
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 23 June 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1101582
Background: The effectiveness of intravenous lidocaine infusion in managing acute and chronic pain following breast surgery has been a topic of debate. This meta-analysis aims to assess the impact of perioperative intravenous lidocaine on the relief of postoperative pain among patients undergoing breast surgery.
Methods: A systematic search of databases was conducted to identify randomized controlled trials (RCTs) that compared the effects of intravenous lidocaine infusion with placebo or routine care in patients undergoing breast surgery. The primary outcome of interest was the occurrence of chronic post-surgical pain (CPSP) at the longest follow-up. Meta-analyses, incorporating trial sequential analysis, were performed using a random-effects model to assess the overall effect.
Results: A total of twelve trials, involving 879 patients, were included in the analysis. Perioperative intravenous lidocaine demonstrated a significant reduction in the incidence of CPSP at the longest follow-up (risk ratio [RR] 0.62, 95% confidence interval [CI] 0.48-0.81; P = 0.0005; I2 = 6%). Trial sequential analysis (TSA) indicated that the cumulative z curve crossed the trial sequential monitoring boundary for benefit, providing sufficient and conclusive evidence. Furthermore, intravenous lidocaine was associated with decreased opioid consumption and a shorter length of hospital stay.
Conclusion: Perioperative intravenous lidocaine is effective in relieving acute and CPSP in patients undergoing breast surgery.
Systematic review registration: https://inplasy.com/, identifier INPLASY2022100033.
Breast surgery is a widely performed procedure worldwide, with a significant number of patients experiencing moderate to severe acute pain (30-50%) (1, 2)and developing chronic post-surgical pain (CPSP) (25-68%) (3, 4).CPSP, characterized by persistent or worsening pain in the breast region lasting for at least 3 months after surgery (5, 6), can have detrimental effects on emotional well-being, functional abilities, quality of life, and impose substantial financial burdens on healthcare systems (4, 7). The pathophysiology of CPSP involves mechanisms such as traumatic nerve injury, neuroinflammation, and central neuronal sensitization (8). The conventional approach to managing postoperative pain relies heavily on opioids, which carries the risk of adverse effects including respiratory depression, addiction, and even mortality (9). To address these challenges, multimodal analgesic strategies have been proposed to alleviate both acute and chronic postoperative pain following breast surgery (10).
Lidocaine, being used originally as an antiarrhythmic agent, has been found to possess antinociceptive (11), anti-inflammatory (12) and anti-hyperalgesia (13) properties, making it a potentially useful drug for relieving postoperative pain. The systemic administration of lidocaine has shown efficacy in relieving neuropathic pain (14). Previous meta-analyses have demonstrated the effectiveness of intravenous lidocaine in reducing postoperative pain and opioid consumption in patients undergoing spine (15) and abdomen surgery (16, 17). However, the efficacy of intravenous lidocaine specifically for breast surgery has not been extensively evaluated due to limitations such as small sample sizes and conflicting findings from individual studies (18–22). Therefore, we conducted this meta-analysis to assess the efficacy of intravenous lidocaine in breast surgery patients. Our hypothesis was that perioperative intravenous lidocaine could alleviate both acute postoperative pain and chronic persistent post-surgical pain (CPSP) following breast surgery.
This meta-analysis was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (23) and is reported in compliance with the updated PRISMA 2020 statement guideline (24). The protocol was registered on International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY2022100033) (https://inplasy.com/).
A systematic electronic search was conducted in PubMed, Embase, and the Cochrane Library from their inception until September 20, 2022. The search strategy employed in PubMed was as follows: (lidocaine OR lignocaine OR xylocitin OR xylocaine OR lidocainum) AND (breast OR mastectomy OR mammaplasty). No restrictions were applied during the search process. In addition, the reference lists of the retrieved studies and previous reviews were examined to identify any additional potentially eligible trials for inclusion in the analysis.
The initial records were imported into EndNote software (Clarivate Analytics), and duplicate records were removed. Two authors (JL and JH) independently reviewed the titles and abstracts of the records to determine their relevance. The records were categorized as included, excluded, or requiring further evaluation. In cases where there was uncertainty, the full-text articles were obtained for further assessment of eligibility. Any disagreements regarding the inclusion of a trial were resolved through discussion between the authors.
The inclusion criteria for studies in this meta-analysis were as follows: (1) the study population consisted of adult patients undergoing breast surgery; (2) the intervention involved perioperative intravenous lidocaine; (3) a comparison group receiving either a control intervention or placebo was present; and (4) the study design was a randomized controlled trial (RCT). The primary outcome of interest was the occurrence of chronic post-surgical pain (CPSP) at the longest follow-up. Secondary outcomes included acute postoperative pain, morphine consumption during and after surgery, administration of postoperative rescue analgesics, postoperative nausea and vomiting (PONV), quality of recovery, and length of hospital stay.
