- 1School of Medicine, Nankai University, Tianjin, China
- 2Department of General Surgery, The First Medical Center, Chinese PLA General Hospital, Beijing, China
- 3Department of Radiotherapy, The Fifth Medical Center, Chinese PLA General Hospital, Beijing, China
Background: Immune checkpoint inhibitors (ICIs) have shown promising prospects in locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC) immunotherapy, but their efficacy in neoadjuvant settings remains unclear. This study aimed to assess the efficacy and safety of integrating programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors into neoadjuvant chemotherapy (NACT) of GC/GEJC treatment.
Methods: PubMed, Cochrane Library, Embase, ClinicalTrials.gov, and main oncology conference databases were systematically searched up to 19 November 2022, and randomized controlled trials (RCTs) and cohort studies that evaluated the efficacy and safety of PD-1/PD-L1 inhibitors plus NACT were included. The main outcomes were pathological complete response (pCR), major pathological response (MPR), R0 resection rate, and treatment-related adverse events (TRAEs).
Results: A total of 753 patients from 20 prospective studies were included in this meta-analysis. The pooled pCR and MPR rates from studies reporting were 21.7% [95% confidence interval (CI), 18.1%–25.5%] and 44.0% (95% CI, 34.1%–53.8%), respectively. The pooled incidence rate of total TRAEs was 89.1% (95% CI, 82.7%–94.3%), and the incidence rate of grade 3 to 4 TRAEs was 34.4% (95% CI, 17.8%–66.5%). The pooled R0 resection rate was reported to be 98.9% (95% CI, 97.0%–99.9%). Subgroup analysis has not found significant differences in efficacy and safety among different PD-1/PD-L1 inhibitors. Moreover, the efficacy in patients with positive PD-L1 expression (combined positive score ≥1) was comparable with that in the entire study population [pCR, 22.5% vs. 21.2% (p > 0.05); MPR, 48.6% vs. 43.7% (p > 0.05)].
Conclusion: This systematic review and meta-analysis found that PD-1/PD-L1 inhibitors combined with NACT for locally advanced GC/GEJC were well tolerated and may confer therapeutic advantages. The integration of ICIs into NACT has shown the potential for application in any PD-L1 expression population.
1 Introduction
Gastric cancer (GC) is one of the most common malignant tumors, which ranks fifth of the incidence rate and fourth of the mortality, with more than 1 million estimated new cases annually (1). East Asia is a region with a high incidence rate of GC. Unlike a higher proportion of early GC in Japan and South Korea, about 80% of patients suffer from advanced GC (AGC) due to inappropriate dietary habits and low prevalence of early cancer screening, which cause a serious health burden on life in China (2, 3). At present, many studies have proved that the combination of gastrectomy and perioperative therapy like chemotherapy, targeted therapy, or immunotherapy can prolong the survival and improve the quality of life of patients with AGC, bringing prospects and opportunities for the investigation of optimized treatment (4, 5).
Helicobacter pylori infection is the most well-described risk factor for GC. Other risk factors include male sex, older age, smoking, alcohol consumption, high salt intake, low vegetables and fruits intake, and low socioeconomic status (6). New molecular classifications, such as The Cancer Genome Atlas classification, divide GC into four subtypes: EBV, MSI, CIN, and GS, which could better reflect tumor characteristics and show prognostic values than that based on morphological or histopathological features (7). Alteration of the tumor microenvironment (TME) has also been widely reported in GC with upregulated programmed cell death ligand 1 (PD-L1) expression observed, which correlated with tumor immune escape (8). As GC is a highly heterogeneous malignant disease and the therapeutic effect is still unsatisfactory, it is of great significance to elucidate the pathogenesis and explore better treatment means for GC.
In recent years, as the representative of immunotherapy, programmed cell death 1 (PD-1)/PD-L1 inhibitors have become a superior option for treating AGC. The mechanism of action is briefly through selective blockade of the immune checkpoints of the T-cell surface to activate T cells for effective anti-tumor response (9). Most perspectives considered that the combination of immunotherapy and chemotherapy could synergistically enhance the anti-cancer effect. This might be due to the increased recognition and elimination of the tumor by the host immune system with the combination of drugs, as well as reduced immunosuppression of the TME and enhanced antitumor efficacy (10, 11). Some authoritative randomized controlled trials (RCTs) like Checkmate-649 (12) and Attraction-4 (13) also proved that the combination of PD-1/PD-L1 inhibitors and chemotherapy in unresectable AGC has significantly improved disease control rate and brought long-term survival benefits compared with chemotherapy alone. This high-level evidence makes chemotherapy combined with immunotherapy become the first-line treatment modality for unresectable AGC (14).
Since the MAGIC study (15) established the predominance of neoadjuvant chemotherapy (NACT) in the treatment of locally AGC (LAGC), this preoperative treatment modality has been widely recognized for its satisfied therapeutic effect on reducing tumor stage, inhibiting tumor micrometastasis, increasing R0 resection rate, and even achieving pathological complete response (pCR) and acquiring long-term survival benefits (16). The important value of immunotherapy in AGC draws surgeons’ attention to applying it in the neoadjuvant treatment of LAGC. Teng et al. (17) first reported the clinical use of NACT and immunotherapy for patients with LAGC, and the results of this phase II single-arm study demonstrated that patients who accepted sintilimab in combination with CapeOX regimen acquired 97.2% R0 resection rate and 19.4% pCR rate, which seemed superior to the results of neoadjuvant CapeOX regimen only in the previous studies (18, 19). In the last 2 years, an increasing number of prospective studies have started to focus on the effect of NACT combined with immunotherapy and obtained initial results. Therefore, we summarized the current literatures and conducted this meta-analysis in an attempt to comprehensively analyze the therapeutic safety and clinicopathological evaluation of NACT combined with immunotherapy for LAGC and to provide a reference for surgeons to rationally select the effective treatment modalities.
2 Methods
2.1 Literature search strategy
The systematic literature search was conducted in PubMed, Cochrane Library, Embase, ClinicalTrials.gov, and several main oncology conference databases, including American Society of Clinical Oncology (ASCO) Meeting, European Society for Medical Oncology (ESMO) Meeting, and American Association for Cancer Research (AACR) Meeting up to 19 November 2022. Search terms using the medical subject headings (MeSH) were as follows: (“immunotherapy” [MeSH] OR “immune checkpoint inhibitors” [MeSH] OR “nivolumab” OR “pembrolizumab” OR “camrelizumab” OR “sintilimab” OR “toripalimab” OR “tislelizumab” OR “atezolizumab” OR “durvalumab” OR “avelumab”) AND (“neoadjuvant therapy” [MeSH] OR “perioperative period” [MeSH]) AND (“esophagogastric junction” [MeSH] OR “stomach neoplasm” [MeSH] OR “gastroesophageal cancer” OR “gastroesophageal junction cancer”). We also searched unpublished studies and ongoing clinical trials of neoadjuvant immunochemotherapy in locally advanced GC/GEJC. All studies were limited to English language and human subjects.
2.2 Inclusion criteria
Inclusion criteria according to the PICOS principle was listed as follows: patients: patients with newly diagnosed resectable locally advanced (T1N1-3M0 or T2-3NanyM0) GC/GEJC; intervention: administrated with NACT combined with PD-1/PD-L1 inhibitors; and outcomes: efficacy and security indicators, including pathological complete response (pCR), major pathological response (MPR), R0 resection rate, treatment-related adverse events (TRAEs), and grade 3 to 4 TRAEs. pCR and MPR are both graded according to Becker criteria of Tumor Regression Grade (TRG). Studies design: RCTs, non-RCTs, and prospective cohort studies.
