
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 18 July 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1109068
 Lili Wu1†
Lili Wu1† Yaolin Xu2,3,4†
Yaolin Xu2,3,4† Yuhong Zhou5
Yuhong Zhou5 Zhaochong Zeng1
Zhaochong Zeng1 Yue Fan6
Yue Fan6 Dansong Wang2
Dansong Wang2 Wenchuan Wu2,3,4
Wenchuan Wu2,3,4 Xi Guo5
Xi Guo5 Minzhi Lv7
Minzhi Lv7 Yuxiu Ouyang8
Yuxiu Ouyang8 Shisuo Du1*
Shisuo Du1* Wenhui Lou2,3,4*
Wenhui Lou2,3,4*Background: While adjuvant chemotherapy has been established as standard practice following radical resection of pancreatic ductal adenocarcinoma (PDAC), the role of adjuvant radiation therapy (RT) and which patients may benefit remains unclear.
Methods: This retrospective study included PDAC patients who received pancreatic surgery from April 2012 to December 2019 in Zhongshan Hospital Fudan University. Patients with carcinoma in situ, distant metastasis, and without adjuvant chemotherapy were excluded. Cox proportional hazards modeling of survival were constructed to find potential prognostic factors. Propensity score matching (PSM) and exploratory subgroup analyses were used to create a balanced covariate distribution between groups and to investigate therapeutic effect of radiotherapy in certain subgroups.
Results: A total of 399 patients were finally included, 93 of them receiving adjuvant chemoradiotherapy (C+R+) and 306 of them receiving chemotherapy only. Patients in C+R+ group were more likely to be male patients with T3-4 disease. Lymph node metastases was the only negative prognostic factor associated with overall survival (OS). Additional adjuvant RT was not associated with an OS benefit both before and after PSM. Surprisingly, a trend towards improved OS with RT among patients with either T4, N2 disease or R1 resection becomes significant in patients alive more than 1 year after surgery.
Conclusion: Adjuvant RT was not associated with an OS benefit across all patients, though did show a possible OS benefit for the subgroup with T4N2 disease or R1 resection at 1 year after surgery.
Pancreatic ductal adenocarcinoma (PDAC) is one of the malignancies with the poorest prognosis, with an estimated 5-year survival around 10% (1). In China, the estimated numbers of new cases and deaths per year were 125,000 and 122,000, respectively (2, 3). Nowadays, PDAC has been recognized as a systemic disease and multimodality management is highly recommended in Chinese Society of Clinical Oncology (CSCO), National Comprehensive Cancer Network (NCCN), and European Society for Medical Oncology (ESMO) practice guidelines for pancreatic cancer (4–7).
Adjuvant chemotherapy has been standard-of-care for PDAC after curative surgery. Modified fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX) and nab-paclitaxel plus gemcitabine have been shown to significantly improve survival outcomes of PDAC patients after surgery (8–10). For those who could not tolerate the side effects of combined regimens, S-1 or gemcitabine (GEM) alone was alternative treatment and could also prolonged overall survival after surgical resection (11). However, whether additional adjuvant radiotherapy would bring survival benefit remained controversial.
Previous Gastrointestinal Tumor Study Group (GITSG) 9173 trial and some retrospective studies had demonstrated that chemoradiotherapy (CRT) showed relatively good local control and brought survival benefit for PDAC patients after surgical treatment compared with those without any adjuvant treatment (12–15). Meanwhile, the Radiation Therapy Oncology Group (RTOG) 0848, 9704 trials, and two other prospective studies had all indicated promising efficacy of either 5-FU or GEM based CRT in adjuvant setting among PDAC patients (16–21).
When directly comparing adjuvant CRT with chemotherapy alone, the results of EORTC-40013-22012/FFCD-9203/GERCOR and ESPAC-1 trials were contradictory. Karin et al. administrated either GEM based CRT or GEM to pancreatic head cancer patients after R0 resection of primary tumor. Median disease-free survival (DFS) and overall survival (OS) showed no difference between groups, while first local recurrence was less frequent in CRT group (22). However, the results of ESPAC-1 indicated that CRT group (including CRT alone and CRT + chemotherapy) was associated with poorer OS comparing with no CRT group (including observation and chemotherapy alone) (23, 24).
