- 1Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan
- 2Department of Pathology, The University of Tokyo Hospital, Tokyo, Japan
- 3Department of Orthopedic Surgery, The University of Tokyo Hospital, Tokyo, Japan
- 4Department of Rehabilitation Medicine, Saitama Medical University Hospital, Saitama, Japan
Background: Glioblastoma is a malignant tumor, and its prognosis is as poor as 1.5 to 2 years. Most cases recur within one year even under the standard treatment. The majority of recurrences are local, and in rare cases, metastasize mostly within the centra nervous system. Extradural metastasis of glioma is exceedingly rare. Here, we present a case of vertebral metastasis of glioblastoma.
Case presentation: We present a 21-year-old man post total resection of the right parietal glioblastoma, diagnosed with lumbar metastasis. He originally presented with impaired consciousness and left hemiplegia and underwent gross total resection of the tumor. Given the diagnosis of glioblastoma, he was treated with radiotherapy combined with concurrent and adjuvant temozolomide. Six months after tumor resection, the patient presented with severe back pain, and was diagnosed as metastatic glioblastoma on the first lumbar vertebrae. Posterior decompression with fixation and postoperative radiotherapy were conducted. He went on to receive temozolomide and bevacizumab. However, at 3 months after the diagnosis of lumbar metastasis, further disease progression was noted, and his care was transitioned to best supportive care. Comparison on copy number status between primary and metastatic lesions on methylation array analysis revealed more enhanced chromosomal instability including 7p loss, 7q gain and 8 gain in the metastatic lesion.
Conclusion: Based upon the literature review and our case, younger age of initial presentation, multiple surgical interventions, and long overall survival seem to be the risk factors of vertebral metastasis. As the prognosis of glioblastoma improves over time, its vertebral metastasis is seemingly more common. Therefore, extradural metastasis should be kept in mind in the treatment of glioblastoma. Further, detailed genomic analysis on multiple paired specimens is mandated to elucidate the molecular mechanisms of vertebral metastasis.
1 Introduction
Glioblastoma is a malignant tumor, classified as grade 4 in World Health Organization (WHO) classification of central nervous system (CNS) tumors 2021, and its prognosis is as poor as 1.5 to 2 years (1, 2). Most cases recur within one year even under the standard treatment of surgical resection, radiation therapy and chemotherapy. The vast majority of recurrences are local, and in rare cases, metastatic mostly within the CNS. Extradural metastasis is considered exceedingly rare due to the presence of blood brain barrier (3). Here, we report a case of vertebral metastasis of glioblastoma.
This study was reported in agreement with principles of the CARE guidelines (4). Written informed consent was obtained from the individual and the patient’s legal guardian for the publication of any potentially identifiable images or data included in this article.
2 Case description
2.1 Clinical course
A 21-year-old man post total resection of the right parietal glioblastoma was diagnosed with lumber metastasis. At the age of 20, without any significant family history or past medical history, he presented to a local emergency department with impaired consciousness and left hemiplegia. Computed tomography (CT) showed intracranial hemorrhage in the right parietal lobe, and hematoma evacuation was conducted. Dilated veins were observed around the hematoma and the patient was diagnosed with intracranial hemorrhage from venous hemangioma. Five months after the operation, he began to complain of headache, and magnetic resonance imaging (MRI) showed an enhanced lesion in the right hemisphere which was rapidly increasing in size. Partial removal of the mass and external decompression was conducted. Then he was referred to our institute for resection of the remaining mass and adjuvant therapy. Gross total resection of the tumor, placement of carmustine wafer in the resection cavity and cranioplasty were conducted at our institute (Figure 1). Pathology was consistent with glioblastoma, isocitrate dehydrogenase (IDH)-wildtype. The patient was ambulatory post-operatively. Radiotherapy combined with concurrent and adjuvant temozolomide (75mg/m2/day) per the Stupp regimen was conducted. Intensity Modulated Radiation Therapy (IMRT) of 60Gy was conducted at the edematous area surrounding the tumor, and IMRT of 50Gy at the tumor removal site. Four cycles of temozolomide (150mg/m2/day) were administered during the maintenance phase. At the same time, bevacizumab (10mg/kg) was administered twice, two weeks apart.