2.3 Data extraction
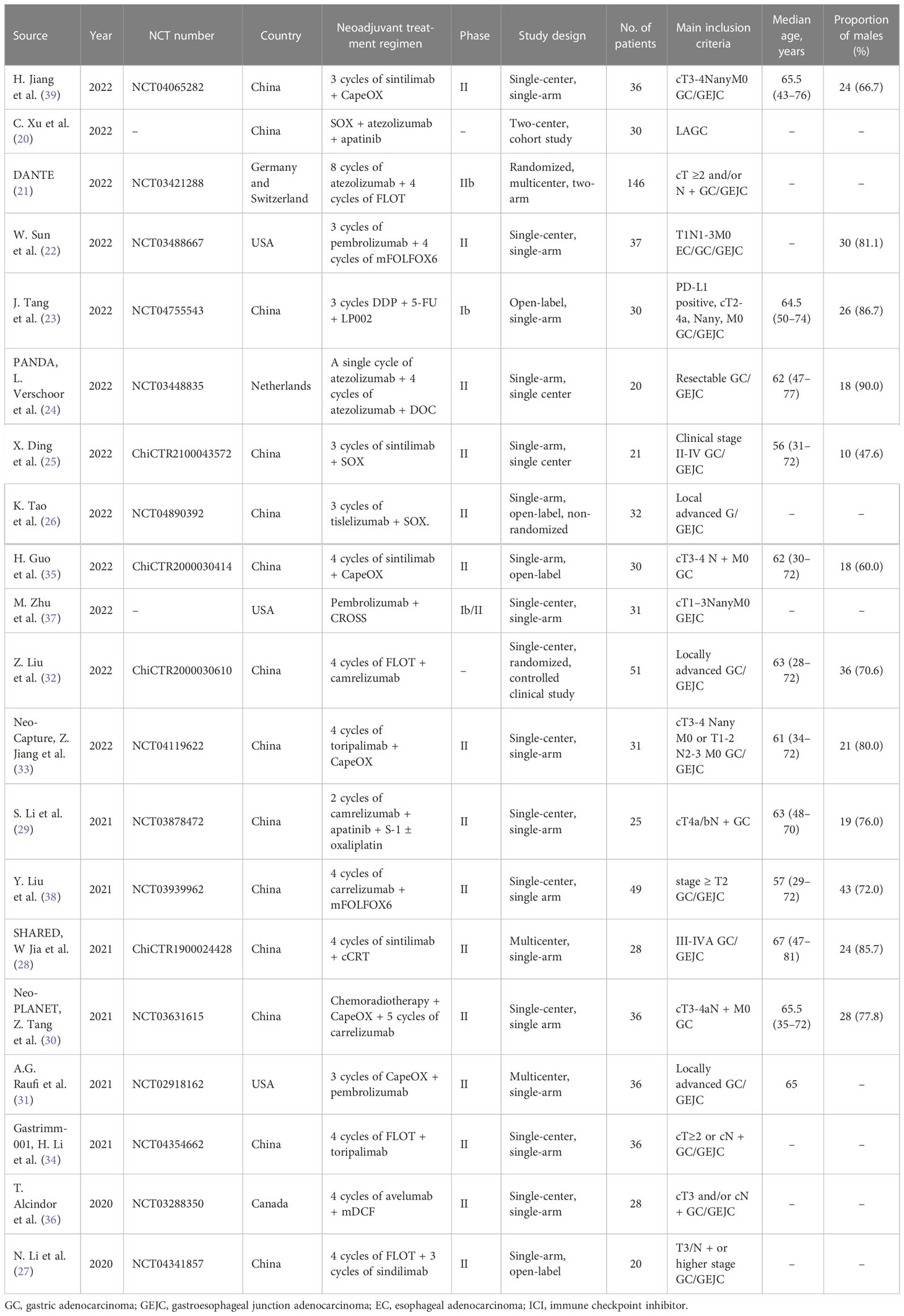
Two authors (HC and ZY) independently carried out the literature selected and data extraction, and disagreements were resolved by discussing with the third author (BW). The following information was extracted: name of clinical trials, name of first author, number of clinical trials, publication year, region, tumor site, neoadjuvant treatment regimen, type of PD-1/PD-L1 inhibitors, study phase, study design, number of patients, main inclusion criteria, main outcome indicators, median age, proportion of males, resection rate, R0 resection rate, pCR rate, MPR rate, incidence of TRAEs, incidence of grade 3 to 4 TRAEs, incidence of serious adverse events (SAE), PD-L1 expression level (detected before neoadjuvant therapy), and mismatch repair (MMR) status.
2.4 Quality assessment and risk of bias
The Cochrane ROBINS-I tool was applied to assess the quality and bias of included studies, which incorporating assessment for bias in the domains of selection, attrition, detection, performance, and reporting (Supplementary Figure 1). Two reviewers (JH and LS) estimated the quality of studies independently, and disagreements were discussed with the third investigator. Publication bias was evaluated by Begg’s funnel plots and Egger’s test.
2.5 Statistical analysis
The meta-analysis was carried out using R software (version 4.2.1). Because most of studies were single-arm trails, five transformation approaches (PRAW, PAS, PFT, PLN, and PLOGIT) were conducted, and the approach by which the lowest heterogeneity was achieved was adopted for further analysis. Moreover, the heterogeneity among studies was evaluated by the Cochrane’s Q test and I2 statistical; if the heterogeneity was negligible (I2 < 50%, p > 0.1), the fixed-effects model was adopted; and if the heterogeneity was significant (I2 > 50%, p < 0.1), the random-effects model was adopted. A p-value < 0.05 was considered statistically significant.
3 Results
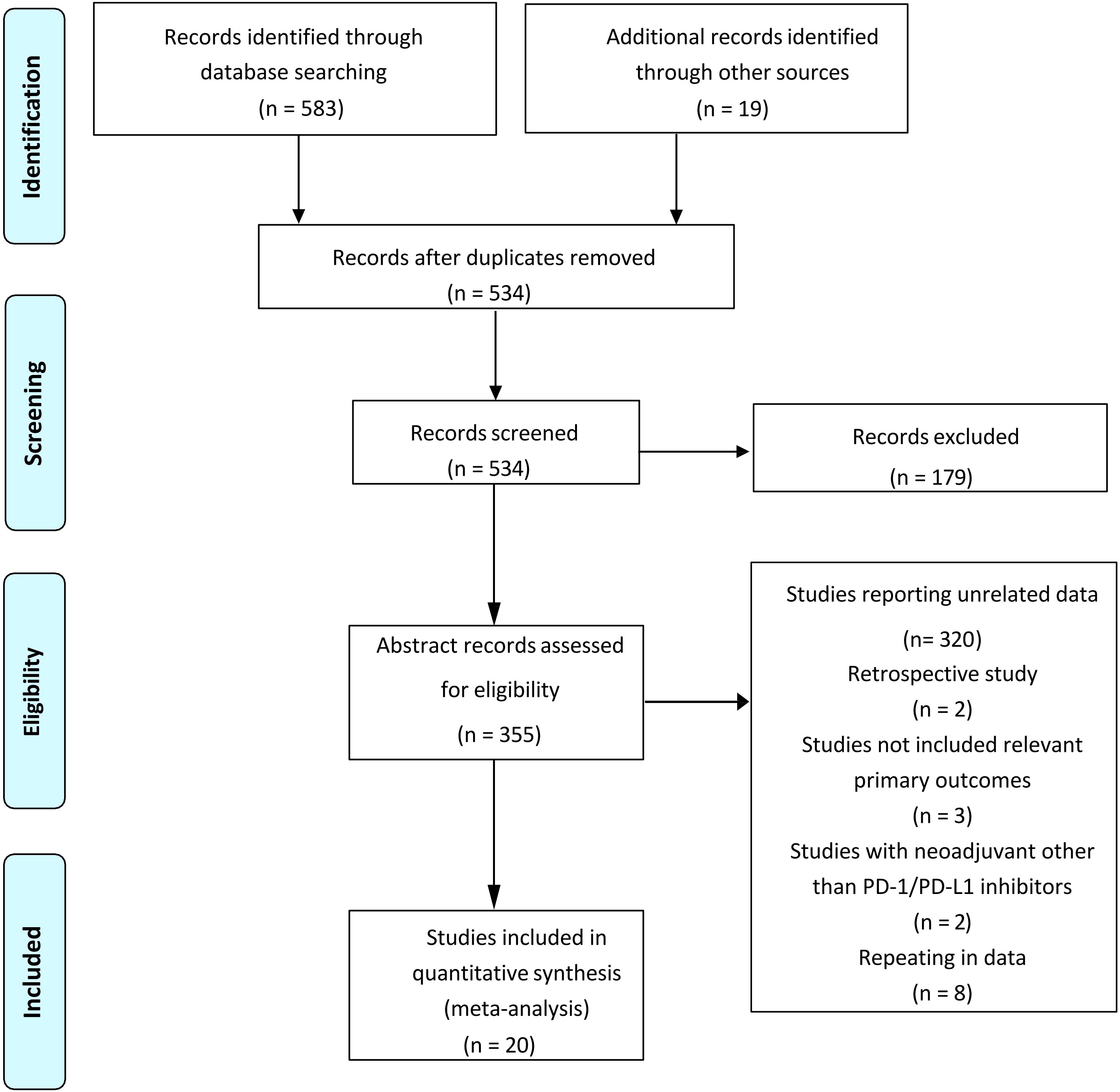
The original search identified a total of 602 publications, and the study retrieval process is depicted in Figure 1. Twenty studies met the inclusion criteria, including a total number of 753 patients, were included in this meta-analysis (17, 20–38). Of these, 15 studies reported that patients received neoadjuvant immunotherapy combined with chemotherapy, which our meta-analysis mainly focused on. Another five studies reported patients who received neoadjuvant immunochemotherapy with radio therapy or anti-VEGF drugs. The characteristics of all studies are listed in Table 1. Publication bias was estimated by Begg’s funnel plot and Egger’s test and showed overall limited, as illustrated in Supplementary Figure 2.