One of the reasons for these discrepancies may be patient selection for adjuvant CRT. Which specific patient subgroups could benefit from adjuvant CRT remained unknown. We previously reported that S-1 based CRT showed promising efficacy and well-tolerated in terms of adverse effect in resected PDAC patients with high-risk pathologic feature (including positive resection margin, pathologic T3-4 and/or N1-2 disease, peripancreatic fat invasion, microvascular invasion, and perineural invasion) (25). Some other recent retrospective studies with either single center cohort or public database indicated possible benefit of CRT for PDAC patients with T3 disease, lymph node metastases, and R1 resection (15, 26–28). Meanwhile, failure to adhere to radiotherapy protocol among different medical centers may have also had an impact on the survival outcome (29). As a high-volume pancreatic cancer center with experienced radiation therapists, we conducted the present retrospective cohort study to further explore the therapeutic effect of adjuvant CRT in PDAC patients after surgery.
This study was reviewed and approved by the ethics committee of Zhongshan Hospital Fudan University. We included patients who underwent curative-intent surgery between 1 April 2012 and 31 December 2019 and were pathologically diagnosed as pancreatic ductal adenocarcinoma. R1 resection was defined as a positive margin within less than 1 mm according to the 8th American Joint Committee on Cancer (AJCC) manual. Patients with carcinoma in situ (Tis) or distant metastases, and those not receiving adjuvant chemotherapy were excluded. All the patients were restaged pathologically according to the 8th AJCC TNM classification. OS was calculated as the time from surgery to death or last follow-up. DFS was calculated as the time from surgery to disease progression. The follow-up duration was from the surgery date to 1 July 2022. All the medical information and time of survival were obtained from medical records and telephone interviews. Physical examinations, complete blood count (CBC), liver function, kidney function, and CA 19-9 radioimmunoassay were tested before the start of treatment and repeated every cycle of chemotherapy. Contrast-enhanced computed tomography and/or magnetic resonance imaging were performed every other cycle of chemotherapy and 3 weeks after CRT. Evaluations of treatment response according to the radiology reports were done by the same oncologist in a blinded manner. This study was approved by the ethical committee of our hospital (B2022-249R) and all patients have signed informed consent forms before we collected their medical records for researching purpose.
Data analyses were performed by R project 4.2.1 for Windows and Rstudio 2022.07.1. Normality and homogeneity of variance were tested by Shapiro-Wilk test and Levene’s test. Categorical variables were reported as frequencies and percentages. Continuous variables conforming to normal distribution were presented by means and standard error, others were described as medians and Inter-Quartile Range. The baseline characteristics between different groups were compared using Fisher’s precision probability test for categorical variables, using Wilcoxon rank sum test for continuous variables, respectively. Propensity score matching (PSM) was performed with “MatchIt” packages using R project. A 1:1 ratio propensity score matching study group was created using the nearest neighbor matching method with a 0.6 caliper. Survival curves were drawn with the method of Kaplan–Meier, and log-rank test and landmark analyses was used to compare the survival outcome of different groups. Cox proportional hazards model was used to estimate the hazard ratio of death. The significant statistical variables (p<0.1) in univariate Cox regression analysis were incorporated into the multivariate analysis to identify the independent prognostic factors for survival. And forest plot was performed to show the outcome of subgroup analysis.
A total of 399 patients were incorporated in the total cohort and were stratified by whether receiving adjuvant radiotherapy or not. Flowchart of patient selection were illustrated in Figure 1 and patients’ clinicopathologic characteristics were listed in Table 1. There were 306 patients receiving adjuvant chemotherapy only (C+R-) and 93 patients receiving both adjuvant chemotherapy and radiotherapy (C+R+). Patients in C+R+ groups were more likely to be male patients (Male, C+R- vs C+R+, 55.9% vs 72%, p=0.006) with pathologic T3-4 disease (T3-4, C+R- vs C+R+, 15.3% vs 28%, p=0.008). Other factors, including N stage and tumor markers, didn’t differ significantly between two groups.