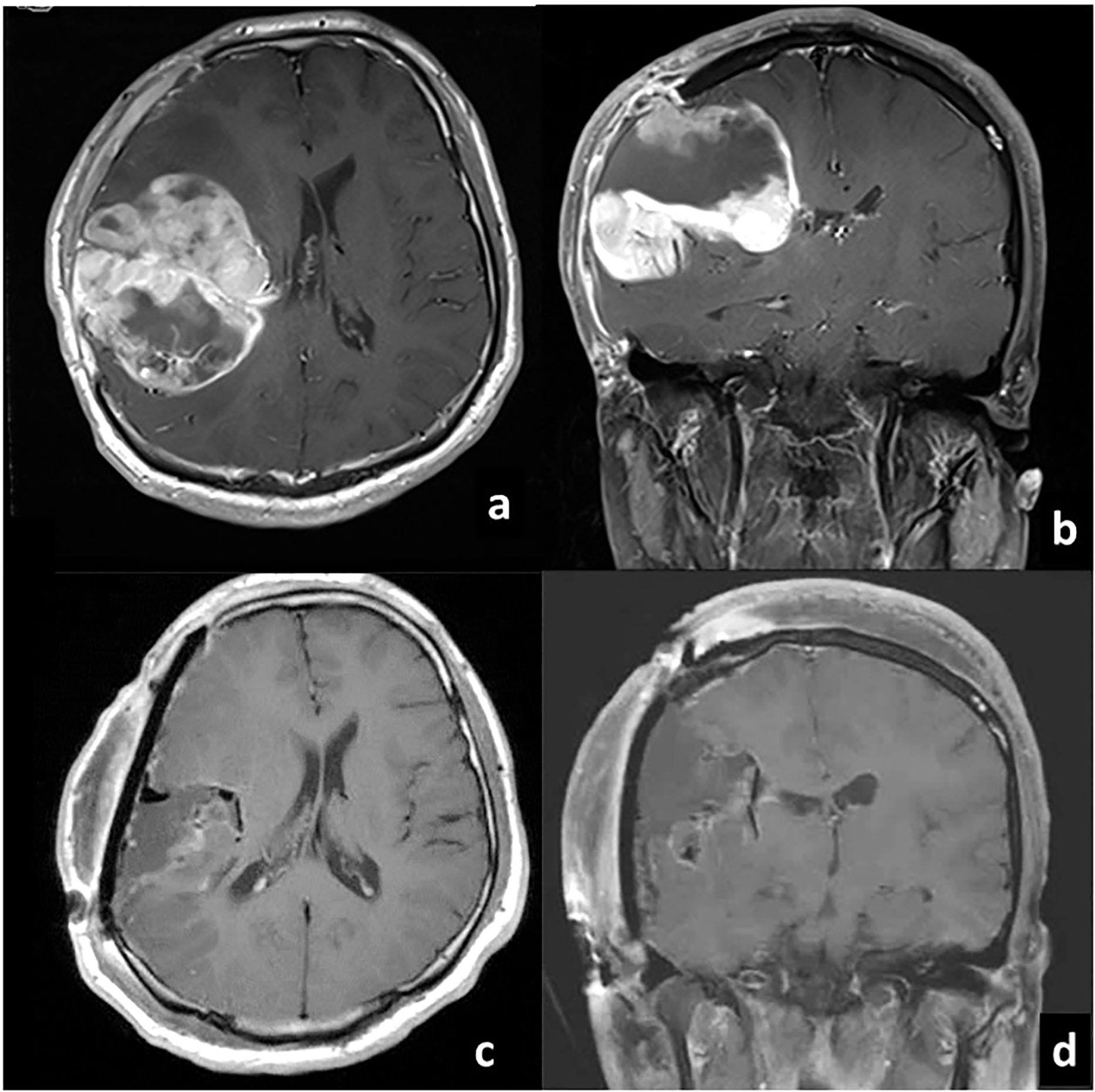
Figure 1 Gadolinium-enhanced T1-weighted MRI before (A) axial, (B) coronal and after (C) axial, (D) coronal) gross total resection of glioblastoma at our institute.
At 6 months after the resection of tumor at our institute (14 months after the first operation), the patient presented with severe back pain. CT showed an osteolytic mass on the body of the first lumbar vertebrae and MRI showed an enhanced lesion constricting the spinal canal (Figure 2). There was no spinal metastasis. The patient did not present symptoms of spinal cord compression such as bladder and rectal disturbance or lower extremities paralysis. Brain MRI showed no intracranial recurrence, and whole spine MRI and whole-body CT showed no other lesions. Needle biopsy of the mass on the first lumbar vertebrae showed densely infiltrating spindle-shaped cells with eosinophilic cytoplasm accompanied with regions of necrosis, which was consistent with glioblastoma (Figure 3). The cells were positive for glial fibrillary acidic protein (GFAP), and its MIB-1 index was 30%. Posterior decompression with fixation and postoperative radiotherapy of 30Gy were conducted to relieve the pain. He further received one more cycle of temozolomide (150mg/m2/day) and two more administrations of bevacizumab (10mg/kg, two weeks apart). Three months after the diagnosis of lumbar metastasis, however, disease progression with multiple metastasis to the lymph nodes, the lungs, and the liver was found, prompting the transition to best supportive care.
