- 1Key Laboratory of Basic and Applied Research of Zhuang Medicine Prescriptions, Guangxi University of Traditional Chinese Medicine, Nanning, Guangxi, China
- 2School of Second Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Zhuang Yao Pharmaceutical Engineering and Technology Research Center, Guangxi University of Traditional Chinese Medicine, Nanning, Guangxi, China
Gastric carcinomas have high morbidity and mortality. It produces no noticeable symptoms in the early stage while causing complex complications in its advanced stage, making treatment difficult. Palliative therapy aims to relieve the symptoms of cancer patients and focuses on improving their quality of life. At present, five palliative therapies for advanced gastric carcinomas are offered: resection, gastrojejunostomy, stenting, chemotherapy, and radiotherapy. In recent years, palliative therapy has been used in the clinical treatment of advanced gastric carcinomas and related complications because of its efficacy in gastric outlet obstruction and gastric bleeding. In the future, multimodal and interdisciplinary palliative therapies can be applied to control general symptoms to improve patients’ condition, prolong their lifespan and improve their quality of life.
1 Introduction
Gastric carcinoma is a malignant tumor that starts from the epithelial tissue of the gastric mucosa. It is one of the most common malignant tumors in the world and the fifth leading cause of cancer-related death. Its mortality among tumors ranks fourth in the world and it has burdened economic development and global health care (1, 2). Early gastric carcinomas incur no obvious symptoms, and the most common clinical symptoms in the advanced stage are non-specific weight loss, persistent abdominal pain, dysphagia, hematemesis, anorexia, nausea, early satiety, and dyspepsia. Patients with advanced diseases in local areas or metastatic disease usually have obvious symptoms such as abdominal pain, potential ascites, weight loss, fatigue, and possible gastric outlet obstruction (3). The incidence of gastric carcinomas has obvious regional and gender differences. The morbidity rate of gastric carcinomas in men is twice that of women and the Asia-Pacific region has a high concentration of gastric carcinomas incidence in men, while Asia holds the highest incidence of this disease in women (4). It is estimated that by 2040, the number of new cancer cases worldwide will exceed 28.4 million, an increase of 47% compared with 2020. The increase is more significant in developing countries and the pressure of treating cancer will intensify (5, 6).
The current standard treatment for gastric carcinomas is mainly surgery, which delays disease progression and reduces tumor recurrence by directly resecting the patient’s tumor, lymph nodes, and damaged organs (7). Statistics show that the five-year survival rate for patients receiving radical resection for early gastric carcinomas is up to 90% (8), but recurrence and metastasis can still be observed in 20-60% of patients (9, 10). Since there are no noticeable symptoms early in the onset of gastric carcinomas, 80% of patients were in the advanced stage when they were diagnosed, and the prognosis was extremely poor because they missed the right time for treatment (11, 12). Complications such as intestinal obstruction, anemia, dumping syndrome, and gastrointestinal dysfunction may occur after surgical treatment. Although postoperative radiotherapy and chemotherapy can effectively prevent the recurrence of postoperative metastasis of gastric carcinomas, it often brings unbearable side effects, such as adverse reactions in the gastrointestinal tract and bone marrow suppression (13). The main goal of palliative therapy is to improve patients’ quality of life, and it is widely used in the treatment of advanced tumors as it relieves the symptoms of patients and solves their physical, psychological, and social problems, to improve the life of patients and their families (14). We focus on the application of palliative therapy in advanced gastric carcinomas, including the clinical application of these therapies for different advanced gastric carcinomas and related complications, with the aim to provide new plans for palliative therapy in the treatment of advanced gastric carcinomas and promote its application.
2 Significance of palliative therapy
The World Health Organization defines palliative care as a patient, family, or caregiver-centered medical service that focuses on the optimal management of painful symptoms (15). The long-term and repeated hospitalization, surgery, radiotherapy, multiple chemotherapy, and symptomatic treatment have seriously affected the psychological state of the patients. Palliative therapy is conducive to the smooth implementation of anti-cancer treatment and the best effect of treatment plans, especially for patients with gastric carcinomas chemotherapy (16). Relevant researchers believe that earlier active palliative therapy helps better control the general symptoms, prolong the survival of patients, and improve their living quality (17). At present, the main methods of palliative therapy for patients with gastric carcinomas include palliative resection (18), gastrojejunostomy (19), stent placement (20), chemotherapy (21) and radiotherapy (22).
3 Palliative gastrectomy
Palliative resection of gastric carcinomas refers to the surgery conducted to release complications and relieve symptoms for patients who cannot receive radical gastrectomy for various reasons, or for patients with potentially life-threatening complications, such as gastrointestinal bleeding, perforation, or obstruction (23). For patients with metastatic gastric carcinomas who have received first-line treatment, palliative resection can directly remove the obstruction, prevent the occurrence of critical complications such as gastric perforation and gastric bleeding, and effectively relieve symptoms such as obstruction, bleeding, pain and nausea, and might prolong the survival of patients when distant lymph node metastasis, liver metastasis, pelvic planting, peritoneal spread, invasion of surrounding organs occur (24, 25). A clinical study has found that palliative gastrectomy exerts a significant impact on the survival and short-term survival rate of patients with advanced gastric carcinomas (26). In addition, the surgical complications are controllable (27). Compared with patients who did not undergo palliative gastrectomy, the overall survival rate of those who received the palliative resection was significantly higher (28, 29). It was found (30) that among patients with locally advanced diseases without distant metastases, those who underwent palliative gastrectomy had a higher short-term survival rate. Relevant data of palliative total gastrectomy (PTG) for stage IV proximal gastric carcinomas suggested that compared with no operation, laparotomy and jejunostomy, the PTG of stage IV proximal gastric carcinomas better prolongs the survival time and improves the quality of life (31, 32). However, despite the feasibility and safety of PTG, it is necessary to select appropriate patients with stage IV proximal gastric carcinomas for this therapy and thoughtful preparations should be made during the perioperative period (33).
In addition, related studies suggest that age is closely related to the survival rate after palliative gastrectomy (34). Hsu et al. (35) found that in palliative gastrectomy, factors such as younger age (below 58 years old), better preoperative nutritional status, less lymph node involvement and postoperative chemotherapy had a significant impact on the improvement of postoperative survival rate. In the gastric carcinomas trial conducted by Dutch scholars (36), it was found that patients younger than 70 years old with only one metastatic site had longer overall survival after palliative resection and could benefit significantly from palliative gastrectomy. Some researchers also proposed the combination of palliative resection and chemotherapy. A recent clinical observation reported that a patient with advanced metastatic gastric cancer who received Nivolumab combined chemotherapy after palliative gastrectomy had no recurrence 32 months after surgery, and the patient achieved complete remission of symptoms and prolonged survival (37). Seo HS et al. (38) found that the 3-year survival rate of stage IV gastric cancer patients undergoing palliative gastrectomy after chemotherapy was higher than that of patients undergoing palliative gastrectomy alone (61.1 vs 16.2%, p = 0.003). These studies have shown that the combination of palliative resection and chemotherapy can improve the survival rate of patients compared with monotherapy (39).
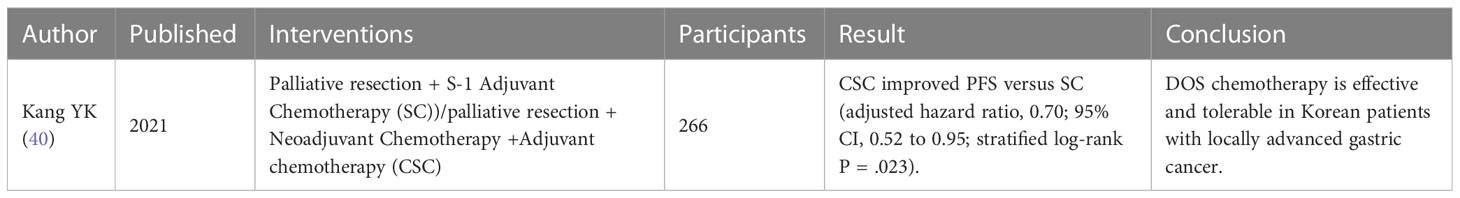
Compared with gastric carcinomas patients without palliative resection, the general symptoms of patients after palliative gastrectomy were effectively relieved, and the short-term survival rate and postoperative survival time were improved (32). Although gastric carcinomas resection can alleviate the general symptoms of patients to a certain extent and improve the span and quality of their life, it is still necessary to pay attention to factors such as the age of the patient, the metastatic site of gastric carcinomas, lymphatic involvement, and patient’s preoperative nutrition because they also affect the prognosis of gastric carcinomas resection (35). In future studies, further clinical studies should be conducted on the related factors, and the post-resection chemotherapy and the combination of palliative resection and chemotherapy. Recent clinical trials of palliative gastrectomy on palliative treatment of advanced GC are shown in Table 1.
