- 1Department of Neurosurgery, Keio University School of Medicine, Tokyo, Japan
- 2Department of Neurosurgery, Kawasaki Municipal Hospital, Kawasaki-ku, Kanagawa, Japan
Objective: The goal of schwannoma resection is to control the tumor while preserving neurological function. Schwannomas have a variable postoperative growth pattern, therefore preoperative prediction of a schwannoma’s growth pattern is favorable. This study aimed to examine the relationship between preoperative neutrophil-to-lymphocyte ratio (NLR) and postoperative recurrence and retreatment in patients with schwannoma.
Methods: We retrospectively examined 124 patients who underwent schwannoma resection in our institution. Associations between preoperative NLR, other patient and tumor characteristics, and tumor recurrence and retreatment were analyzed.
Results: Median follow-up was 2569.5 days. Postoperative recurrence occurred in 37 patients. Recurrence that required retreatment occurred in 22. Treatment-free survival (TFS) was significantly shorter in patients with NLR ≥2.21 (P = 0.0010). Multivariate Cox proportional hazards regression showed that NLR and neurofibromatosis type 2 were independent predictors of retreatment (P = 0.0423 and 0.0043, respectively). TFS was significantly shorter in patients with NLR ≥2.21 in the following subgroups: sporadic schwannoma, primary schwannoma, schwannoma ≥30 mm in size, subtotal resection, vestibular schwannoma, and postoperative recurrence.
Conclusions: Preoperative NLR ≥2.21 before surgery was significantly associated with retreatment after schwannoma resection. NLR may be a novel predictor of retreatment and assist surgeons in preoperative surgical decision making.
Introduction
Schwannomas are benign tumors that originate from Schwann cells of the cranial and peripheral nerves, and have a variable growth pattern (1). Clinical outcome after their surgical resection is related to extent of removal (2–6), which in the future could be assisted by new imaging modalities besides classic magnetic resonance imaging (MRI) (7). The goal of schwannoma resection is to control the tumor while preserving neurological function (8–11). Accurate preoperative prediction of a schwannoma’s growth pattern might assist surgeons with clinical decision making regarding aggressiveness of resection and need for adjuvant radiotherapy. Ki-67 is a commonly used proliferative marker for several types of tumors; however, it cannot be evaluated before surgery and its significance in schwannoma is controversial (3, 5).
Inflammation promotes tumor development throughout all stages of tumorigenesis (12). Systemic inflammation and immune system activation is broadly reflected by the neutrophil-to-lymphocyte ratio (NLR), an inexpensive, easily measured, and readily available blood test. A high NLR has been associated with worse overall survival in many solid malignant tumors (13–17). A relationship between NLR and refractory intracranial benign tumor has also been demonstrated in other studies (18–22). In a previous study, we showed that preoperative NLR ≥2.6 was significantly associated with shorter progression-free survival in all grades of meningioma, including World Health Organization grade I (22). This study aimed to examine the relationship between preoperative NLR and postoperative recurrence and retreatment in patients with schwannoma.
Methods
Study design and clinical data
We retrospectively reviewed 270 patients who underwent surgical schwannoma resection in our institution from February 2010 to February 2018. The study received institutional review board approval (reference number, 20050002) and all patients provided written informed consent. Patients who received steroids or immunosuppressive drugs before preoperative laboratory testing, those with systemic infection, and with a history of malignancy were excluded. We also excluded those with incomplete clinical, laboratory, or radiological data.
The following data were obtained from the medical records: age at time of surgery, sex, neurofibromatosis 2 (NF2) status, tumor origin, primary/recurrent tumor, solid/cystic tumor, brain compression, neurological symptoms, and extent of removal. Tumor origin was determined using gadolinium-enhanced T1-weighted MRI. Extent of removal was determined using MRI after surgery.
MRI was performed every 6 to 12 months after surgery. In patients who underwent gross total resection (GTR), tumor recurrence was defined as the appearance of new tumor at the surgical site. In patients who underwent subtotal resection (STR), recurrence was defined as residual tumor growth ≥2 mm. Recurrence-free survival (RFS) was defined as the time from the date of surgery to the date of tumor recurrence or last imaging follow-up. Because not all schwannoma recurrences require treatment, treatment-free survival (TFS), a relatively new measure of disease control (23), was also evaluated. TFS was defined as the time from the date of surgery to the date of retreatment decision or last follow-up.
Laboratory data
Absolute neutrophil, lymphocyte, monocyte, and platelet counts and concentrations of albumin, C-reactive protein, and fibrinogen were routinely obtained before surgery and the following inflammatory parameters calculated (24–29): NLR, lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and prognostic nutritional index (PNI). PNI was calculated using the following formula: (10 × albumin concentration) + (0.005 × lymphocyte count).
Statistical analysis
Statistical analyses were performed using JMP 16 software (SAS Institute, Cary, NC, USA). Continuous variables are expressed as means with standard deviation and were compared using the Mann–Whitney U test. Categorical variables are expressed as numbers with percentage and were compared using Fisher’s exact test. RFS and TFS were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards regression were used to evaluate the influence of variables on RFS and TFS. Receiver operating characteristic (ROC) curves were constructed to determine optimal cut-off values for each variable. P <0.05 was considered significant.