3.1 Evaluation of efficacy outcomes
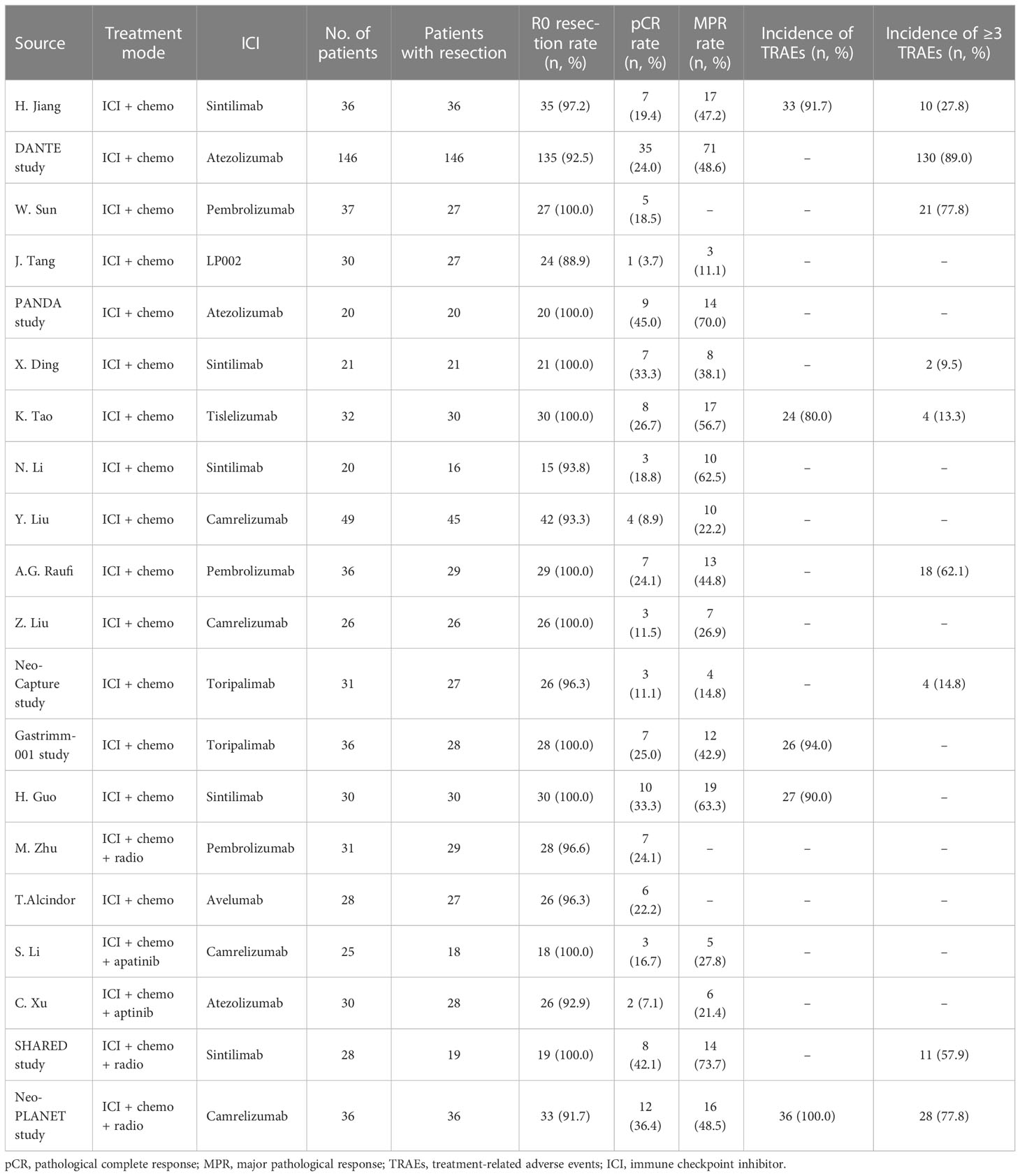
The efficacy- and safety-related outcomes were extracted from eligible studies and illustrated in Table 2. The data of pCR rate, MPR rate, and R0 resection rate were available in 14, 12, and 14 studies, respectively. The pooled rates of pCR and MPR from trials using neoadjuvant immunotherapy plus chemotherapy were 21.7% [95% confidence interval (CI), 18.1%–25.5%] and 44.0% (95% CI, 34.1%–53.8%), respectively. The pooled R0 resection rate was 98.9% (95% CI, 97.0%–99.9%). Only patients who received neoadjuvant immunotherapy combined with chemotherapy were included in the pooled analysis, and the results of the addition of neoadjuvant radiotherapy or anti-VEGF drug, apatinib, were analysis separately.
No significant heterogeneity was observed among results of pCR rates (I2 = 31%, p = 0.13, Figure 2), and fixed-effects model was adopted. Meanwhile, a high level of heterogeneity was observed for MPR (I2 = 78%, p < 0.01) and R0 resection rate (I2 = 59%, p < 0.01). Sensitivity analysis did not find the source of heterogeneity of MPR rates, whereas, after sensitivity analysis and DANTE study were excluded, the heterogeneity of R0 resection rate was decreased (Supplementary Figure 3).
