 10.1097/JTO.0b013e318227816a
4. Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol (2021) 157:103194. doi: 10.1016/j.critrevonc.2020.103194
5. Karachaliou N, Rosell R. Targeted treatment of mutated EGFR-expressing non-small-cell lung cancer: Focus on erlotinib with companion diagnostics. Lung Cancer (Auckl). (2014) 5:73–9. doi: 10.2147/LCTT.S50671
6. Batra U, Nathany S, Sharma M, Mehta A, Dhanda S, Jose JT. Machine learning-based algorithm demonstrates differences in del19 and L858R EGFR subgroups in non-small cell lung cancer: A single center experience. Am J Transl Res (2022) 14(4):2677–84.
7. Bae J, Choi YS, Cho G, Jang SJ. The patient-derived cancer organoids: Promises and challenges as platforms for cancer discovery. Cancers (Basel). (2022) 14(9):2144. doi: 10.3390/cancers14092144
8. Qiang Y, Yao N, Zuo F, Qiu S, Cao X, Zheng W. Tumor organoid model and its pharmacological applications in tumorigenesis prevention. Curr Mol Pharmacol (2022). doi: 10.2174/1874467215666220803125822
9. Burkhart RA, Baker LA, Tiriac H. Testing susceptibility of patient-derived organoid cultures to therapies: Pharmacotyping. Methods Mol Biol (2018) 1787:253–61. doi: 10.1007/978-1-4939-7847-2_19
10. Kim SY, Kim SM, Lim S, Lee JY, Choi SJ, Yang SD, et al. Modeling clinical responses to targeted therapies by patient-derived organoids of advanced lung adenocarcinoma. Clin Cancer Res (2021) 27(15):4397–409. doi: 10.1158/1078-0432.CCR-20-5026
11. Shi Y, Au JSK, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol (2014) 9(2):154–62. doi: 10.1097/JTO.0000000000000033
12. Svaton M, Pešek M. Successful therapy of Czech patients with ROS1 translocation by crizotinib. Klin Onkol. (2016) 29(1):63–5. doi: 10.14735/amko201663
13. Cheng YI, Gan YC, Liu D, Davies MPA, Li WM, Field JK. Potential genetic modifiers for somatic EGFR mutation in lung cancer: A meta-analysis and literature review. BMC Cancer. (2019) 19(1):1068. doi: 10.1186/s12885-019-6317-6
14. Tapia C, Savic S, Bihl M, Rufle A, Zlobec I, Terracciano L, et al. [EGFR mutation analysis in non-small-cell lung cancer : Experience from routine diagnostics]. Pathologe (2009) 30(5):384–92. doi: 10.1007/s00292-009-1141-4
15. Chen H, Gotimer K, De Souza C, Tepper CG, Karnezis AN, Leiserowitz GS, et al. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol Oncol (2020) 157(3):783–92. doi: 10.1016/j.ygyno.2020.03.026
16. Chen J, Chu X, Zhang J, Nie Q, Tang W, Su J, et al. Genomic characteristics and drug screening among organoids derived from non-small cell lung cancer patients. Thorac Cancer. (2020) 11(8):2279–90. doi: 10.1111/1759-7714.13542
17. Li L, Selaru FM. Patient-derived functional organoids as a personalized approach for drug screening against hepatobiliary cancers. Adv Cancer Res (2022) 156:319–41. doi: 10.1016/bs.acr.2022.01.011
18. Baik CS, Wu D, Smith C, Martins RG, Pritchard CC. Durable response to tyrosine kinase inhibitor therapy in a lung cancer patient harboring epidermal growth factor receptor tandem kinase domain duplication. J Thorac Oncol (2015) 10(10):e97–99. doi: 10.1097/JTO.0000000000000586
19. Gallant JN, Sheehan JH, Shaver TM, Bailey M, Lipson D, Chandramohan R, et al. EGFR kinase domain duplication (EGFR-KDD) is a novel oncogenic driver in lung cancer that is clinically responsive to afatinib. Cancer Discovery (2015) 5(11):1155–63. doi: 10.1158/2159-8290.CD-15-0654
20. Pan YH, Jiao L, Lin CY, Lu CH, Li L, Chen HY, et al. Combined treatment with metformin and gefitinib overcomes primary resistance to EGFR-TKIs with EGFR mutation via targeting IGF-1R signaling pathway. Biologics (2018) 12:75–86.
21. Huber L, Birk R, Rotter N, Aderhold C, Lammert A, Jungbauer F, et al. Effect of small-molecule tyrosine kinase inhibitors on PDGF-AA/BB and PDGFRα/β expression in SCC according to HPV16 status. Anticancer Res (2020) 40(2):825–35. doi: 10.21873/anticanres.14014
22. Francies HE, Barthorpe A, McLaren-Douglas A, Barendt WJ, Garnett MJ. Drug sensitivity assays of human cancer organoid cultures. Methods Mol Biol (2019) 1576:339–51. doi: 10.1007/7651_2016_10
Keywords: cancer, organoid, genetic analysis, lung cancer, case report
Citation: Pan Y, Cui H and Song Y (2023) Organoid drug screening report for a non-small cell lung cancer patient with EGFR gene mutation negativity: A case report and review of the literature. Front. Oncol. 13:1109274. doi: 10.3389/fonc.2023.1109274
Received: 27 November 2022; Accepted: 30 January 2023;
Published: 16 February 2023.
Edited by:
Edwin Roger Parra, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Vera Luiza Capelozzi, University of São Paulo, BrazilCopyright © 2023 Pan, Cui and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongbin Song, aGVieHdrQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.