Results
Patient characteristics and laboratory data
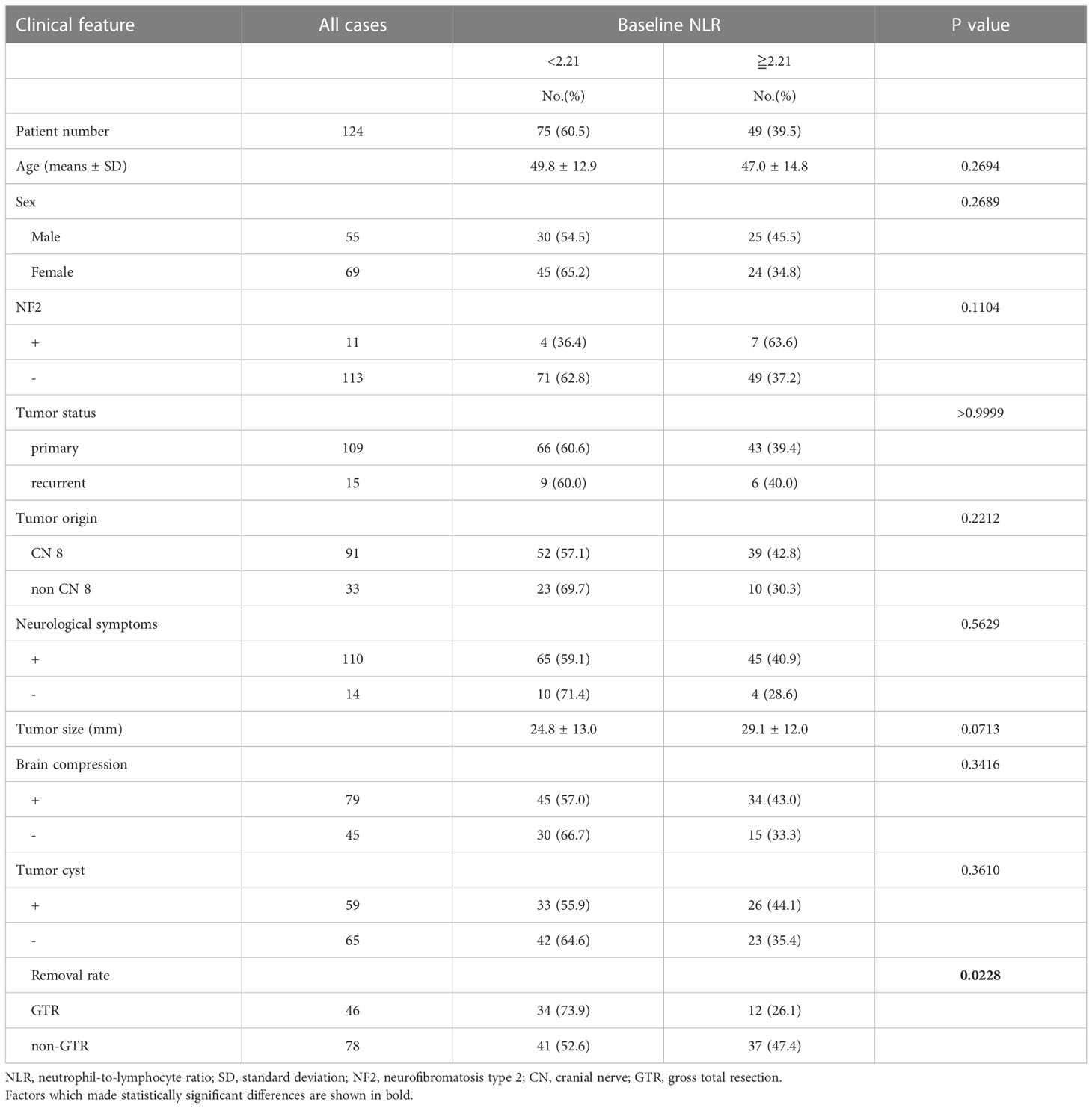
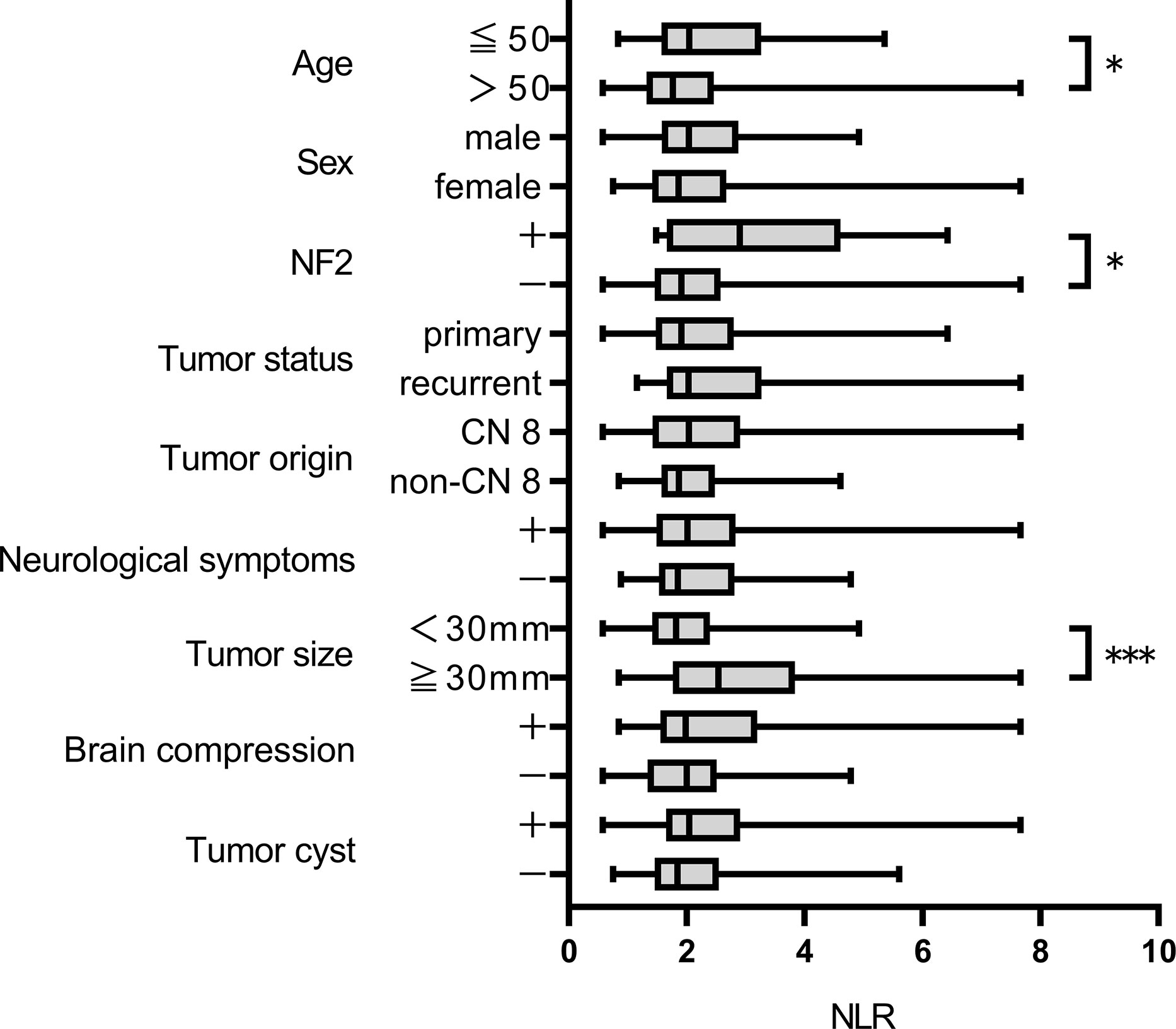
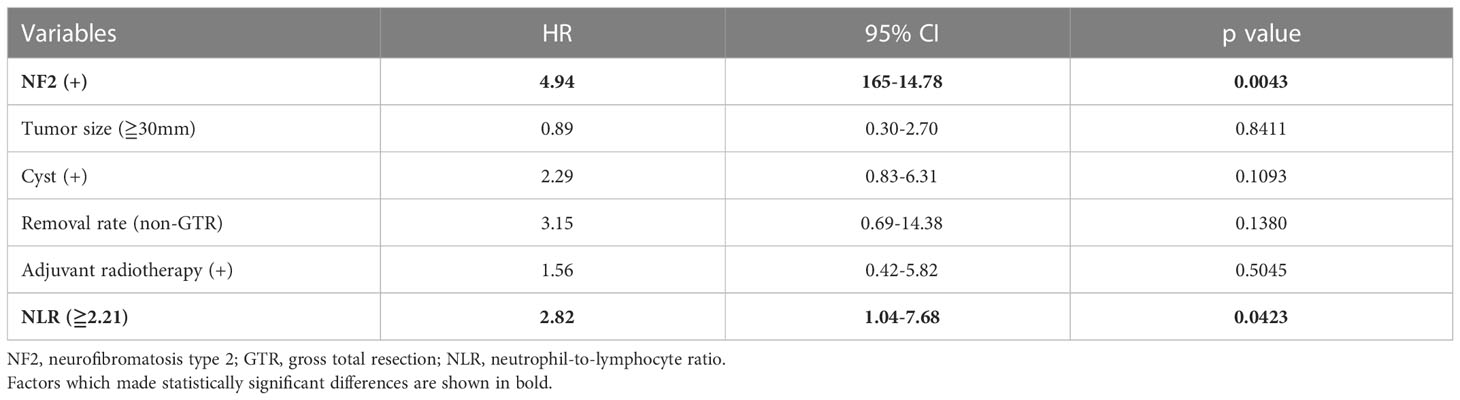
After excluding 146 patients based on criteria, 124 patients were included for analysis. Median follow-up was 2569.5 days. Postoperative recurrence occurred in 37 patients; recurrence that required retreatment occurred in 22. ROC curves were constructed for each variable using two outcomes, recurrence and retreatment. The optimal NLR cut-off value for recurrence and retreatment was 2.03 and 2.21, respectively. The area under the curve for recurrence and retreatment was 0.6039 and 0.7273, respectively (Supplementary Figure 1). Table 1 and Supplementary Table 1 show patient and tumor characteristics overall and with patients stratified by NLR cut-off value. The stratified groups were similar except for extent of resection. As shown in Figure 1, NLR value was significantly higher in younger patients and those with NF2 and tumor size ≥30 mm. NLR value was significantly higher in the retreatment group than the no recurrence and the recurrence but no retreatment groups. This suggests that NLR might be an important predictor of retreatment. NLR did not significantly differ between the no recurrence and the recurrence but no retreatment groups (Figure 2).