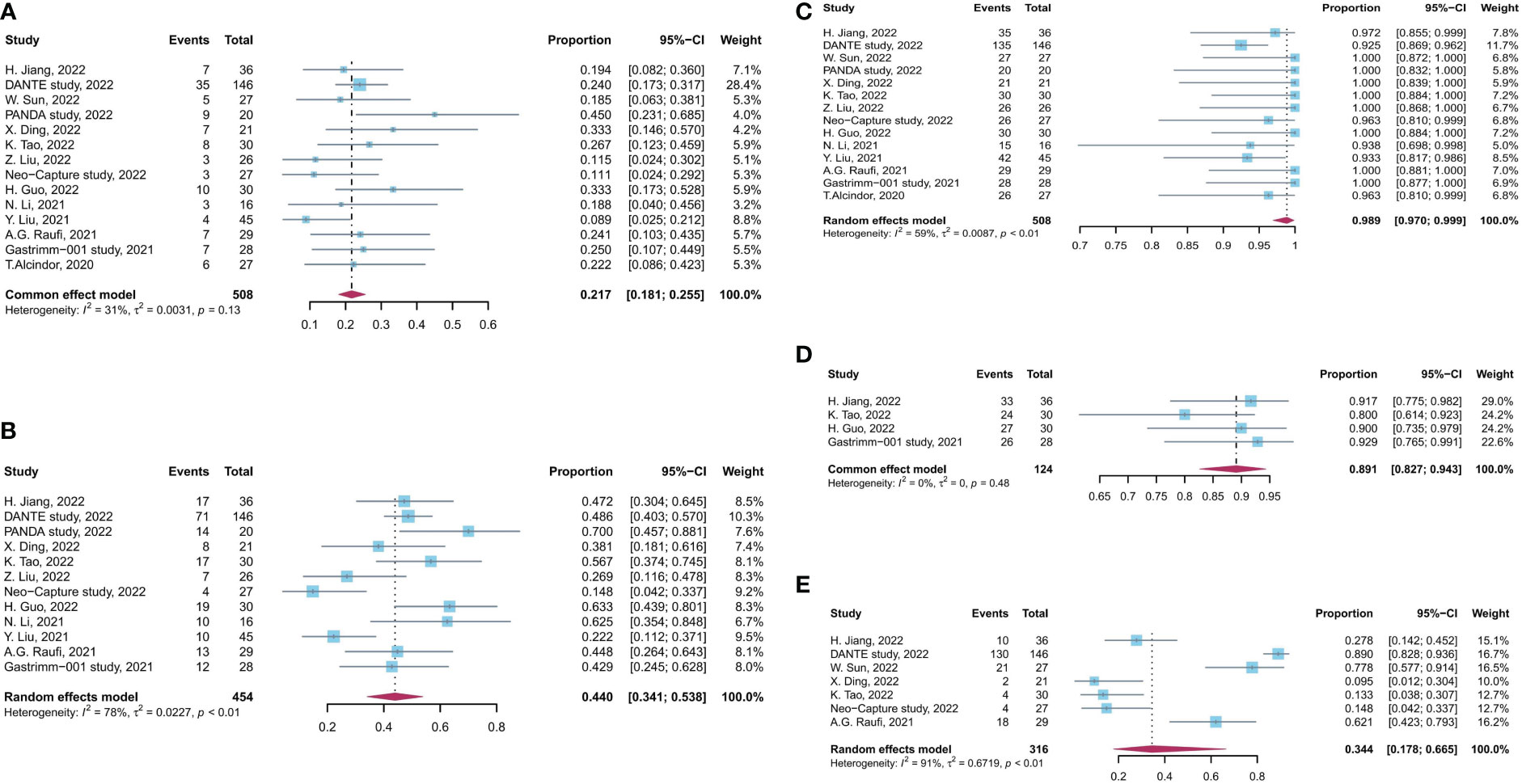
Figure 2 Forest plot of the efficacy and safety evaluation of neoadjuvant immunochemotherapy for locally advanced GC/GEJC. (A) pCR rate; (B) MPR rate; (C) R0 resection rate; (D) incidence of TRAEs; (E) incidence of grade 3 to 4 TRAEs. pCR, complete pathological response; MPR, major pathological response; TRAEs, treatment-related adverse event; GC, gastric adenocarcinoma; GEJC, gastroesophageal junction adenocarcinoma.
3.2 Safety of neoadjuvant immunotherapy
The incidence rate of TRAEs was used to assess the safety of neoadjuvant immunotherapy combined with chemotherapy. The pooled incidence of TRAEs was 89.1% (95% CI, 82.7%–94.3%), based on four studies with available data. The pooled incidence rate of grade 3 to 4 TRAEs was 34.4% (95% CI, 17.8%–66.5%), supported by seven studies with available data (I2 = 31%, p = 0.13, Figure 2). There is a significant heterogeneity for grade 3 to 4 TRAEs (I2 = 91%, p < 0.01), and sensitivity analysis did not find the source of heterogeneity (Supplementary Figure 3). No significant heterogeneity was observed among results of TRAEs.
3.3 Subgroup analysis
Subgroup analysis was conducted on the basis of PD-1/PD-L1 inhibitor types and PD-L1 expression levels separately. Subgroup analysis has not found significant differences in efficacy and safety among different PD-1/PD-L1 inhibitors (Figure 3). Patients who received sintilimab- or atezolizumab-based neoadjuvant immunochemotherapy had a relatively better MPR rate compared with those who received camrelizumab- or toripalimab-based treatment (total, p < 0.01). For the incidence of grade 3 to 4 TRAEs, patients treated with sintilimab, tisleizumab, and toripalimab had a relatively lower incidence than those treated with atezolizumab or pembrolizumab (total, p < 0.01; Supplementary Figure 4), which were consistent with results in pairwise comparisons. No difference in R0 resection rate, pCR rate, or TRAE rate was found in subgroup analysis by PD-1/PD-L1 inhibitor types (Supplementary Figure 4).
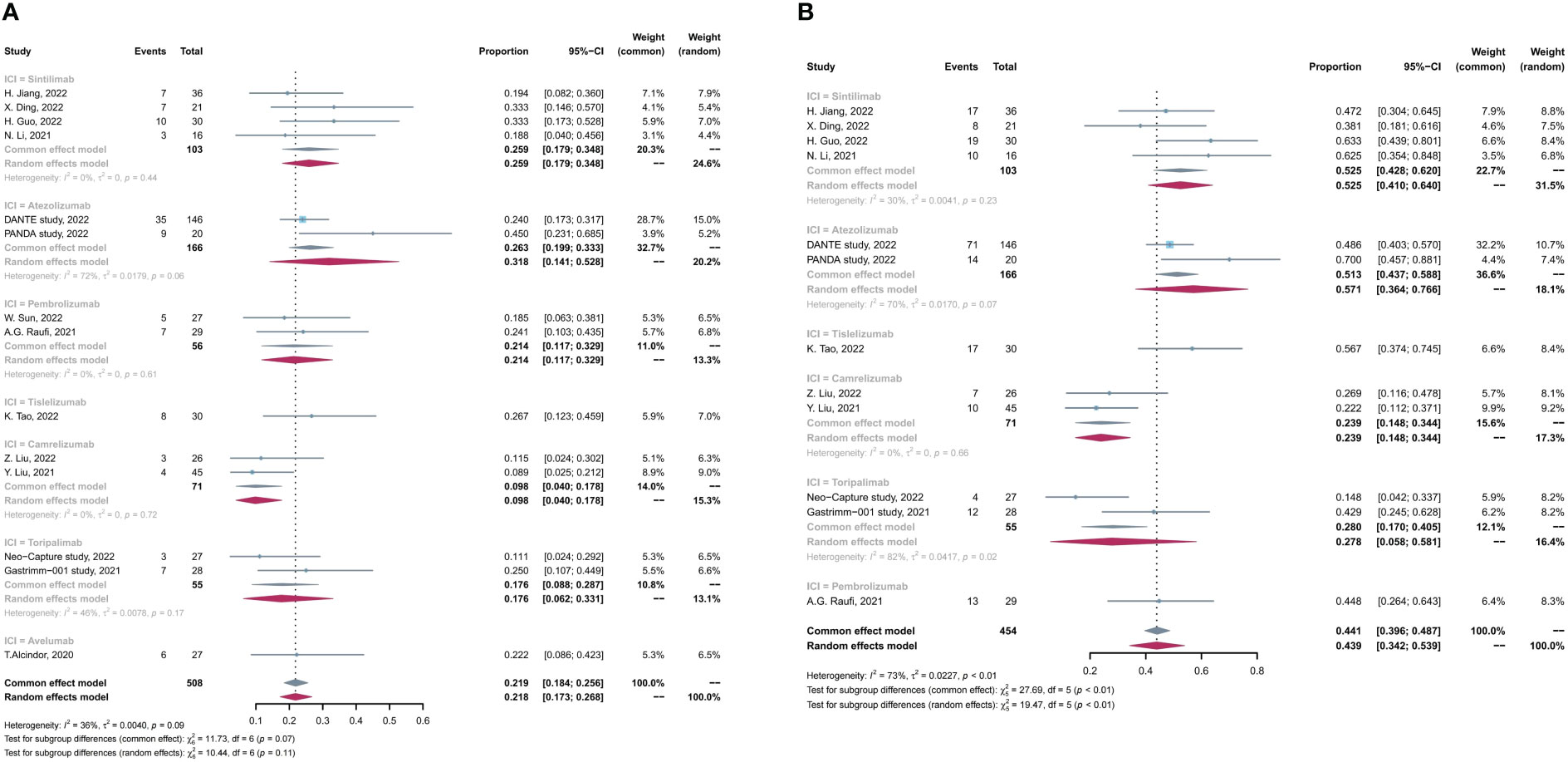
Figure 3 Subgroup analysis based on PD-1/PD-L1 inhibitors for (A) pCR rate and (B) MPR rate. pCR, complete pathological response rate; MPR, major pathological response rate.