The Kaplan-Meier estimator were used to compare survival outcome between C+R-and C+R+ groups. As shown in Figure 2A, the addition of adjuvant radiotherapy to resection and adjuvant chemotherapy was not associated with an increase in OS among all PDAC patients (mOS, C+R- vs C+R+, 34.7 vs 29.9 months, p=0.4). Cox proportional-hazards model were constructed to further investigate potential prognostic factors among PDAC patients with surgical treatment and adjuvant chemotherapy. The univariate and multivariate Cox regression analysis demonstrated that regional lymph node metastases was an independent prognostic factor (p=0.04; N0 as reference; N2, hazard ratio [HR]=2.71, 95% confidential index [CI] 1.21-6.07, p=0.015) and preoperative carbohydrate antigen 125 [CA 125] level seemed to associated with OS (p=0.061; CA 125<20 U/ml as reference; CA 125≥20 U/ml, HR 1.59, 95% CI 0.85-3.8). Other details of Cox proportional model was summarized in Table 2.
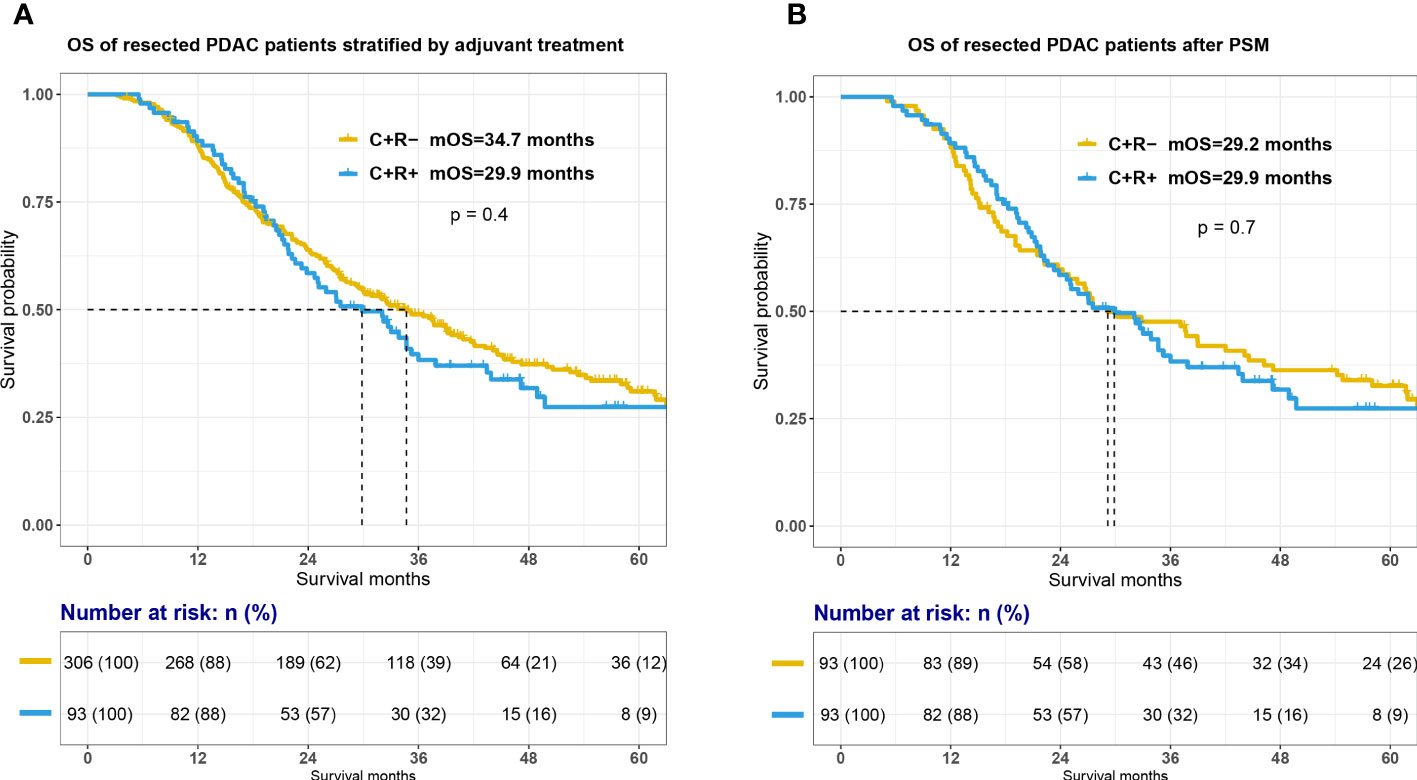
Figure 2 Survival between C+R- and C+R+ groups before and after propensity score matching. Figure showed the survival curves between two groups before (A) and after propensity score matching (B). OS, overall survival; C+R-, receiving adjuvant chemotherapy only; C+R+, receiving both adjuvant chemotherapy and radiotherapy; PSM, propensity score matching.