Survival analysis
As shown in Supplementary Figure 2, RFS was significantly shorter in patients with NF2 than in patients without NF2 (p <0.0001); RFS did not significantly differ between patients stratified by NLR using a cut-off value of 2.03 (p = 0.088). TFS was significantly shorter in patients with NF2 and in those who underwent STR (p <0.0001 and p = 0.0040, respectively; Figure 3A, B). TFS was significantly shorter in patients with NLR ≥2.21 (p = 0.0010; Figure 3C).
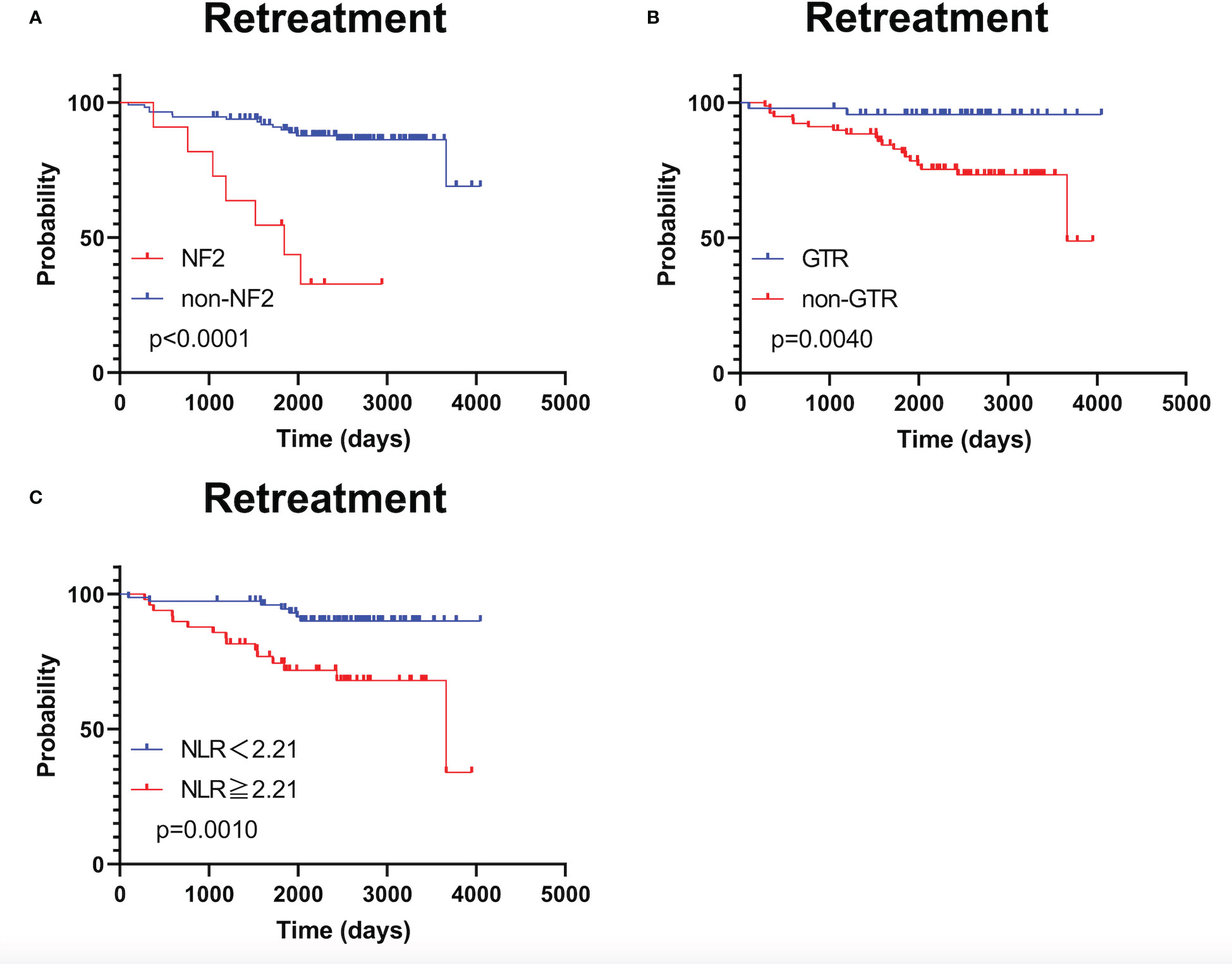
Figure 3 Kaplan–Meier treatment-free survival curves with patients stratified according to neurofibromatosis type 2 status (A), extent of tumor removal (B), and optimal neutrophil-to-lymphocyte ratio cut-off value (C).
