
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 February 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1099514
This article is part of the Research Topic Video-Assisted Surgery in Oncology View all 13 articles
Background: The safety, feasibility, and prognosis of sleeve lobectomy by minimally invasive surgery (MIS) remain to be validated. The purpose of this study was to investigate outcomes in real-world patients receiving minimally invasive sleeve lobectomy in a balanced large cohort.
Methods: Between January 2013 and December 2018, 578 consecutive patients undergoing sleeve resection at a high-volume center were retrospectively analyzed. Surgical and oncologic outcomes were compared between MIS and thoracotomy patients after propensity-score matching (PSM).
Results: MIS sleeve lobectomy was increasingly used as a time-trend in real-world. Before PSM, the MIS group had smaller tumor size, more T2-stage cases, and more right upper lobe sleeve lobectomies compared to the Open group. After 1:4 PSM by patient demographics and tumoral characteristics, 100 cases of MIS and 338 cases of Open sleeve lobectomy were further analyzed. Although median operation time was longer in the MIS group than in the Open group (170.5 minutes vs.149.5 minutes, P < 0.001), patients in MIS group had significantly less estimated intraoperative blood loss (100 ml vs. 200 ml, P = 0.003), shorter drainage duration (5 days vs. 6 days, P = 0.027) and less amount of drainage (1280 ml vs. 1640 ml, P < 0.001) after surgery. Complete resection rate, combined angioplasty, number of dissected lymph nodes, post-operative length of stay, postoperative morbidity and mortality rate, and application of adjuvant therapy were similar between the two matched groups. Conversion to open thoracotomy was necessary in 13.6% patients, but with similar perioperative outcomes compared to Open cases except for longer operation time. More lower lobe sleeve lobectomies were accomplished via robot-assisted thoracoscopic surgery than via video-assisted thoracoscopic surgery (40.0% vs. 12.0%, P = 0.017) in MIS patients. Five-year overall survivals (MIS vs. Open: 72.7% vs. 64.4%, P = 0.156) and five-year progression-free survivals (MIS vs. Open: 49.2% vs. 50.5%, P = 0.605) were similar between the two matched groups.
Conclusions: MIS sleeve lobectomy is associated with similar or even better perioperative results and oncologic outcomes to open thoracotomy. Conversion to thoracotomy does not compromise perioperative outcomes. Robot surgery may be preferable for more complex sleeve resections.
Lung cancer is currently one of the leading causes of cancer death in the world (1). Non-small cell lung cancers (NSCLCs) can be clinically divided into centrally located and peripheral ones according to their position in the lung. In 1933, Graham performed the first successful pneumonectomy (2) for a centrally located lung cancer. However, pneumonectomy is associated with high mortality and morbidity. A selected group of centrally located tumors can be completely resected by using bronchoplastic techniques with anastomosis of one lobar bronchus to the other to preserve lung parenchyma. These so-called sleeve resections were reported for the first time by Clement Price Thomas in 1956 (3). Compared to pneumonectomy, sleeve lobectomy has been shown to be associated with less morbidity and mortality but similar or even better long-term survival if the tumor could be completely removed (4–10). It has thus become the preferred surgical procedure for centrally located NSCLC, whenever technically feasible and when complete resection can be achieved (11).
Minimally invasive surgery (MIS), including video-assisted thoracoscopic surgery (VATS) and robot-assisted thoracoscopic surgery (RATS), is the preferred approach in the current guidelines for the surgical management of early-stage NSCLC (12). Its advantage over open thoracotomy includes less pain, decreased postoperative complications, less impaired pulmonary function, and better quality of life and compliance to adjuvant therapies after surgery (13–16). And similar oncologic outcomes in lymph node dissection and long-term survival have been demonstrated in MIS and open surgery (17, 18). But most sleeve lung resections are still accomplished via conventional open thoracotomy, as they are technically more demanding and are often applied in locally advanced tumors. Although Santambrogio et al. (19) reported the first successful case of VATS sleeve lobectomy in 2002, up till now, there have been only a few single-institutional reports with small numbers of cases showing its feasibility technically (20–30). Although the conversion rates reported in these series were generally higher than in standard lobectomy, converted cases were either excluded or their outcomes not studied in the previous reports. Most reported cases were accomplished via VATS, with very few RATS cases included (27, 29). Since most of the MIS sleeve lobectomies were done in recent years, follow-up time of MIS patients was unanimously short in these series. Therefore, the safety and efficacy of MIS in sleeve lobectomy for NSCLC remains largely unknown.
Our study thus aimed to find out the results of MIS for sleeve lobectomy in a real-world setting, with special attention paid to its potential benefits and surgical outcomes in conversion cases, and to the unique advantages of RATS.
Patients with centrally located primary NSCLC receiving bronchial sleeve resection with or without pulmonary artery angioplasty at the Shanghai Chest Hospital between January 2013 and December 2018 were retrospectively identified from the institutional database. The study was approved by the Institutional Review Board of Shanghai Chest Hospital (No. KS(Y) 21268). Informed consent was waived as only de-characterized data were used for the study.
Designed as a real-world study, all consecutive patients receiving sleeve lobectomy for potentially resectable primary NSCLC via either MIS or Open thoracotomy were included. Exclusion criteria were concomitant carina resection or reconstruction of great vessels such as superior vena cava, patients with metastatic disease, small cell lung cancer, or benign diseases. All patients were confirmed of having central lung cancer by bronchoscopy. Pretreatment evaluation included chest computed tomography (CT) scan, brain magnetic resonance imaging, neck, and abdominal ultrasonography, bone scintigraphy, or positron emission tomography. Tumor stage was re-classified according to the 8th Edition TNM Classification of Malignant Tumors (31).
Patients were divided into two groups according to the planned surgery: the MIS group and the Open group. The approach was chosen according to the surgeons’ decision. Those converted to open thoracotomy during the operation were included in the MIS group, using an intention-to-treat (ITT) analysis.