In order to balance confounding factors which might affect survival outcome, PSM was performed by a 1:1 ratio. The potentially adjusting variables were based on the results of cox regression analysis, which included AJCC 8th N stage (p=0.04) and preoperative CA 125 level (p=0.061). A total of 93 PDAC patients with C+R- adjuvant treatment were matched with 93 patients in C+R+ group in the total cohort. The baseline characteristics between the two groups after PSM were listed in Supplemental Table 1. All adjusting variables were comparable after PSM. However, additional adjuvant radiotherapy still did not improve OS of PDAC patients after surgical resection and adjuvant chemotherapy (Figure 2B; mOS, C+R- vs C+R+, 29.2 vs 29.9 months, p=0.7).
Further subgroup analyses were conducted to identify patients who may benefit from additional adjuvant radiotherapy. Disappointingly, forest plot (Supplemental Figure 1) did not show any survival advantage of extra radiotherapy in any of the subgroups.
Based on Cox analyses, our previous prospective research and some retrospective studies, PDAC patients after curative surgery and with high-risk pathologic features may have better survival outcome with adjuvant radiotherapy (25–28, 30). We sought to examine the potential benefit of adjuvant radiotherapy among the highest-risk patients and therefore patients with either T4N2 disease or R1 resection were retrieved from the total cohort. Among this high-risk subgroup, there was a trend toward improved OS when adjuvant RT was administered (Figure 3A; mOS, C+R- vs C+R+, 19.5 vs 29.7 months, p=0.098). The curves diverge following the 1-year time point and landmark survival analysis for patients surviving beyond this point surprisingly demonstrated a significant difference as seen in Figure 3B (mOS for patients alive 1 year after surgical resection, 21.2 vs 47.2 months for the C+R- vs C+R groups, respectively, p=0.0088), although it must be noted that numbers for comparison were low (33 C+R- patients, 17 C+R+ patients).
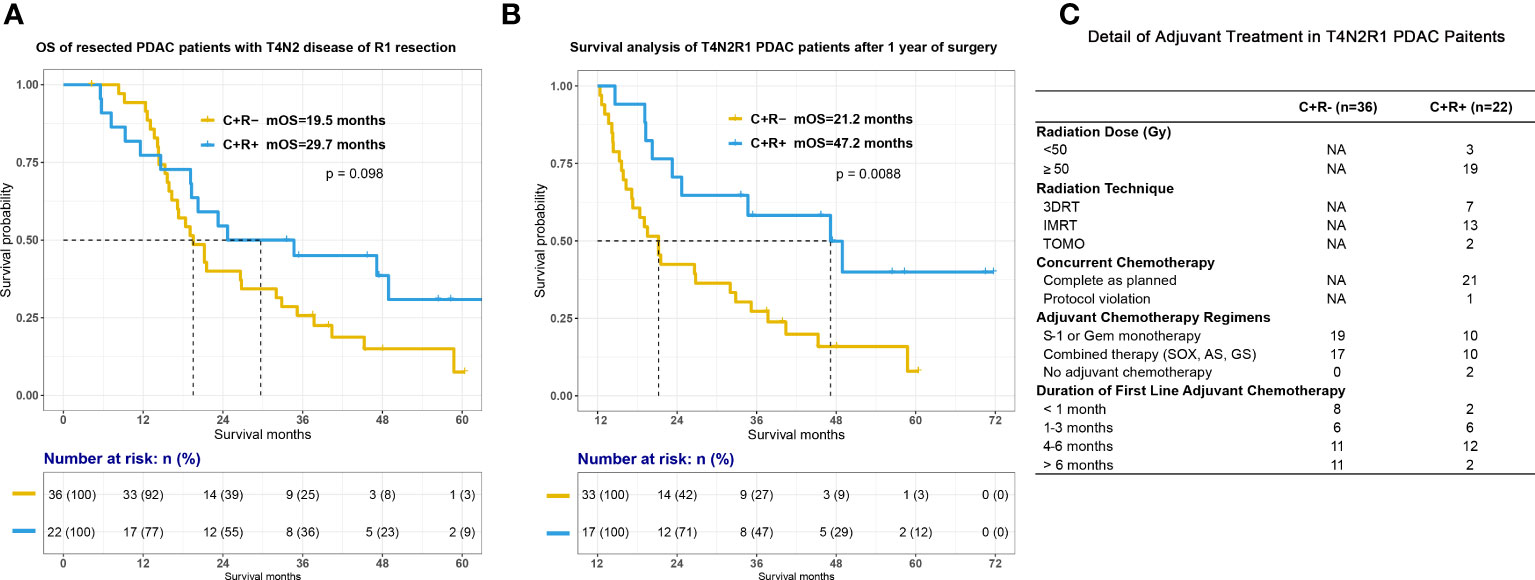
Figure 3 Survival between C+R- and C+R+ groups in PDAC patients with either T4, N2 disease or R1 resection. Figure showed the survival curves between two groups after surgery (A) and 1 year after surgical treatment (B). (C) Detail of adjuvant treatment among T4N2R1 patients. T4N2R1, classified as pathologic T4 or N2 staging or R1 resection; Gy, gray; 3DRT, three-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; TOMO, tomotherapy; Gem, gemcitabine; SOX, S-1 plus oxaliplatin; AS; nabpaclitaxel plus S-1; GS, gemcitabine plus S-1.