Subsequently, we conducted subgroup analysis on the basis of PD-L1 expression levels, and four studies reported the data of efficacy in positive PD-L1 expression [defined as combined positive score (CPS) ≥1] subgroup. As shown in Figure 4, the pCR and MPR rates in patients with positive PD-L1 was comparable with those in the entire included patients with any PD-L1 expression levels (pCR, 22.5% in positive PD-L1 vs. 21.2% in any PD-L1, p > 0.05; MPR 48.6% in positive PD-L1 vs. 43.7% in any PD-L1, p > 0.05).
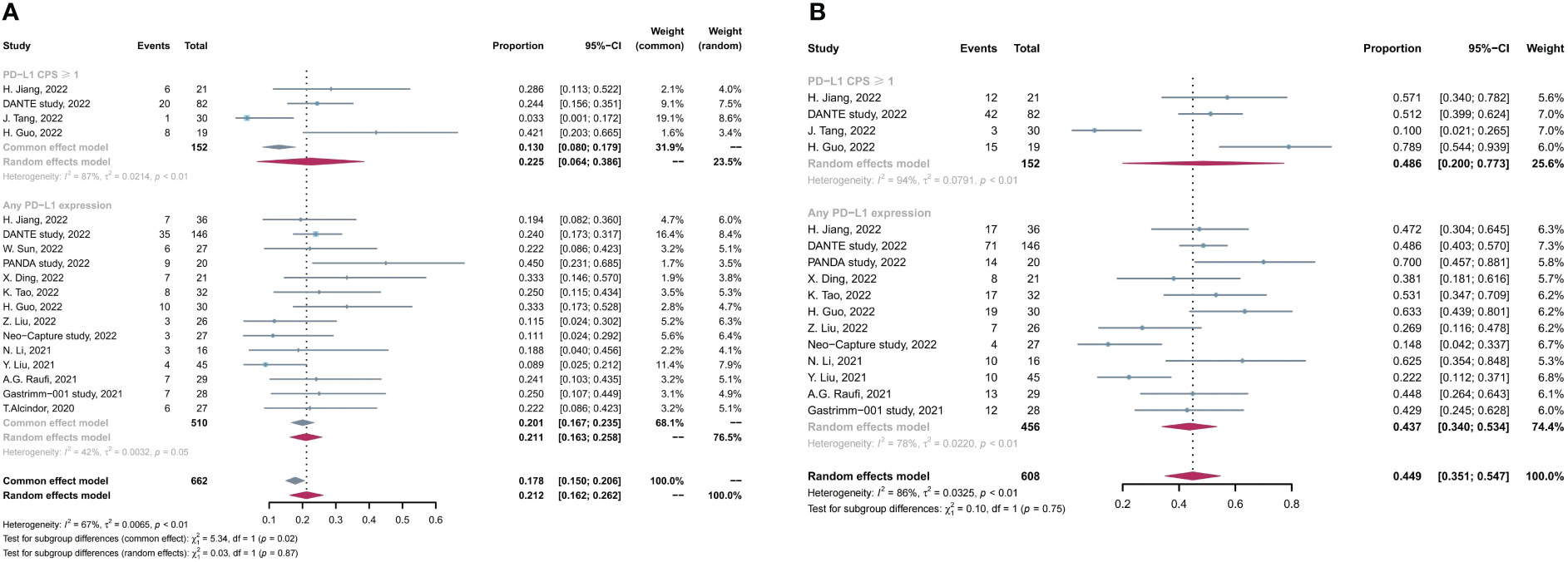
Figure 4 Subgroup analysis based on PD-L1 expression levels for (A) pCR rate and (B) MPR rate. pCR, complete pathological response rate; MPR, major pathological response rate.
Furthermore, we additionally analyzed the efficacy and safety of neoadjuvant immunochemotherapy plus radiotherapy or anti-VEGF drug (apatinib) in locally advanced GC/GEJC (Supplementary Figure 5). In pairwise comparisons, compared with neoadjuvant immunochemotherapy only, neoadjuvant immunochemotherapy plus radiotherapy improved MPR rate (p = 0.01), but did not improve pCR rate (p > 0.05), and correlated with a higher incidence of grade 3 to 4 TRAEs (p < 0.01). Moreover, neoadjuvant immunochemotherapy plus apatinib was correlated with worse MPR rate than neoadjuvant immunochemotherapy only (p < 0.01) or neoadjuvant immunochemotherapy plus radiotherapy (p < 0.01).
4 Discussion
The combination of NACT and immunotherapy brings infinite possibility for elevating therapeutic effects for patients with locally advanced GC/GEJC. In this systematic review and meta-analysis, we summarized the efficacy and safety data of medication in the latest prospective studies to figure out the potential value for further application.
The major feature of neoadjuvant therapy was the possibility of tumor downstaging, which might achieve MPR or even pCR for sensitive individuals. These pathologically favorable responses have been shown to benefit patients in terms of long-term overall survival (OS) and disease-free survival (DFS) (40, 41). Therefore, pCR and MPR could be used as key indicators in the selection of optimal regimens. Previous studies have demonstrated that the pCR rate and MPR rate for patients with LAGC who received NACT was mostly 5.6%–16% (16, 42, 43) and 21.2%–27.6% (44, 45). In the present study, we found that the pooled pCR rate is 21.7% and that the pooled MPR rate is 44.0% in patients with LAGC who received NACT combined with immunotherapy, which seemed superior to that in patients who received NACT alone in previous studies. Currently, three studies have reported encouraging results that NACT combined with immunotherapy performed better in short-term outcomes than single NACT in LAGC treatment (Supplementary Table 1), and six relevant clinical trials are going (Supplementary Table 2). For further research, it is necessary to combine multi-omics techniques to predict the sensitive populations (46–48) and to discover clinical predictors associated with better pathologic responses (49), so that the therapeutic effect of LAGC will ultimately improve.