Univariate and multivariate analyses
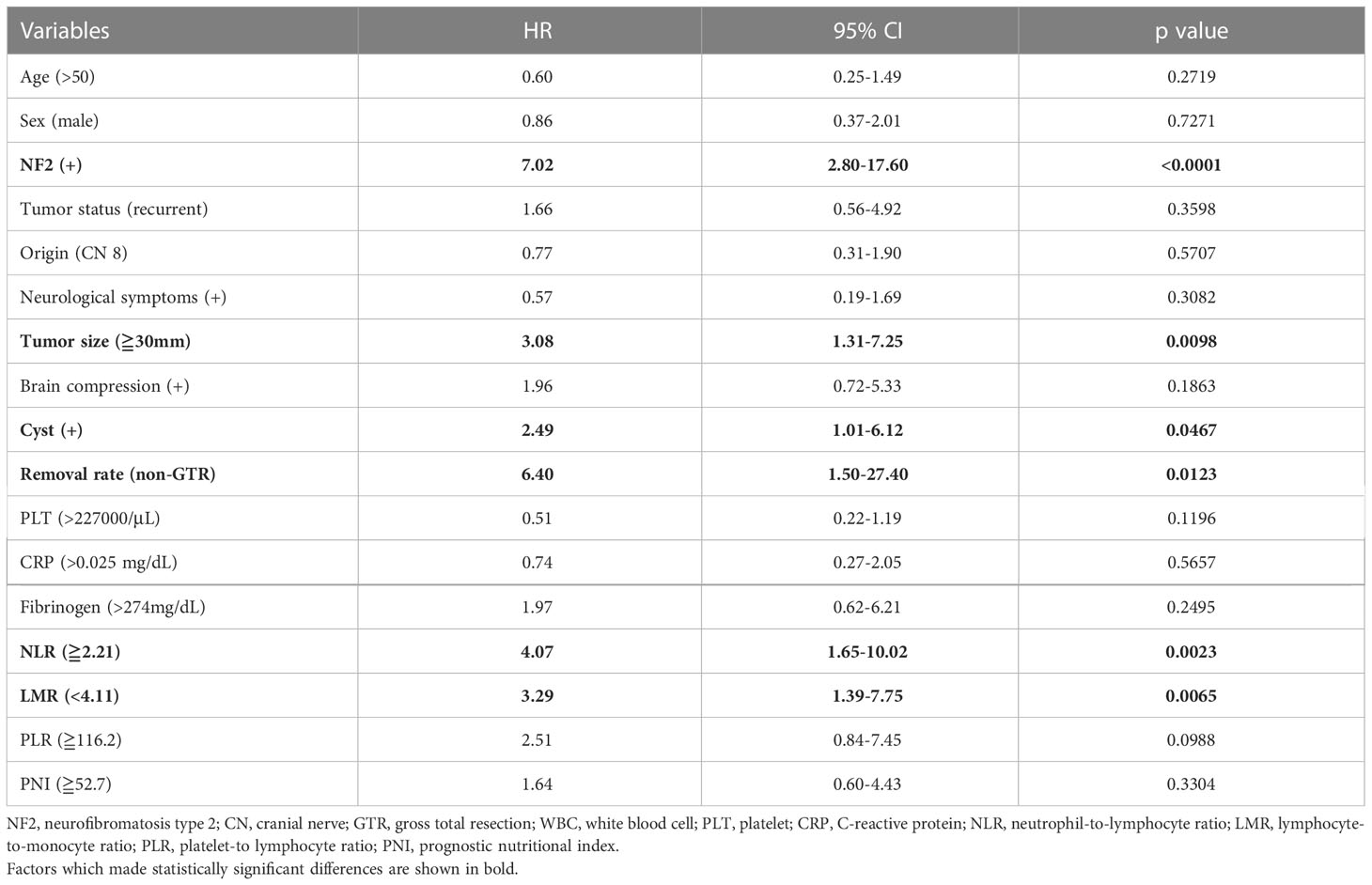
Because NLR was associated with TFS but not RFS, univariate and multivariate Cox proportional hazards regression was performed to investigate the influence of variables on TFS (Tables 2, 3). Although LMR was also associated with shorter TFS, NLR had a higher statistical power than LMR. The multivariate analysis showed that NF2 and NLR ≥2.21 were independent predictors of retreatment (P = 0.0043 and 0.0423, respectively).
Subgroup analysis
As shown in Figure 4, TFS was significantly shorter in patients with NLR ≥2.21 in the following subgroups: sporadic schwannoma, primary schwannoma, schwannoma ≥30 mm in size, STR, vestibular schwannoma, and postoperative recurrence. Subgroup analysis of NF2 patients was not performed because the sample size was small.