4 Gastrojejunostomy
Gastrojejunostomy (GJ) is a standard palliative operation for gastric outlet obstruction (GOO), a common complication of gastric carcinomas, and is also the preferred treatment for malignant GOO in patients with advanced gastric carcinomas (41). Relevant clinical trials have demonstrated that the incidence of stent-related complications, re-obstruction, and re-intervention of gastrojejunostomy was low (42). Ouchi K et al. (43) investigated 95 patients to study the indicators of quality of life such as survival and hospital-free survival (HFS) of patients undergoing palliative gastric carcinomas surgery. The results showed that patients who received palliative therapies such as GJ and total gastrectomy had a moderate postoperative survival rate in the absence of peritoneal dissemination and adjacent peritoneal metastases. In improving the success rate of GJ, Prager LP et al. (44) conducted a study on the establishment of complete suture of GJ in machine-assisted Roux-en-Y gastric bypass and found that machine-assisted surgery can shorten the operation and improve suture, which is conducive to the recovery of the incision and the decrease of the risk of gastrojejunostomy stenosis as it reduces scar stenosis at the anastomotic point. In a retrospective study, investigators found that patients older than 80 years had a higher incidence of delayed gastric emptying and subsequent aspiration pneumonitis after GJ. Patients with gastric carcinomas induced mGO0 had an extremely poor prognosis, and the median survival time after gastrojejunostomy was less than 2 months (45), which suggests that gastrojejunostomy is not the best choice for elderly patients with gastric carcinomas.
Traditional GJ uses upper midline or subcostal incision, its cut is large and the safety and efficacy are low (46). With the advancement of laparoscopic techniques, recent studies have found that endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ) had a smaller incision, fewer postoperative adverse events, and was safer and more effective (47–49). Boghossian M et al. (50) compared the efficacy of endoscopic ultrasound-guided gastroenterostomy (EUS-GE), duodenal stent (DS) placement, and surgical gastrojejunal (SGJ) therapies in the treatment of malignant GOO. It was found that compared with DS and SGJ, EUS-GE had a higher clinical success rate in the treatment of malignant GOO, lower incidence of serious adverse events and 30-day mortality, and shorter hospitalization. On W et al. (51) analyzed patients with gastric outlet obstruction who underwent continuous endoscopic ultrasound-guided gastroenterostomy (EUS-GJ) between August 2018 and March 2021, and found that the success rate of surgery was as high as 92%, the mean score of the gastric outlet obstruction scoring system was improved compared with SGJ (2.52 vs 0.68, p<0.01), and only 8% of patients reported adverse events, all of which were due to improper stent deployment and incurred gastric defect that requires endoscope to repair. This indicated that EUS-GJ could effectively improve the symptoms of patients with gastric outlet obstruction, and was safer and more efficient. When EUS-GJ is selected in future clinical practices, experienced endoscopists should be appointed to reduce the incidence of adverse events and the rate of reoperation.
The effect of GJ is not as obvious as that of total gastrectomy, but for patients with GOO, GJ can effectively improve their nutrition, avoid further obstruction, and reduce the occurrence of complications (52). Clinically, the overall condition of the patient should be comprehensively evaluated, the surgical standard should be defined, unnecessary morbidity should be avoided, cost-effective treatment should be achieved, and effective palliative therapy and suitable surgical methods should be selected (53). For patients with long life expectancy and good physical status, GJ is a preferred palliative therapy with a low rate of reintervention (54). It should be noted that the prognosis of GJ in elderly cancer patients is poor, and the survival time is short. The hospital should inform patients and their families of the risks, and ensure smooth communication between doctors and patients (55). In addition, with the advancement of science and technology, machine assistance such as endoscopes and ultrasound in surgery to improve precision, shorten the operation time, reduce the incidence of adverse events, relieve the pain, and promote the standardization of the operation (56). However, there are few relevant clinical studies at present, and palliative surgery research on this aspect can be increased in the future. Recent clinical trials of gastrojejunostomy on palliative treatment of advanced GC are shown in Table 2.
5 Endoscopic stent placement
In patients with in-progress and advanced gastric carcinomas, obstructions, such as gastric outlet obstruction and malignant intestinal obstruction are common complications (58). The main symptoms include pain, nausea, vomiting, abdominal distension, and decreased oral intake, which can lead to dehydration and malnutrition, undermining the quality of life of patients (59). ES is a palliative therapy for gastric carcinomas induced GOO with the significant advantage of a non-invasive approach (60). The stent can directly clear the digestive tract obstruction caused by gastric carcinomas minimize the pain of the patient and helps resume the patient’s normal diet (61) and improve their lives. Compared with GJ, ES has lower postoperative morbidity. However, secondary obstructions may reoccur due to tumor growth in ES, and the risk of re-operative intervention is higher than that of GJ, thus ES is preferred in elderly patients with advanced gastric carcinomas or multi-organ dysfunction (62, 63).
Self-expanding metal stent (SEMS) is the most common type of expandable stent characterized by softness and good expandability, and it is safe and effective for various gastrointestinal strictures (64). Jin et al. (65) investigated 207 patients who underwent metal stent implantation for gastric carcinomas induced GOO and it was found that the clinical success rate of stent implantation in all patients was 97%, the cumulative survival time reached 153 days, and the incidence of serious complications was only 1%, which indicated that metal stent implantation has clinical efficacy in the palliative therapy of GOO in patients with gastric carcinomas. In a study of the effect of endoscopic placement of SEMS or open gastric division gastrojejunostomy on the quality of life, researchers found that stenting can relieve the symptoms of pyloric obstruction in the short term and significantly improve the quality of life of patients (66). Krishnamoorthi et al. (67) compared the clinical success rate, GOO recurrence rate and safety indicators among duodenal SEMS, GJ and EUS-GE, and the results have shown that the clinical curative effect and clinical success rate of the three were similar. EUS-GE had a lower GOO recurrence rate (P=0.0036), and duodenal SEMS had a lower rate of procedure-related bleeding but a higher rate of reintervention (P=0.041). Other studies have also noted that SEMS placement is the optimal treatment for malignant GOO because it is less invasive. SEMS placement is more effective than palliative GJ in terms of earlier time to restoration of oral intake and a shorter hospital stay for patients with malignant gastric outlet obstruction (68, 69). The researchers compared the effects of GJ and SEMS as two palliative therapies for malignant GOO. They found that both methods were effective, but SEMS had a more significant short-term effect and thus it was more suitable for gastric carcinomas patients with short expected survival time (70). In terms of complication research, some researchers used the Ponsky-Gauderer technique to insert a ConfloTM PEG tube into the second part of the duodenum. The patient had an excellent tolerance of enteral nutrition and effective symptom relief and regained some weight without postoperative complications (71).
The palliative therapy of malignant outlet obstruction with stent implantation can significantly improve patients’ life, and this treatment has a high success rate especially for elderly patients with advanced gastric carcinomas and multiple organ dysfunction. Most of the implanted stents currently used in clinical use are metal stents. Metal stents are less invasive and thus can be further promoted. Endoscopic ultrasound-guided stent implantation is even safer, and it allows patients to diet after surgery and shortens their hospitalization. However, for obstruction caused by tissue or tumor growth, stent implantation has a higher reoperation rate and reintervention rate than gastrojejunostomy, therefore, it is more suitable for patients with shorter life expectancy. In addition, current studies suggest that few complications of stent implantation are identified and only limited relevant clinical research data have been collected, which means more samples should be involved in future research. Recent clinical trials of endoscopic stent placement on palliative treatment of advanced GC are shown in Table 3.