Data extraction was performed by JH and confirmed independently by other authors (JL and JTY). We used a predefined data extraction form (Excel, Microsoft Corporation, USA) to collect the following information: first author, year of publication, country, population, ASA classification, surgical procedure, number of patients, intervention (route, dosage, and duration of lidocaine), comparison, and outcomes.
Two authors (JL and JH) assessed risk of bias independently, using the Cochrane risk-of-bias tool (25) including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Each item was rated as low, unclear, or high risk of bias. Trials with ≥1 key domains at high risk of bias were considered as at high risk of bias; trials with all key domains at low risk of bias were classified into low risk of bias; otherwise, they were considered to be at unclear risk of bias.
The certainty of evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (26) system. The assessment considered factors such as risk of bias, inconsistency, indirectness, imprecision, and publication bias. Based on these criteria, the quality of evidence was categorized as very low, low, moderate, or high. The GRADE Profiler (version 3.6, GRADE pro) was utilized to construct a summary table presenting the findings.
Summary statistics were reported as relative risks (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs) with 95% CIs for continuous outcomes. Pooled data were analyzed using random-effects models based on the intention-to-treat principle. Heterogeneity across the trials were evaluated by Cochrane Q test (P < 0.1) and the quantitative I2 statistic (I2 >50%) (27, 28). Regardless of heterogeneity, outcome data were synthesized using a random-effects model. Subgroup analyses of CPSP were conducted based on the duration of follow-up. Publication bias was assessed visually using a funnel plot and also evaluated using Begg’s and Egger’s tests (29, 30). Statistical significance was considered at a two-sided P-value less than 0.05, unless otherwise specified. All statistical analyses were performed using RevMan 5.4 (Nordic Cochrane Centre) and Stata version 12.0 (Stata Corp LP).
Interim analyses in a single trial can increase the risk of type I error (false-positive results). To avoid this issue, monitoring boundaries can be implemented to determine whether a trial should be terminated early based on a sufficiently small P-value indicating the anticipated effect or futility. Similarly, meta-analysis with small sample sizes may increase the type I error results due to the sparse data and repetitive testing of accumulating data (31). Trial sequential analysis (TSA) is a new statistical method to addressing these challenges. It can generate the monitoring boundaries, required information size, and futility boundaries to determine whether the evidence in a meta-analysis is reliable and conclusive. If the cumulative Z curve reaches the required information size (RIS) line or enters the trial sequential monitoring boundary, it indicates that sufficient evidence to achieve the anticipated effect of intervention and no further trials are need. If the Z curve does not cross any of the boundaries and has not reach the RIS, there is insufficient evidence to draw conclusion. We used TSA to estimate the RIS for this meta-analysis. Parameters for calculating RIS include type I error (α = 0.05, two-sided), type II error (β= 0.20, power of 80%), the control event proportions, and the RR reduction of 20% for primary outcomes. Trial Sequential Analysis Viewer version 0.9 Beta was used for these analyses (32).
