- 1Dana-Farber Cancer Institute, Boston, MA, United States
- 2Harvard Medical School, Department of Medicine, Boston, MA, United States
- 3Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, United States
- 4Robert Wood Johnson Medical School, New Brunswick, NJ, United States
- 5Earle A. Chiles Research Institute, Providence Cancer Institute, Portland, OR, United States
- 6Virginia Mason Cancer Institute, Seattle, WA, United States
- 7Beth Israel Deaconess Medical Center, Boston, MA, United States
- 8Rush University Medical Center, Department of Internal Medicine, Chicago, IL, United States
- 9Ankyra Therapeutics, Boston, MA, United States
- 10Massachusetts General Hospital, Boston, MA, United States
- 11Laura and Isaac Perlmutter Cancer Center, New York University Langone Medical Center, New York, NY, United States
Introduction: High-dose interleukin-2 (HD IL-2) and pembrolizumab are each approved as single agents by the U.S. F.D.A. for the treatment of metastatic melanoma. There is limited data using the agents concurrently. The objectives of this study were to characterize the safety profile of IL-2 in combination with pembrolizumab in patients with unresectable or metastatic melanoma.
Methods: In this Phase Ib study, patients received pembrolizumab (200 mg IV every 3 weeks) and escalating doses of IL-2 (6,000 or 60,000 or 600,000 IU/kg IV bolus every 8 hours up to 14 doses per cycle) in cohorts of 3 patients. Prior treatment with a PD-1 blocking antibody was allowed. The primary endpoint was the maximum tolerated dose (MTD) of IL-2 when co-administered with pembrolizumab.
Results: Ten participants were enrolled, and 9 participants were evaluable for safety and efficacy. The majority of the evaluable participants (8/9) had been treated with PD-1 blocking antibody prior to enrollment. Patients received a median of 42, 22, and 9 doses of IL-2 in the low, intermediate, and high dose cohorts, respectively. Adverse events were more frequent with increasing doses of IL-2. No dose limiting toxicities were observed. The MTD of IL-2 was not reached. One partial response occurred in 9 patients (11%). The responding patient, who had received treatment with an anti-PD-1 prior to study entry, was treated in the HD IL-2 cohort.
Discussion: Although the sample size was small, HD IL-2 therapy in combination with pembrolizumab appears feasible and tolerable.
Clinical trial registration: ClinicalTrials.gov, identifier NCT02748564.
Introduction
Since the 1990s, it has been known that responses to high dose interluekin-2 (HD IL-2) in melanoma patients are low in frequency but exceptionally durable (1, 2). According to an analysis of 270 melanoma patients who participated in 8 clinical trials between 1985 and 1993, the overall response rate was 16%; importantly, nearly half (47%) of the responding patients survived 5 years or more (1). Due to the associated toxicity, mainly hypotension and capillary leak syndrome, HD IL-2 therapy is administered in the inpatient setting. Most patients are admitted for 5-6 days in the hospital to receive up to 14 doses of HD IL-2 per cycle, as tolerated. In contrast to HD IL-2, immune checkpoint inhibitors, especially PD-1 inhibitors, are generally well-tolerated and have favorable toxicity profiles. Therefore, in the modern era of front-line immune checkpoint inhibitor therapies, the application of HD IL-2 has become more limited, and it is typically reserved as a salvage therapy for patients whose cancer fails to respond to PD-1 inhibition.
Salvage therapy with HD IL-2 has been studied in patients with immune checkpoint-refractory disease in a registry called PROCLAIM, in which data were captured prospectively during HD IL-2 treatment. Among melanoma patients in the PROCLAIM registry who were treated with HD IL-2 after prior treatment with ipilimumab or an anti-PD1 antibody, the response rates were 21% (11 of 52 patients) and 22.5% (9 of 40 patients), respectively (3, 4). These response rates are comparable to historical controls (1, 2); these data suggest that HD IL-2 treatment retains its effectiveness in patients whose melanoma is refractory to ipilimumab or anti-PD-1 treatment. According to the PROCLAIM registry data, toxicity was manageable, although 1 of 57 patients who received prior anti-PD-1 developed pneumonitis requiring steroid therapy, suggesting that HD IL-2 can re-activate checkpoint inhibitor-like toxicity on occasion (4). Patients received an average of 8-9 doses of IL-2 per cycle, as expected, indicating that overall, these patients tolerated HD IL-2 as well as historical controls.