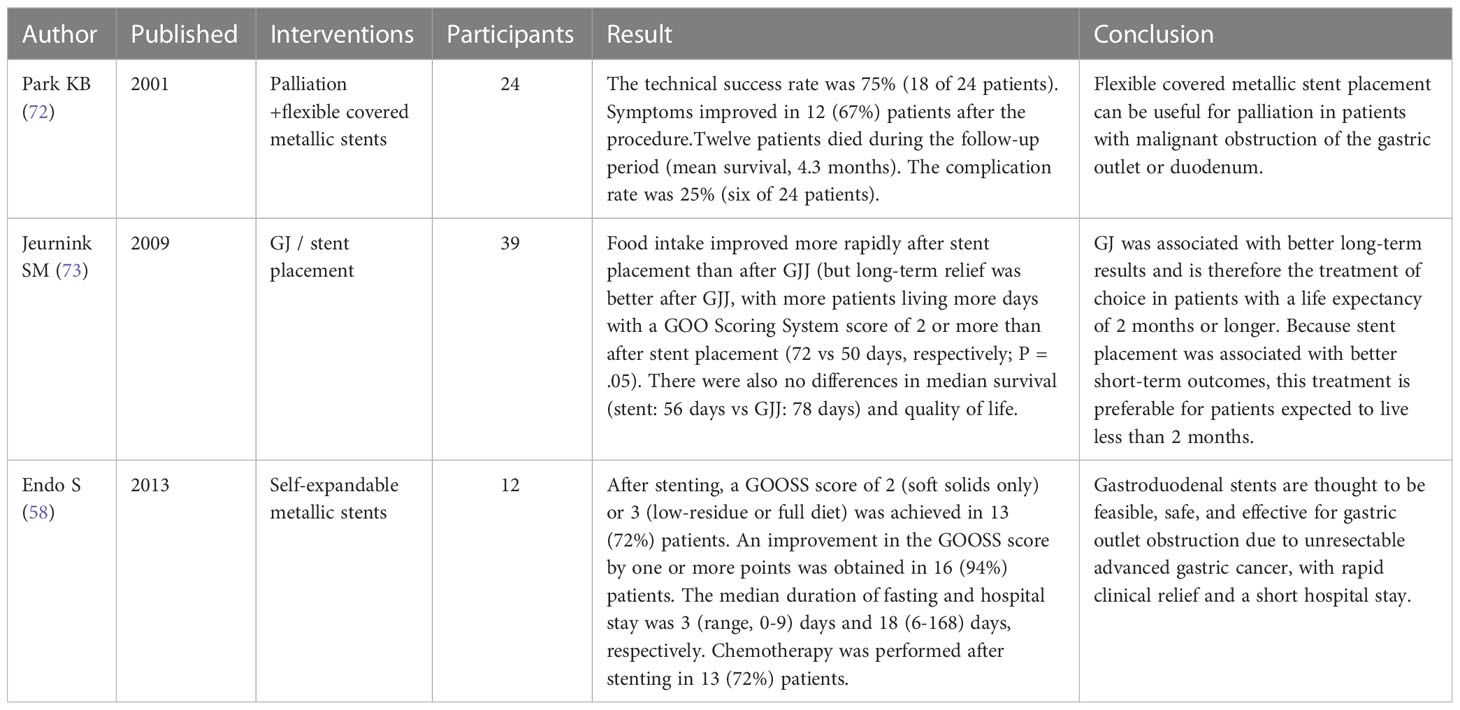
Table 3 Recent clinical trials of endoscopic stent placement on palliative treatment of advanced GC.
6 Palliative chemotherapy
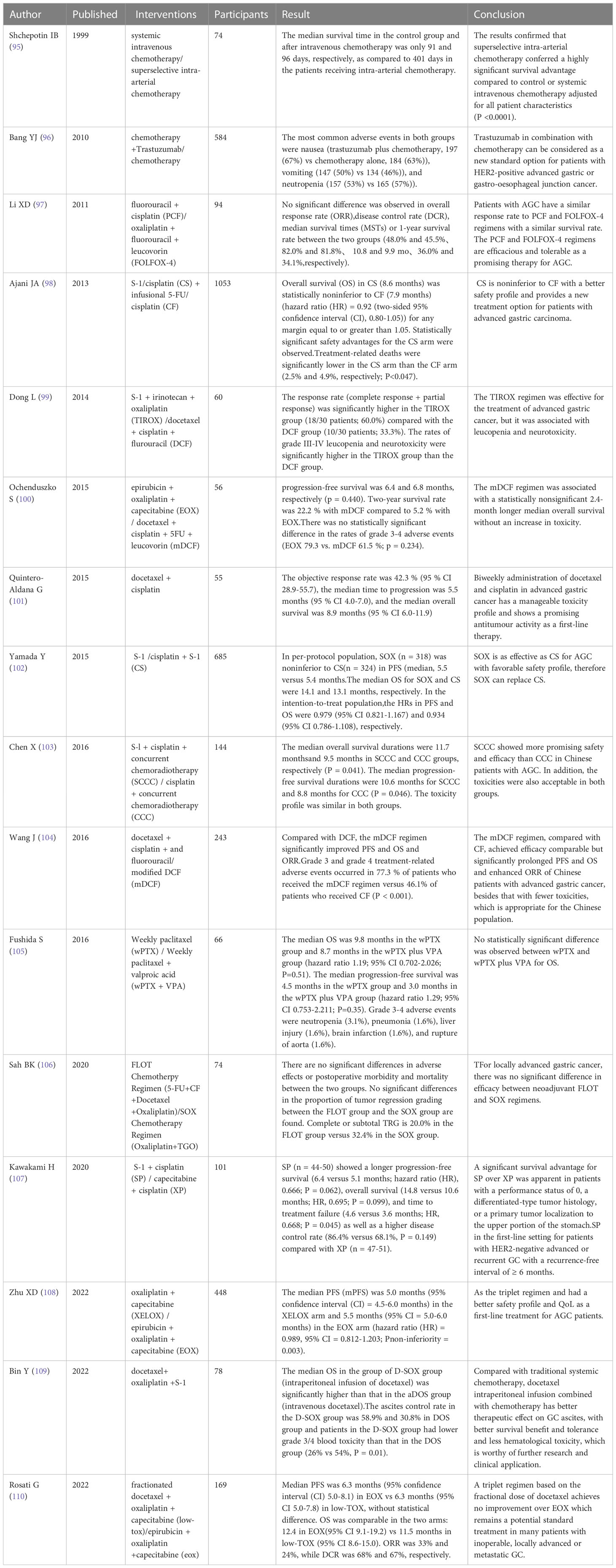
For patients with unresectable advanced gastric carcinomas, recurrent gastric carcinomas, and metastatic gastric carcinomas, systemic chemotherapy has become the standard treatment. Systemic chemotherapy is a kind of palliative therapy that aims to provide symptom relief and prolong survival (74). Treatment guidelines recommend a combination of palliative chemotherapy and chemotherapy drugs as first-line treatment because the therapy is non-invasive (75). For patients in the progression of gastric carcinomas, chemotherapy can reduce the postoperative recurrence rate (76). For patients with advanced gastric carcinomas, early palliative therapy combined with standard chemotherapy can deliver benefits to themselves, their families and society as the chemotherapy after surgery has a more significant impact on their survival (77). Currently, the most widely accepted standard palliative chemotherapy regimen worldwide is the combination of 5-fluorouracil (5-FU) and platinum analogs, in which 5-FU can be replaced by S-1 or capecitabine, and cisplatin can be replaced by oxaliplatin (74). Countries in East Asia typically use standard chemotherapy regimens of S-1 or capecitabine & oxaliplatin based on the ACTS-GC (78) or CLASSIC (79) trials for palliative chemotherapy.
Research data have proved that palliative chemotherapy for advanced gastric carcinomas can improve the survival rate of patients, reduce the burden of locally advanced tumors, and relieve common clinical symptoms such as pain, dysphagia, and malignant obstruction (80). Results of a multicenter study in Japan showed that patients with stage Il or Ill gastric carcinomas who underwent gastrectomy with extended (D2) lymph node dissection had 10% higher overall survival after adjuvant S-1 therapy than those who just underwent surgery (80.1% VS 70.1%) (78, which indicates that S-1 is an effective adjuvant therapy for patients with D2 resection of locally advanced gastric carcinomas in East Asia (81).
In addition to standardized palliative chemotherapy regimens, new palliative chemotherapy regimens are also explored. Japan Clinical Oncology Group (JCOG) conducted a multicenter phase II trial to evaluate the safety and efficacy of preoperative chemotherapy S-1&cisplatin combined with gastrectomy in plastic gastritis (type 4) or large ulcero-invasive-type (type 3) gastric carcinomas patients. The results showed that the treatment completion rate of this therapy reached 73.5%, higher than the threshold of 45%, and treatment-related death (TRD) was 2%, confirming that the therapy was safe and effective (81). Zhu YJ et al. (82) found that acupuncture therapy helped relieve symptom burden and reduce toxic and side effects in patients receiving palliative chemotherapy.
Peritoneal metastasis refers to the formation of peritoneal tumors in the abdominal cavity, and GC patients with peritoneal metastasis generally have a poor prognosis (83). In the past few decades, many techniques have been applied for the treatment of GC patients with peritoneal metastasis, such as repeated intraperitoneal chemotherapy combined with systemic chemotherapy, and pressurized intraperitoneal aerosol chemotherapy (PIPAC), with considerable clinical efficacy (84). Several phase II trials and one phase III trial conducted by Kobayashi et al. (85) confirmed that repeated intraperitoneal chemotherapy based on taxanes combined with systemic chemotherapy had little toxicity in the treatment of gastric carcinomas with peritoneal metastases, and the 1-year overall survival rate of patients ranged 67% to 91%, suggesting the safety and feasibility. Recent studies also found that pressurized intraperitoneal aerosol chemotherapy was a safe and feasible treatment with low in-hospital morbidity and mortality, and it could effectively improve the quality of life of patients with peritoneal metastases (86). Alyami et al. (87) performed a retrospective analysis on patients with gastric carcinomas peritoneal metastasis (PM) treated with PIPAC. The results indicated that the overall complication rate and major complication rate (CTCAE-III, IV) was 6.1% and 3.1%, and 14.3% of the patients could receive tumor resection during the treatment, which shows that PIPAC is a safe and effective drug delivery system for the treatment of PM in gastric carcinomas. Based on the retrospective analysis, it was found that for the majority of GC patients with unresectable PM, PIPAC was a well-tolerated option providing good quality of life stability in terms of symptom relief (88). These studies provide new ideas for the chemotherapy of gastric carcinoma peritoneal metastasis. In future research, we can further strengthen the exploration of new technologies to identify their potential and possibilities. For example, these chemotherapy methods can be used as a “neoadjuvant” strategy to qualify those unfit GC patients for gastric carcinomas resection.