The initial search resulted in a total of 1270 records, out of which 119 duplicates were removed. Following the screening of titles and abstracts, the full texts of the remaining 21 articles were assessed for eligibility. Ultimately, twelve randomized controlled trials (RCTs) met the inclusion criteria and were included in the meta-analysis (6, 18–22, 33–38) (Figure 1).
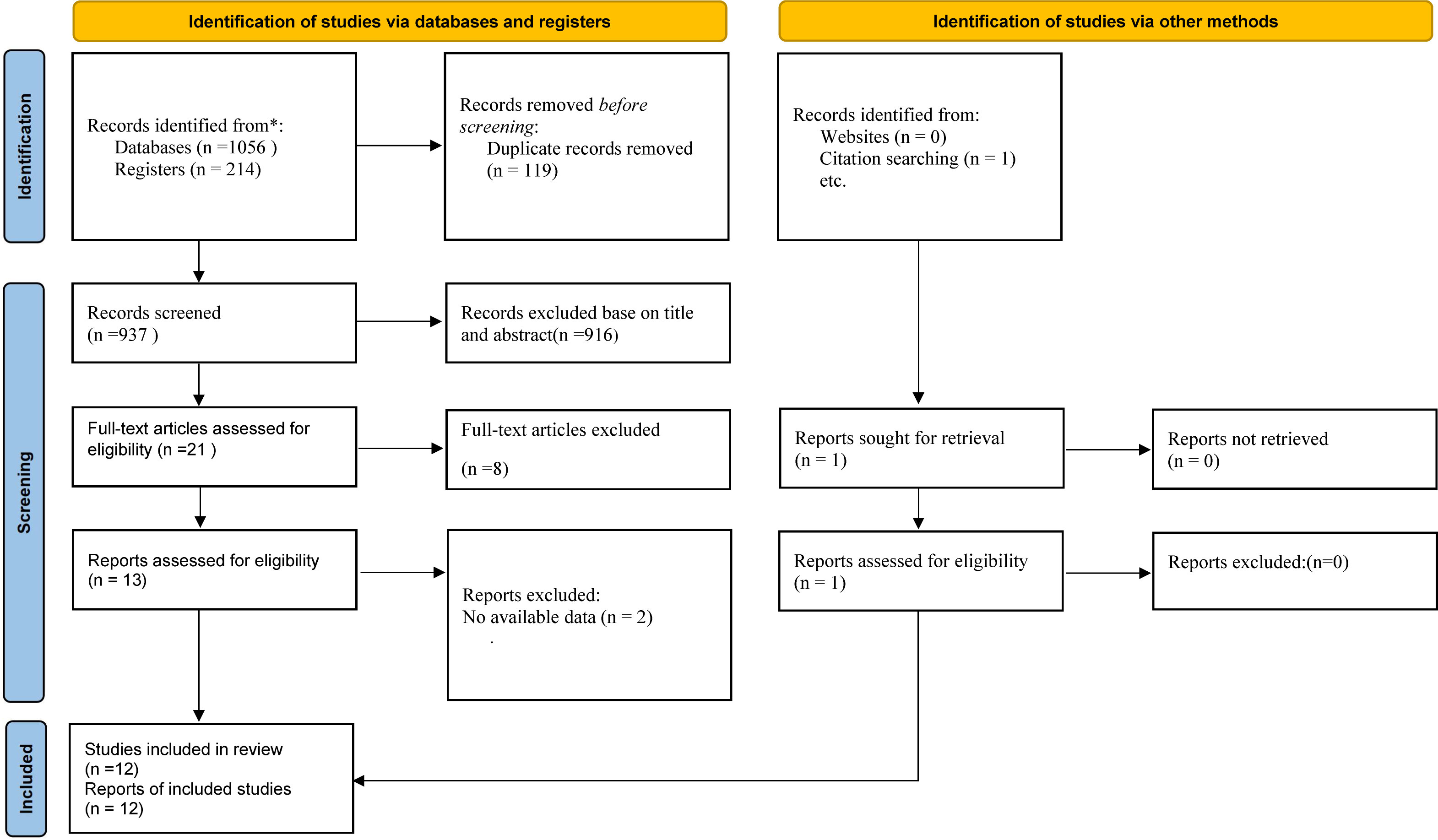
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram summarizing literature screening process.
The main characteristics of the included trials are summarized in Table 1. These trials were published between 2012 and 2022. The sample size ranged from 37 to 150, with a total of 879 participants. Among the included trials, three trials were multi-center (22, 35, 37), the remaining nine were single-center (6, 18–21, 33, 34, 36, 38). All trials except Choi et al. (33) recruited patients undergoing breast cancer surgery. All trials’ route of administration of lidocaine are intravenous injection except Toner et al. (37) used postoperative subcutaneous lidocaine for 12 hours after completion of surgery. The dosage of continuous lidocaine infusion ranges from 1.5mg/kg/h to 2mg/kg/h throughout the breast surgery. The continuous lidocaine infusion was stopped at 1 hour after the start of surgery (20), at the starting (22) or end of skin closure (33), 1 hour after the surgical closure (18, 35), before transferring patients into the recovery room (21), 2 hours after arrival in the recovery room (19, 34), the end of surgery (6, 38), 1 hour (36) and 12 hours after surgery (37).
Details of risks of bias across the included trials are presented in Figure 2. Out of the twelve trials, seven were classified as having a low risk of bias (6, 18, 21, 22, 35, 37, 38), while the remaining five trials were categorized as being at unclear risk of bias (19, 20, 33, 34, 36). Nine trials reported the generation of an adequate randomized sequence (6, 18, 20–22, 35–38), and three trials provided information on appropriate allocation concealment (20, 33, 36).
Seven trials included in this meta-analysis provided data on CPSP at the longest follow-up. Pooled analysis suggested that intravenous lidocaine significantly reduced the incidence of CPSP at the longest follow-up (seven trials; RR 0.62, 95% CI 0.48-0.81; P = 0.0005; Figure 3; Table 2). There was no significant heterogeneity observed across the studies (I2 = 6%). These findings remained consistent when subgroup analyses were performed based on the follow-up time of CPSP (Supplementary Material Figure S1). The trial sequential analysis (TSA) indicated that the cumulative Z curve crossed both the conventional boundary and the trial sequential monitoring boundary for benefit, establishing sufficient and conclusive evidence (Figure 4 and Supplementary Material Figure S2).