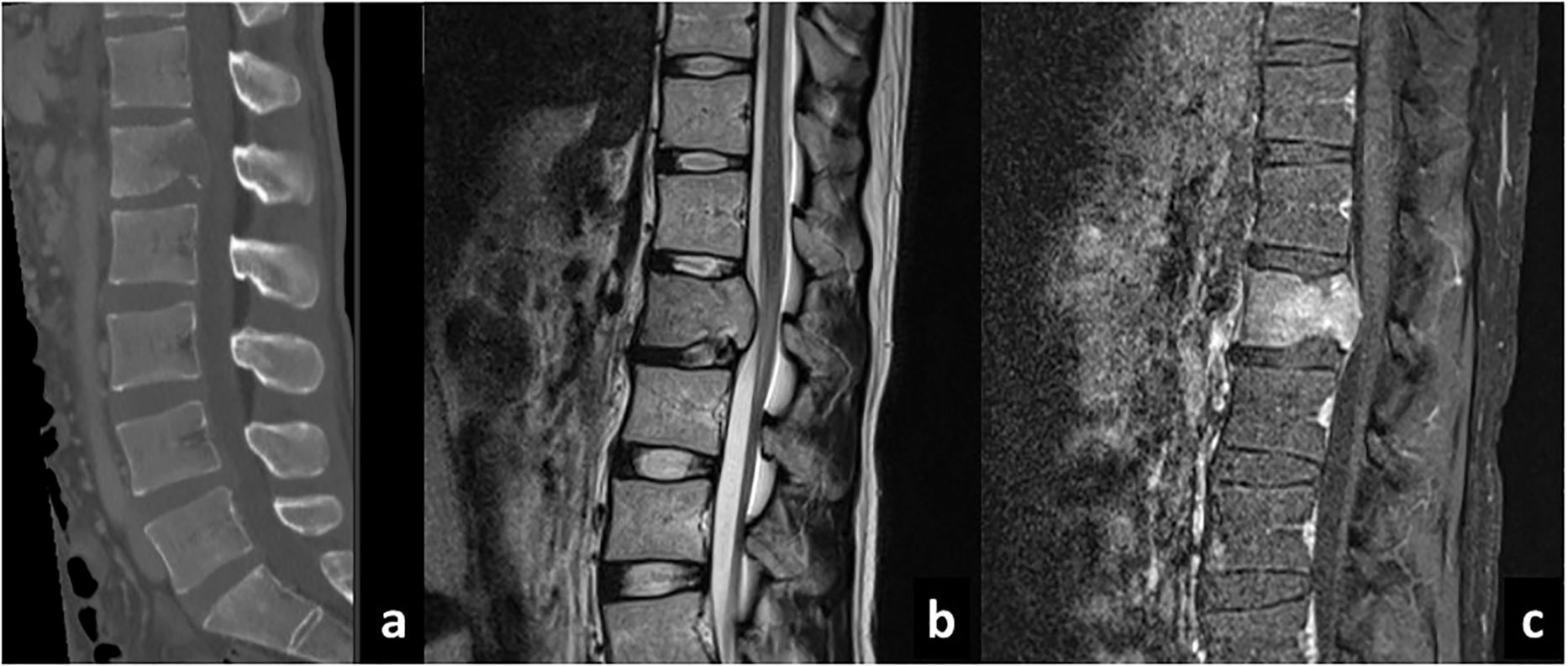
Figure 2 Sagittal CT (A), T2-weighted (B), and gadolinium-enhanced T1-weighted (C) MRI sequences of the spine showing development of metastatic lesion.
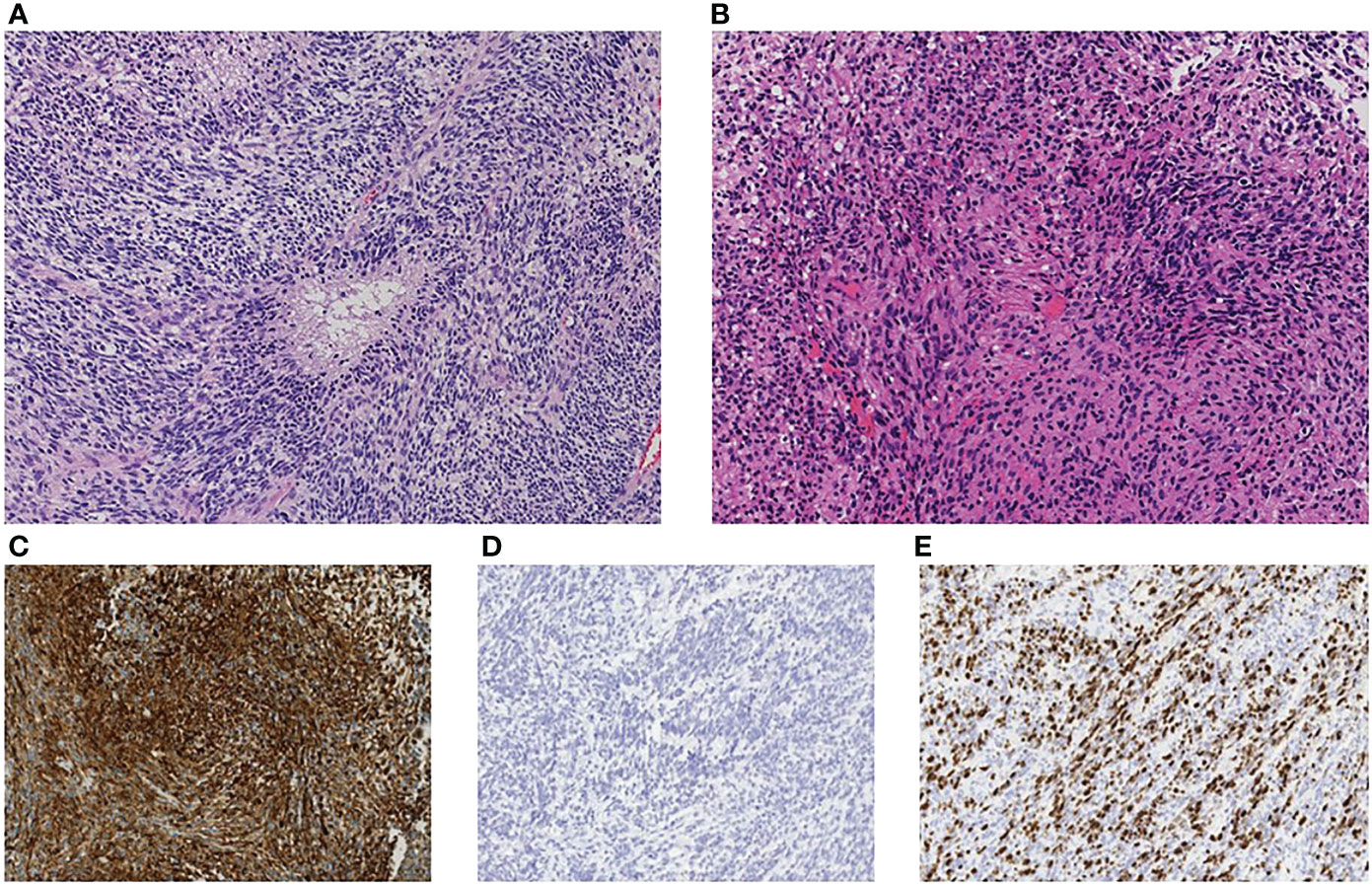
Figure 3 Immunohistopathological investigation showed densely infiltrating spindle-shaped cells with eosinophilic cytoplasm accompanied with regions of necrosis, consistent with glioblastoma (A) primary site, (B) lumbar metastasis). The cells were positive for glial fibrillary acidic protein (GFAP) (C) and negative for mutant isocitrate dehydrogenase 1 (IDH1-R132H) (D). MIB-1 labeling index was 30% (E). The original magnification was x200 (A–E).