The effectiveness of palliative chemotherapy in elderly patients with gastric carcinomas has been receiving much attention. Available data suggest that combination chemotherapy can be an option for elderly patients with metastatic gastric carcinomas classified as not frail according to Comprehensive Geriatric Assessment (CGA) (89). ShengLi He et al. studied the use of capecitabine “rhythmic” chemotherapy in elderly patients with gastric carcinomas and they found that the disease control rate (DCR) of chemotherapy exceeded 50% (95% CI 25.7–67.8) at 8 weeks, and there was no report of IV hematological toxicity or febrile neutropenia, suggesting that palliative chemotherapy is safe and effective in patients with advanced gastric carcinomas (90). Liao et al. divided 306 patients receiving palliative chemotherapy into two groups at the age of 70 years and compared the clinical demographics, prognosis and hematological toxicity of chemotherapy between the two groups. It was found that the progression-free survival and overall survival were the same and the incidence of hematological toxicity was similar (91), which suggests that age is not a limiting condition for palliative chemotherapy in elderly patients with metastatic gastric carcinomas.
Palliative chemotherapy can improve the patient’s symptoms and quality of life, and is considered a safe and promising treatment for gastric carcinomas (92). In addition to studying existing palliative chemotherapy regimens, research should also be done on the role of new palliative regimens that combine palliative chemotherapy with other treatments for patients with advanced gastric carcinomas (93). The condition of patients with advanced gastric carcinomas is complex and they are often complicated with peritoneal metastasis, thus age and physical condition should be considered in the selection of palliative chemotherapy. Research on the selection of intraperitoneal chemotherapy regimens, follow-up treatment after failure of initial treatment, conversion surgery after control of peritoneal metastases and its survival effect are remains insufficient,and there is still room for improvement (94). PIPAC may be a feasible option for relieving symptoms of unresectable PM but more random test research is needed. For elderly and frail patients, their overall condition should be accessed and lower-intensity palliative chemotherapy can be used. Further prospective, randomized, controlled trials can be conducted in the future to verify the results. Recent clinical trials of chemotherapy on palliative treatment of advanced gastric cancer are shown in Table 4.
7 Palliative radiotherapy
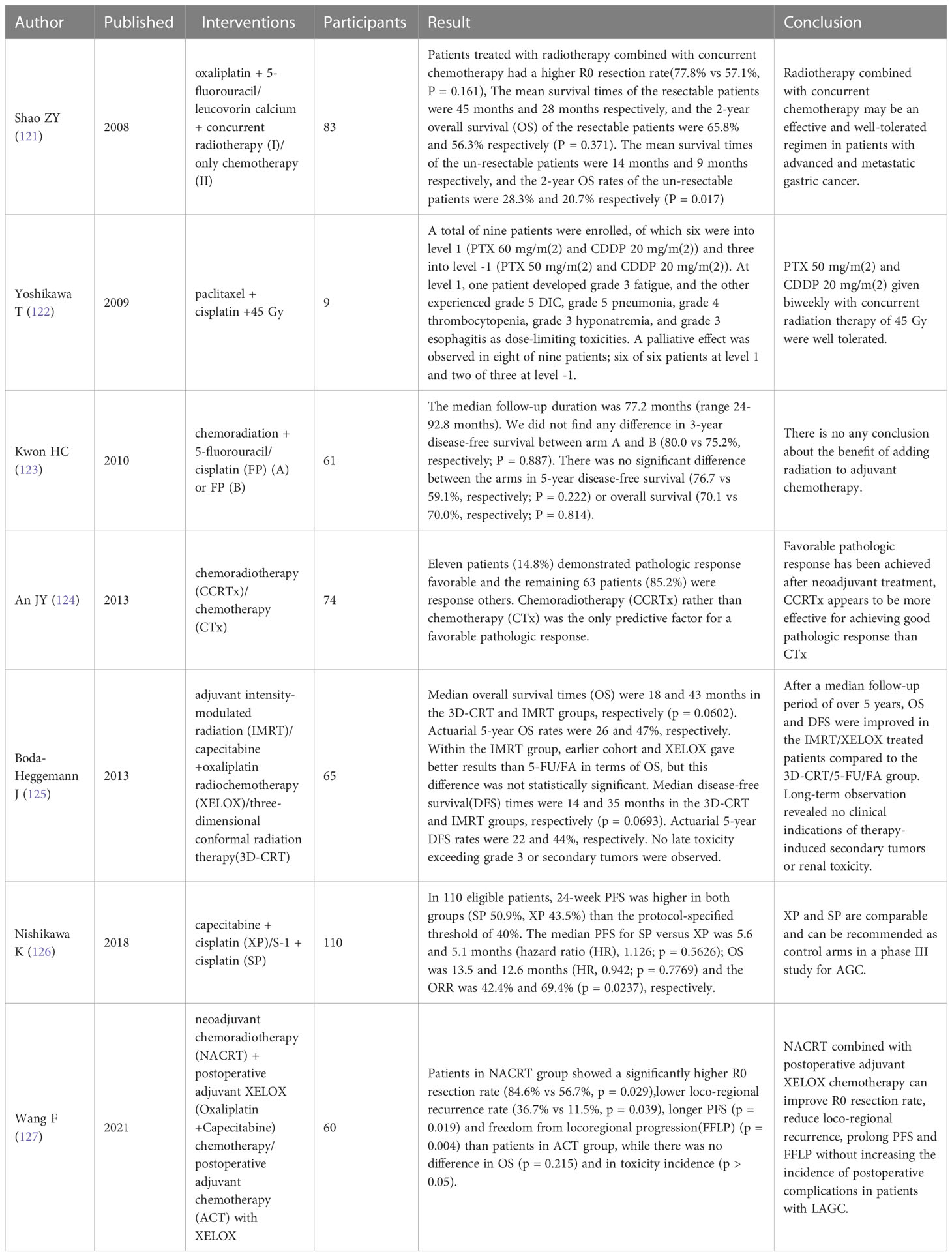
Palliative radiotherapy refers to the application of radiotherapy to the primary tumor and metastases of advanced malignant tumors to relieve pain, improve symptoms, and prolong life (111). Palliative radiotherapy for advanced GC patients is safe and effective. It is well-tolerated by patients and can greatly improve the quality of life of patients with poor prognoses (112).
RT has high efficacy for local gastric carcinomas, and its total effective rate of treating bleeding, pain and obstructive symptoms can reach 74%, 67% and 68% respectively. RT works for elderly patients who are not suitable for chemotherapy, patients with poor performance status, or patients receiving chemotherapy (113). RT has the most obvious curative effect in the treatment of gastric bleeding (114). Studies have found that for patients with unresectable advanced gastric carcinomas, RT is minimally invasive which can achieve hemostasis in a short time and effectively treat gastric carcinomas with gastric bleeding and obstruction (115). In addition, multi-center studies have confirmed that palliative radiotherapy had considerably improved mildly toxic anemia (116).
Although radiation therapy has a certain effect on gastric hemostasis, the mechanism is not clear, and the radiation dose needs to be further determined (117). The researchers used low-dose 6Gy to treat gastric bleeding, and the results suggest that LSP-RT (low-dose, short-course palliative RT) for the treatment of GC bleeding is safe, effective, and reproducible in the case of rebleeding. This treatment may help improve the quality of life of patients (118). Asakura et al. used RT at a dose of 30 Gy to moderate bleeding in advanced gastric carcinomas, achieving hemostasis in 73% of patients. Considering the median actuarial time to rebleeding (3.3 months), this tiered dose regimen appears to be adequate, especially for patients with poor prognoses (119).
Hemorrhage and obstruction are common complications in patients with advanced gastric carcinomas. In clinical practice, RT is used to treat bleeding and obstruction in advanced gastric carcinomas (120). However, the number of clinical observations on the relationship between radiation dose and rebleeding is relatively small, and more randomized controlled trials are needed TO determine the optimal dosage and distribution scheme. Recent clinical trials of radiotherapy on palliative treatment of advanced gastric cancer are shown in Table 5.