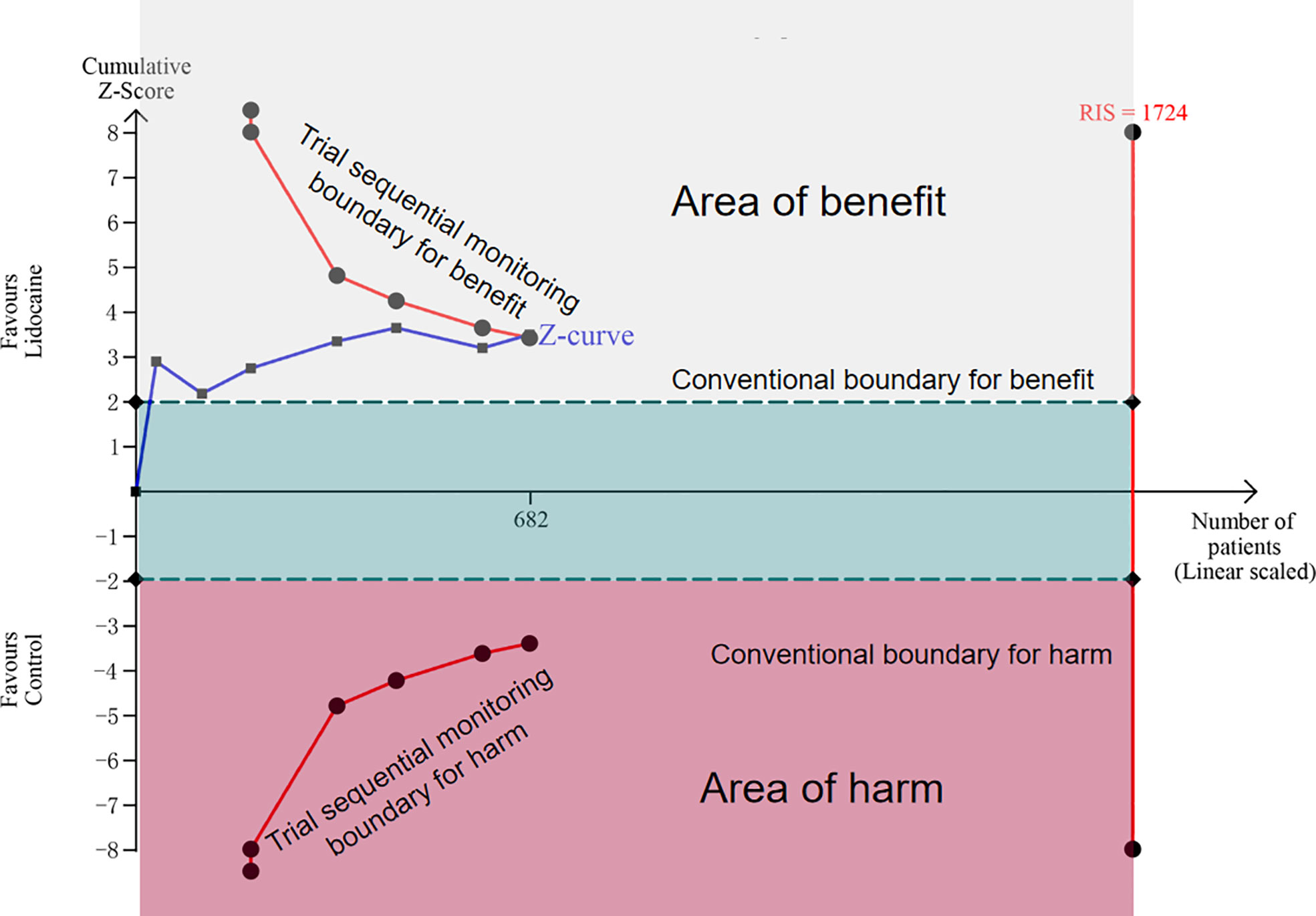
Figure 4 Trial sequential analysis for chronic post-surgical pain at the longest follow up (scaled trial distance). Trial sequential analysis of seven trials (black filled squares) illustrating that the cumulative Z curve crossed the conventional boundary and the trial sequential monitoring boundary for benefit, establishing sufficient and conclusive evidence. A diversity-adjusted required information size of 1724 patients were calculated using α = 0.05 (two-sided), β= 0.20 (power of 80%), an anticipated relative risk reduction of 20%, and an event proportion of 32.75% in the control group.
This meta-analysis examined the postoperative pain scores assessed using Visual Analogue Scale or Numerical Rating Scale at rest and during movement (Table 2). The pooled analysis demonstrated that postoperative pain scores at rest were significantly lower in the lidocaine group compared to the control group at 2h (three trials; MD -0.73; 95% CI -1.00 to -0.46; P < 0.00001; I2 = 12%), 4h (three trials; MD -1.03; 95% CI -1.40 to -0.65; P < 0.00001; I2 = 0%), 48h (four trials; MD -0.45; 95% CI -0.67 to -0.23; P < 0.0001; I2 = 0%), and 72h (three trials; MD -0.59; 95% CI -0.99 to -0.20; P < 0.003; I2= 29%; Figure 5). Similarly, postoperative pain scores during movement were significantly lower in the lidocaine group at 2h (MD -0.63; 95% CI -0.91 to -0.35; P < 0.00001; Figure 6). Although no statistically significant difference was observed in pain scores between the two groups at 24 hours after surgery, there was a trend towards improved pain control in the intravenous lidocaine group.