The role of CRT in the adjuvant setting for pancreatic cancer remains debatable due to the conflicting results from different clinical trials and retrospective studies. The reasons behind may be difference in indications of CRT and treatment protocols. While adjuvant chemotherapy has been a standard treatment for PDAC patients after curative surgery, it was critical to identify whom would benefit from additional adjuvant radiotherapy. Thus, we conducted the present retrospective study to investigate the therapeutic effect of additional adjuvant radiotherapy in PDAC patients who received surgical treatment of primary pancreatic lesion and adjuvant chemotherapy after surgery. A total of 399 patients were included, 93 of them receiving adjuvant chemoradiotherapy and 306 of them receiving adjuvant chemotherapy only. Patients in C+R+ group were more likely to be male patients with T3-4 disease. Lymph node metastases was the only negative prognostic factor associated with OS. Additional adjuvant radiotherapy did not improve OS both before and after PSM. Further subgroup analyses showed that PDAC patients with either T4N2 disease or R1 resection have increased OS with radiotherapy at 1 year after surgical treatment.
It was reasonable that no difference in OS was found between C+R- and C+R+ in total cohort. Based on CSCO and NCCN guidelines, adjuvant radiotherapy was recommended only in patients with features that portend high risk for local recurrence, for example, positive resection margin and regional lymph node metastases (6, 7). However, a high proportion of patients in this study appear to have had relatively early-stage disease (>50% stage I/>90% stage I-II in the C+R-group; 38.8% stage I/>85% stage I-II in the C+R+ group) and negative margins (93.7%). Based on our previous research and some retrospective studies, CRT showed promising efficacy in resected PDAC patients with high-risk pathologic features, including positive resection margin, pathologic T3-4 or N1-2 disease, peripancreatic fat invasion, microvascular invasion, and perineural invasion (25–28, 30). Thus, we conducted further subgroup analyses and surprisingly found that patients with T4, N2 stage or R1 resection had better OS with additional radiotherapy at 1 year after surgical resection. This result was similar with those of PREOPANC Trial, which demonstrating that neoadjuvant chemoradiotherapy shown survival advantage for resectable and borderline resectable PDAC patients after 1 year from diagnosis when compared with upfront surgery (31). As we known, biological nature should be taken into consideration when talking about resectability of pancreatic cancer. Some patients could have disease progression even after aggressive surgery and (neo)adjuvant treatments and died within 1 year after diagnosis. By conducting landmark analyses with a cutoff point at 1 year after surgical treatment, we may be able to reduce the confounding effect of these biologically unresectable PDAC patients. Besides, the underlying mechanism about poor response to radiation was nebulous. Previous study has demonstrated that genetic alterations and tumor microenvironment of individual patients could impact the response to radiation therapy. Thus, further research, which shall include the genetic and microenvironment detail of individual PDAC patients into survival analyses, is needed to reveal potential mechanism (32).