8 Conclusions and directions for future development
The occurrence of GC is occult, and the best way to improve advanced gastric carcinomas is prevention based on early screening. When gastric carcinomas progress to advanced stage, early and active palliative therapy as well as multi-modal and interdisciplinary methods should be applied to control general symptoms to improve patients’ condition, their survival and their quality of life. When palliative resection is used to treat advanced gastric carcinomas, attention should be paid to the age of the patient, the metastatic site and the number of cancers. If the tumor is limited to only one metastatic site and the patient is younger than 70 years old, patients can have a good prognosis with resection. Stenting can quickly relieve the obstruction of patients and improve their quality of life in a short period, but the complications of stenting and the risk of reoperation should be noted. Further well-designed randomized clinical studies can be conducted to compare different techniques related to palliative surgery, and different palliative therapy methods can also be used in combination to improve the treatment effect of palliative therapy.
Author contributions
AH and Y-ZP were responsible for the study concept and design, as co-corresponding author. X-FL and Y-HL drafted the manuscript together, equal contribution, listed as co-first author, and all authors revised it for important intellectual content. The work reported in the paper has been performed by the authors, unless clearly specified in the text. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Guangxi University of Chinese Medicine School Xinglin young talents (2022C035), Innovation Team of Guangxi University of Chinese Medicine for Inheriting Gui School of Traditional Chinese Medicine - Innovation Team for Clinical Research and Application of Du-related Diseases in Zhuang Medicine (2022A003), and Qihuang Scholar Cultivation Program of Guangxi (Y-Z P). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We thank AH and Y-ZP helpful suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep (2019) 21(8):67. doi: 10.1007/s11912–019–0820–4
2. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci (2020) 21(11):4012. doi: 10.3390/ijms21114012
3. Dreznik A, Purim O, Idelevich E, Kundel Y, Sulkes J, Sulkes A, et al. Gastric cancer: biology and clinical manifestations in Israel. J Surg Oncol (2012) 105(3):316–22. doi: 10.1002/jso.22078
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open (2021) 4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708
6. Bray F, Parkin DM, African Cancer Registry Network. Cancer in sub–Saharan Africa in 2020: A review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol (2022) 23(6):719–28. doi: 10.1016/S1470–2045(22)00270–4
7. Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, et al. The therapeutic survival benefit of splenic hilar nodal dissection for advanced proximal gastric cancer invading the greater curvature. Ann Surg Oncol (2019) 26(3):829–35. doi: 10.1245/s10434–018–07122–9
8. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140–6736(20)31288–5
9. Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer (2011) 14(4):301–16. doi: 10.1007/s10120–011–0085–6
10. Rivera F, Vega–Villegas ME, López–Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev (2007) 33(4):315–24. doi: 10.1016/j.ctrv.2007.01.004
11. Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience (2018) 12:883. doi: 10.3332/ecancer.2018.883
12. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003–15: A pooled analysis of 17 population–based cancer registries. Lancet Glob Health (2018) 6(5):e555–67. doi: 10.1016/S2214–109X(18)30127–X
13. Tanaka Y, Fujii S, Kusaka T, Kokuryu H. Gastric mucosal carcinoma with pyloric stenosis. Intern Med (2021) 60(5):807–8. doi: 10.2169/internalmedicine.5924–20
14. Radbruch L, De Lima L, Knaul F, Wenk R, Ali Z, Bhatnaghar S, et al. Redefining palliative care–a new consensus–based definition. J Pain Symptom Manage (2020) 60(4):754–64. doi: 10.1016/j.jpainsymman.2020.04.027
15. Piñeros M, Znaor A, Mery L, Bray F. A global cancer surveillance framework within noncommunicable disease surveillance: Making the case for population–based cancer registries. Epidemiol Rev (2017) 39(1):161–9. doi: 10.1093/epirev/mxx003
16. Deo SVS, Kumar N, Rajendra VKJ, Kumar S, Bhoriwal SK, Ray M, et al. Palliative surgery for advanced cancer: Clinical profile, spectrum of surgery and outcomes from a tertiary care cancer centre in low–Middle–Income country. Indian J Palliat Care (2021) 27(2):281–5. doi: 10.25259/IJPC_399_20
17. Harada K, Zhao M, Shanbhag N, Baba H, Ajani JA. Palliative care for advanced gastric cancer. Expert Rev Anticancer Ther (2020) 20(7):575–80. doi: 10.1080/14737140.2020.1781620
18. Samarasam I, Chandran BS, Sitaram V, Perakath B, Nair A, Mathew G. Palliative gastrectomy in advanced gastric cancer: is it worthwhile? ANZ J Surg (2006) 76(1–2):60–3. doi: 10.1111/j.1445–2197.2006.03649.x
19. Del Piano M, Ballarè M, Montino F, Todesco A, Orsello M, Magnani C, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc (2005) 61(3):421–6. doi: 10.1016/s0016–5107(04)02757–9
20. Espinel J, Sanz O, Vivas S, Jorquera F, Muñoz F, Olcoz J, et al. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc (2006) 20(7):1083–7. doi: 10.1007/s00464–005–0354–8
21. Cunningham SC, Schulick RD. Palliative management of gastric cancer. Surg Oncol (2007) 16(4):267–75. doi: 10.1016/j.suronc.2007.08.004
22. Izuishi K, Mori H. Recent strategies for treating stage IV gastric cancer: Roles of palliative gastrectomy, chemotherapy, and radiotherapy. J Gastrointestin Liver Dis (2016) 25(1):87–94. doi: 10.15403/jgld.2014.1121.251.rv2
23. Peng W, Ma T, Xu H, Wu Z, Wu C, Sun G. Survival benefits of palliative gastrectomy in stage IV gastric cancer: A propensity score matched analysis. J Gastrointest Oncol (2020) 11(2):376–85. doi: 10.21037/jgo.2020.01.07
24. Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: A systematic review and meta–analysis. BMC Cancer (2013) 13:577. doi: 10.1186/1471–2407–13–577
25. Park JY, Yu B, Park KB, Kwon OK, Lee SS, Chung HY. Impact of palliative gastrectomy in patients with incurable gastric cancer. Med (Kaunas) (2021) 57(3):198. doi: 10.3390/medicina57030198
26. Kim DY, Joo JK, Park YK, Ryu SY, Kim YJ, Kim SK, et al. Is palliative resection necessary for gastric carcinoma patients? Langenbecks Arch Surg (2008) 393(1):31–5. doi: 10.1007/s00423–007–0206–1
27. Chen XJ, Chen GM, Wei YC, Yu H, Wang XC, Zhao ZK, et al. Palliative gastrectomy versus gastrojejunostomy for advanced gastric cancer with outlet obstruction: A propensity score matching analysis. BMC Cancer (2021) 21(1):188. doi: 10.1186/s12885–021–07904–7
28. Dittmar Y, Voigt R, Heise M, Rabsch A, Jandt K, Settmacher U. Indikationen und ergebnisse der palliativen magenresektion beim fortgeschrittenen magenkarzinom (Indications and results of palliative gastric resection in advanced gastric carcinoma). Zentralbl Chir (2009) 134(1):77–82. doi: 10.1055/s–0028–1098793
29. Cowling J, Gorman B, Riaz A, Bundred JR, Kamarajah SK, Evans RPT, et al. Peri–operative outcomes and survival following palliative gastrectomy for gastric cancer: A systematic review and meta–analysis. J Gastrointest Cancer (2021) 52(1):41–56. doi: 10.1007/s12029–020–00519–4
30. Amaral L, Mendes J, Martins P, Bernardo L, Quintanilha R, Santos V, et al. Survival benefits of palliative gastric cancer resection – a regional center experience. Hepatogastroenterology (2012) 59(117):1651–6. doi: 10.5754/hge10201
31. Lupaşcu C, Andronic D, Ursulescu C, Vasiluţă C, Raileanu G, Georgescu ST, et al. Palliative gastrectomy in patients with stage IV gastric cancer–our recent experience. Chirurgia (Bucur) (2010) 105(4):473–6.