The administration of perioperative intravenous lidocaine was found to be associated with a significant reduction in remifentanil consumption during surgery (MD -187.40; 95% CI -238.37 to -136.44; P < 0.00001; Table 2) without significant heterogeneity (I2 = 0%; Supplementary Material Figure S3). Additionally, it was observed that lidocaine resulted in decreased morphine consumption at 24 hours after breast surgery (MD -0.78; 95% CI -1.04 to -0.52; P < 0.00001; Table 2), with no significant heterogeneity (I2 = 0%; Supplementary Material Figure S4). However, there were no significant differences in morphine consumption during surgery between the lidocaine and placebo groups (Supplementary Material Figure S5).
The pooled estimates revealed that there was no significant difference in the incidence of postoperative nausea or vomiting (PONV) between the lidocaine and placebo groups (RR 0.95, 95% CI 0.69-1.31; P = 0.75; Supplementary Material Figure S6). Similarly, there was no significant difference in the administration of rescue analgesics within 24 hours after surgery between the lidocaine and placebo groups (RR 0.95, 95% CI 0.84-1.06; P = 0.33; Supplementary Material Figure S7). However, the length of hospital stay was significantly shorter in the lidocaine group (MD -1.11; 95% CI -2.12 to -0.1; P < 0.003; I2 = 0%; Supplementary Material Figure S8). Furthermore, the quality of postoperative recovery within 24 hours after surgery was comparable between the lidocaine and placebo groups (Supplementary Material Figure S9).
Four trials reported intravenous lidocaine associated side effects (19, 22, 37, 38). No toxicity cases were found in trials performed by Terkawi et al. (19), Khan et al. (22) and Wei et al. (38). However, Toner et al. (37) specifically monitored the occurrence of toxicity for 12 hours and reported that three patients in the lidocaine group and one patient in the control group experienced a metallic taste. Due to the limited amount of data available, pooled analyses were not conducted for these side effects.
The GRADE evidence profiles for the outcomes are presented in Supplementary Material Table S1. The level of evidence according to GRADE is classified as moderate for chronic postoperative pain scores (CPSP), acute postoperative pain scores at most time points, and remifentanil consumption during surgery. For other outcomes, such as postoperative nausea and vomiting (PONV) and hospital stay, the level of evidence is categorized as low.
Visual inspection suggested that the funnel plot for CPSP appear to be asymmetrical. But no publication bias was detected in formal statistical tests (Begg´s test, P = 0.764; Egger´s test, P = 0.401; Supplementary Material Figure S10).
Our comprehensive and systematic meta-analysis examined the available literature and revealed several key findings. Firstly, perioperative intravenous lidocaine demonstrated a significant reduction in chronic postoperative pain (CPSP) following breast surgery. This beneficial effect was consistent across subgroup analyses and was further supported by trial sequential analysis (TSA). Secondly, perioperative intravenous lidocaine exhibited positive effects in alleviating acute pain after breast surgery. Thirdly, the use of perioperative intravenous lidocaine resulted in reduced opioid consumption during and 24 hours after surgery. Fourthly, patients receiving perioperative intravenous lidocaine experienced a shorter hospital stay compared to those who did not. Lastly, there was no observed benefit of perioperative intravenous lidocaine on the quality of recovery within 24 hours after surgery. These findings provide valuable insights into the potential benefits of perioperative intravenous lidocaine in breast surgery patients.
In a previous meta-analysis conducted by Chang et al. (39), they explored the potential benefits of perioperative intravenous lidocaine in postoperative pain management. However, their study was limited by a small sample size, including only four randomized controlled trials (RCTs) involving 167 patients. Consequently, their findings lacked sufficient statistical power to draw conclusive results regarding the effects of intravenous lidocaine on acute pain after breast surgery. In contrast, our current meta-analysis overcomes this limitation by including a larger pool of evidence comprising 12 RCTs with a total of 879 patients. The increased statistical power provided by our larger sample size allows for more robust conclusions to be drawn. Our findings demonstrate significant benefits of perioperative intravenous lidocaine in reducing the incidence of chronic postoperative pain (CPSP). To ensure a conservative estimate, we employed trial sequential analysis (TSA), which further supported the sufficiency and conclusiveness of the evidence. Inconsistent with Chang et al. (39), our pooled analyses showed significant benefits of intravenous lidocaine on alleviating acute pain (2h, 4h, 48h, 72h at rest) after breast surgery. In addition, we found perioperative intravenous lidocaine was associated with reduced opioid consumption during and 24h after breast surgery. Lastly, we expanded our assessment to include various recovery indices such as the quality of postoperative recovery, postoperative nausea and vomiting (PONV), and hospital stay, in order to provide a comprehensive evaluation of the effects of intravenous lidocaine compared to the control group.