General anesthesia and double-lumen tube intubation were used in all patients. Open thoracotomy was performed using a postero-lateral incision at the fourth or fifth intercostal space. MIS was accomplished via one, two, three, or four-port VATS or RATS according to surgeons’ preference. Frozen section was performed intraoperatively in all patients to assess the resected bronchial margins. In patients with poor pulmonary function or severe comorbidity, pneumonectomy was usually avoided even if the bronchial margin was positive, and postoperative radiotherapy would be recommended to these patients. Most of the surgeons in our institution chose to do the running sutures using non-absorbable thread in MIS and Open sleeve lobectomy. But a few surgeons preferred absorbable thread to do interrupted sutures in open cases. Angioplasty would be added if the pulmonary artery trunk was also invaded by the tumor. In open sleeve cases, some surgeons preferred covering the anastomosis with muscle or pericardium flap or thymus. However, we do not routinely cover the anastomosis with any tissues in the MIS sleeve cases. We routinely did bronchoscopy right after finishing the bronchial anastomosis to control the anastomosis.
After surgery, adjuvant chemotherapy was recommended to patients with histologically proven stage II or III diseases who did not receive neoadjuvant therapy before surgery. Adjuvant radiation would also be suggested to patients with positive resection margin or pathological N2 disease. Patients were followed every three months after treatment in the first two years and 6-12 months afterwards. These routinely included serum tumor markers, chest CT scan, neck, and abdominal ultrasonography. Brain magnetic resonance imaging, bone scintigraphy, or positron emission tomography was conducted when disease progression was suspected.
Patients’ demographics, tumoral characteristics, and treatment outcomes were compared between the two groups. Overall survival (OS) was defined as the duration from the date of operation to death of any cause or the date of last follow-up. Progression-free survival (PFS) was defined as the duration from the date of operation to the date of progress or death of any cause or the date of last follow-up.
Continuous variables were expressed as mean ± standard deviations (SD) if normally distributed, otherwise were exhibited as median with interquartile range (IQR). Student t-test or Mann-Whitney test was used for comparison. Comparison of categorical variables was performed by Chi-Squared test or Fisher’s exact test when appropriate. Survival curves were plotted using the Kaplan-Meier method. Log-rank test was used to compare survivals between different groups. As the baseline characteristics in the MIS and Open cases were not balanced, a propensity-score matched (PSM) analysis was performed with R version 4.2.0. A 1:4 matching was performed by potential confounding factors including sex, age, body mass index (BMI), forced expiratory volume in one second (FEV1), percentage of diffusing capacity of the lung for carbon monoxide (DLCO%), comorbidity, smoking history, tumor size, tumor location, clinical T and N stage, histological classification, and neoadjuvant chemotherapy. Patients having MIS were ordered and sequentially matched to the nearest unmatched patients having thoracotomy. Surgical and postoperative outcomes were then compared between the matched groups. Univariable analysis was performed using the Cox univariate model to assess the impact of potential risk factors on survival and disease-progression. Multivariable analysis was performed with a Cox proportional model, using the enter method. The variables would be included into the multivariable analysis if their P-values were less than 0.05 in univariable analysis. Statistical significance was defined as P < 0.05 throughout the study.
Between January 2013 and December 2018, 692 consecutive patients underwent sleeve lobectomy at the Shanghai Chest Hospital. Based on the exclusion criteria, 114 cases were excluded, leaving 578 patients for analysis, 103 (17.8%) in the MIS group and 475 (82.2%) in the Open group. The MIS group included 20 RATS cases and 83 VATS cases (Figure 1). There was an obvious trend toward increasing use of MIS in sleeve lobectomy patients during the study period (7.7% in 2013 to 36% in 2018), as shown in Figure 2. Conversion to thoracotomy was found necessary in 14 patients (13.6%) due to difficult tumor or hilar lymph nodes, dense adhesion, or unexpected intraoperative bleeding. Details of patient demographics and oncologic characteristics are listed in Table 1. Demographic characteristics were similar between the MIS and the Open groups before PSM. There was no difference in patient age, sex, comorbidity or functional status between the MIS and the Open group. The proportion of patients receiving neoadjuvant therapy (10.7% vs. 16.0%, P = 0.224) were also similar. However, right upper lobe sleeve lobectomy accounted for only 47.2% of the cases in the Open group, while it was 59.2% in the MIS group (P = 0.026). There was only one (0.9%) case of sleeve bilobectomy in the MIS group but 17(3.6%) in the Open group. The MIS group also had more patients with smaller lesions and thus more cT2 tumors (94.2% vs. 85.9%, P = 0.022). The mean diameter of tumor was 32 mm in the MIS group and 37 mm in the Open group (P = 0.002).