TRAE is widely used clinical indicators to evaluate the safety of perioperative treatment, and it is common in immunotherapy (50). Chemotherapy combined with immunotherapy has become the first-line treatment in AGC. The ATTRACTION-4 study in Asian population showed a comparable safety profile in the nivolumab-chemotherapy group compared with that in the placebo-chemotherapy group, with neutropenia being the most common grade 3 to 4 adverse event (13). The ORIENT-16 study in the Chinese population found that the incidence rate of grade ≥3 TRAEs in the sintilimab-chemotherapy group was comparable with that in the chemotherapy group (59.8% vs. 52.5%, p = 0.063) (51). A meta-analysis comparing the safety of different combinations of immunotherapy and chemotherapy regimens showed that the incidence rate of TRAE ≥3 grade was similar between PD-1 inhibitor with oxaliplatin-based chemotherapy and that with cisplatin-based chemotherapy (RR = 0.86, 95% CI, 0.66–1.12), whereas, among patients with oxaliplatin-based chemotherapy, the incidence rate of TRAE ≥3 times was comparable between those combining nivolumab and those combining sintilimab (52). When immunotherapy was applied in neoadjuvant therapy, a meta-analysis found a higher incidence of TRAEs in neoadjuvant ICI plus chemotherapy compared with neoadjuvant immunotherapy, in which the most common all-grade TRAEs were neutropenia, nausea, and alopecia; high-grade TRAEs were neutropenia, anemia, and aspartate aminotransferase and aspartate aminotransferase (AST) elevation; moreover, the low rates of treatment-related surgical delays and deaths further confirm the safety and feasibility of neoadjuvant immunotherapy (53). In present study, we found that the overall TRAE rate was 89.1%, and the incidence rate of grade 3 to 4 TRAEs was 34.4% in patients treated with NACT plus immunotherapy. With regard to dosing and perioperative mortality, four deaths among patients with atezolizumab combining FLOT (4/141, 3%) were reported by DANTE study (21). Moreover, Raufi et al. (54) reported that two of the three cases with grade 5 TRAEs among patients treated with pembrolizumab plus neoadjuvant CapeOX might attributable to treatment; no deaths were observed in the remaining patients during the dosing and perioperative period.
Furthermore, a significant heterogeneity was observed in grade 3 to 4 TRAEs in different ICI treatments (Figure 2E). Atezolizumab and pembrolizumab were correlated with a relatively high grade 3 to 4 TRAE rate, whereas the rate was relatively low in sintilimab, tislelizumab, and toripalimab. Detailed information of grade 3 to 4 TRAEs has been listed in Supplementary Table 3, and, partially, the difference can be attributed to different type of ICIs. Apart from the type of PD-1/PD-L1 inhibitors, different chemotherapy regimens may influence the effectiveness of the perioperative treatment (55). There have been controversial regarding the differences in terms of efficacy and safety for different regimen of NACT. Studies by Taieb et al. and Sah et al. reported that no statistically difference was observed in effectiveness between FLOT regimen and SOX regimen (56, 57). However, Grizzi et al. found that patients administrated with FLOT had better OS and DFS than those administrated with SOX (58). Studies included in this meta-analysis incorporate several NACT regimens, including SOX, CapeOX, FLOT, mFOLFOX6, and DOC. We have performed additional subgroup analysis by different chemotherapy regimens and illustrated that SOX performed better in pCR rate and MPR rate and correlated with lower incidence of grade 3 to 4 TRAEs than other regimens (Supplementary Figure 6). Moreover, different combination of ICI and chemotherapy regimen may affect the effectiveness of treatment, but the limited number of studies could not support relevant analysis, which need further investigation to make individualized treatment regimen for patients.
PD-L1 expression levels was considered to be associated with the effectiveness of PD-1/PD-L1 inhibitors (59); however, whether individuals with low PD-L1 expression could benefit from immunotherapy remains controversial. A study by Zhao et al. reported that patients with PD-L1 low expression could not benefit from ICI treatment (60). However, the Checkmate-649 and ORIENT-16 studies have illustrated that all-treated patients with advanced GC/GEJC had significant better OS and PFS, no matter what the expression levels of PD-L1 are (12). These findings also supported by the recent studies that the combining of ICIs and chemotherapy could improve the prognosis of patients with GC/GEJC (61, 62). Our results were consistent with the findings above. There are eight trials included that reported the PD-L1 status, and patients with positive PD-L1 status (CPS ≥ 1) had a comparable pCR and MPR compared with all-treated patients, which supported those who are administrated with PD-1/PD-L1 inhibitors regardless of the PD-L1 status in GC/GEJC neoadjuvant immunochemotherapy; further large-scale studies are needed.
Several studies have illustrated that the addition of radiotherapy (28, 30) or anti-VEGF drugs (20, 29) improved the effectiveness of neoadjuvant therapy. Our study reported that, compared with the addition of radiotherapy or anti-VEGF drugs with single neoadjuvant immunochemotherapy, the combination with radiotherapy did improve MPR rate but had a higher incidence of TRAEs. We even found that the combination of neoadjuvant immunochemotherapy with apatinib had worse MPR than neoadjuvant immunochemotherapy only. However, the results were relatively not stable only in the two studies for the addition of neoadjuvant radiotherapy and anti-VEGF drugs.
There are several limitations to this meta-analysis. First, some included studies are with conference abstract without available full text. Second, not every clinical trial reported all outcomes, and some have not yet reached their endpoint, which may affect the stability of the results. Third, the prognostic endpoints, like OS and DFS, were not included in this meta-analysis, because most studies are ongoing trials. Furthermore, most studies are single-arm clinical trials and those compared neoadjuvant immunochemotherapy with NACT are limited. Thus, large-sample, multicenter, and RCT studies are needed to evaluate these results. The variations in NACT regimens, study design, clinical parameters, and MMR status all contribute to heterogeneity.
5 Conclusions
This systematic review and meta-analysis found that PD-1/PD-L1 inhibitors combined with NACT for locally advanced GC/GEJC were well tolerated and may confer therapeutic advantages. The integration of ICIs into NACT has shown the potential for application in any PD-L1 expression population.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
Concept and Design: BW, ZY, HC, WL, and SW. Collection and assembly of data: BC, WL, and LS. Data analysis and interpretation: GL, JH, LC, ZY, HC, and SW. Manuscript writing: ZY, HC, and SW. Manuscript revision: ZY, HC, and SW. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Basic Research Program of China (2019YFB1311505) and by the National Natural Science Foundation of China (82073192, 82273231). Beijing Science and Technology Program (Z221100007422125).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1103320/full#supplementary-material
Abbreviations