Concurrent therapy with immune checkpoint inhibitor plus HD IL-2 has been explored in clinical trials for treatment of melanoma and renal cell carcinoma recently, based on reported synergy between IL-2 based therapy and checkpoint blockade in preclinical models (5). We previously conducted a small prospective clinical trial of concurrent HD IL-2 plus ipilimumab (6). We found that 1 of 9 patients with melanoma responded to this combination. There were no new safety signals observed; however, expected IL-2-related side effects such as liver and kidney injury were more prolonged than usual, and systemic corticosteroids were required to treat immune-related adverse events in one-third of the subjects. Combining IL-2 with PD-1 is more attractive than ipilimumab, owing to the better safety profile and antitumor activity of PD-1 blockade in patients with melanoma. It is known that IL-2 production can be suppressed by the actions of the PD-1 checkpoint (7), and that modulation by PD-1 inhibitors can reverse this anergy (5). In humans with melanoma undergoing anti-PD-1 therapy, response is correlated with proliferation of intra-tumoral lymphocytes (8). We hypothesized that these tumor-infiltrating lymphocytes could be stimulated by IL-2 therapy. In the present study, we treated patients with unresectable or metastatic melanoma with the standard dose of pembrolizumab and escalating doses of IL-2 to determine the safety of the combination and to select a dose of IL-2 for further study in combination with anti-PD-1 therapy.
Methods
Patient selection
Adults with histologically confirmed unresectable stage III and IV melanoma and ECOG performance status 0-1 and normal cardiopulmonary and renal function were enrolled at three academic centers between 2017 and 2019. Main exclusion criteria were ocular melanoma, active brain metastases, active autoimmune disease, and use of concurrent systemic immunosuppressive therapy. Patients who had received prior treatment with IL-2 were excluded. All other prior therapies, including pembrolizumab, were allowed. The clinical protocol was approved by all local institutional review board (IRB) prior to patient accrual. All patients gave written informed consent to be treated.
Design
In this Phase Ib study, cohorts of 3 patients were treated with pembrolizumab (200 mg IV every 3 weeks) and escalating doses of IL-2 (6,000 or 60,000 or 600,000 IU/kg IV bolus every 8 hours). The primary endpoint was the (MTD) of IL-2 when co-administered with pembrolizumab. Ten participants were enrolled, and 9 participants were evaluable for safety and efficacy.
Treatment
Patients received pembrolizumab (200 mg flat dose IV) every 3 weeks, and a course IL-2 beginning with the second cycle of pembrolizumab (Figure 1). Each course of IL-2 consisted of 2 cycles of up to 14 doses each, as tolerated. After up to 3 courses of IL-2 plus pembrolizumab, patients could continue to receive pembrolizumab monotherapy for up to two years. Cohorts of 3 patients received IL-2 in a low, medium, or high dose (6,000, 60,000, or 600,000 IU/kg) IV bolus every 8 hours for up to 14 doses, as tolerated. A physical examination and laboratory tests (including CBC with differential and comprehensive metabolic profile and thyroid tests) were done at screening and every 3 weeks. Safety assessments were performed daily during hospitalization for IL-2 therapy. Adverse events (AEs) were evaluated and graded using NCI Common Toxicity Criteria v4.0. Dose reductions were not permitted for either drug. Both drugs were held and/or discontinued for high grade autoimmune toxicity. No intra-patient dose escalation was allowed. Imaging for tumor assessment was performed every 12 weeks. Response was assessed using RECIST criteria version 1.1 (9). Patients were considered evaluable for safety and efficacy if they received at least one dose of each study drug. The primary endpoint was defined as the (MTD) of IL-2 in combination with pembrolizumab. More than half of patients treated with high-dose IL-2 experience transient grade 3 toxicities; thus, dose limiting toxicity (DLT) was defined as any treatment-related grade 3 event that occurred during the first 6 weeks of study treatment and did not resolve to grade 2 or less within 14 days of onset. In addition, any treatment-related grade 4 or 5 toxicity that occurred during the first 6 weeks of study treatment was considered a DLT, with exceptions for specific reversible grade 4 events that are expected with IL-2 therapy: hypotension, decreased urine output, pulmonary edema, cytokine release syndrome and venous access complications. Descriptive statistics were used for clinical outcome and safety reporting. Blood samples were collected on all subjects for correlative analysis and tumor biopsies were optional. The protocol included a plan for an expansion to treat 3 additional subjects at the MTD for a total of 6 subjects treated at the MTD, and a Phase 2 portion with a target sample size of 48 patients; however, the sponsor terminated the study due to slow enrollment before the Phase 2 portion could begin.
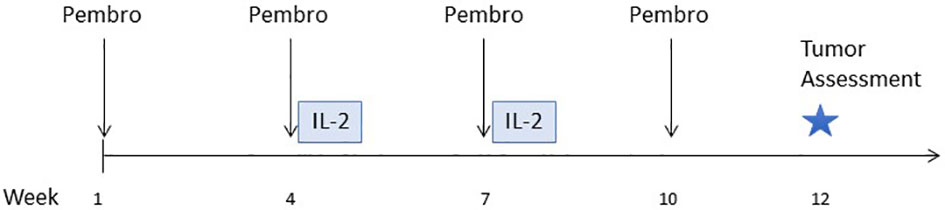
Figure 1 Study schema. Treatment began with pembrolizumab only for the first cycle. IL-2 therapy (2 cycles of up to 14 doses each) was given immediately following the second and third cycles of pembrolizumab.