2.2 Epigenetic analysis
This study was approved by the Ethics Committee of the University of Tokyo (#G10028). DNA was extracted from FFPE tissue samples and analyzed using the Illumina Infinium Human Methylation EPIC Bead Chip array according to the manufacturer’s instructions. All DNA methylation analyses were performed using R version 4.1.0. Methylation values were calculated as β values. The following filtering criteria were applied: removal of probes overlapping with single-nucleotide polymorphisms, those mapped to chromosomes X and Y, or the Illumina control probes. Copy number alterations were calculated using signal data from the methylation array using the conumee Bioconductor package version 1.26.0.
Primary and metastatic samples had overall copy number status in common such as chromosome 1p loss, 16 loss and 22q loss; however, the metastatic sample had more prominent copy number alterations, including newly acquired 4 gain, 7p loss, 7q gain, 8 gain, 13q gain, 19q gain, and 20 loss (Figure 4).
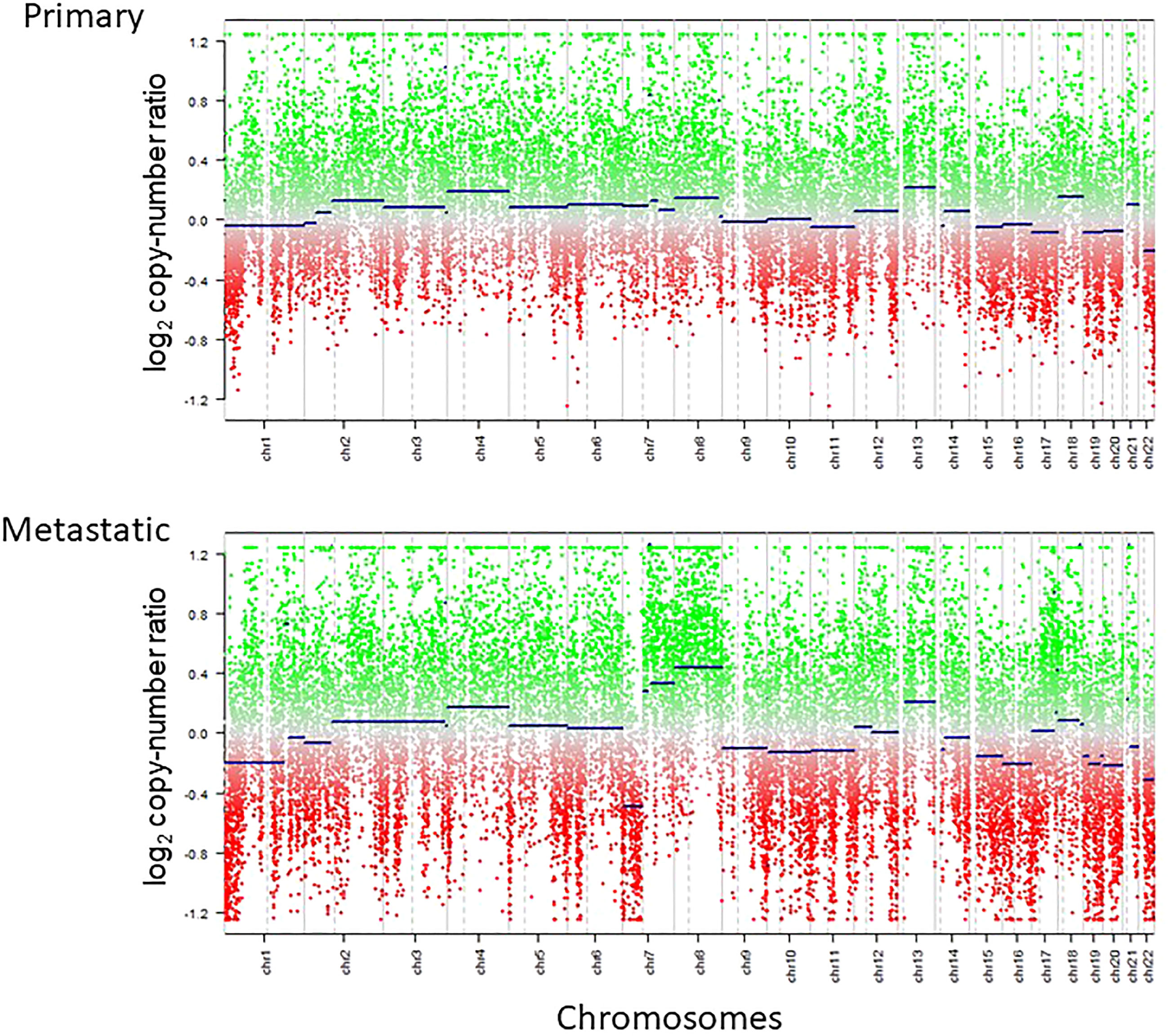
Figure 4 Copy number profiles of primary and metastatic lesions calculated by methylation array are shown. Copy number baseline imbalance was enhanced for the metastatic lesion compared with the primary lesion.