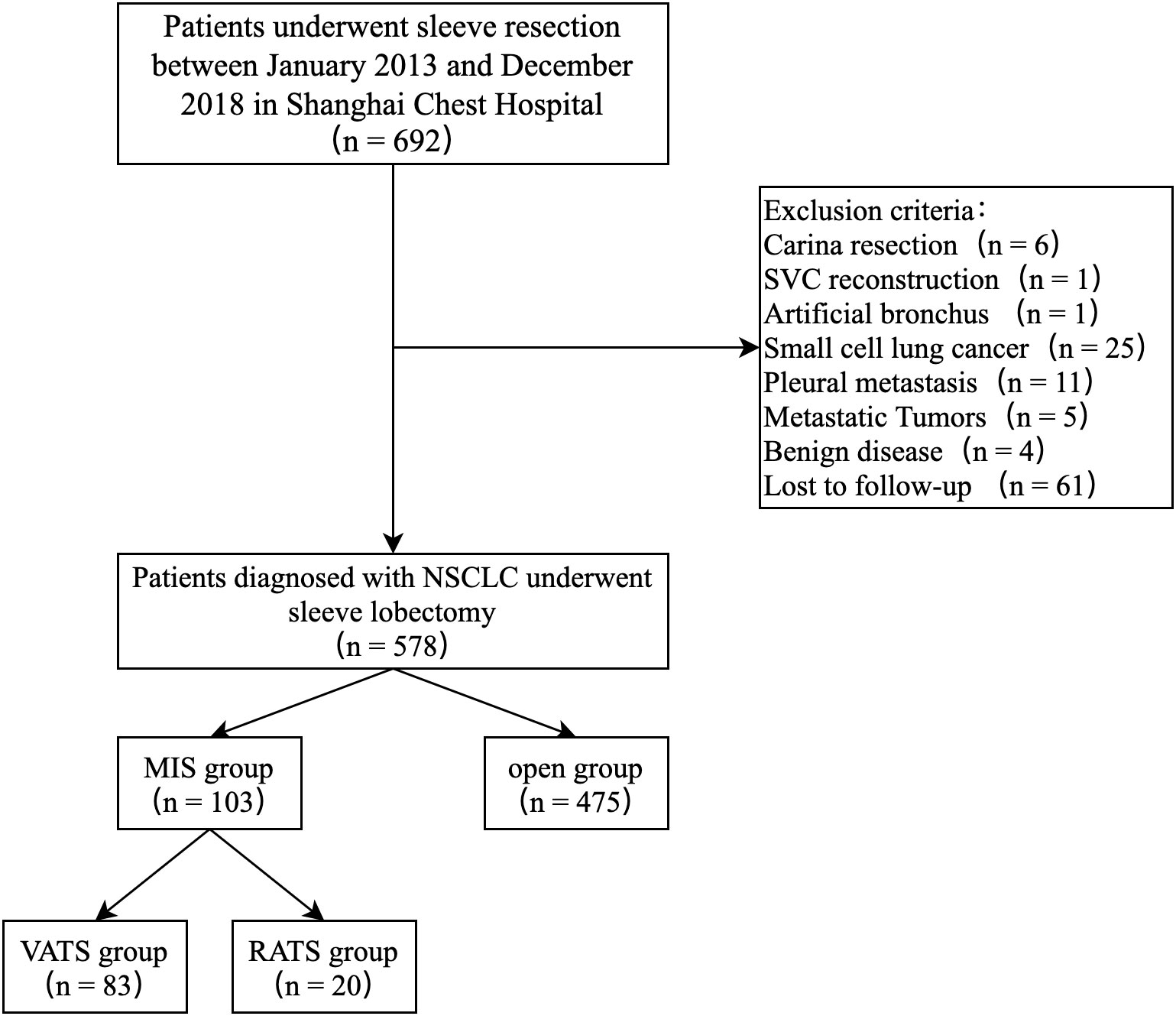
Figure 1 Study population flow diagram — patients who underwent sleeve lobectomy between 2013 and 2018.
In the unmatched cohort, median operation time was 171 minutes in the MIS group and 151 minutes in the Open group (P < 0.001). However, rate of angioplasty (7.8% vs. 8.4%, P = 0.983), R0 resection (84.5% vs. 84.6%, P = 0.966) and median number of harvested lymph nodes (15 vs. 15, P = 0.445) were similar between the two groups. The MIS group had shorter drainage duration (5 days vs. 6 days, P = 0.004), less drainage amount (1270 ml vs. 1670 ml, P < 0.001) and shorter length of postoperative hospitalization (7 days vs. 8 days, P = 0.007) compared to the Open group. In term of postoperative complications, there was only one patient diagnosed with bronchopleural fistula (BPF) after surgery in the MIS group who recovered after conservative treatment. Regarding the late complications, there was 1 patient in the MIS group and 2 in the Open group experiencing bronchial stenosis after surgery. These patients received balloon dilation via bronchoscopy. We did not see any patients with late dehiscence postoperatively. Five patients in the Open group died due to bronchopleural fistula or pulmonary infection, and one in the MIS group died due to empyema and sepsis during postoperative hospitalization. The in-hospital mortality was not significantly different (MIS vs. Open, 1.0% vs. 1.1%, P = 0.940).
After PSM, 100 MIS and 338 Open patients were retained for further analysis. There was no longer any significant difference in patient demographics or tumor characteristics between the two matched groups (Table 1). Potential confounders like tumor size, tumor location, tumor stage, and proportion of patients receiving neoadjuvant therapy before surgery were well-balanced after PSM. In the matched cohort, median operation time in the MIS group was still longer than in the Open group (170.5 minutes [IQR, 134–224.5] vs.149.5 minutes [IQR, 128–179], P < 0.001). However, the estimated intraoperative blood loss was significantly less in the MIS group (100 ml [IQR, 100–200] vs. 200 ml [IQR, 100–200], P = 0.003). There was no difference in complete resection rate, number of total or mediastinal lymph nodes dissected between the two groups. After surgery, chest drainage duration (5 days [IQR, 4–7] vs. 6 days [IQR, 5–7], P = 0.027) was significantly shorter, and total amount of drainage (1280 ml [IQR, 957.5–1695] vs. 1640 ml [IQR, 1200–2307.5], P < 0.001) was significantly less in the MIS group than in the Open group. Postoperative ICU stay and length of stay in hospital was similar (Table 2) between two groups. The overall postoperative complication rate was 18.0% in the MIS group and 20.7% in the Open group, which was also similar (Table 2). The BPF rate was 0% in the MIS group and 1.5% in the Open group in the matched cohort.
Perioperative outcomes were also compared between the conversion patients and the Open group. The conversion cases had longer operation time than the Open group (190.5 minutes [IQR, 153.25–306.25] vs. 151 minutes [IQR, 127–179], P = 0.004). However, intraoperative blood loss, number of total and mediastinal lymph nodes dissected, postoperative ICU stay, postoperative drainage duration or amount, postoperative length of hospitalization, or postoperative complication rates were similar between the two groups. No conversion cases died during hospitalization (Table 3).