32. Wu P, Wang P, Ma B, Yin S, Tan Y, Hou W, et al. Palliative gastrectomy plus chemotherapy versus chemotherapy alone for incurable advanced gastric cancer: A meta–analysis. Cancer Manag Res (2018) 10:4759–71. doi: 10.2147/CMAR.S179368
33. Zhang JZ, Lu HS, Huang CM, Wu XY, Wang C, Guan GX, et al. Outcome of palliative total gastrectomy for stage IV proximal gastric cancer. Am J Surg (2011) 202(1):91–6. doi: 10.1016/j.amjsurg.2010.05.014
34. Huang KH, Wu CW, Fang WL, Chen JH, Lo SS, Wang RF, et al. Palliative resection in noncurative gastric cancer patients. World J Surg (2010) 34(5):1015–21. doi: 10.1007/s00268–010–0467–7
35. Hsu JT, Liao JA, Chuang HC, Chen TD, Chen TH, Kuo CJ, et al. Palliative gastrectomy is beneficial in selected cases of metastatic gastric cancer. BMC Palliat Care (2017) 16(1):19. doi: 10.1186/s12904–017–0192–1
36. Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ, Dutch Gastric Cancer Group. Value of palliative resection in gastric cancer. Br J Surg (2002) 89(11):1438–43. doi: 10.1046/j.1365–2168.2002.02220.x
37. Dai P, Rao X, Zhang X, Qiu E, Wu G, Lin Y, et al. Case report: Complete remission of a patient with metastatic gastric cancer treated with nivolumab combined with chemotherapy after palliative surgery. Front Immunol (2022) 13:908558. doi: 10.3389/fimmu.2022.908558
38. Seo HS, Song KY, Jung YJ, Park SM, Jeon HM, Kim W, et al. Radical gastrectomy after chemotherapy may prolong survival in stage IV gastric cancer: A Korean multi–institutional analysis. World J Surg (2018) 42(10):3286–93. doi: 10.1007/s00268–018–4635–5
39. Tokunaga M, Makuuchi R, Miki Y, Tanizawa Y, Bando E, Kawamura T, et al. Surgical and survival outcome following truly palliative gastrectomy in patients with incurable gastric cancer. World J Surg (2016) 40(5):1172–7. doi: 10.1007/s00268–015–3398–5
40. Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: A phase III study of neoadjuvant docetaxel, oxaliplatin, and s–1 plus surgery and adjuvant s–1 versus surgery and adjuvant s–1 for resectable advanced gastric cancer. J Clin Oncol (2021) 39(26):2903–13. doi: 10.1200/JCO.20.02914
41. Min SH, Son SY, Jung DH, Lee CM, Ahn SH, Park DJ, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res (2017) 93(3):130–6. doi: 10.4174/astr.2017.93.3.130
42. Bian SB, Shen WS, Xi HQ, Wei B, Chen L. Palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer: A meta–analysis comparison of gastrojejunostomy with endoscopic stenting. Chin Med J (Engl) (2016) 129(9):1113–21. doi: 10.4103/0366–6999.180530
43. Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol (1998) 69(1):41–4. doi: 10.1002/(sici)1096–9098(199809)69:1<41::aid–jso8>3.0.co,2–k
44. Prager LP, Huff ML, Alfieri SE, Sujka JA. A fully stapled technique for gastrojejunal anastomosis creation in robotic Roux-en-Y gastric bypass. Langenbecks Arch Surg 407(8):3311–4. doi: 10.1007/s00423-022-02634-1
45. Yao S, Kita R, Ishikawa K, Kumode T, Tanino K, Mizuno R, et al. Is palliative gastrojejunostomy for malignant gastric outlet obstruction an optimal choice for very elderly patients? A report from a long–lived country. Ann Surg Oncol (2023) 30(1):313–21. doi: 10.1245/s10434-022-12599-6
46. Itoi T, Baron TH, Khashab MA, Tsuchiya T, Irani S, Dhir V, et al. Technical review of endoscopic ultrasonography–guided gastroenterostomy in 2017. Dig Endosc (2017) 29(4):495–502. doi: 10.1111/den.12794
47. Chen YI, Kunda R, Storm AC, Aridi HD, Thompson CC, Nieto J, et al. EUS–guided gastroenterostomy: A multicenter study comparing the direct and balloon–assisted techniques. Gastrointest Endosc (2018) 87(5):1215–21. doi: 10.1016/j.gie.2017.07.030
48. McCarty TR, Garg R, Thompson CC, Rustagi T. Efficacy and safety of EUS–guided gastroenterostomy for benign and malignant gastric outlet obstruction: A systematic review and meta–analysis. Endosc Int Open (2019) 7(11):E1474–82. doi: 10.1055/a–0996–8178
49. Ojima T, Nakamori M, Nakamura M, Katsuda M, Hayata K, Yamaue H. Laparoscopic gastrojejunostomy for patients with unresectable gastric cancer with gastric outlet obstruction. J Gastrointest Surg (2017) 21(8):1220–5. doi: 10.1007/s11605–017–3387–0
50. Boghossian MB, Funari MP, De Moura DTH, McCarty TR, Sagae VMT, Chen YI, et al. EUS–guided gastroenterostomy versus duodenal stent placement and surgical gastrojejunostomy for the palliation of malignant gastric outlet obstruction: A systematic review and meta–analysis. Langenbecks Arch Surg (2021) 406(6):1803–17. doi: 10.1007/s00423–021–02215–8
51. On W, Huggett MT, Young A, Pine J, Smith AM, Tehami N, et al. Endoscopic ultrasound guided gastrojejunostomy in the treatment of gastric outlet obstruction: multi-centre experience from the United Kingdom. Surg Endosc (2022). doi: 10.1007/s00464-022-09692-y
52. Yoshida Y, Fukutomi A, Tanaka M, Sugiura T, Kawata N, Kawai S, et al. Gastrojejunostomy versus duodenal stent placement for gastric outlet obstruction in patients with unresectable pancreatic cancer. Pancreatology (2017) 17(6):983–9. doi: 10.1016/j.pan.2017.09.011
53. Binmoeller KF, Shah JN. Endoscopic ultrasound–guided gastroenterostomy using novel tools designed for transluminal therapy: A porcine study. Endoscopy (2012) 44(5):499–503. doi: 10.1055/s–0032–1309382
54. Miyazaki Y, Takiguchi S, Takahashi T, Kurokawa Y, Makino T, Yamasaki M, et al. Treatment of gastric outlet obstruction that results from unresectable gastric cancer: Current evidence. World J Gastrointest Endosc (2016) 8(3):165–72. doi: 10.4253/wjge.v8.i3.165
55. Giordano S, Victorzon M. Laparoscopic roux–En–Y gastric bypass in elderly patients (60 years or older): A meta–analysis of comparative studies. Scand J Surg (2018) 107(1):6–13. doi: 10.1177/1457496917731183
56. Tyberg A, Kats D, Choi A, Gaidhane M, Nieto J, Kahaleh M. Endoscopic ultrasound guided gastroenterostomy: What is the learning curve? J Clin Gastroenterol (2021) 55(8):691–3. doi: 10.1097/MCG.0000000000001400
57. Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, et al. Short–term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS–02–RCT). Ann Surg (2019) 270(6):983–91. doi: 10.1097/SLA.0000000000003217
58. Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, et al. Efficacy of endoscopic gastroduodenal stenting for gastric outlet obstruction due to unresectable advanced gastric cancer: A prospective multicenter study. J Surg Oncol (2014) 109(3):208–12. doi: 10.1002/jso.23486
59. Potz BA, Miner TJ. Surgical palliation of gastric outlet obstruction in advanced malignancy. World J Gastrointest Surg (2016) 8(8):545–55. doi: 10.4240/wjgs.v8.i8.545
60. Hong J, Chen Y, Li J, Hu P, Chen P, Du N, et al. Comparison of gastrojejunostomy to endoscopic stenting for gastric outlet obstruction: An updated systematic review and meta–analysis. Am J Surg (2022) 223(6):1067–78. doi: 10.1016/j.amjsurg.2021.10.038
61. Sterpetti AV, Fiori E, Sapienza P, Lamazza A. Complications after endoscopic stenting for malignant gastric outlet obstruction: A cohort study. Surg Laparosc Endosc Percutan Tech (2019) 29(3):169–72. doi: 10.1097/SLE.0000000000000656
62. Khamar J, Lee Y, Sachdeva A, Anpalagan T, McKechnie T, Eskicioglu C, et al. Gastrojejunostomy versus endoscopic stenting for the palliation of malignant gastric outlet obstruction: A systematic review and meta–analysis. Surg Endosc (2022). doi: 10.1007/s00464-022-09572-5
63. Haga Y, Hiki N, Kinoshita T, Ojima T, Nabeya Y, Kuwabara S, et al. Treatment option of endoscopic stent insertion or gastrojejunostomy for gastric outlet obstruction due to gastric cancer: A propensity score–matched analysis. Gastric Cancer (2020) 23(4):667–76. doi: 10.1007/s10120–020–01040–0
64. Majumder S, Buttar NS, Gostout C, Levy MJ, Martin J, Petersen B, et al. Lumen–apposing covered self–expanding metal stent for management of benign gastrointestinal strictures. Endosc Int Open (2016) 4(1):E96–E101. doi: 10.1055/s–0041–108195
65. Kim JH, Song HY, Shin JH, Hu HT, Lee SK, Jung HY, et al. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: Primary gastric carcinoma versus pancreatic carcinoma. AJR Am J Roentgenol (2009) 193(1):241–7. doi: 10.2214/AJR.08.1760