ICI, immune checkpoint inhibitor; GC/GEJC, gastric or gastroesophageal junction adenocarcinoma; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; MPR, major pathological response; TRAE, treatment-related adverse event; CPS, combined positive score; RCT, randomized controlled trial; GC, Gastric cancer; advanced gastric cancer, AGC; locally advanced gastric cancer, LAGC; ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; AACR, American Association for Cancer Research; CI, confidence interval; MMR, mismatch repair; SAE, serious adverse event; AST, aspartate aminotransferase; OS, overall survival; DFS, disease-free survival; EC, esophageal adenocarcinoma.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Yang X, Zhang T, Zhang H, Sang S, Chen H, Zuo X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030: comparison with Japan, south Korea, and Mongolia. biomark Res (2021) 9(1):84. doi: 10.1186/s40364-021-00340-6
3. Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, et al. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res (2021) 33(2):168–80. doi: 10.21147/j.issn.1000-9604.2021.02.05
4. Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol (2017) 39(7):1010428317714626. doi: 10.1177/1010428317714626
5. Sugawara K, Kawaguchi Y, Seto Y, Vauthey JN. Multidisciplinary treatment strategy for locally advanced gastric cancer: A systematic review. Surg Oncol (2021) 38:101599. doi: 10.1016/j.suronc.2021.101599
6. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657
7. Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B. Comprehensive molecular characterization of gastric adenocarcinoma. Nature (2014) 513(7517):202–9. doi: 10.1038/nature13480
8. Dai Z, Zhang J, Wu Q, Fang H, Shi C, Li Z, et al. Intestinal microbiota: a new force in cancer immunotherapy. Cell Commun Signal (2020) 18(1):90. doi: 10.1186/s12964-020-00599-6
9. Li X, Xu J, Xie J, Yang W. Research progress in targeted therapy and immunotherapy for gastric cancer. Chin Med J (Engl). (2022) 135(11):1299–313. doi: 10.1097/CM9.0000000000002185
10. Wu J, Waxman DJ. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett (2018) 419:210–21. doi: 10.1016/j.canlet.2018.01.050
11. Salas-Benito D, Pérez-Gracia JL, Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E, et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discovery (2021) 11(6):1353–67. doi: 10.1158/2159-8290.CD-20-1312
12. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
13. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6
14. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). (2021) 41(8):747–95. doi: 10.1002/cac2.12193
15. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med (2006) 355(1):11–20. doi: 10.1056/NEJMoa055531
16. Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: A phase III study of neoadjuvant docetaxel, oxaliplatin, and s-1 plus surgery and adjuvant s-1 versus surgery and adjuvant s-1 for resectable advanced gastric cancer. J Clin Oncol (2021) 39(26):2903–13. doi: 10.1200/JCO.20.02914
17. Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer (2022) 10(3). doi: 10.1136/jitc-2021-003635
18. Terazawa T, Matsuyama J, Goto M, Kawabata R, Endo S, Imano M, et al. A phase II study of perioperative capecitabine plus oxaliplatin therapy for clinical SS/SE N1-3 M0 gastric cancer (OGSG 1601). Oncologist (2020) 25(2):119–e208. doi: 10.1634/theoncologist.2019-0601
19. Xie BW, Zang L, Ma JJ, Sun J, Yang X, Wang ML, et al. [Safety and effectiveness of oxaliplatin combined with capecitabine or oxaliplatin combined with s-1 neoadjuvant chemotherapy in the treatment of advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. (2021) 24(2):138–44. doi: 10.3760/cma.j.cn.441530-20200721-00433
20. Xu C, Xie X, Kang N, Jiang H. Neoadjuvant PD-1 inhibitor and apatinib combined with s-1 plus oxaliplatin for locally advanced gastric cancer patients: a multicentered, prospective, cohort study. J Cancer Res Clin Oncol (2022). doi: 10.1007/s00432-022-04302-9
21. Al-Batran S-E, Lorenzen S, Thuss-Patience PC, Homann N, Schenk M, Lindig U, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German gastric cancer group and Swiss SAKK. J Clin Oncol (2022) 40(16_suppl):4003. doi: 10.1200/JCO.2022.40.16_suppl.4003
22. Sun W, Saeed A, Al-Rajabi RMT, Kasi A, Veeramachaneni NK, Al-Kasspooles MF, et al. A phase II study of perioperative mFOLFOX plus pembrolizumab combination in patients with potentially resectable adenocarcinoma of the esophageal, gastroesophageal junction (GEJ) and stomach. J Clin Oncol (2022) 40(16). doi: 10.1200/JCO.2022.40.16_suppl.4047
23. Tang J, Huang J, Zhang B, Qi L, Dao X, Wang L, et al. Perioperative chemotherapy with LP002, an anti-PD-L1 antibody, in patients with resectable gastric and gastroesophageal junction cancer: A prospective, open-label, phase ib trial. J Clin Oncol (2022) 40(16). doi: 10.1200/JCO.2022.40.16_suppl.4041
24. Verschoor YL, Kodach L, Van Den Berg J, Van Sandick JW, Van Dieren J, Balduzzi S, et al. Neoadjuvant atezolizumab plus docetaxel/oxaliplatin/capecitabine in non-metastatic gastric and gastroesophageal junction adenocarcinoma: The PANDA trial. J Clin Oncol (2022) 40(16). doi: 10.1200/JCO.2022.40.16_suppl.4059
25. Ding X, Li B, Xue Q, Cai M, Cui J, Wang B, et al. Perioperative sintilimab combination with SOX for resectable locally advanced gastric/gastroesophageal junction cancer(GC/GEJC): Initial findings of a single-arm phase II trial. J Clin Oncol (2022) 40(4 SUPPL). doi: 10.1200/JCO.2022.40.4_suppl.294
26. Tao K, Yin Y, Lin Y, Li W, Li R, Liu W, et al. Neoadjuvant PD-1 inhibitor tislelizumab combined with s-1 plus oxaliplatin in patients with local advanced gastric cancer or gastroesophageal junction adenocarcinoma: Interim results of a single-arm, phase II trial. J Clin Oncol (2022) 40(4 SUPPL). doi: 10.1200/JCO.2022.40.4_suppl.300
27. Li N, Li Z, Fu Q, Zhang B, Zhang J, Wan X, et al. Phase II study of sintilimab combined with FLOT regimen for neoadjuvant treatment of gastric or gastroesophageal junction (GEJ) adenocarcinoma. Ann Oncol (2020) 31:S1302. doi: 10.1016/j.annonc.2020.10.181
28. Wei J, Lu X, Liu Q, Fu Y, Liu S, Yang J, et al. SHARED: Efficacy and safety of sintilimab in combination with concurrent chemoradiotherapy (cCRT) in patients with locally advanced gastric (G) or gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol (2021) 39(15_suppl):4040. doi: 10.1200/JCO.2021.39.15_suppl.4040
29. Li S, Yu W, Xie F, Liu Z, Lv W, Shi D, et al. A prospective, phase II, single-arm study of neoadjuvant/conversion therapy with camrelizumab, apatinib, s-1 ± oxaliplatin for locally advanced cT4a/bN+ gastric cancer. J Clin Oncol (2021) 39(15_suppl):4061. doi: 10.1200/JCO.2021.39.15_suppl.4061
30. Tang ZQ, Wang Y, Liu D, Yu YY, Cui YH, Tang C, et al. 1385P phase II study of neoadjuvant camrelizumab combined with chemoradiation for locally advanced proximal gastric cancer (Neo-PLANET, NCT03631615). Ann Oncol (2021) 32:S1049. doi: 10.1016/j.annonc.2021.08.1494
31. Raufi AG, Lee S, May M, Del Portillo A, Sender N, Ana SS, et al. Phase II trial of perioperative pembrolizumab plus capecitabine and oxaliplatin followed by adjuvant pembrolizumab for resectable gastric and gastroesophageal junction (GC/GEJ) adenocarcinoma. Cancer Res (2022) 82(12). doi: 10.1158/1538-7445.AM2022-CT009
32. Liu Z, Liu N, Zhou Y, Niu Z, Jiang H, Zhu Y, et al. Efficacy and safety of camrelizumab combined with FLOT versus FLOT alone as neoadjuvant therapy in patients with resectable locally advanced gastric and gastroesophageal junction adenocarcinoma who received D2 radical gastrectomy: Data update. J Clin Oncol (2022) 40(16). doi: 10.1200/JCO.2022.40.16_suppl.e16044