Results
Patient characteristics
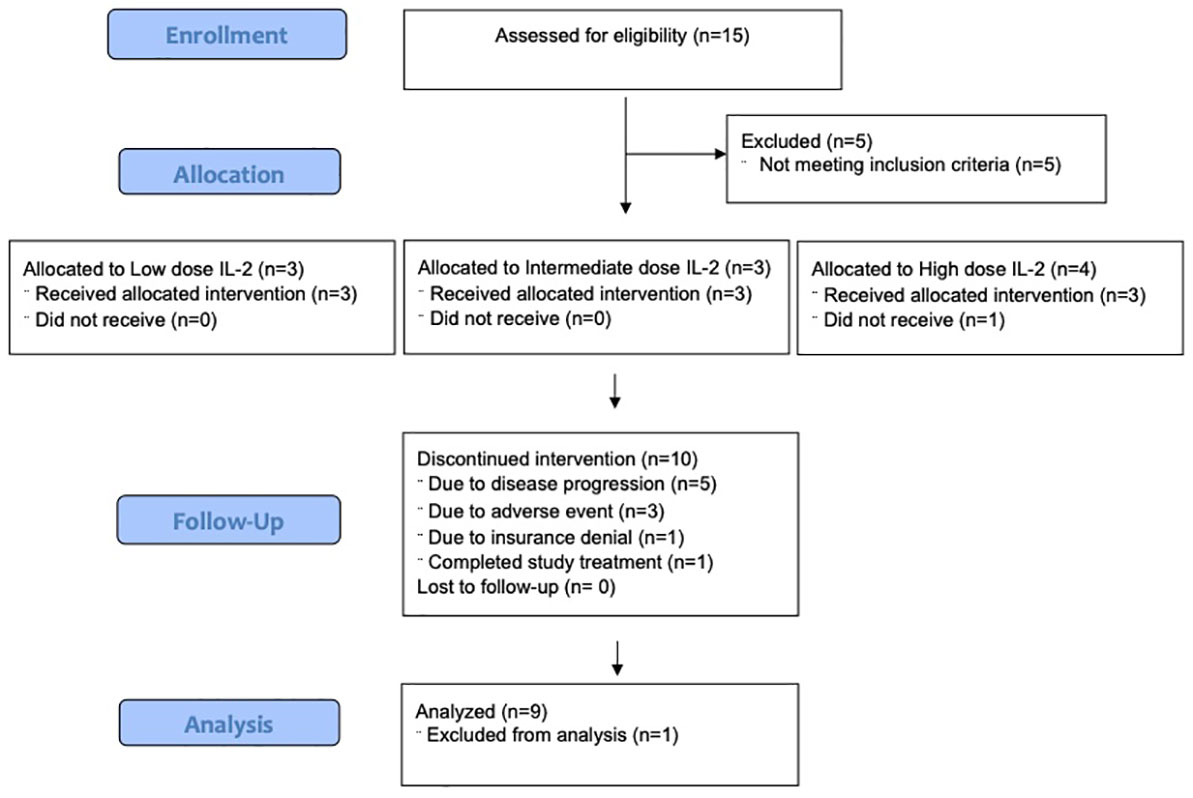
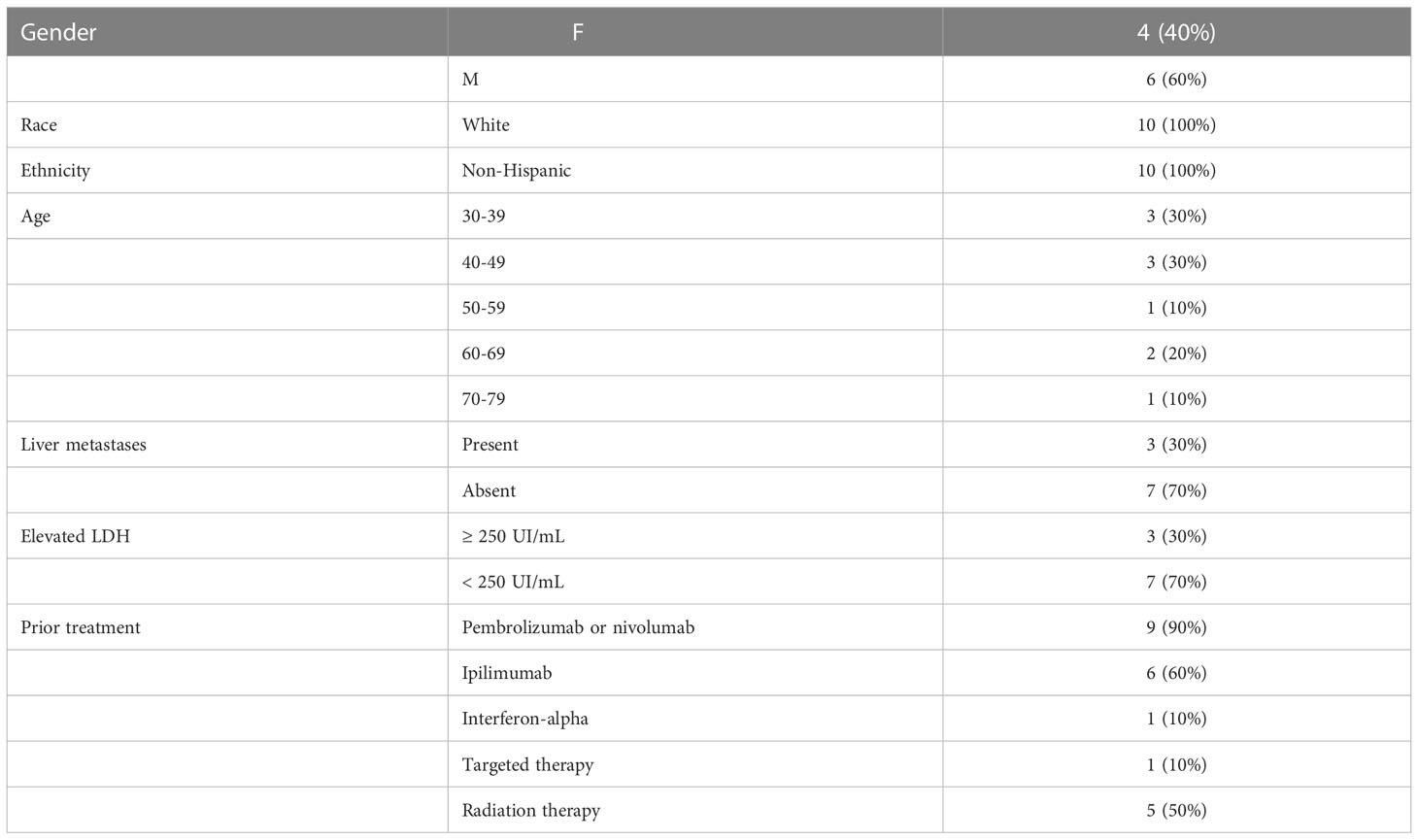
Fifteen patients were consented. Five patients did not meet eligibility criteria. Ten patients were enrolled, and 9 patients were evaluable for safety and efficacy (Figure 2). One patient began the first cycle of treatment but was unable to receive IL-2 due to denial of health insurance coverage, so the subject was removed from study. Baseline patient characteristics of the 10 patients who enrolled are summarized in Table 1. Most patients (9/10) received anti-PD-1 prior to study entry. Patients received a median of 42, 22, and 9 doses of IL-2, and a median of 8, 5, and 2 doses of pembrolizumab in the low, intermediate, and high dose cohorts, respectively (Table 2). One patient completed 2 years of treatment on study.