The benefits of perioperative intravenous lidocaine in postoperative pain management can be attributed to three theoretical mechanisms: its analgesic (11), anti-inflammatory (12) and anti-hyperalgesia (13) properties, which have been previously described. The analgesic effect of lidocaine is primarily achieved through the blockade of voltage-gated sodium channels, resulting in a reversible inhibition of action potential propagation (40). Lidocaine also blocks potassium currents, which are important regulators of resting potential in neural transmission. By modulating these channels, lidocaine exerts its analgesic effects (11, 39). In terms of anti-inflammatory properties, neuro-inflammation plays a crucial role in the development of chronic postoperative pain. Lidocaine has been shown to down-regulate nuclear factor-kappa B and protein kinase C, leading to a decrease in neutrophil recruitment and a reduction in the release of pro-inflammatory cytokines such as IL-4 and IL-6 (41, 42). This anti-inflammatory action contributes to the attenuation of pain. Microglia, a type of immune cell in the central nervous system, are believed to be involved in nociceptive transmission (42). Lidocaine can directly act on microglia by inhibiting the increase of intracellular calcium (43), which may further contribute to its analgesic and anti-inflammatory effects. Furthermore, lidocaine exhibits anti-hyperalgesia properties by blocking N-methyl-D-aspartate (NMDA) receptors. NMDA receptors are particularly implicated in the transmission of pathological pain signals. By blocking these receptors, lidocaine helps reduce hyperalgesia, thereby providing additional pain relief (11, 44). These mechanisms collectively contribute to the overall efficacy of perioperative intravenous lidocaine in mitigating postoperative pain and its associated complications.
Our findings have significant implications for clinical practice. Although our meta-analysis revealed positive effects of perioperative intravenous lidocaine in alleviating acute and chronic pain after breast surgery, caution should be exercised regarding the widespread use of intravenous lidocaine in clinical practice. One primary concern is the uncertain safety profile of intravenous lidocaine in managing postoperative pain. Among the trials included in this meta-analysis, only four reported adverse events associated with intravenous lidocaine. Due to the limited availability of data, we were unable to provide a comprehensive estimate of the adverse outcomes. Therefore, further high-quality, large-scale, prospective, multicenter trials are required to clarify the safety profile of intravenous lidocaine in reducing pain after breast surgery. Another important consideration is the narrow therapeutic window and potential toxicity of lidocaine (45), Clinicians should remain mindful of the possibility of lidocaine toxicity and strictly adhere to recommended doses and duration when using lidocaine. Recent guidelines on the management of intravenous lidocaine recommend an initial dose of no more than 1.5 mg/kg administered over a 10-minute period, calculated based on the patient’s ideal body weight. Considering the pharmacokinetic characteristics of lidocaine, even with continuous intravenous infusion at a dose of 3 mg/kg/hour, the resulting plasma concentration is 2.6 μg/ml (45), which remains below the toxic range of 8-12 μg/ml (46). There is data to support safe administration to 2 mg/kg/hour (47). The elimination half-life of lidocaine typically ranges from 90 to 120 minutes in most patients. However, this half-life may be prolonged in obese patients (48)or in patients with hepatic injury or congestive heart failure (11, 49). In such cases, there is a potential risk of lidocaine accumulation with continuous infusion, leading to intoxication. Therefore, it is crucial for clinicians to individualize lidocaine therapy based on the specific characteristics and needs of each patient.
This meta-analysis possesses a significant strength as it was registered in INPLASY and meticulously reported following the updated PRISMA guidelines. In order to enhance the robustness of our findings, we employed Trial Sequential Analysis (TSA) to assess the impact of intravenous lidocaine on chronic postsurgical pain (CPSP) following breast surgery. However, our meta-analysis does have certain limitations.
Firstly, although no substantial statistical heterogeneity was observed, the variation in patient ages across the included studies, as well as differences in anesthetic techniques, may influence the reliability of the results. Secondly, according to the GRADE system, the majority of evidence for both primary and secondary outcomes was determined to be of low to moderate quality.Lastly, due to limited data available from the included trials, we were unable to calculate the effect of the duration of intravenous lidocaine administration on CPSP and other outcomes.