Patient characteristics and perioperative outcomes were further compared between VATS and RATS cases (Table 4). Operation time, intraoperative blood loss, lymph node dissection, postoperative drainage, postoperative length of hospitalization and overall postoperative complication rates were similar between the two groups. However, significantly more lower lobe sleeve lobectomies were accomplished via RATS than via VATS (40.0% vs. 12.0%, P = 0.017).
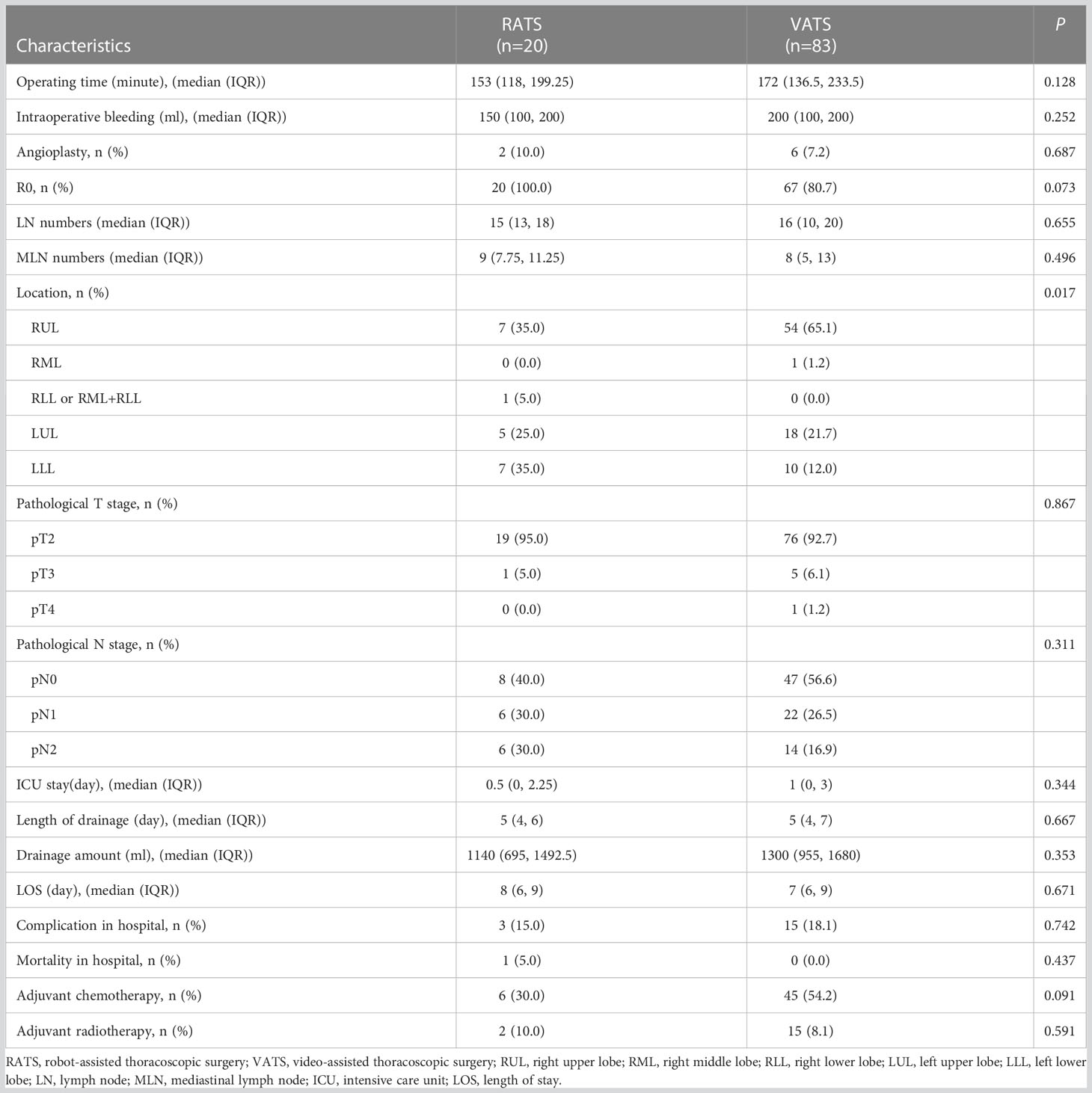
Table 4 Perioperative outcomes between robot-assisted thoracic surgery and video-assisted thoracic surgery groups in unmatched cohort.
The median follow-up time was 31 months in the Open group and 42 months in the MIS group before PSM. Five-year OS rate in the MIS group was significantly better than in the Open group (73.5% vs. 60.6%, P = 0.039) before PSM (Figure 3A). Five-year PFS rate was 47.9% in the MIS group and 50.7% in the Open group without significant difference before PSM (Figure 3B). As showed in Figure 3C, five-year OS remained better in the MIS group compared with the Open group after PSM, although without statistical significance (72.7% vs. 64.4%, P = 0.156). Five-year PFS was similar after PSM, 49.2% in the MIS group and 50.5% in the Open group (Figure 3D). To determine whether surgical approach would have any impact on OS and PFS, univariable and multivariable analyses were performed in the entire cohort (Supplemental Table S1). The multivariable results showed that surgical approach was not associated with OS or PFS in sleeve lobectomy patients (Figure 4). Interestingly, we also found that there was no significant difference in five-year OS rates (60.7% vs. 63.1%, P = 0.763) or PFS rates (39.1% vs. 49.9%, P = 0.205) between margin positive group and margin negative group. When we delved into the database, we found that 37(41.6%) patients received postoperative radiotherapy (PORT) in margin positive group for local control, but only 47(9.6%) patients in the margin negative group received PORT.