3 Discussion
In this case illustration, we present an extremely rare case of glioblastoma which metastasized extracranially to the lumbar vertebra during the course of treatment. Glioblastoma was not believed to metastasize outside of the CNS until Davis first reported glioma meningeal metastasis in 1928 (5, 6). Extradural metastasis of glioblastoma is as rare as less than 2% (3). Dense dura around intracranial sinuses preventing tumor cell penetration and lack of a nurturing stroma in other organs to facilitate the survival of glioblastoma cells are some biological obstacles that prevent glioblastoma cells to infiltrate outside the CNS (7). Also, overall low median survival causes patients to die from intracranial hypertension or other complications before extracranial metastasis develop (6).
Vertebral metastasis is exceedingly rare; to the best of our knowledge, there has been 59 cases reported in the literature (Supplementary Tables 1, 2) (6, 8–58). Mean age of the 59 patients is 43.9 years (range, 11-70 years), which is much younger than the average age of patients diagnosed with glioblastoma. Moreover, in most cases, glioblastoma metastasis to the vertebral body is accompanied by metastasis to other locations such as the lung, lymph nodes, other bones and the liver. As for the cases with sufficient data, the mean overall survival of the cases is 28.0 months (range, 1-139 months) after the initial diagnosis of glioblastoma, and 8.5 months (range, 0-48 months) after the diagnosis of vertebral metastasis. Overall survival in these cases is longer than the average prognosis of glioblastoma. This suggests that long-surviving glioblastoma patients provide glioblastoma cells adequate time to cause extradural metastasis.
Considering the biological obstacles that prevent glioblastomas from infiltrating outside of the CNS, it can be speculated that deposition of tumor cells into the blood stream or excision of the dura due to surgical interventions may attribute to extracranial metastasis. As for the 59 reported cases, the mean number of surgeries conducted prior to vertebral metastasis was 1.5 (range, 0-5). In our case, the patient first presented with intracranial hemorrhage and the tumor was hypervascular enough to be misdiagnosed for venous hemangioma, allowing tumor cells to be feasibly deposited into the blood stream. Also, multiple surgeries including external decompression were conducted causing defects of the dura and the skull, which could have facilitated the extradural infiltration of glioblastoma cells. Based upon the literature and our case, younger age of initial presentation of glioblastoma and multiple surgical interventions seem to be the risk factors of vertebral metastasis of glioblastoma, though we still lack further statistical evaluation.
Little has been known about the genetic and epigenetic changes seen in the extracranial metastasis of glioblastoma. Copy number alterations have been known to be enhanced at the metastatic site compared with the primary site, in parallel with mutational burden, in systemic cancers such as lung, hepatocellular and urothelial malignancies among others (59–61). This is deemed to primarily reflect the clonal evolution and selection of the tumor cells at the primary site with the gain of metastatic potential (62, 63). Previously, a case of GBM with osseous metastasis was shown to harbor additional copy number alterations plus mutations compared with the intracranial tumor (64). In concordance, our case demonstrated the increased fluctuation of the copy number baseline (Figure 4), highly suggestive of the existence of tumor cells with the acquisition of metastatic capability. This is still an under-investigated finding in cases with metastatic glioblastoma and needs to be investigated in a larger cohort of samples.
Due to the improvement in the prognosis of the disease, vertebral metastasis is suspected to be encountered more commonly. Therefore, extradural metastasis of glioblastoma must be included in differential diagnoses in treating patients with glioblastoma. Further studies with detailed genomic analysis on multiple paired tumor specimens are warranted to unravel the molecular mechanisms of vertebral metastasis.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: This is a case report. All the information is stored in the medical chart. Requests to access these datasets should be directed to STan,c3RhbmFrYUBtLnUtdG9reW8uYWMuanA=.
Ethics statement
The studies involving human participants were reviewed and approved by The University of Tokyo. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual and the patient’s legal guardian for the publication of any potentially identifiable images or data included in this article.