66. Fiori E, Crocetti D, Sapienza P, Cirocchi R, Sterpetti AV, Miccini M, et al. Palliative surgery or metallic stent positioning for advanced gastric cancer: Differences in QOL. Med (Kaunas) (2021) 57(5):428. doi: 10.3390/medicina57050428
67. Krishnamoorthi R, Bomman S, Benias P, Kozarek RA, Peetermans JA, McMullen E, et al. Efficacy and safety of endoscopic duodenal stent versus endoscopic or surgical gastrojejunostomy to treat malignant gastric outlet obstruction: Systematic review and meta–analysis. Endosc Int Open (2022) 10(6):E874–97. doi: 10.1055/a–1794–0635
68. Park CH, Park JC, Kim EH, Chung H, An JY, Kim HI, et al. Impact of carcinomatosis and ascites status on long–term outcomes of palliative treatment for patients with gastric outlet obstruction caused by unresectable gastric cancer: Stent placement versus palliative gastrojejunostomy. Gastrointest Endosc (2015) 81(2):321–32. doi: 10.1016/j.gie.2014.06.024
69. Laitamäki M, Tyrväinen T, Lehto JT, Laukkarinen J, Ukkonen M. Endoscopic duodenal stenting is efficient, but has higher rate of reoperations than gastrojejunostomy in palliative treatment for gastric outlet obstruction. Langenbecks Arch Surg (2022) 407(6):2509–15. doi: 10.1007/s00423–022–02565–x
70. Jang SH, Lee H, Min BH, Kim SM, Kim HS, Carriere KC, et al. Palliative gastrojejunostomy versus endoscopic stent placement for gastric outlet obstruction in patients with unresectable gastric cancer: A propensity score–matched analysis. Surg Endosc (2017) 31(10):4217–23. doi: 10.1007/s00464–017–5480–6
71. Dresner SM, Wayman J, Lovel T, Hayes N, Griffin SM. Percutaneous endoscopic duodenostomy: The relief of obstruction in advanced gastric carcinoma. Palliat Med (1999) 13(2):165–7. doi: 10.1191/026921699670950414
72. Park KB, Do YS, Kang WK, Choo SW, Han YH, Suh SW, et al. Malignant obstruction of gastric outlet and duodenum: Palliation with flexible covered metallic stents. Radiology (2001) 219(3):679–83. doi: 10.1148/radiology.219.3.r01jn21679
73. Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): A multicenter randomized trial. Gastrointest Endosc (2010) 71(3):490–9. doi: 10.1016/j.gie.2009.09.042
74. Ohtsu A. Chemotherapy for metastatic gastric cancer: Past, present, and future. J Gastroenterol (2008) 43(4):256–64. doi: 10.1007/s00535–008–2177–6
75. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow–up. Ann Oncol (2016) 27(suppl 5):v38–v49. doi: 10.1093/annonc/mdw350
76. Minami Y, Kanemura S, Oikawa T, Suzuki S, Hasegawa Y, Miura K, et al. Associations of cigarette smoking and alcohol drinking with stomach cancer survival: A prospective patient cohort study in Japan. Int J Cancer (2018) 143(5):1072–85. doi: 10.1002/ijc.31408
77. Yang K, Liu K, Zhang WH, Lu ZH, Chen XZ, Chen XL, et al. The value of palliative gastrectomy for gastric cancer patients with intraoperatively proven peritoneal seeding. Med (Baltimore) (2015) 94(27):e1051. doi: 10.1097/MD.0000000000001051
78. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with s–1, an oral fluoropyrimidine (published correction appears in n engl J med. N Engl J Med (2007) 357(18):1810–20. doi: 10.1056/NEJMoa072252
79. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open–label, randomised controlled trial. Lancet (2012) 379(9813):315–21. doi: 10.1016/S0140–6736(11)61873–4
80. Kim TH, Ahn MS, Choi YW, Kang SY, Choi JH, Lee HW, et al. Analysis of treatment outcomes according to the cycles of adjuvant chemotherapy in gastric cancer: A retrospective nationwide cohort study. BMC Cancer (2022) 22(1):948. doi: 10.1186/s12885–022–10006–7
81. Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, et al. Phase II study of preoperative chemotherapy with s–1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol (2013) 107(7):741–5. doi: 10.1002/jso.23301
82. Zhu YJ, Wu XY, Wang W, Chang XS, Zhan DD, Diao DC, et al. Acupuncture for quality of life in gastric cancer patients undergoing adjuvant chemotherapy. J Pain Symptom Manage (2022) 63(2):210–20. doi: 10.1016/j.jpainsymman.2021.09.009
83. Wang Z, Chen JQ, Liu JL, Tian L. Issues on peritoneal metastasis of gastric cancer: An update. World J Surg Oncol (2019) 17(1):215. doi: 10.1186/s12957–019–1761–y
84. Grass F, Vuagniaux A, Teixeira–Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg (2017) 104(6):669–78. doi: 10.1002/bjs.10521
85. Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer (2017) 20(Suppl 1):111–21. doi: 10.1007/s10120–016–0662–9
86. Horvath P, Yurttas C, Baur I, Steidle C, Reymond MA, Girotti PNC, et al. Current medical care situation of patients in Germany undergoing pressurized intraperitoneal aerosol chemotherapy (PIPAC). Cancers (Basel) (2022) 14(6):1443. doi: 10.3390/cancers14061443
87. Alyami M, Bonnot PE, Mercier F, Laplace N, Villeneuve L, Passot G, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur J Surg Oncol (2021) 47(1):123–7. doi: 10.1016/j.ejso.2020.05.021
88. Li Z, Wong LCK, Sultana R, Lim HJ, Tan JW, Tan QX, et al. A systematic review on quality of life (QoL) of patients with peritoneal metastasis (PM) who underwent pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum (2022) 7(2):39–49. doi: 10.1515/pp–2021–0154
89. Kim HS, Kim JH, Kim JW, Kim BC. Chemotherapy in elderly patients with gastric cancer. J Cancer (2016) 7(1):88–94. doi: 10.7150/jca.13248
90. He S, Shen J, Hong L, Niu L, Niu D. Capecitabine "metronomic" chemotherapy for palliative treatment of elderly patients with advanced gastric cancer after fluoropyrimidine–based chemotherapy. Med Oncol (2012) 29(1):100–6. doi: 10.1007/s12032–010–9791–x
91. Liao PW, Cheng SB, Chou CW, Lin HC, Lin CH, Chen TC, et al. Chemotherapy for metastatic gastric cancer: Does age matter? A single–center, retrospective, real–world study. Clin Med Insights Oncol (2022) 16:11795549221123617. doi: 10.1177/11795549221123617
92. Cainap C, Vlad C, Seicean A, Balacescu O, Seicean R, Constantin AM, et al. Gastric cancer: Adjuvant chemotherapy versus chemoradiation. A clinical point of view. J BUON (2019) 24(6):2209–19.
93. Coccolini F, Fugazzola P, Ansaloni L, Sartelli M, Cicuttin E, Leandro G, et al. Advanced gastric cancer: The value of systemic and intraperitoneal chemotherapy. Acta BioMed (2018) 89(8–S):104–9. doi: 10.23750/abm.v89i8–S.7904
94. Zurleni T, Gjoni E, Altomare M, Rausei S. Conversion surgery for gastric cancer patients: A review. World J Gastrointest Oncol (2018) 10(11):398–409. doi: 10.4251/wjgo.v10.i11.398
95. Shchepotin IB, Chorny V, Hanfelt J, Evans SR. Palliative superselective intra–arterial chemotherapy for advanced nonresectable gastric cancer. J Gastrointest Surg (1999) 3(4):426–31. doi: 10.1016/s1091–255x(99)80060–2
96. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2–positive advanced gastric or gastro–oesophageal junction cancer (ToGA): A phase 3, open–label, randomised controlled trial (published correction appears in lancet. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140–6736(10)61121–X
97. Li XD, Shen H, Jiang JT, Zhang HZ, Zheng X, Shu YQ, et al. Paclitaxel based vs oxaliplatin based regimens for advanced gastric cancer. World J Gastroenterol (2011) 17(8):1082–7. doi: 10.3748/wjg.v17.i8.1082
98. Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, et al. Combination of cisplatin/S–1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5–fluorouracil in the first–line advanced gastric cancer study. Eur J Cancer (2013) 49(17):3616–24. doi: 10.1016/j.ejca.2013.07.003
99. Dong L, Li J, Lou XP, Miao JH, Lu P, Chang ZW, et al. Comparison of short–term efficacy and safety of TIROX and DCF regimens for advanced gastric cancer. J Int Med Res (2014) 42(3):737–43. doi: 10.1177/0300060513510657
100. Ochenduszko S, Puskulluoglu M, Konopka K, Fijorek K, Urbanczyk K, Budzynski A, et al. Comparison of efficacy and safety of first–line palliative chemotherapy with EOX and mDCF regimens in patients with locally advanced inoperable or metastatic HER2–negative gastric or gastroesophageal junction adenocarcinoma: A randomized phase 3 trial. Med Oncol (2015) 32(10):242. doi: 10.1007/s12032–015–0687–7