Safety
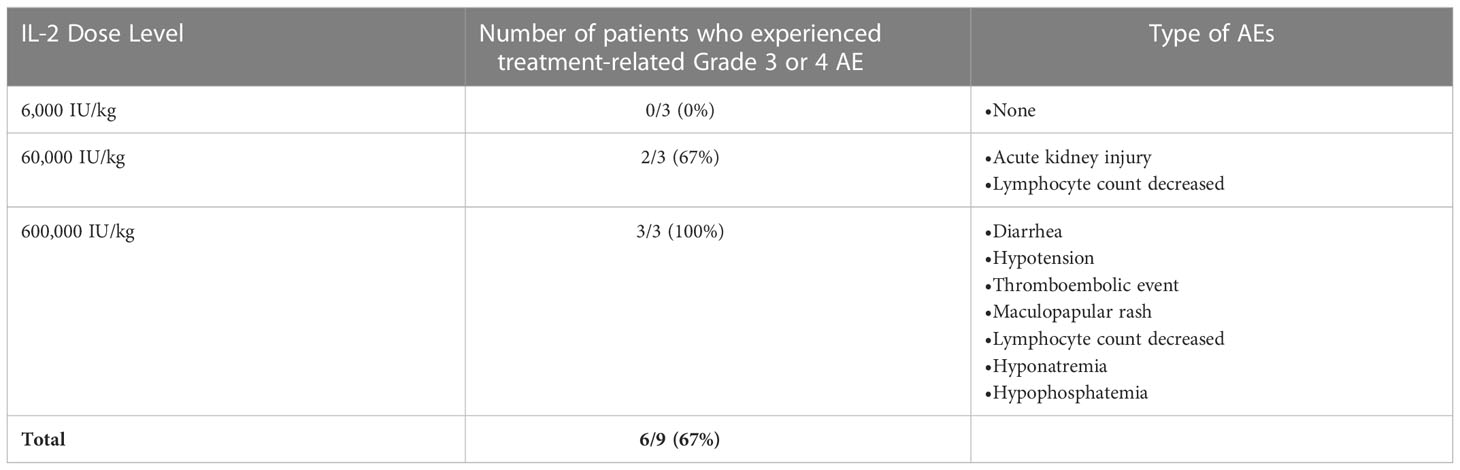
There were no treatment-related deaths. Six of the 9 evaluable participants (67%) experienced 19 grade 3 or 4 treatment-related adverse events (Table 3). There were more adverse events in the higher doses of IL-2 (Table 3). There were no (DLTs). Three patients discontinued both study drugs due to adverse events, which included a patient with nausea at the intermediate dose of IL-2 (60,000 IU/kg), and a patient with rash, which occurred at the high dose of IL-2 (600,000 IU/kg). A third patient, also treated at the high dose of IL-2, discontinued due to a combination of adrenal insufficiency and dyspnea not otherwise specified, and was treated with reater than 40 mg prednisone daily and inhaled steroids. No patient required steroids greater than 40 mg daily to treat an adverse event.