There were obviously some limitations of our study. First, this was a single institution retrospective study conducted in a high-volume pancreatic cancer center. We have specialized pancreatic disease multidisciplinary team (MDT) with experienced radiation therapists. Our research and previous studies have showed that high volume center with MDT could contribute to appropriate clinical decision making, lower mortality rate of pancreatectomies and better survival outcome (33–35). Meanwhile, we should also take into account the quality of radiotherapy which could have a significant impact on therapeutic effect of CRT, since Abrams et al. had demonstrated that failure to adherence to radiation therapy protocol was associated with poorer survival outcome in PDAC patients (29). Thus, the conclusion may not be able to generalized to other hospitals. Second, our results may need to be interpreted with attention that discrepancies in treatment protocol, especially adjuvant chemotherapy regimens, could affect survival outcome of PDAC patients after surgery. PRODIGE-24 has revealed the superiority of mFOLFIRINOX regimen compared to gemcitabine in terms of DFS and OS (8). Although we have summarized details of adjuvant treatment of T4N2R1 patients as Figure 3C, we were not able to collect all information about adjuvant treatment in other patients in this study. We have no idea if additional adjuvant radiotherapy could still offer survival benefit when combined adjuvant chemotherapy regimens, such as mFOLFIRINOX, nabpaclitaxel plus gemcitabine, capecitabine plus gemcitabine, were administrated in the process of adjuvant CRT. Third, we did not show the comparison of DFS in the result part. The median DFS for all included patients was around 16.4 months. We did compare DFS and the recurrence patterns between groups and the analyses showed no difference in DFS in both all included patients and T4N2R1 patients (Supplemental Figure 3). The reason why we did not highlight DFS is that some of the patients who received surgery between 2017 to 2019 were not able to complete their routine 3-months-follow-up in either our hospital or local medical centers on time due to the pandemic in the past 3 years in China. Although we have collected the event of disease progression through telephone follow-up, DFS was not precise enough to describe the actual survival outcome when compared with OS.
To our knowledge, further clinical trials should be undertaken in the following issues: 1) identifying potential subgroups who could benefit from adjuvant radiotherapy and also those with poor biological features and dismal prognosis even with aggressive treatment; 2) what is the best treatment combination, including chemotherapy regimens, treatment sequence, radiotherapy technique etc.
Additional adjuvant RT was not associated with an OS benefit across all included PDAC patients receiving surgery and adjuvant chemotherapy, though did show a possible OS benefit for the subgroup with T4N2 disease or R1 resection at 1 year after surgery.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the ethical committee of our hospital (B2022-249R) and all patients have signed informed consent forms before we collected their medical records for researching purpose. The patients/participants provided their written informed consent to participate in this study.