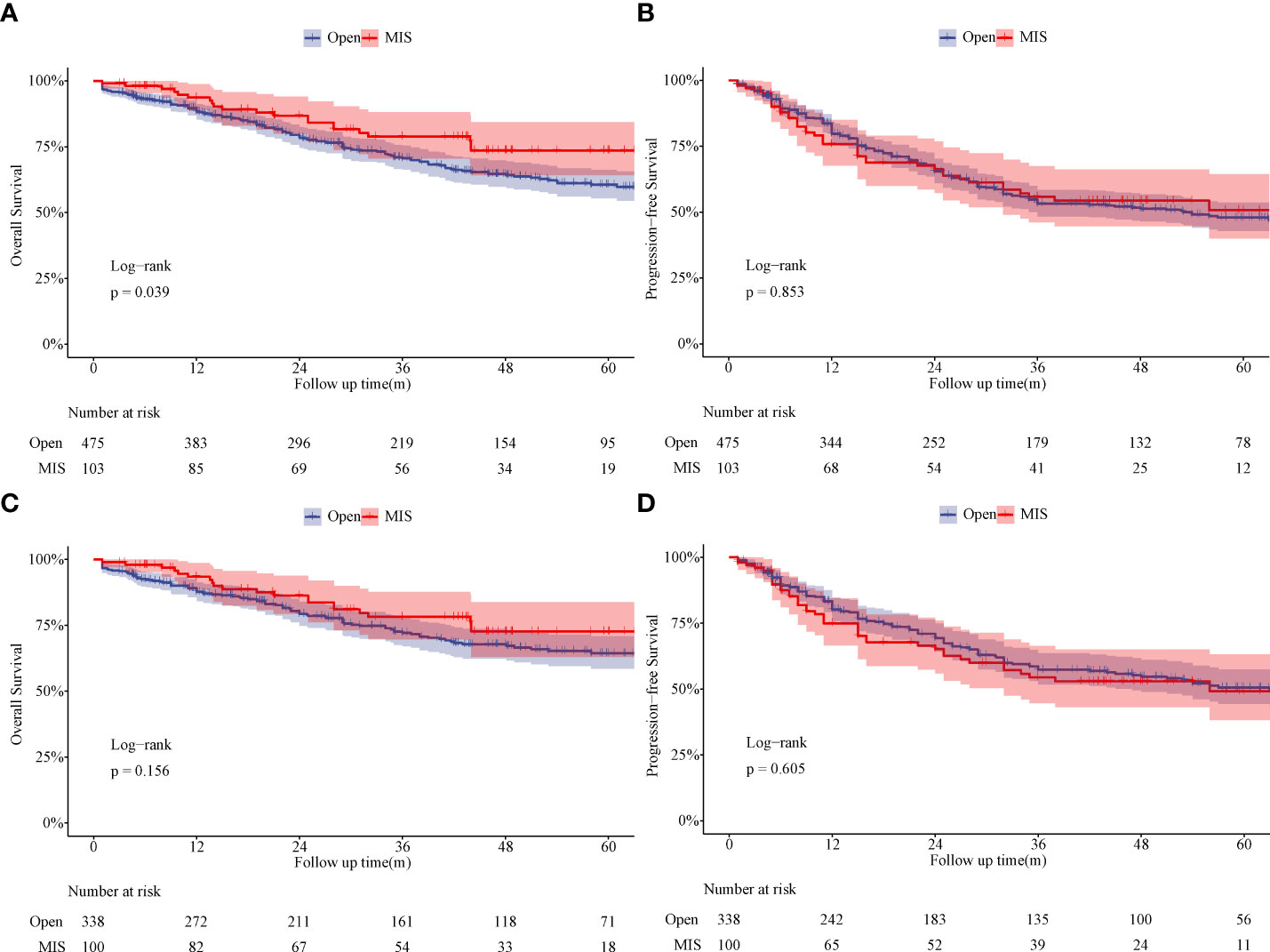
Figure 3 Comparison of overall survival and progression-free survival between the Open group and MIS group. (A) Comparison of overall survival between the Open group and MIS group (unmatched). (B) Comparison of progression-free survival between the Open group and MIS group (unmatched). (C) Comparison of overall survival between the Open group and MIS group (matched). (D) Comparison of progression-free survival between the Open group and MIS group (matched).