101. Quintero–Aldana G, Jorge M, Grande C, Salgado M, Gallardo E, Varela S, et al. Phase II study of first–line biweekly docetaxel and cisplatin combination chemotherapy in advanced gastric cancer. Cancer Chemother Pharmacol (2015) 76(4):731–7. doi: 10.1007/s00280–015–2839–z
102. Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus s–1 with cisplatin plus s–1 in chemotherapy–naïve patients with advanced gastric cancer. Ann Oncol (2015) 26(1):141–8. doi: 10.1093/annonc/mdu472
103. Chen X, Li W, Sun L, Liu Y, Liu S, Ma R. S–l combined with cisplatin plus concurrent chemoradiotherapy versus cisplatin plus concurrent chemoradiotherapy for Chinese patients with advanced gastric cancer: A multi–centre randomized controlled trial. Clin Transl Oncol (2016) 18(7):672–6. doi: 10.1007/s12094–015–1416–6
104. Wang J, Xu R, Li J, Bai Y, Liu T, Jiao S, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first–line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer (2016) 19(1):234–44. doi: 10.1007/s10120–015–0457–4
105. Fushida S, Kinoshita J, Kaji M, Oyama K, Hirono Y, Tsukada T, et al. Paclitaxel plus valproic acid versus paclitaxel alone as second– or third–line therapy for advanced gastric cancer: A randomized phase II trial. Drug Des Devel Ther (2016) 10:2353–8. doi: 10.2147/DDDT.S110425
106. Sah BK, Zhang B, Zhang H, Li J, Yuan F, Ma T, et al. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun (2020) 11(1):6093. doi: 10.1038/s41467–020–19965–6
107. Kawakami H, Fujitani K, Matsuyama J, Akamaru Y, Tamura S, Endo S, et al. Comparison of s–1–cisplatin every 5 weeks with capecitabine–cisplatin every 3 weeks for HER2–negative gastric cancer (recurrent after s–1 adjuvant therapy or chemotherapy–naïve advanced): Pooled analysis of HERBIS–2 (OGSG 1103) and HERBIS–4A (OGSG 1105) trials. Int J Clin Oncol (2020) 25(9):1635–43. doi: 10.1007/s10147–020–01711–z
108. Zhu XD, Huang MZ, Wang YS, Feng WJ, Chen ZY, He YF, et al. XELOX doublet regimen versus EOX triplet regimen as first–line treatment for advanced gastric cancer: An open–labeled, multicenter, randomized, prospective phase III trial (EXELOX). Cancer Commun (Lond) (2022) 42(4):314–26. doi: 10.1002/cac2.12278
109. Bin Y, Lan D, Bao W, Yang H, Zhou S, Huang F, et al. SOX combined with intraperitoneal perfusion of docetaxel compared with DOS regimen in the first–line therapy for advanced gastric cancer with malignant ascites: A prospective observation. Trials (2022) 23(1):211. doi: 10.1186/s13063–022–06143–w
110. Rosati G, Cella CA, Cavanna L, Codecà C, Prisciandaro M, Mosconi S, et al. A randomized phase III study of fractionated docetaxel, oxaliplatin, capecitabine (low–tox) vs epirubicin, oxaliplatin and capecitabine (eox) in patients with locally advanced unresectable or metastatic gastric cancer: The lega trial. Gastric Cancer (2022) 25(4):783–93. doi: 10.1007/s10120–022–01292–y
111. Williams GR, Manjunath SH, Butala AA, Jones JA. Palliative radiotherapy for advanced cancers: Indications and outcomes. Surg Oncol Clin N Am (2021) 30(3):563–80. doi: 10.1016/j.soc.2021.02.007
112. Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Med (Baltimore) (2014) 93(22):e118. doi: 10.1097/MD.0000000000000118
113. Tey J, Soon YY, Koh WY, Leong CN, Choo BA, Ho F, et al. Palliative radiotherapy for gastric cancer: A systematic review and meta–analysis. Oncotarget (2017) 8(15):25797–805. doi: 10.18632/oncotarget.15554
114. Kondoh C, Shitara K, Nomura M, Takahari D, Ura T, Tachibana H, et al. Efficacy of palliative radiotherapy for gastric bleeding in patients with unresectable advanced gastric cancer: A retrospective cohort study. BMC Palliat Care (2015) 14:37. doi: 10.1186/s12904–015–0034–y
115. Takeda K, Sakayauchi T, Kubozono M, Katagiri Y, Umezawa R, Yamamoto T, et al. Palliative radiotherapy for gastric cancer bleeding: A multi–institutional retrospective study. BMC Palliat Care (2022) 21(1):52. doi: 10.1186/s12904–022–00943–2
116. Saito T, Kosugi T, Nakamura N, Wada H, Tonari A, Ogawa H, et al. Treatment response after palliative radiotherapy for bleeding gastric cancer: A multicenter prospective observational study (JROSG 17–3). Gastric Cancer (2022) 25(2):411–21. doi: 10.1007/s10120–021–01254–w
117. Tey J, Zheng H, Soon YY, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: A phase II trial. Cancer Med (2019) 8(4):1447–58. doi: 10.1002/cam4.2021
118. Kawabata H, Uno K, Yasuda K, Yamashita M. Experience of low–dose, short–course palliative radiotherapy for bleeding from unresectable gastric cancer. J Palliat Med (2017) 20(2):177–80. doi: 10.1089/jpm.2016.0141
119. Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, et al. Palliative radiotherapy in symptomatic locally advanced gastric cancer: A phase II trial. Cancer Med (2019) 8(4):1447–58. doi: 10.1002/cam4.2021
120. Hiramoto S, Kikuchi A, Tetsuso H, Yoshioka A, Kohigashi Y, Maeda I. Efficacy of palliative radiotherapy and chemo–radiotherapy for unresectable gastric cancer demonstrating bleeding and obstruction. Int J Clin Oncol (2018) 23(6):1090–4. doi: 10.1007/s10147–018–1317–0
122. Yoshikawa T, Tsuburaya A, Hirabayashi N, Yoshida K, Nagata N, Kodera Y, et al. A phase I study of palliative chemoradiation therapy with paclitaxel and cisplatin for local symptoms due to an unresectable primary advanced or locally recurrent gastric adenocarcinoma. Cancer Chemother Pharmacol (2009) 64(6):1071–7. doi: 10.1007/s00280–009–0963–3
123. Kwon HC, Kim MC, Kim KH, Jang JS, Oh SY, Kim SH, et al. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol (2010) 6(4):278–85. doi: 10.1111/j.1743–7563.2010.01331.x
124. An JY, Kim HI, Cheong JH, Hyung WJ, Kim CB, Noh SH. Pathologic and oncologic outcomes in locally advanced gastric cancer with neoadjuvant chemotherapy or chemoradiotherapy. Yonsei Med J (2013) 54(4):888–94. doi: 10.3349/ymj.2013.54.4.888
125. Boda–Heggemann J, Weiss C, Schneider V, Hofheinz RD, Haneder S, Michaely H, et al. Adjuvant IMRT/XELOX radiochemotherapy improves long–term overall– and disease–free survival in advanced gastric cancer. Strahlenther Onkol (2013) 189(5):417–23. doi: 10.1007/s00066–013–0309–2
126. Nishikawa K, Tsuburaya A, Yoshikawa T, Kobayashi M, Kawada J, Fukushima R, et al. A randomised phase II trial of capecitabine plus cisplatin versus s–1 plus cisplatin as a first–line treatment for advanced gastric cancer: Capecitabine plus cisplatin ascertainment versus s–1 plus cisplatin randomised PII trial (XParTS II). Eur J Cancer (2018) 101:220–8. doi: 10.1016/j.ejca.2018.06.026
127. Wang F, Qu A, Sun Y, Zhang J, Wei B, Cui Y, et al. Neoadjuvant chemoradiotherapy plus postoperative adjuvant XELOX chemotherapy versus postoperative adjuvant chemotherapy with XELOX regimen for local advanced gastric cancer–a randomized, controlled study. Br J Radiol (2021) 94(1124):20201088. doi: 10.1259/bjr.20201088
Keywords: gastric carcinomas, palliative therapy, application, treatment planning, research progress
Citation: Luo X-F, Luo Y-H, Zhao X-Y, Lin X-T, Li W-L, Jie J, Wu D, Fang G, Pang Y-Z and Huang A (2023) Application and progress of palliative therapy in advanced gastric carcinomas. Front. Oncol. 13:1104447. doi: 10.3389/fonc.2023.1104447
Received: 21 November 2022; Accepted: 16 February 2023;
Published: 09 March 2023.
Edited by:
Bin Xiong, Zhongnan Hospital, Wuhan University, ChinaReviewed by:
Jin Zhou, The First Affiliated Hospital of Soochow University, ChinaBurcu Yazıcıoğlu, Haliç University, Türkiye
Tushar Mishra, All India Institute of Medical Sciences Bhubaneswar, India
Copyright © 2023 Luo, Luo, Zhao, Lin, Li, Jie, Wu, Fang, Pang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-zhou Pang, cGFuZ3l6QGd4dGNtdS5lZHUuY24=; An Huang, YW5jbGVfaEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Xiao-fan Luo
Xiao-fan Luo Ye-hao Luo2†
Ye-hao Luo2† Xin-yi Zhao
Xin-yi Zhao An Huang
An Huang