LW: project administration, data curation, investigation, methodology. YX: project administration, data curation, investigation, writing – original draft. YZ: investigation. ZZ: funding acquisition, investigation, methodology, project administration, resources. YF: investigation. DW: investigation. WW: project administration, data curation, investigation. XG: investigation. ML: software, visualization, methodology. YO: investigation. SD: funding acquisition, investigation, writing – review & editing. WL: funding acquisition, investigation, writing – review & editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Nature Science Foundation of China (81773220, 82073479, 82273382、81827807、81972218、81972257、82103409、82272929、82173116), Shanghai ShenKang Hospital Development Centre Project (SHDC2020CR2017B), Program of Shanghai Academic/Technology Research Leader (23XD1400600), China Postdoctoral Science Foundation (2021M690037), Shanghai Sailing Program (21YF1407100), Science and Technology Planning Project of Yunnan Province (202305AF150148), and Shanghai Municipal Health Commission (20224Y0307). The funding agencies had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1109068/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, et al. Advances in the epidemiology of pancreatic cancer: trends, risk factors, screening, and prognosis. Cancer Lett (2021) 520:1–11. doi: 10.1016/j.canlet.2021.06.027
4. Seufferlein T, Bachet J, Van Cutsem E, Rougier P. Pancreatic adenocarcinoma: esmo–esdo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2012) 23:i33–40. doi: 10.1093/annonc/mds224
5. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26:v56–68. doi: 10.1093/annonc/mdv295
6. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19:439–57. doi: 10.6004/jnccn.2021.0017
7. Cui J, Jiao F, Li Q, Wang Z, Fu D, Liang J, et al. Chinese Society of clinical oncology (csco): clinical guidelines for the diagnosis and treatment of pancreatic cancer. J Natl Cancer Center. (2022). doi: 10.1016/j.jncc.2022.08.006
8. Conroy T, Hammel P, Hebbar M, Ben AM, Wei AC, Raoul JL, et al. Folfirinox or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med (2018) 379:2395–406. doi: 10.1056/NEJMoa1809775
9. Tempero MA, Reni M, Riess H, Pelzer U, O'Reilly EM, Winter JM, et al. Apact: phase iii, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-p/g) vs gemcitabine (g) for surgically resected pancreatic adenocarcinoma. J Clin Oncol (2019) 37:4000. doi: 10.1200/JCO.2019.37.15_suppl.4000
10. Conroy T, Castan F, Lopez A, Turpin A, Ben Abdelghani M, Wei AC, et al. Five-year outcomes of folfirinox vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol (2022) 8:1571–8. doi: 10.1001/jamaoncol.2022.3829
11. Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of s-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (jaspac 01). Lancet (2016) 388:248–57. doi: 10.1016/S0140-6736(16)30583-9
12. Kalser MH, Ellenberg SS. Pancreatic cancer. adjuvant combined radiation and chemotherapy following curative resection. Arch Surg (1985) 120:899–903. doi: 10.1001/archsurg.1985.01390320023003
13. Gastrointestnal TSG. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer (1987) 59:2006–10. doi: 10.1002/1097-0142(19870615)59:12<2006::AID-CNCR2820591206>3.0.CO;2-B
14. Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the johns hopkins hospital-mayo clinic collaborative study. Ann Surg Oncol (2010) 17:981–90. doi: 10.1245/s10434-009-0743-7
15. Rutter CE, Park HS, Corso CD, Lester-Coll NH, Mancini BR, Yeboa DN, et al. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: an analysis of the national cancer data base. Cancer (2015) 121:4141–9. doi: 10.1002/cncr.29652
16. Demols A, Peeters M, Polus M, Honore P, Boterberg T, Gay F, et al. Adjuvant gemcitabine and concurrent continuous radiation (45 gy) for resected pancreatic head carcinoma: a multicenter belgian phase ii study. Int J Radiat Oncol Biol Phys (2005) 62:1351–6. doi: 10.1016/j.ijrobp.2005.01.043
17. Blackstock AW, Mornex F, Partensky C, Descos L, Case LD, Melin SA, et al. Adjuvant gemcitabine and concurrent radiation for patients with resected pancreatic cancer: a phase ii study. Br J Cancer (2006) 95:260–5. doi: 10.1038/sj.bjc.6603270
18. Regine WF, Winter KW, Abrams R, Safran H, Hoffman JP, Konski A, et al. Rtog 9704 a phase iii study of adjuvant pre and post chemoradiation (crt) 5-fu vs. gemcitabine (g) for resected pancreatic adenocarcinoma. J Clin Oncol (2006) 24:4007. doi: 10.1200/jco.2006.24.18_suppl.4007
19. Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. Jama (2008) 299:1019–26. doi: 10.1001/jama.299.9.1019
20. Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the u.s. intergroup/rtog 9704 phase iii trial. Ann Surg Oncol (2011) 18:1319–26. doi: 10.1245/s10434-011-1630-6
21. Abrams RA, Winter KA, Safran H, Goodman KA, Regine WF, Berger AC, et al. Results of the nrg oncology/rtog 0848 adjuvant chemotherapy question-erlotinib+gemcitabine for resected cancer of the pancreatic head: a phase ii randomized clinical trial. Am J Clin Oncol (2020) 43:173–9. doi: 10.1097/COC.0000000000000633
22. Van Laethem JL, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized eortc-40013-22012/ffcd-9203/gercor phase ii study. J Clin Oncol (2010) 28:4450–6. doi: 10.1200/JCO.2010.30.3446
23. Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet (2001) 358:1576–85. doi: 10.1016/s0140-6736(01)06651-x
24. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med (2004) 350:1200–10. doi: 10.1056/NEJMoa032295
25. Wu L, Xu Y, Zeng Z, Chen Y, Zhou Y, Wang D, et al. Efficacy and safety of s-1 based adjuvant chemoradiotherapy for resected pancreatic ductal adenocarcinoma with high-risk pathological feature: a prospective, single-arm, interventional study. J Pancreatology (2022) 5:18–26. doi: 10.1097/JP9.0000000000000084
26. Naffouje SA, Sabesan A, Kim DW, Carballido E, Frakes J, Hodul P, et al. Adjuvant chemoradiotherapy in resected pancreatic ductal adenocarcinoma: where does the benefit lie? a nomogram for risk stratification and patient selection. J Gastrointest Surg (2021) 26(2):376–86. doi: 10.1007/s11605-021-05130-x
27. Shi X, Peng J, Jiang H, Gao Y, Wang W, Zhou F. Impact of adjuvant chemoradiotherapy on survival of resected pancreatic adenocarcinoma cancer: a surveillance, epidemiology and end results (seer) analysis. Front Oncol (2021) 11:651671. doi: 10.3389/fonc.2021.651671
28. Xing J, Yang B, Hou X, Jia N, Gong X, Li X, et al. Prognostic factors and effect of adjuvant chemoradiation following chemotherapy in resected pancreatic cancer patients with lymph node metastasis or r1 resection. Front Oncol (2021) 11:660215. doi: 10.3389/fonc.2021.660215
29. Abrams RA, Winter KA, Regine WF, Safran H, Hoffman JP, Lustig R, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in rtog 9704–a phase iii trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys (2012) 82:809–16. doi: 10.1016/j.ijrobp.2010.11.039
30. Merchant NB, Rymer J, Koehler EA, Ayers GD, Castellanos J, Kooby DA, et al. Adjuvant chemoradiation therapy for pancreatic adenocarcinoma: who really benefits? J Am Coll Surg (2009) 208:829–838, 838-841. doi: 10.1016/j.jamcollsurg.2008.12.020
31. Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the dutch randomized preopanc trial. J Clin Oncol (2022) 40:1220–30. doi: 10.1200/JCO.21.02233
32. Javadrashid D, Baghbanzadeh A, Derakhshani A, Leone P, Silvestris N, Racanelli V, et al. Pancreatic cancer signaling pathways, genetic alterations, and tumor microenvironment: the barriers affecting the method of treatment. Biomedicines (2021) 9:373. doi: 10.3390/biomedicines9040373
33. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med (2011) 364:2128–37. doi: 10.1056/NEJMsa1010705
34. Chau Z, West JK, Zhou Z, McDade T, Smith JK, Ng SC, et al. Rankings versus reality in pancreatic cancer surgery: a real-world comparison. Hpb (Oxford) (2014) 16:528–33. doi: 10.1111/hpb.12171
Keywords: pancreatic ductal adenocarcinoma, radiotherapy, chemotherapy, surgery, overall survival
Citation: Wu L, Xu Y, Zhou Y, Zeng Z, Fan Y, Wang D, Wu W, Guo X, Lv M, Ouyang Y, Du S and Lou W (2023) Additional adjuvant radiotherapy improves survival at 1 year after surgical treatment for pancreatic cancer patients with T4, N2 disease, positive resection margin, and receiving adjuvant chemotherapy. Front. Oncol. 13:1109068. doi: 10.3389/fonc.2023.1109068
Received: 27 November 2022; Accepted: 27 June 2023;
Published: 18 July 2023.
Edited by:
James C. L. Chow, University of Toronto, CanadaReviewed by:
Antonella Argentiero, National Cancer Institute Foundation (IRCCS), ItalyCopyright © 2023 Wu, Xu, Zhou, Zeng, Fan, Wang, Wu, Guo, Lv, Ouyang, Du and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shisuo Du, ZHUuc2hpc3VvQHpzLWhvc3BpdGFsLnNoLmNu; Wenhui Lou, bG91Lndlbmh1aUB6cy1ob3NwaXRhbC5zaC5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.