Efficacy
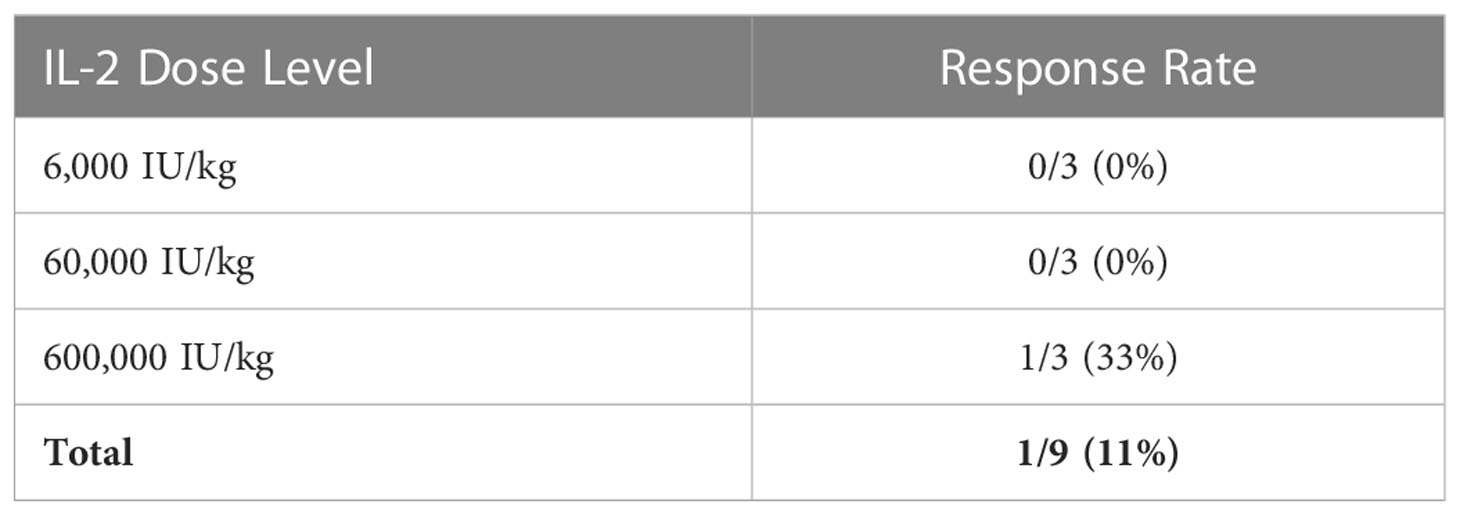
In the 9 evaluable participants, there was 1 partial response (11%; Table 4). Four participants had stable disease (44%) and 4 had progressive disease (44%). The partial response was observed in the patient who discontinued high dose IL-2 due to grade 3 rash, as above. His previous treatments consisted of nivolumab followed by anti-LAG-3 therapy, which ended 10 months and 1 month prior to study entry, respectively. The median follow-up time was 20.4 months. Two patients had durable progression-free survival, lasting 33 and 54+ months (Figure 3). At the time of this analysis, 8/9 participants have expired. The median overall survival (OS) was 20.4 months, with 1-year and 2-year OS rates of 6/9 (67%) and 4/9 (44%), respectively. One participant who had a reduction in tumor burden that was classified as stable disease remains alive 4.5 years after study entry. He had received ipilimumab in the adjuvant setting, but he did not receive treatment with PD-1 checkpoint antibodies prior to study entry. Only one fresh tumor biopsy was collected. Although blood samples were collected on all patients, samples from only 5 subjects could be located at the time of data analysis. Thus, no correlative samples were analyzed.