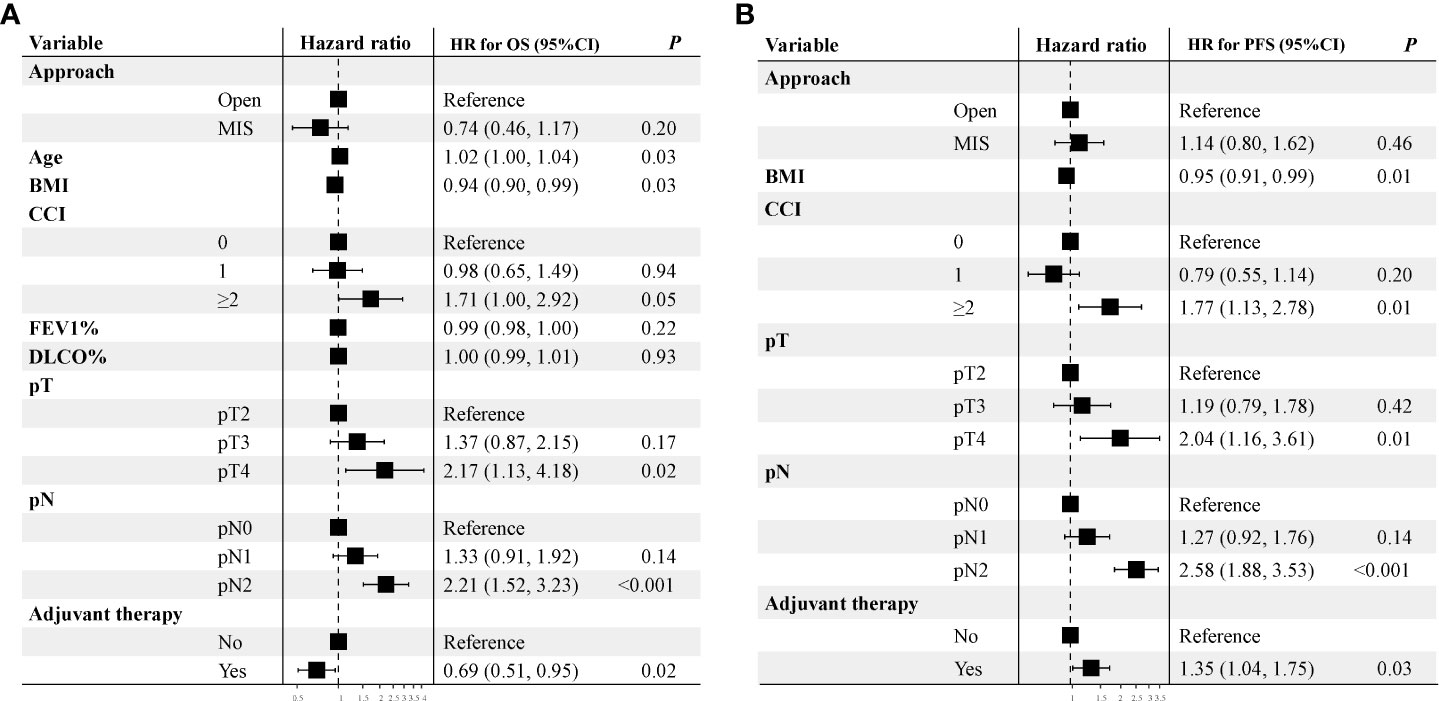
Figure 4 Multivariable analysis of overall survival and progression-free survival of unmatched cohort. A hazard ratio more than 1 implies a higher risk of overall survival and progression-free survival after sleeve lobectomy.
In this real-world study, only 17.8% of the sleeve resections for NSCLC were performed by MIS. MIS including both VATS and RATS were increasingly used during the study period, although it was more often used for smaller tumors and relatively simpler right upper lobe sleeve resections. Our study showed that in a matched cohort, intraoperative blood loss and postoperative drainage after MIS sleeve lobectomy was significantly less than after open surgery, although operation time for sleeve lobectomy by MIS was about 20 minutes longer than by open surgery. And overall mortality and morbidity were comparable between the two groups. The conversion cases had similar postoperative outcomes compared to the Open cases. What is more, there was no significant difference between the MIS group and the Open group in OS or PFS. And surgical approach was not associated with long-term outcomes in multivariable analysis for survivals.
In the real-world, the application of MIS for sleeve lobectomy was still much less often used than open thoracotomy even at a high-volume thoracic surgery center. According to the annual report of the Shanghai Chest Hospital, the overall MIS rate for lung surgery was over 95% (32), but the MIS rate for sleeve lobectomy was only 17.8% in our study during the same time period. As a technically demanding procedure, surgeons tended to perform MIS sleeve lobectomy for smaller tumors in earlier stages or less complex right upper lobe sleeve lobectomy, as was shown in our study. However, pulmonary function and comorbidity had no influence on patient selection. Unlike most previous reports, patients with neoadjuvant therapies before surgery or requiring angioplasty were also included in our study. Even with such more difficult MIS cases (10.7% after neoadjuvant therapy and 7.8% of angioplasty), perioperative results and long-term survivals were not compromised or even better after MIS sleeve lobectomy than after open surgery in the real world.
To reduce potential selection bias, we performed PSM and ITT analysis to validate our findings. In the matched cohort, although operation time by MIS was around 20 minutes longer than that by open surgery, it did not bring any additional complication compared to open surgery. This was further supported by the very low rate of anastomotic complications, especially BPF, which occurred similarly between the MIS group and the Open group (1.0% vs. 1.9%). On the other hand, intraoperative blood loss and postoperative amount of drainage in the MIS group were significantly less than in the Open group, indicating that MIS sleeve lobectomy could render uncompromised recovery and carries with it certain benefits in selected patients with centrally located NSCLC. Recently a database study showed that the VATS approach was associated with shorter length of stay and decreased morbidity in sleeve lobectomy cases (33), which was consistent with our findings.
In this study, there were fourteen cases intended to receive MIS but were converted to open surgery. The conversion rate was 13.6%, which was similar to the 4.5%–21.1% conversion rates in the other published MIS sleeve lobectomy studies (28–30, 33). Unfortunately, none of those studies reported the surgical outcomes in converted cases. Our results showed that although operation time was longer in the conversion cases than open surgery, intraoperative blood loss, postoperative drainage, length of hospital stays, and postoperative complication rates were similar between the two groups. No conversion patient died after surgery during hospital stay. Our results suggested that conversion to thoracotomy during the operation did not bring additional risks to the patients. It is thus safe and feasible to start sleeve lobectomy minimally invasively in well selected patients.
Robotic surgery has gradually become an integrated part of MIS. However, whether RATS had any advantages in sleeve lobectomy remains to be explored. Among all sleeve lobectomies, right upper lobe is the most straight forward. The lower lobe sleeve lobectomies are comparatively more complex because of the anastomosis angles and greater size discrepancy between the proximal and distal bronchi. In addition to significantly more right upper sleeve lobectomies in the MIS group than in the Open group (59.2% vs. 47.2%), percentage of right upper sleeve lobectomy was the highest in VATS cases (65.1%) but was the lowest (35.0%) in RATS cases. Meanwhile 40.0% lower lobe sleeve lobectomies were done via RATS, but only 12.0% of them were done via VATS. This was in consistency with the findings in Qiu’s study in which lower lobe sleeve lobectomies were most often accomplished via RATS than via VATS or open thoracotomy (26.5% vs. 21.9% vs. 16.7%) (29). There are two potential explanations for this. First, RATS is more flexible and feasible than VATS. The three-dimensional and magnified vision and the dexterous robotic arms are helpful in more demanding cases. Second, surgeons favoring robotic surgery may be more experienced in MIS and in handling anastomotic difficulties. According to our previous study, short-term and mid-term outcomes after RATS sleeve lobectomy were comparable to open surgery (27). Therefore, RATS may be an important alternative in complex MIS surgery such as lower lobe sleeve lobectomy.