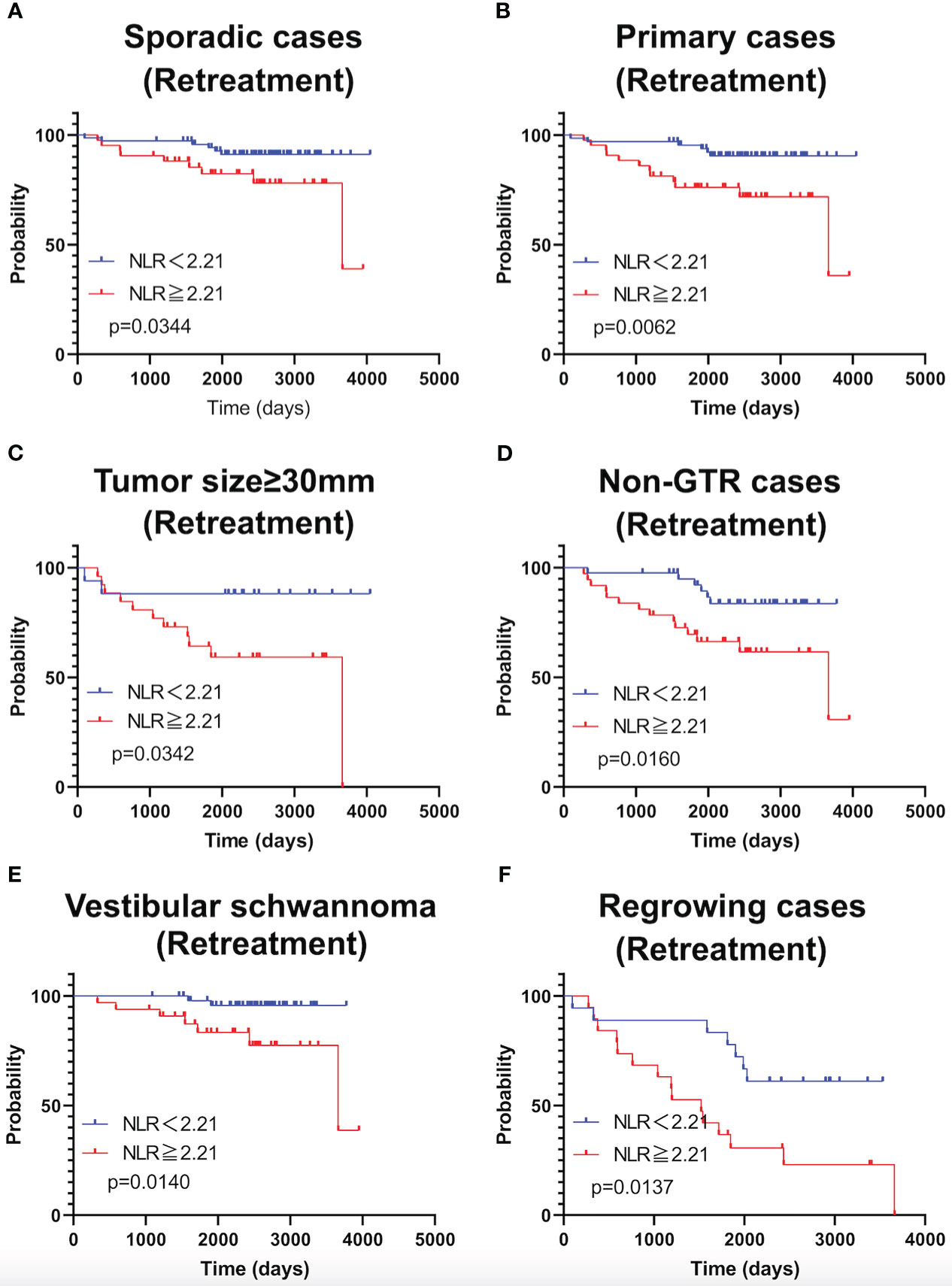
Figure 4 Kaplan-Meier curves of TFS related to sporadic cases (A), primary cases (B), large tumor (≥30mm) (C), Non-GTR cases (D), cases with vestibular schwannomas (E), and growing cases (F) are shown.
Discussion
In this study, preoperative NLR was an independent predictor of postoperative schwannoma recurrence that required retreatment. It was also applicable to a subgroup of vestibular schwannomas. Ki-67, S100, p53, microvessel density, and macrophage colony stimulating factor have been previously reported as biomarkers with prognostic value in schwannoma (5, 30–32). Histological inflammation and angiogenesis play a role in growth of sporadic and NF2-related vestibular schwannoma (33). CD163+ tumor-associated macrophages in particular have a supportive effect on schwannoma growth (31, 34–36). However, histopathological biomarkers cannot be evaluated preoperatively and therefore cannot contribute to early surgical decision making. In contrast, NLR can be easily calculated using preoperative blood testing. Although a few previous studies have evaluated serum and radiological prognostic factors associated with inflammatory status, only one has demonstrated that NLR is an important predictor of the natural history in schwannomas (18). However, this study did not examine postoperative growth pattern in surgical cases. Although dynamic positron emission tomography can predict inflammation in schwannomas (37), routine use of such a specialized and expensive imaging modality before surgery is not practical.
Based on the natural history of schwannomas, a subset of schwannomas do not exhibit growth after diagnosis (38–40). Similarly, some schwannomas grow slowly or transiently after surgery, while others grow rapidly and require retreatment. It is important to evaluate continuous tumor growth needing active treatment strategies such as surgery and radiotherapy. TFS, which is survival without the need of treatment for recurrence, may allow us to identify distinct prognostic group of schwannomas. Preoperative identification of those with a shorter TFS would assist surgeons and clinicians with treatment decision making. Our findings suggest that preoperative NLR ≥2.21 is a predictor of shorter TFS after surgery and that STR without adjuvant radiotherapy may be enough for those with a lower NLR. This surgical strategy has a merit in preserving neurological function (8). NLR may be essential in order to provide an evidence-based treatment recommendation for the patient with schwannomas.