33. Jiang Z, Xie Y, Wang B, Zhu Y, Ke Y, Zhang W, et al. Oxaliplatin and capecitabine (XELOX) plus toripalimab as perioperative treatment for locally advanced gastric or gastro-esophageal junction adenocarcinoma (Neo-capture): A single-arm, phase 2 study. J Clin Oncol (2022) 40(16). doi: 10.1200/JCO.2022.40.16_suppl.e16001
34. Li H, Deng J, Ge S, Zang F, Zhang L, Ren P, et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol (2021) 39(15_suppl):4050. doi: 10.1200/JCO.2021.39.15_suppl.4050
35. Guo H, Ding P, Sun C, Yang P, Tian Y, Liu Y, et al. Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: A single-arm, open-label, phase II trial. Front Oncol (2022) 12:927781. doi: 10.3389/fonc.2022.927781
36. Alcindor T, Opu T, Elkrief A, Khosrow-Khavar F, Mueller CL, Cools-Lartigue J, et al. Phase II trial of perioperative chemotherapy + avelumab in locally advanced gastroesophageal adenocarcinoma: Preliminary results. J Clin Oncol (2021) 39(15_suppl):4046. doi: 10.1200/JCO.2021.39.15_suppl.4046
37. Zhu M, Chen C, Foster NR, Hartley C, Mounajjed T, Salomao MA, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res (2022) 28(14):3021–31. doi: 10.1158/1078-0432.CCR-22-0413
38. Liu Y, Han G, Li H, Zhao Y, Li Z, Zhuang J, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: Updated results of efficacy and safety. J Clin Oncol (2021) 39(15_suppl):4036. doi: 10.1200/JCO.2021.39.15_suppl.4036
39. Jiang J, Wang Y, Gao Y, Sugimura H, Minervini F, Uchino J, et al. Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res (2022) 11(2):277–94. doi: 10.21037/tlcr-22-75
40. Levenson G, Voron T, Paye F, Balladur P, Debove C, Chafai N, et al. Tumor downstaging after neoadjuvant chemotherapy determines survival after surgery for gastric adenocarcinoma. Surgery (2021) 170(6):1711–7. doi: 10.1016/j.surg.2021.08.021
41. Wang T, Wang N, Zhou H, Zhou A, Jin J, Chen Y, et al. Long-term survival results of patients with locally advanced gastric cancer and pathological complete response after neoadjuvant chemotherapy and resection. Transl Cancer Res (2020) 9(2):529–35. doi: 10.21037/tcr.2019.11.37
42. Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with s-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol (2021) 22(8):1081–92. doi: 10.1016/S1470-2045(21)00297-7
43. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol (2016) 17(12):1697–708. doi: 10.1016/S1470-2045(16)30531-9
44. Chen Y, Wei K, Liu D, Xiang J, Wang G, Meng X, et al. A machine learning model for predicting a major response to neoadjuvant chemotherapy in advanced gastric cancer. Front Oncol (2021) 11:675458. doi: 10.3389/fonc.2021.675458
45. Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg (2011) 253(5):934–9. doi: 10.1097/SLA.0b013e318216f449
46. Wang W, Peng Y, Feng X, Zhao Y, Seeruttun SR, Zhang J, et al. Development and validation of a computed tomography-based radiomics signature to predict response to neoadjuvant chemotherapy for locally advanced gastric cancer. JAMA Netw Open (2021) 4(8):e2121143. doi: 10.1001/jamanetworkopen.2021.21143
47. Huang W, Zhan D, Li Y, Zheng N, Wei X, Bai B, et al. Proteomics provides individualized options of precision medicine for patients with gastric cancer. Sci China Life Sci (2021) 64(8):1199–211. doi: 10.1007/s11427-021-1966-4
48. Li Z, Gao X, Peng X, May Chen MJ, Li Z, Wei B, et al. Multi-omics characterization of molecular features of gastric cancer correlated with response to neoadjuvant chemotherapy. Sci Adv (2020) 6(9):eaay4211. doi: 10.1126/sciadv.aay4211
49. Bausys A, Ümarik T, Luksta M, Reinsoo A, Rackauskas R, Anglickiene G, et al. Impact of the interval between neoadjuvant chemotherapy and gastrectomy on short- and long-term outcomes for patients with advanced gastric cancer. Ann Surg Oncol (2021) 28(8):4444–55. doi: 10.1245/s10434-020-09507-1
50. Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer. (2019) 19(1):559. doi: 10.1186/s12885-019-5785-z
51. Xu J, Jin Y, Liu Y, Zhou H, Wang Y. Abstract CT213: ORIENT-16: Sintilimab plus XELOX vs placebo plus XELOX as 1st line treatment for unresectable advanced gastric and GEJ adenocarcinoma. Cancer Res (2019) 79(13_Supplement):CT213–CT. doi: 10.1158/1538-7445.AM2019-CT213
52. Guo X, Yang B, He L, Sun Y, Song Y, Qu X. PD-1 inhibitors plus oxaliplatin or cisplatin-based chemotherapy in first-line treatments for advanced gastric cancer: A network meta-analysis. Front Immunol (2022) 13:905651. doi: 10.3389/fimmu.2022.905651
53. Xu J, Wu Y, Xu Y, Qiu Y, Li X, Song Y, et al. Safety of neoadjuvant immunotherapy in resectable cancers: A meta-analysis. Front Immunol (2022) 13:802672. doi: 10.3389/fimmu.2022.802672
54. Raufi AG, Lee S, May M, Portillo AD, Sender N, Ana SS, et al. Abstract CT009: Phase II trial of perioperative pembrolizumab plus capecitabine and oxaliplatin followed by adjuvant pembrolizumab for resectable gastric and gastroesophageal junction (GC/GEJ) adenocarcinoma. Cancer Res (2022) 82(12_Supplement):CT009–CT. doi: 10.1158/1538-7445.AM2022-CT009
55. Lumish MA, Ku GY. Approach to resectable gastric cancer: Evolving paradigm of neoadjuvant and adjuvant treatment. Curr Treat Options Oncol (2022) 23(7):1044–58. doi: 10.1007/s11864-021-00917-1
56. Taieb J, Moehler M, Boku N, Ajani JA, Yañez Ruiz E, Ryu MH, et al. Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: Current status and future perspectives. Cancer Treat Rev (2018) 66:104–13. doi: 10.1016/j.ctrv.2018.04.004
57. Sah BK, Zhang B, Zhang H, Li J, Yuan F, Ma T, et al. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun (2020) 11(1):6093. doi: 10.1038/s41467-020-19965-6
58. Grizzi G, Petrelli F, Di Bartolomeo M, Viti M, Texeira Moraes M, Luciani A, et al. Preferred neoadjuvant therapy for gastric and gastroesophageal junction adenocarcinoma: a systematic review and network meta-analysis. Gastric Cancer. (2022) 25(5):982–7. doi: 10.1007/s10120-022-01314-9
59. Takei S, Kawazoe A, Shitara K. The new era of immunotherapy in gastric cancer. Cancers (Basel). (2022) 14(4):1054. doi: 10.3390/cancers14041054
60. Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol (2022) 40(4):392–402. doi: 10.1200/JCO.21.01862
61. Dubois M, Liscia N, Brunetti O, Ziranu P, Lai E, Argentiero A, et al. The role of immune checkpoint inhibitors in the treatment sequence of advanced gastric or gastro-esophageal junction cancer: A systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol (2022) 173:103674. doi: 10.1016/j.critrevonc.2022.103674
Keywords: gastric adenocarcinoma, gastroesophageal junction adenocarcinoma, PD-1/PD-L1 inhibitors, immune checkpoint inhibitors, neoadjuvant immunotherapy
Citation: Yuan Z, Cui H, Wang S, Liang W, Cao B, Song L, Liu G, Huang J, Chen L and Wei B (2023) Combining neoadjuvant chemotherapy with PD-1/PD-L1 inhibitors for locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: A systematic review and meta-analysis. Front. Oncol. 13:1103320. doi: 10.3389/fonc.2023.1103320
Received: 20 November 2022; Accepted: 09 January 2023;
Published: 26 January 2023.
Edited by:
Zhibo Yan, Qilu Hospital, Shandong University, ChinaReviewed by:
Chen Huang, Shanghai General Hospital, ChinaKaixiong Tao, Huazhong University of Science and Technology, China
Gang Ji, Fourth Military Medical University, China
Copyright © 2023 Yuan, Cui, Wang, Liang, Cao, Song, Liu, Huang, Chen and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Wei, d2VpYm9AdmlwLjE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Zhen Yuan
Zhen Yuan Hao Cui
Hao Cui Shuyuan Wang1,3†
Shuyuan Wang1,3† Bo Cao
Bo Cao Bo Wei
Bo Wei