The existing evidence strongly indicates that perioperative intravenous lidocaine administration effectively reduces both acute and chronic pain following breast surgery.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JL and JH: Study conception and design; acquisition of and analysis the data; drafting of the article; Revising it critically for important intellectual content. J-TY: Acquisition and analysis of data; statistical reviewer; revising the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1101582/full#supplementary-material
1. Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain (2006) 7(9):626–34. doi: 10.1016/j.jpain.2006.02.007
2. Fecho K, Miller NR, Merritt SA, Klauber-Demore N, Hultman CS, Blau WS. Acute and persistent postoperative pain after breast surgery. Pain Med (2009) 10(4):708–15. doi: 10.1111/j.1526-4637.2009.00611.x
3. Bruce J, Thornton AJ, Powell R, Johnston M, Wells M, Heys SD, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain (2014) 155(2):232–43. doi: 10.1016/j.pain.2013.09.028
4. Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat (2018) 167(1):157–69. doi: 10.1007/s10549-017-4485-0
5. Schneiderhan J, Clauw D, Schwenk TL. Primary care of patients with chronic pain. Jama (2017) 317(23):2367–8. doi: 10.1001/jama.2017.5787
6. Xia M, Wei Q, Zhang Q, Jiang H. Effect of intravenous lidocaine on chronic postoperative pain in patients undergoing breast cancer surgery: a prospective, double-blind, randomized, placebo-controlled clinical trial. Ann Transl Med (2022) 10(14):803. doi: 10.21037/atm-22-3522
7. Visnjevac O, Matson B. Postmastectomy pain syndrome: an unrecognized annual billion dollar national financial burden. J Pain (2013). doi: 10.1016/j.jpain.2013.01.487
8. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology (2018) 129(3):590–607. doi: 10.1097/ALN.0000000000002238
9. Skolnick P. The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol (2018) 58:143–59. doi: 10.1146/annurev-pharmtox-010617-052534
10. Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain (London England) (2015) 19(4):451–65. doi: 10.1002/ejp.567
11. Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesthesia (2019) 123(3):335–49. doi: 10.1016/j.bja.2019.06.014
12. Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anaesth (2000) 93(3):858–75. doi: 10.1097/00000542-200009000-00038
13. Kawamata M, Takahashi T, Kozuka Y, Nawa Y, Nishikawa K, Narimatsu E, et al. Experimental incision-induced pain in human skin: effects of systemic lidocaine on flare formation and hyperalgesia. Pain (2002) 100(1-2):77–89. doi: 10.1016/S0304-3959(02)00233-6
14. Tremont-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth Analg (2005) 101(6):1738–49. doi: 10.1213/01.ANE.0000186348.86792.38
15. Licina A, Silvers A. Perioperative intravenous lidocaine infusion for postoperative analgesia in patients undergoing surgery of the spine: systematic review and meta-analysis. Pain Med (2022) 23(1):45–56. doi: 10.1093/pm/pnab210
16. Wei S, Yu-Han Z, Wei-Wei J, Hai Y. The effects of intravenous lidocaine on wound pain and gastrointestinal function recovery after laparoscopic colorectal surgery. Int Wound J (2020) 17(2):351–62. doi: 10.1111/iwj.13279
17. Li J, Wang G, Xu W, Ding M, Yu W. Efficacy of intravenous lidocaine on pain relief in patients undergoing laparoscopic cholecystectomy: a meta-analysis from randomized controlled trials. Int J Surg (London England) (2018) 50:137–45. doi: 10.1016/j.ijsu.2018.01.001
18. Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain (2012) 28(7):567–72. doi: 10.1097/AJP.0b013e31823b9cc8
19. Terkawi AS, Durieux ME, Gottschalk A, Brenin D, Tiouririne M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: a double-blind, placebo-controlled randomized trial. Reg Anesth Pain Med (2014) 39(6):472–7. doi: 10.1097/AAP.0000000000000140
20. Couceiro T, Lima LC, Burle LMC, Valença MM. Intravenous lidocaine for postmastectomy pain treatment: randomized, blind, placebo controlled clinical trial. Braz J Anesthesiology (English Edition) (2015) 65(3):207–12. doi: 10.1016/j.bjane.2014.05.017
21. Kim MH, Lee KY, Park S, Kim SI, Park HS, Yoo YC. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: a prospective, randomized, double-blind, comparative clinical trial. PloS One (2017) 12(3):e0173026. doi: 10.1371/journal.pone.0173026
22. Khan JS, Hodgson N, Choi S, Reid S, Paul JE, Hong NJL, et al. Perioperative pregabalin and intraoperative lidocaine infusion to reduce persistent neuropathic pain after breast cancer surgery: a multicenter, factorial, randomized, controlled pilot trial. J Pain (2019) 20(8):980–93. doi: 10.1016/j.jpain.2019.02.010
23. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3. Cochrane (2022). Available at: https://www.training.cochrane.org/handbook
24. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed) (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
29. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
30. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed) (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
31. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol (2008) 61(1):64–75. doi: 10.1016/j.jclinepi.2007.03.013
32. Thorlund K EJ, Wetterslev J, Brok J, et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research (2016).
33. Choi SJ, Kim MH, Jeong HY, Lee JJ. Effect of intraoperative lidocaine on anesthetic consumption, and bowel function, pain intensity, analgesic consumption and hospital stay after breast surgery. Korean J Anesthesiol (2012) 62(5):429–34. doi: 10.4097/kjae.2012.62.5.429
34. Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: a double-blind, placebo-controlled randomized trial. Pain Physician (2015) 18(2):E139–146.