Previous studies suggested that oncological outcomes after MIS might be similar to open surgery in patients with NSCLC needing sleeve lobectomy. But one of the major limitations in most such studies was the relatively short follow-up time in MIS patients, being 24–36.8 months in previous published reports (28–30). This was mostly because MIS sleeve lobectomies were often accomplished more recently, with open cases in earlier years as historical controls. The median follow-up time of the MIS group reached 42 months in our study. And it is by far the longest follow-up time in MIS sleeve lobectomy patients, with a control Open group during the same time period. OS turned out to be significantly better in the MIS group than in the Open group (73.5% vs. 60.6%, P = 0.039), probably because of more smaller tumors in MIS patients. Although OS in the MIS group was still better than in the Open group after PSM, PFS was similar between the two groups both before (47.9% vs. 50.7%, P = 0.853) and after PSM (49.2% vs. 50.5%, P = 0.605). Together with similar R0 resection rates and numbers of lymph node dissected, our results indicated that oncological outcomes after minimally invasive sleeve lobectomy were at least non-inferior to those after open thoracotomy. There have been studies showing that MIS approach could reduce level of cytokine responses and lead to better immune function than open surgery (34, 35). Hopefully with increasing experience in MIS, sleeve lobectomy patients may have benefit in both perioperative recovery as well as prolonged survival in the future.
There were certain limitations in our study. First, our study was retrospective in nature. Unknown confounding factors like surgeons’ preference and expertise would still influence the results even though PSM was used to diminish potential impact from patient conditions and tumor characteristics. However, this study included all consecutive patients receiving sleeve lobectomy for potentially resectable primary NSCLC, using an ITT analysis. Our study results clearly revealed the surgical and oncological outcomes of MIS in the real world. Second, all patients included in this study were treated at a single institution, which has a very high surgical volume and has more experience in MIS for lung cancers. It would thus be interesting to use external data to validate our findings on the efficacy of MIS sleeve lobectomy. Third, the detailed information on conversion to pneumonectomy could not be accurately retrieved due to the retrospective nature of the study. However, we found that the positive margin did not compromise the long-term survival of sleeve lobectomy patients, probably because of the role of postoperative radiation for local control.
In conclusion, MIS sleeve lobectomy is still a technically demanding procedure currently. Nonetheless, it is safe and feasible in experienced hands, with similar or even better surgical and oncologic outcomes compared to open surgery in well-selected patients. And RATS may be preferable for more difficult sleeve cases. Conversion to thoracotomy does not compromise perioperative recovery of the patients. Therefore, it does little harm to try sleeve lobectomy minimally invasively first.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Shanghai Chest Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
(I) Conception and design: TC, WZ, WF. (II) Administrative support: WF. (III) Provision of study materials or patients: TC, CJ. (IV) Collection and assembly of data: WZ, TC. (V) Data analysis and interpretation: All authors. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MC declared a shared parent affiliation with the authors TC, WZ, CJ, LJ, YW, YL and WF to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1099514/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. CA Cancer J Clin (1974) 24:238–42. doi: 10.3322/canjclin.24.4.238
4. Deslauriers J, Grégoire J, Jacques LF, Piraux M, Guojin L, Lacasse Y. Sleeve lobectomy versus pneumonectomy for lung cancer: A comparative analysis of survival and sites or recurrences. Ann Thorac Surg (2004) 77:1152–6. doi: 10.1016/j.athoracsur.2003.07.040
5. Ludwig C, Stoelben E, Olschewski M, Hasse J. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg (2005) 79:968–73. doi: 10.1016/j.athoracsur.2004.08.062
6. Takeda S-I, Maeda H, Koma M, Matsubara Y, Sawabata N, Inoue M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: Trends over time and 20-year institutional experience. Eur J Cardiothorac Surg (2006) 29:276–80. doi: 10.1016/j.ejcts.2005.12.017
7. Okada M, Yamagishi H, Satake S, Matsuoka H, Miyamoto Y, Yoshimura M, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg (2000) 119:814–9. doi: 10.1016/S0022-5223(00)70018-3
8. Pagès P-B, Mordant P, Renaud S, Brouchet L, Thomas P-A, Dahan M, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg (2017) 153:184–195.e3. doi: 10.1016/j.jtcvs.2016.09.060
9. Abdelsattar ZM, Shen KR, Yendamuri S, Cassivi S, Nichols FC, Wigle DA, et al. Outcomes after sleeve lung resections versus pneumonectomy in the united states. Ann Thorac Surg (2017) 104:1656–64. doi: 10.1016/j.athoracsur.2017.05.086
10. Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: Optimal management strategy using decision analysis techniques. Ann Thorac Surg (2003) 76:1782–8. doi: 10.1016/s0003-4975(03)01243-8
11. Park JS, Yang HC, Kim HK, Kim K, Shim YM, Choi YS, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol (2010) 5:517–20. doi: 10.1097/JTO.0b013e3181d0a44b
12. National comprehensive cancer network (NCCN) clinical practice guidelines in oncology: Non-small cell lung cancer (2022). Available at: https://www.nccn.org.