Author contributions
AM and STan contributed to conception and design of the study. AM organized the database. AM and HT performed the statistical analysis. AM and STan wrote the first draft of the manuscript. HT wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1101552/full#supplementary-material
References
1. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol (2009) 10:459–66. doi: 10.1016/S1470-2045(09)70025-7
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
3. Ruff MW, Bhargav AG, Raghunathan A. A case of epidural glioblastoma metastasis presenting with a cervical myelopathy, torticollis, and l’hermitte’s phenomenon. Brain Tumor Pathol (2018) 35:181–5. doi: 10.1007/s10014-018-0319-y
4. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
6. Wu W, Zhong D, Zhao Z, Wang W, Li J, Zhang W. Postoperative extracranial metastasis from glioblastoma: a case report and review of the literature. World J Surg Oncol (2017) 15:231. doi: 10.1186/s12957-017-1300-7
7. Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol (2011) 105:261–73. doi: 10.1007/s11060-011-0575-8
8. Winkelman NW Jr., Cassel C, Schlesinger B. Intracranial tumors with extracranial metastases. J Neuropathol Exp Neurol (1942) 11:149–68. doi: 10.1097/00005072-195204000-00004
9. Wisiol ES, Handler S, French LA. Extracranial metastases of a glioblastoma multiforme. J Neurosurg (1962) 19:186–94. doi: 10.3171/jns.1962.19.3.0186
10. Nigogosyan G, de la Pava S, Pickren JW. Brain tumor with extracranial metastases. report of two cases. Arch Neurol (1962) 6:300–6. doi: 10.1001/archneur.1962.00450220042007
11. Smith DR, Hardman JM, Earle KM. Contiguous glioblastoma multiforme and fibrosarcoma with extracranial metastasis. Cancer (1969) 24:270–6. doi: 10.1002/1097-0142(196908)24:2<270::AID-CNCR2820240210>3.0.CO;2-5
12. Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg (1969) 31:50–8. doi: 10.3171/jns.1969.31.1.0050
13. Anzil AP. Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case Rep J Neurosurg (1970) 33:88–94. doi: 10.3171/jns.1970.33.1.0088
14. Takeda F, Handa IK, Aiba T, Kawabuchi J, Fukai K. Autopsy case of malignant glioma with extreneural metastases. Shinkei Kenkyu No Shimpo (1971) 15:720–30.
15. Cooper PR, Budzilovich GN, Berczeller PH, Lieberman A, Battista A. Metastatic glioma associated with hypercalcemia. report of two cases. J Neurosurg (1974) 40:255–9. doi: 10.3171/jns.1974.40.2.0255
16. Hulbanni S, Goodman PA. Glioblastoma multiforme with extraneural metastases in the absence of previous surgery. Cancer (1976) 37:1577–83. doi: 10.1002/1097-0142(197603)37:3<1577::AID-CNCR2820370348>3.0.CO;2-0
17. Schatzki SC, McIlmoyle G, Lowis S. Diffuse osteoblastic metastases from an intracranial glioma. AJR Am J Roentgenol (1977) 128:321–3. doi: 10.2214/ajr.128.2.321
18. Slowik F, Balogh I. Extracranial spreading of glioblastoma multiforme. Zentralblatt fur Neurochirurgie (1980) 41:57–68.
19. Dietz R, Burger L, Merkel K, Schimrigk K. Malignant gliomas - glioblastoma multiforme and astrocytoma III-IV with extracranial metastases report of two cases. Acta Neurochir (1981) 57(1–2):99–105. doi: 10.1007/BF01665120
20. Sadik AR, Port R, Garfinkel B, Bravo J. Extracranial metastasis of cerebral glioblastoma multiforme: case report. Neurosurgery (1984) 15:549–51. doi: 10.1227/00006123-198410000-00014
21. Friedman JH, Liu HM, Spremulli E, Calabresi P. Distant metastases from a malignant glioma: unusual complications associated with treatment of a glioblastoma: distant metastases and focal white matter degeneration. J Neurol Neurosurg Psychiatry (1987) 50(2):237–8. doi: 10.1136/jnnp.50.2.237
22. Haddon M, Slavin JD, Spencer RP. Multiple bone metastases in a patient with glioblastoma multiforme. Clin Nucl Med (1989) 14(1):13–4. doi: 10.1097/00003072-198901000-00004
23. Lampl Y, Eshel Y, Gilad R, Sarova-Pinchas I. Glioblastoma multiforme with bone metastase and cauda equina syndrome. J Neurooncol (1990) 8(2):167–72. doi: 10.1007/BF00177841
24. Myers T, Egelhoff J, Myers M. Glioblastoma multiforme presenting as osteoblastic metastatic disease: case report and review of the literature. AJNR Am J Neuroradiol (1990) 11:802–3.
25. Chesnut RM, Abitbol JJ, Chamberlain M, Marshall LF. Vertebral collapse with quadraparesis due to metastatic gliobla multiforme: case report and review of the literature. J Neurooncol (1993) 16:135–40. doi: 10.1007/BF01324700
26. Mihara F, Ikeda M, Rothman MI, Numaguchi Y, Kristt D. Vertebral body metastasis of glioblastoma multiforme with epidural mass formation. contrast-enhanced MRI study. Clin Imaging (1994) 18:386–9. doi: 10.1016/0899-7071(94)90011-6
27. Kleinschmidt-Demasters BK. Diffuse bone marrow metastases from glioblastoma multiforme: the role of dural invasion. Hum Pathol (1996) 27(2):197–201. doi: 10.1016/S0046-8177(96)90376-7
28. Moriyama T, Kataoka H, Seguchi K, Nabeshima K, Kawano H, Goya T, et al. Establishment and characterization of a new human glioblastoma cell line (MGM-1) with highly motile phenotype. Hum Cell (1997) 10(1):105–10.
29. Solau-Gervais E, Flipo RM, Cotten A, Lecomte-Houcke M, Delcambre B. Metastasis from a glioblastoma and staphylococcus aureus spondylitis in the same vertebral body. Rev Rhum Engl Ed (1998) 65(1):75–6.