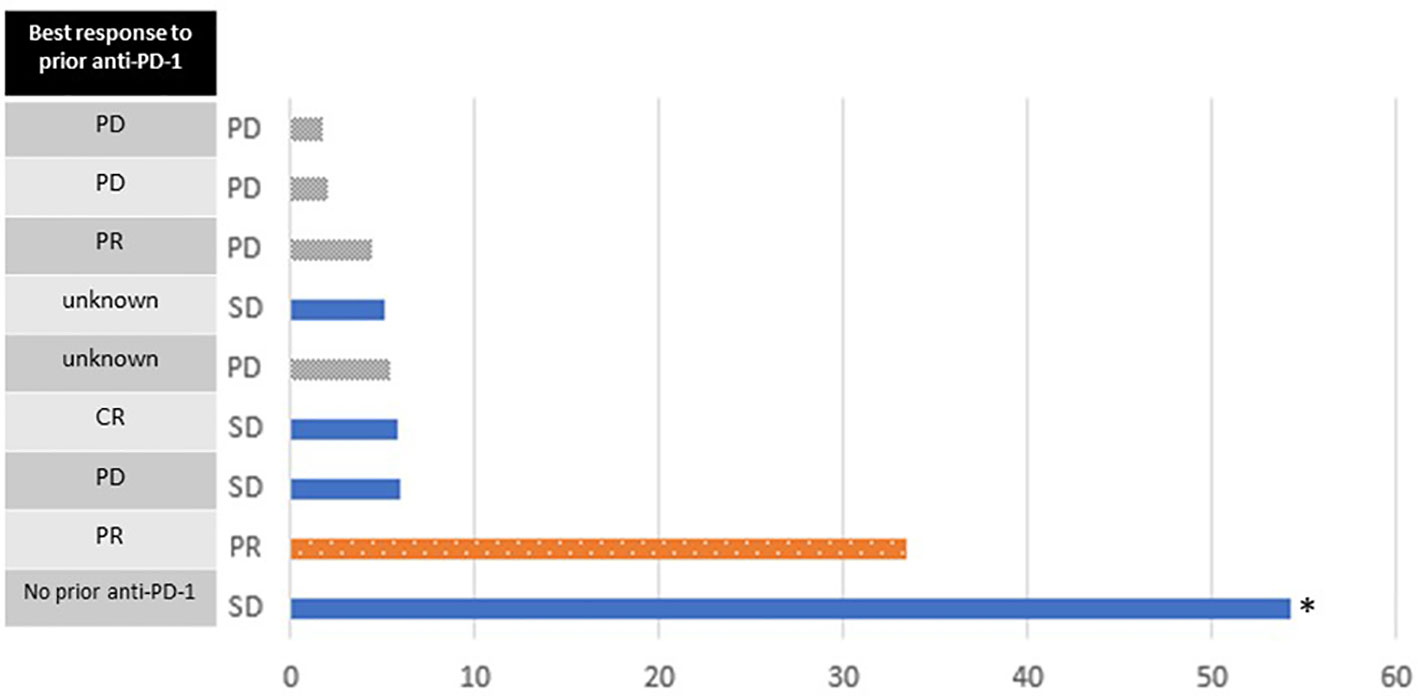
Figure 3 Swimmer plot of progression-free survival (months). Bars are colored according to the best response on study. Best response to prior anti-PD-1 treatment is shown on the left. The asterisk indicates data censored at the time of last follow-up.
Discussion
In this dose-escalation study of 9 evaluable patients, we demonstrated that treatment with a range of doses of IL-2 concurrent with pembrolizumab is feasible. No new safety signals were identified. The number of patients who experienced one or more serious treatment-related AEs increased with increasing doses of IL-2, which was an expected finding. There was one response observed (11%) in a patient with PD-1 refractory melanoma who was treated with HD IL-2. Another patient had stable disease that was durable for more than 4.5 years (censored at the time of last follow-up), without the need for further treatment. However, as he did not have prior PD-1 therapy, the control of his disease may be attributable to the pembrolizumab alone. This exploratory study was designed to determine the safety and feasibility of combination IL-2 and pembrolizumab, but the sample size was too small to make definitive conclusions on clinical responses. Patients in this study generally skewed younger than the average melanoma patient, and all but one patient had received anti-PD-1 therapy prior to study entry, reflecting the typical patient population that would be considered for salvage HD IL-2 therapy in the modern era.