13. Swanson SJ, Herndon JE, D’Amico TA, Demmy TL, McKenna RJ, Green MR, et al. Video-assisted thoracic surgery lobectomy: Report of CALGB 39802–a prospective, multi-institution feasibility study. J Clin Oncol (2007) 25:4993–7. doi: 10.1200/JCO.2007.12.6649
14. Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg (2008) 85:S719–728. doi: 10.1016/j.athoracsur.2007.09.056
15. Cao C, Manganas C, Ang SC, Yan TD. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg (2012) 1:16–23. doi: 10.3978/j.issn.2225-319X.2012.04.18
16. Lim E, Batchelor TJP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NEJM Evidence (2022) 1:EVIDoa2100016. doi: 10.1056/EVIDoa2100016
17. Scott WJ, Allen MS, Darling G, Meyers B, Decker PA, Putnam JB, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: A secondary analysis of data from the American college of surgeons oncology group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg (2010) 139:976–81. doi: 10.1016/j.jtcvs.2009.11.059
18. Lee PC, Nasar A, Port JL, Paul S, Stiles B, Chiu Y-L, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg (2013) 96:951–60. doi: 10.1016/j.athoracsur.2013.04.104
19. Santambrogio L, Cioffi U, De Simone M, Rosso L, Ferrero S, Giunta A. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: A case report. Chest (2002) 121:635–6. doi: 10.1378/chest.121.2.635
20. Schmid T, Augustin F, Kainz G, Pratschke J, Bodner J. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg (2011) 91:1961–5. doi: 10.1016/j.athoracsur.2010.08.079
21. Gonzalez-Rivas D, Fernandez R, Fieira E, Rellan L. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: First report. J Thorac Cardiovasc Surg (2013) 145:1676–7. doi: 10.1016/j.jtcvs.2013.02.052
22. Gonzalez-Rivas D, Delgado M, Fieira E, Fernandez R. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg (2014) 3:E2. doi: 10.3978/j.issn.2225-319X.2014.03.13
23. Liu L, Mei J, Pu Q, Ma L. Thoracoscopic bronchovascular double sleeve lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg (2014) 46:493–5. doi: 10.1093/ejcts/ezu103
24. Pan X, Gu C, Wang R, Zhao H, Shi J, Chen H. Initial experience of robotic sleeve resection for lung cancer patients. Ann Thorac Surg (2016) 102:1892–7. doi: 10.1016/j.athoracsur.2016.06.054
25. Mahtabifard A, Fuller CB, McKenna RJ. Video-assisted thoracic surgery sleeve lobectomy: A case series. Ann Thorac Surg (2008) 85:S729–732. doi: 10.1016/j.athoracsur.2007.12.001
26. Zhou S, Pei G, Han Y, Yu D, Song X, Li Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg (2015) 10:116. doi: 10.1186/s13019-015-0318-6
27. Gu C, Pan X, Chen Y, Yang J, Zhao H, Shi J. Short-term and mid-term survival in bronchial sleeve resection by robotic system versus thoracotomy for centrally located lung cancer. Eur J Cardiothorac Surg (2018) 53:648–55. doi: 10.1093/ejcts/ezx355
28. Yang Y, Mei J, Lin F, Pu Q, Ma L, Liu C, et al. Comparison of the short- and long-term outcomes of video-assisted thoracoscopic surgery versus open thoracotomy bronchial sleeve lobectomy for central lung cancer: A retrospective propensity score matched cohort study. Ann Surg Oncol (2020) 27:4384–93. doi: 10.1245/s10434-020-08805-y
29. Qiu T, Zhao Y, Xuan Y, Qin Y, Niu Z, Shen Y, et al. Robotic sleeve lobectomy for centrally located non-small cell lung cancer: A propensity score-weighted comparison with thoracoscopic and open surgery. J Thorac Cardiovasc Surg (2020) 160:838–846.e2. doi: 10.1016/j.jtcvs.2019.10.158
30. Xie D, Deng J, Gonzalez-Rivas D, Zhu Y, Jiang L, Jiang G, et al. Comparison of video-assisted thoracoscopic surgery with thoracotomy in bronchial sleeve lobectomy for centrally located non-small cell lung cancer. J Thorac Cardiovasc Surg (2021) 161:403–413.e2. doi: 10.1016/j.jtcvs.2020.01.105
31. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
32. Wang Y, Gu Z, Yao F, Mao T, Wang R, Sun Y, et al. Annual report of thoracic surgery services at the shanghai chest hospital in 2020. Shanghai Chest (2022) 6:3. doi: 10.21037/shc-2021-04
33. Gonzalez M, Chriqui LE, Décaluwé H, Aigner C, Rényi-Vámos F, Opitz I, et al. Sleeve lobectomy in patients with non-small-cell lung cancer: A report from the European society of thoracic surgery database 2021. Eur J Cardiothorac Surg (2022) 62(6):ezac502. doi: 10.1093/ejcts/ezac502
34. Ng CSH, Whelan RL, Lacy AM, Yim APC. Is minimal access surgery for cancer associated with immunologic benefits? World J Surg (2005) 29:975–81. doi: 10.1007/s00268-005-0029-6
Keywords: sleeve lobectomy, minimally invasive surgery, thoracotomy, robot-assisted thoracoscopic surgery, video-assited thoracoscopic surgery
Citation: Chen T, Zhao W, Ji C, Luo J, Wang Y, Liu Y, Weder W and Fang W (2023) Minimally invasive sleeve lobectomy for centrally located lung cancer: A real-world study with propensity-score matching. Front. Oncol. 13:1099514. doi: 10.3389/fonc.2023.1099514
Received: 15 November 2022; Accepted: 19 January 2023;
Published: 01 February 2023.
Edited by:
Jianrong Zhang, The University of Melbourne, AustraliaReviewed by:
Min Cao, Shanghai Jiao Tong University, ChinaCopyright © 2023 Chen, Zhao, Ji, Luo, Wang, Liu, Weder and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wentao Fang, dnd0ZmFuZ0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.