30. Frappaz D, Mornex F, Saint-Pierre G, Ranchere-Vince D, Jouvet A, Chassagne-Clement C, et al. Bone metastasis of glioblastoma multiforme confirmed by fine needle biopsy. Acta Neurochir (Wien) (1999) 141(5):551–2. doi: 10.1007/s007010050342
31. Beauchesne P, Soler C, Mosnier JF. Diffuse vertebral body metastasis from a glioblastoma multiforme: a technetium-99m sestamibi single-photon emission computerized tomography study. J Neurosurg (2000) 93:887–90. doi: 10.3171/jns.2000.93.5.0887
32. Park CC, Hartmann C, Folkerth R, Loeffler JS, Wen PY, Fine HA, et al. Systemic metastasis in glioblastoma may represent the emergence of neoplastic subclones. J Neuropathol Exp Neurol (2000) 59(12):1044–50. doi: 10.1093/jnen/59.12.1044
33. Cervio A, Piedimonte F, Salaberry J, Alcorta SC, Salvat J, Diez B, et al. Bone metastases from secondary glioblastoma multiforme: a case report. J Neurooncol (2001) 52(2):141–8. doi: 10.1023/A:1010629618859
34. Fabi A, Vidiri A, Carapella C, Pace A, Occhipinti E, Caroli F, et al. Bone metastasis from glioblastoma multiforme without central nervous system relapse: a case report. Anticancer Res (2004) 24:2563–5.
35. Rajagopalan V, El Kamar FG, Thayaparan R, Grossbard ML. Bone marrow metastases from glioblastoma multiforme–a case report and review of the literature. J Neurooncol (2005) 72(2):157–61. doi: 10.1007/s11060-004-3346-y
36. Utsuki S, Tanaka S, Oka H, Iwamoto K, Sagiuchi T, Fujii K. Glioblastoma multiforme metastasis to the axis. Case Rep J Neurosurg (2005) 102:540–2. doi: 10.3171/jns.2005.102.3.0540
37. Astner ST, Pihusch R, Nieder C, Rachinger W, Lohner H, Tonn JC, et al. Extensive local and systemic therapy in extraneural metastasized glioblastoma multiforme. Anticancer Res (2006) 26(6c):4917–20.
38. Robert M, Wastie M. Glioblastoma multiforme: a rare manifestation of extensive liver and bone metastases. BioMed Imaging Interv J (2008) 4(1):e3–3. doi: 10.2349/biij.4.1.e3
39. Pham CT, Clarençon F, Ganem G, Cormier E, Guermazi Y, Rose M, et al. Spinal cervical metastasis from a glioblastoma multiform treated by percutaneous vertebroplasty: a case report. J Neuroradiol (2011) 38(5):323–5. doi: 10.1016/j.neurad.2010.08.006
40. Kalokhe G, Grimm SA, Chandler JP, Helenowski I, Rademaker A, Raizer JJ. Metastatic glioblastoma: case presentations and a review of the literature. J Neurooncol (2012) 107(1):21–7. doi: 10.1007/s11060-011-0731-1
41. Blume C, von Lehe M, van Landeghem F, Greschus S, Bostrom J. Extracranial glioblastoma with synchronous metastases in the lung, pulmonary lymph nodes, vertebrae, cervical muscles and epidural space in a young patient - case report and review of literature. BMC Res Notes (2013) 6:290. doi: 10.1186/1756-0500-6-290
42. Hamilton JD, Rapp M, Schneiderhan T, Sabel M, Hayman A, Scherer A, et al. Glioblastoma multiforme metastasis outside the CNS: three case reports and possible mechanisms of escape. J Clin Oncol (2014) 32(22):e80–84. doi: 10.1200/JCO.2013.48.7546
43. Kim W, Yoo H, Shin SH, Gwak HS, Lee SH. Extraneural metastases of glioblastoma without simultaneous central nervous system recurrence. Brain Tumor Res Treat (2014) 2(2):124–7. doi: 10.14791/btrt.2014.2.2.124
44. Khattab MH, Marciscano AE, Lo SS, Lim M, Laterra JJ, Kleinberg LR, et al. Antiangiogenic therapies and extracranial metastasis in glioblastoma: A case report and review of the literature. Case Rep Oncol Med (2015) 2015:431819. doi: 10.1155/2015/431819
45. Undabeitia J, Castle M, Arrazola M, Pendleton C, Ruiz I, Úrculo E. Multiple extraneural metastasis of glioblastoma multiforme. Sist Sanit Navar (2015) 38:157–61. doi: 10.4321/S1137-66272015000100022
46. Starnoni D, Yamgoue Y, Hottinger A, Bartanusz V. Multilevel severe radiculopathy from an extraneural glioblastoma cervical metastasis. Surg Neurol Int (2016) 7:S1028–9. doi: 10.4103/2152-7806.195588
47. Franceschi S, Lessi F, Aretini P, Mazzanti CM, Menicagli M, La Ferla M, et al. Molecular portrait of a rare case of metastatic glioblastoma: somatic and germline mutations using whole-exome sequencing. Neuro Oncol (2016) 18(2):298–300. doi: 10.1093/neuonc/nov314
48. Xu M, Wang Y, Xu J, Yao Y, Yu WX, Zhong P. Extensive therapies for extraneural metastases from glioblastoma, as confirmed with the OncoScan assay. World Neurosurg (2016) 90:698. e697–698.e611. doi: 10.1016/j.wneu.2016.01.074