There are two key problematic features of native IL-2 as a therapeutic agent, which are the short half-life, and the potent activation of regulatory T cells, especially at lower doses. Low doses of the native form of IL-2 have been reported to preferentially expand regulatory T cells (Tregs) which is mediated by the alpha subunit (CD25) of the IL-2 receptor. In a B16-F10 melanoma mouse model, treatment with high and intermediate doses of IL-2 inhibited tumor outgrowth compared to placebo, but treatment with low−dose IL-2 allowed more tumor outgrowth than the placebo (10). An expansion of Tregs was observed in the tumor with low dose IL-2, but not with intermediate or high dose IL-2, possibly owing to the fact that there is abundant expression of high-affinity IL-2 trimeric (CD25–CD122–CD132) receptors on Tregs as compared to effector T cells (11). In the current study, we observed that patients treated at lower doses were able to tolerate more doses of IL-2 (Table 2); however, no responses were observed at the low or intermediate doses of IL-2.
New variants of IL-2 designed to preferentially target CD8+ effector cells are in clinical testing (12, 13). Pegylation is another popular strategy to both extend the half-life of IL-2 and to avoid CD25 signaling. The pegylation confers selectivity for CD122 and CD132, also called the beta and gamma subunits (14, 15). Despite promising preclinical work, the addition of bempegaldesleukin to nivolumab did not result in improvement in response rate or progression-free survival compared to nivolumab alone in a randomized Phase 3 trial in melanoma patients (16, 17). Recent work in a chronic viral infection model demonstrated that IL-2 influences the differentiation of stem-like CD8+ T cells, creating distinct sets of PD-1+CD8+ effector T cells with superior antiviral activity as compared to PD-1 blockade treatment alone. CD25 engagement by IL-2 was shown to be critical for synergy between IL-2 and anti-PD-1 that was observed in these experiments (18), suggesting that the contribution of the alpha subunit of the IL-2 receptor is dynamic and complex, which may provide insight into the difficulty with targeting the IL-2 receptor therapeutically thus far.
Data availability statement
The corresponding author has left the institution; dataset can be available upon request to co-author TS, Director of Clinical Trials Office. Requests to access the datasets should be directed TS,dGtzMTNAY2luai5ydXRnZXJzLmVkdQ==.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board at Rutgers University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AS, BC, HK, JM, WS, and DM, contributed to the study concept, design, analysis and interpretation of data. AS and DM drafted the manuscript. AS, TS, WS, and DM analyzed and interpreted data and drafted the manuscript. AS, BC, JB, PH, CF, JF, HK, JM, and DM were involved in data acquisition. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study received funding from Merck with additional funding from Prometheus. The funder had the following involvement with the study: approval of study design and approval of manuscript for publication.
Acknowledgments
The authors would like to thank the patients who participated in this trial and their families, as well as the study teams at each site, especially Suresh Ramanathan. The authors acknowledge the members of the Cytokine Working Group for their input on the design and conduct of this study and the interpretation of the data, and in particular, the guidance of Dr. Kim Margolin. The authors acknowledge Nancy Gregory, RN of Prometheus for facilitating communication between the sites.
Conflict of interest
AS reports consulting fees from Oncosec and Instil Bio, and research funding to the institution from Biohaven Pharmaceuticals, Replimune, Morphogenesis, Shattuck Laboratories, and Checkmate Pharmaceuticals. BC reports consulting fees from Merck and Sanofi and research funding to the institution from BMS and Clinigen. HK reports being an employee of Ankyra Therapeutics and has received consulting fees from Castle Biosciences and Marengo Therapeutics. JM reports grants/research support – Amgen, AstraZeneca, Bristol Myers Squibb, Novartis, Polynoma, Sanofi, EMD Serono, Immunocore, Incyte, Macrogenics, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; advisory board member – Array BioPharma, Bristol Myers Squibb, EMD Serono, and Sanofi/Regeneron; consultant/independent contractor – Amgen and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA; honorarium – EMD Serono and Pfizer. DM reports consulting fees from BMS, Pfizer, Merck, Alkermes, Inc., EMD Serono, Eli Lilly and Company, Iovance, Eisai, Werewolf Therapeutics, Calithera Biosciences, Synthekine, Inc, Johnson & Johnson, and Aveo. He also reports research funding to the institution from BMS, Merck, Genetech, Pfizer, Elelixis, X4 Pharma, Alkermes, Inc, Checkmate Pharmaceuticals, and CRISPR Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CR, complete response; HD, high-dose; IV, intravenous; IL-2, interleukin-2; MTD, maximum tolerated dose; PR, partial response; PD, progressive disease; PBMC, peripheral blood mononuclear cell; Tregs, regulatory T cells; OS, overall survival.