35. Kendall MC, McCarthy RJ, Panaro S, Goodwin E, Bialek JM, Nader A, et al. The effect of intraoperative systemic lidocaine on postoperative persistent pain using initiative on methods, measurement, and pain assessment in clinical trials criteria assessment following breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Pain Pract (2018) 18(3):350–9. doi: 10.1111/papr.12611
36. van den Heuvel SAS, van der Wal SEI, Bronkhorst EM, Warlé MC, Ronday M, Plat J, et al. Acute cytokine response during breast cancer surgery: potential role of dexamethasone and lidocaine and relationship with postoperative pain and complications - analysis of three pooled pilot randomized controlled trials. J Pain Res (2020) 13:1243–54. doi: 10.2147/JPR.S252377
37. Toner AJ, Bailey MA, Schug SA, Corcoran TB. A pilot multicentre randomised controlled trial of lidocaine infusion in women undergoing breast cancer surgery. Anaesthesia (2021) 76(10):1326–41. doi: 10.1111/anae.15440
38. Wei Q, Xia M, Zhang Q, Wang Z. Effect of intravenous lidocaine infusion on perioperative cellular immunity and the quality of postoperative recovery in breast cancer patients: a randomized controlled trial. Gland Surg (2022) 11(3):599–610. doi: 10.21037/gs-22-134
39. Chang YC, Liu CL, Liu TP, Yang PS, Chen MJ, Cheng SP. Effect of perioperative intravenous lidocaine infusion on acute and chronic pain after breast surgery: a meta-analysis of randomized controlled trials. Pain Pract (2017) 17(3):336–43. doi: 10.1111/papr.12442
40. Ness TJ. Intravenous lidocaine inhibits visceral nociceptive reflexes and spinal neurons in the rat. Anesthesiology (2000) 92(6):1685–91. doi: 10.1097/00000542-200006000-00028
41. Lahat A, Ben-Horin S, Lang A, Fudim E, Picard O, Chowers Y. Lidocaine down-regulates nuclear factor-kappaB signalling and inhibits cytokine production and T cell proliferation. Clin Exp Immunol (2008) 152(2):320–7. doi: 10.1111/j.1365-2249.2008.03636.x
42. Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol (2013) 716(1-3):106–19. doi: 10.1016/j.ejphar.2013.01.072
43. Su D, Gu Y, Wang Z, Wang X. Lidocaine attenuates proinflammatory cytokine production induced by extracellular adenosine triphosphate in cultured rat microglia. Anesth Analg (2010) 111(3):768–74. doi: 10.1213/ANE.0b013e3181e9e897
44. Koppert W, Zeck S, Sittl R, Likar R, Knoll R, Schmelz M. Low-dose lidocaine suppresses experimentally induced hyperalgesia in humans. Anesthesiology (1998) 89(6):1345–53. doi: 10.1097/00000542-199812000-00011
45. Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia (2021) 76(2):238–50. doi: 10.1111/anae.15270
46. Licina A, Silvers A. Perioperative intravenous lignocaine infusion for postoperative pain control in patients undergoing surgery of the spine: protocol for a systematic review and meta-analysis. BMJ Open (2020) 10(10):e036908. doi: 10.1136/bmjopen-2020-036908
47. Eipe N, Gupta S, Penning J. Intravenous lidocaine for acute pain: an evidence-based clinical update. BJA Educ (2016) 16(9):292–8. doi: 10.1093/bjaed/mkw008
48. Abernethy DR, Greenblatt DJ. Lidocaine disposition in obesity. Am J Cardiol (1984) 53(8):1183–6. doi: 10.1016/0002-9149(84)90659-3
Keywords: lidocaine, breast surgery, chronic post-surgical pain, opioid, meta-analysis
Citation: Li J, Huang J, Yang J-t and Liu J-c (2023) Perioperative intravenous lidocaine for postoperative pain in patients undergoing breast surgery: a meta-analysis with trial sequential analysis of randomized controlled trials. Front. Oncol. 13:1101582. doi: 10.3389/fonc.2023.1101582
Received: 18 November 2022; Accepted: 08 June 2023;
Published: 23 June 2023.
Edited by:
Yao Lu, Sun Yat-sen University, ChinaReviewed by:
Hassan Soleimanpour, Tabriz University of Medical Sciences, IranCopyright © 2023 Li, Huang, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-chen Liu, amluZ2NoZW5saXVubkAxNjMuY29t
†The authors have contributed equally as the first authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.