49. Simonetti G, Silvani A, Fariselli L, Hottinger AF, Pesce GA, Prada F, et al. Extra central nervous system metastases from glioblastoma: a new possible trigger event? Neurol Sci (2017) 38(10):1873–5. doi: 10.1007/s10072-017-3036-0
50. Sun Q, Xu R, Xu H, Wang G, Shen X, Jiang H. Extracranial metastases of high-grade glioma: the clinical characteristics and mechanism. World J Surg Oncol (2017) 15(1):181–1. doi: 10.1186/s12957-017-1249-6
51. Ricard JA, Cramer SW, Charles R, Gil Tommee C, Le A, Bell WR, et al. Infratentorial glioblastoma metastasis to bone. World Neurosurg (2019) 131:90–4. doi: 10.1016/j.wneu.2019.07.142
52. Li Z-G, Zheng M-Y, Zhao Q, Liu K, Du J-X, Zhang S-W. Solitary vertebral metastatic glioblastoma in the absence of primary brain tumor relapse: a case report and literature review. BMC Med Imaging (2020) 20(1):89. doi: 10.1186/s12880-020-00488-x
53. Colamaria A, Blagia M, Sacco M, Carbone F. Diffuse vertebral metastases from glioblastoma with vertebroepidural diffusion: a case report and review of the literature. Surg Neurol Int (2021) 12:437. doi: 10.25259/SNI_538_2021
54. den Hartog SJ, van der Kolk A, Bruggink A, Seute T, Wesseling P, Wilbers J. Pathology-proven extradural (“distant”) metastases of gliomas in adults in the Netherlands between 1971 and 2018: a systematic case series. Neurooncol Pract (2021) 8(3):317–24.
55. Noch EK, Sait SF, Farooq S, Trippett TM, Miller AM. A case series of extraneural metastatic glioblastoma at memorial Sloan Kettering cancer center. Neurooncol Pract (2021) 8(3):325–36. doi: 10.1093/nop/npaa083
56. Zhang W, Cai Y, Wang X, Wang X, Li Y, Han G, et al. Bone metastases of glioblastoma: a case report and review of the literature. Front Oncol (2021) 11:705455. doi: 10.3389/fonc.2021.705455
57. Goodwin CR, Liang L, Abu-Bonsrah N, Hdeib A, Elder BD, Kosztowski T, et al. Extraneural glioblastoma multiforme vertebral metastasis. World Neurosurg (2016) 89:578–582 e573. doi: 10.1016/j.wneu.2015.11.061
58. Strong MJ, Koduri S, Allison JA, Pesavento CM, Ogunsola S, Ogunsola O, et al. Bone metastasis from glioblastoma: a systematic review. J Neuro-Oncol (2022) 158:379–92. doi: 10.1007/s11060-022-04025-4
59. Bambury RM, Bhatt AS, Riester M, Pedamallu CS, Duke F, Bellmunt J, et al. DNA Copy number analysis of metastatic urothelial carcinoma with comparison to primary tumors. BMC Cancer (2015) 15:242. doi: 10.1186/s12885-015-1192-2
60. Ouyang L, Lee J, Park CK, Mao M, Shi Y, Gong Z, et al. Whole-genome sequencing of matched primary and metastatic hepatocellular carcinomas. BMC Med Genomics (2014) 7:2. doi: 10.1186/1755-8794-7-2
61. Wang H, Ou Q, Li D, Qin T, Bao H, Hou X, et al. Genes associated with increased brain metastasis risk in non-small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non-small cell lung cancer (Guangdong association study of thoracic oncology 1036). Cancer (2019) 125:3535–44. doi: 10.1002/cncr.32372
62. Tew BY, Legendre C, Schroeder MA, Triche T, Gooden GC, Huang Y, et al. Patient-derived xenografts of central nervous system metastasis reveal expansion of aggressive minor clones. Neuro Oncol (2020) 22:70–83. doi: 10.1093/neuonc/noz137
63. Turajlic S, Xu H, Litchfield K, Rowan A, Horswell S, Chambers T, et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell (2018) 173:595–610 e511. doi: 10.1016/j.cell.2018.03.043
Keywords: glioblastoma, vertebral metastasis, craniotomy, methylation array analysis, copy number alteration, chromosomal instability
Citation: Matsuhashi A, Tanaka S, Takami H, Nomura M, Ikemura M, Matsubayashi Y, Shinoda Y, Yamada K, Sakai Y, Karasawa Y, Takayanagi S and Saito N (2023) Recurrent glioblastoma metastatic to the lumbar vertebra: A case report and literature review: Surgical oncology. Front. Oncol. 13:1101552. doi: 10.3389/fonc.2023.1101552
Received: 18 November 2022; Accepted: 31 January 2023;
Published: 16 February 2023.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Hsin-Hua Lee, Kaohsiung Medical University, TaiwanArchya Dasgupta, Tata Memorial Hospital, India
Chao Li, Guangxi Medical University, China
Copyright © 2023 Matsuhashi, Tanaka, Takami, Nomura, Ikemura, Matsubayashi, Shinoda, Yamada, Sakai, Karasawa, Takayanagi and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shota Tanaka, c3RhbmFrYUBtLnUtdG9reW8uYWMuanA=
 Ako Matsuhashi1
Ako Matsuhashi1 Shota Tanaka
Shota Tanaka Hirokazu Takami
Hirokazu Takami Keisuke Yamada
Keisuke Yamada