References
1. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol (1999) 17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105
2. Alva A, Daniels GA, Wong MKK, Kaufman HL, Morse MA, McDermott DF, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunology Immunother (2016) 65(12):1533–44. doi: 10.1007/s00262-016-1910-x
3. Buchbinder EI, Gunturi A, Perritt J, Dutcher J, Aung S, Kaufman HL, et al. A retrospective analysis of high-dose interleukin-2 (HD IL-2) following ipilimumab in metastatic melanoma. J Immunother Cancer (2016) 4:52. doi: 10.1186/s40425-016-0155-8
4. Buchbinder EI, Dutcher JP, Daniels GA, Curti BD, Patel SP, Holtan SG, et al. Therapy with high-dose interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer (2019) 7(1):49. doi: 10.1186/s40425-019-0522-3
5. West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest (2013) 123(6):2604–15. doi: 10.1172/JCI67008
6. Silk AW, Kaufman HL, Curti B, Mehnert JM, Margolin K, McDermott D, et al. High-dose ipilimumab and high-dose interleukin-2 for patients with advanced melanoma. Front Oncol (2019) 9:1483. doi: 10.3389/fonc.2019.01483
7. Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol (2009) 182(11):6682–9. doi: 10.4049/jimmunol.0900080
8. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515(7528):568–71. doi: 10.1038/nature13954
9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1) Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
10. Zloza A, Dharmadhikari ND, Huelsmann EJ, Broucek JR, Hughes T, Kohlhapp FJ, et al. Low-dose interleukin-2 impairs host anti-tumor immunity and inhibits therapeutic responses in a mouse model of melanoma. Cancer Immunol Immunother (2017) 66(1):9–16. doi: 10.1007/s00262-016-1916-4
11. Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in t(reg) cell function. Nat Immunol (2016) 17(11):1322–33. doi: 10.1038/ni.3540
12. Rosen DB, Kvarnhammar AM, Laufer B, Knappe T, Karlsson JJ, Hong E, et al. TransCon IL-2 β/γ: a novel long-acting prodrug with sustained release of an IL-2Rβ/γ-selective IL-2 variant with improved pharmacokinetics and potent activation of cytotoxic immune cells for the treatment of cancer. J Immunother Cancer (2022) 10(7):e004991. doi: 10.1136/jitc-2022-004991
13. Nirschl CJ, Brodkin HR, Hicklin DJ, Ismail N, Morris K, Seidel-Dugan C, et al. Discovery of a conditionally activated IL-2 that promotes antitumor immunity and induces tumor regression. Cancer Immunol Res (2022) 10(5):581–96. doi: 10.1158/2326-6066.CIR-21-0831
14. Sharma M, Khong H, Fa’ak F, Bentebibel S-E, Janssen LME, Chesson BC, et al. Bempegaldesleukin selectively depletes intratumoral tregs and potentiates T cell-mediated cancer therapy. Nat Commun (2020) 11(1):661. doi: 10.1038/s41467-020-14471-1
15. Ptacin JL, Caffaro CE, Ma L, San Jose Gall KM, Aerni HR, Acuff NV, et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat Commun (2021) 12(1):4785. doi: 10.1038/s41467-021-24987-9
16. Diab A GH, Sandhu SK, Long GV, Ascierto PA, Larkin J, Sznol M, et al. First disclosure of efficacy and safety of bempegaldesleukin (BEMPEG) plus nivolumab (NIVO) vs NIVO monotherapy in advanced melanoma (MEL). European society of medical oncology; 2022 Vol. 33. Paris, France: Annals of Oncology (2022) p. S356–409. doi: 10.1016/annonc/annonc1059
17. Diab A, Tykodi SS, Daniels GA, Maio M, Curti BD, Lewis KD, et al. Bempegaldesleukin plus nivolumab in first-line metastatic melanoma. J Clin Oncol (2021) 39(26):2914–25. doi: 10.1200/JCO.21.00675
Keywords: melanoma, interleukin-2, cytokine, pembrolizumab, combination immunotherapy
Citation: Silk AW, Curti B, Bryan J, Saunders T, Shih W, Kane MP, Hannon P, Fountain C, Felcher J, Zloza A, Kaufman HL, Mehnert JM and McDermott DF (2023) A phase Ib study of interleukin-2 plus pembrolizumab for patients with advanced melanoma. Front. Oncol. 13:1108341. doi: 10.3389/fonc.2023.1108341
Received: 25 November 2022; Accepted: 19 January 2023;
Published: 09 February 2023.
Edited by:
Nihal Ahmad, University of Wisconsin-Madison, United StatesReviewed by:
Diwakar Davar, University of Pittsburgh Medical Center, United StatesUmang Swami, The University of Utah, United States
Copyright © 2023 Silk, Curti, Bryan, Saunders, Shih, Kane, Hannon, Fountain, Felcher, Zloza, Kaufman, Mehnert and McDermott. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann W. Silk, YW5uX3NpbGtAZGZjaS5oYXJ2YXJkLmVkdQ==
 Ann W. Silk
Ann W. Silk Brendan Curti
Brendan Curti Jennifer Bryan
Jennifer Bryan Tracie Saunders3
Tracie Saunders3 Michael P. Kane
Michael P. Kane Andrew Zloza
Andrew Zloza Howard L. Kaufman
Howard L. Kaufman David F. McDermott
David F. McDermott