This study has several limitations. It was retrospective in design and histopathological analysis was not performed. In addition, the relationship between serum inflammatory parameters and histopathological inflammatory status has not been fully elucidated, and our previous study found no association between NLR and histopathological inflammatory cell infiltration in meningioma (22). Moreover, we did not evaluate NLR after surgery, which may also be a useful predictor of recurrence and retreatment. All the cases were benign schwannomas, therefore the relationship between NLR and malignant schwannoma was not assessed. Future prospective studies are warranted to confirm our findings and investigate further.
Conclusions
Preoperative NLR ≥2.21 before surgery was significantly associated with retreatment after schwannoma resection. NLR may be a novel predictor of retreatment and assist surgeons in preoperative surgical decision making.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Keio University Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
KT and RT conceptualized, designed, and performed the study and wrote the manuscript. YK and KK assisted in the acquisition of data. TA and MT assisted with discussion and review of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Dr. Noboru Tsuda at the Department of Pathology, Keio University School of Medicine for technical assistance and Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1099384/full#supplementary-material
Supplementary Figure 1 | Receiver operating characteristic curves for recurrence and retreatment
Supplementary Figure 2 | Kaplan–Meier recurrence-free survival curves with patients stratified according to neurofibromatosis type 2 status and optimal neutrophil-to-lymphocyte ratio cut-off value
References
1. Bin-Alamer O, Bhenderu LS, Palmisciano P, Balasubramanian K, Upadhyay P, Ferini G, et al. Tumors involving the infratemporal fossa: A systematic review of clinical characteristics and treatment outcomes. Cancers (Basel). (2022) 14(21):5420. doi: 10.3390/cancers14215420
2. Fieux M, Zaouche S, Rabaste S, Riche B, Maucort-Boulch D, Tringali S. MRI Monitoring of residual vestibular schwannomas: Modeling and predictors of growth. Otol Neurotol (2020) 41(8):1131–9. doi: 10.1097/MAO.0000000000002742
3. Li J, Deng X, Ke D, Cheng J, Zhang S, Hui X. Risk factors for progression in vestibular schwannomas after incomplete resection: A single center retrospective study. Front Neurol (2021) 12:778590. doi: 10.3389/fneur.2021.778590
4. Park HH, Park SH, Oh HC, Jung HH, Chang JH, Lee KS, et al. The behavior of residual tumors following incomplete surgical resection for vestibular schwannomas. Sci Rep (2021) 11(1):4665. doi: 10.1038/s41598-021-84319-1
5. Fukuda M, Oishi M, Hiraishi T, Natsumeda M, Fujii Y. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection. J Neurosurg (2011) 114(5):1224–31. doi: 10.3171/2010.11.JNS101041
6. Lee WJ, Lee JI, Choi JW, Kong DS, Nam DH, Cho YS, et al. Optimal volume of the residual tumor to predict long-term tumor control using stereotactic radiosurgery after facial nerve-preserving surgery for vestibular schwannomas. J Korean Med Sci (2021) 36(16):e102. doi: 10.3346/jkms.2021.36.e10
7. Umana GE, Ferini G, Harikar MM, Venkataram T, Costanzo R, Scalia G, et al. Detection of "Incidentalomas" on brain and body 68Ga-DOTATOC-PET scans: A retrospective study and case illustration. Anticancer Res (2022) 42(12):5867–73. doi: 10.21873/anticanres.16095
8. Chen Z, Prasad SC, Di Lella F, Medina M, Piccirillo E, Taibah A, et al. The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg (2014) 120(6):1278–87. doi: 10.3171/2014.2.JNS131497
9. Arlt F, Kasper J, Winkler D, Jähne K, Fehrenbach MK, Meixensberger J, et al. Facial nerve function after microsurgical resection in vestibular schwannoma under neurophysiological monitoring. Front Neurol (2022) 13:850326. doi: 10.3389/fneur.2022.850326
10. Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional preservation after planned partial resection followed by gamma knife radiosurgery for Large vestibular schwannomas. World Neurosurg (2015) 84(2):292–300. doi: 10.1016/j.wneu.2015.03.012
11. Palmisciano P, Ferini G, Watanabe G, Conching A, Ogasawara C, Scalia G, et al. Surgical management of craniovertebral junction schwannomas: A systematic review. Curr Oncol (2022) 29(7):4842–55. doi: 10.3390/curroncol29070384
12. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
13. Prabawa IPY, Bhargah A, Liwang F, Tandio DA, Tandio AL, Lestari AAW, et al. Pretreatment neutrophil-to-Lymphocyte ratio (NLR) and platelet-to-Lymphocyte ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev (2019) 20(3):863–8. doi: 10.31557/APJCP.2019.20.3.863
14. Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: A meta-analysis. Med (Baltimore). (2018) 97(49):e13340. doi: 10.1097/MD.0000000000013340
15. Amaral SR, Casal Moura M, Carvalho J, Chaves A, Jesus E, Sousa G. 6P prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in non-small cell lung cancer (NSCLC) treated with immune checkpoint inhibitors. Ann Oncol (2019) 30(suppl 1). doi: 10.1093/annonc/mdz027.004
16. Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, et al. Combined diagnostic efficacy of neutrophil-to-Lymphocyte ratio (NLR), platelet-to-Lymphocyte ratio (PLR), and mean platelet volume (MPV) as biomarkers of systemic inflammation in the diagnosis of colorectal cancer. Dis Markers. (2019) 2019:6036979. doi: 10.1155/2019/6036979
17. Jung MR, Park YK, Jeong O, Seon JW, Ryu SY, Kim DY, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol (2011) 104(5):504–10. doi: 10.1002/jso.21986
18. Kontorinis G, Crowther JA, Iliodromiti S, Taylor WA, Locke R. Neutrophil to lymphocyte ratio as a predictive marker of vestibular schwannoma growth. Otol Neurotol (2016) 37(5):580–5. doi: 10.1097/MAO.0000000000001026
19. Zhang J, He M, Liu Z, Song Y, Wang Y, Liang R, et al. Impact of neutrophil-lymphocyte ratio on long-term outcome in patients with craniopharyngioma. Med (Baltimore). (2018) 97(37):e12375. doi: 10.1097/MD.0000000000012375
20. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol (2018) 11(2):444–9. doi: 10.1016/j.tranon.2018.01.010
21. Marques P, de Vries F, Dekkers OM, van Furth WR, Korbonits M, Biermasz NR, et al. Pre-operative serum inflammation-based scores in patients with pituitary adenomas. Pituitary (2021) 24(3):334–50. doi: 10.1007/s11102-020-01112-5
22. Kuranari Y, Tamura R, Tsuda N, Kosugi K, Morimoto Y, Yoshida K, et al. Prognostic significance of preoperative neutrophil-to-Lymphocyte ratio in patients with meningiomas. Front Oncol (2020) 10:592470. doi: 10.3389/fonc.2020.592470
23. Regan MM, Werner L, Rao S, Gupte-Singh K, Hodi FS, Kirkwood JM, et al. Treatment-free survival: A novel outcome measure of the effects of immune checkpoint inhibition-a pooled analysis of patients with advanced melanoma. J Clin Oncol (2019) 37(35):3350–8. doi: 10.1200/JCO.19.00345
24. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst (2014) 106(6):dju124. doi: 10.1093/jnci/dju124
25. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev (2015) 41(10):971–8. doi: 10.1016/j.ctrv.2015.10.003
26. Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med (2019) 8(9):4135–48. doi: 10.1002/cam4.2281
27. Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, et al. Prognostic nutritional index (PNI) predicts tumor recurrence of very Early/Early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol (2015) 22(13):4138–48. doi: 10.1245/s10434-015-4516-1
28. Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, et al. Preoperative prognostic nutritional index predicts long-term surgical outcomes in patients with esophageal squamous cell carcinoma. World J Surg (2018) 42(7):2199–208. doi: 10.1007/s00268-017-4437-1
29. Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol (2014) 140(9):1537–49. doi: 10.1007/s00432-014-1714-3
30. Li B, Li J, Miao W, Zhao Y, Jiao J, Wu Z, et al. Prognostic analysis of clinical and immunohistochemical factors for patients with spinal schwannoma. World Neurosurg (2018) 120:e617–27. doi: 10.1016/j.wneu.2018.08.135
31. de Vries M, Briaire-de Bruijn I, Malessy MJ, de Bruïne SF, van der Mey AG, Hogendoorn PC. Tumor-associated macrophages are related to volumetric growth of vestibular schwannomas. Otol Neurotol (2013) 34(2):347–52. doi: 10.1097/MAO.0b013e31827c9fbf
32. de Vries WM, Briaire-de Bruijn IH, van Benthem PPG, van der Mey AGL, Hogendoorn PCW. M-CSF and IL-34 expression as indicators for growth in sporadic vestibular schwannoma. Virchows Arch (2019) 474(3):375–81. doi: 10.1007/s00428-018-2503-1
33. Hannan CJ, Lewis D, O'Leary C, Donofrio CA, Evans DG, Roncaroli F, et al. The inflammatory microenvironment in vestibular schwannoma. Neurooncol Adv (2020) 2(1):vdaa023. doi: 10.1093/noajnl/vdaa023
34. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol (2017) 14(7):399–416. doi: 10.1038/nrclinonc.2016.217
35. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol (2009) 86(5):1065–73. doi: 10.1189/jlb.0609385
36. Tamura R, Morimoto Y, Sato M, Kuranari Y, Oishi Y, Kosugi K, et al. Difference in the hypoxic immunosuppressive microenvironment of patients with neurofibromatosis type 2 schwannomas and sporadic schwannomas. J Neurooncol (2020) 146(2):265–73. doi: 10.1007/s11060-019-03388-5
37. Lewis D, Roncaroli F, Agushi E, Mosses D, Williams R, Li KL, et al. Inflammation and vascular permeability correlate with growth in sporadic vestibular schwannoma. Neuro Oncol (2019) 21(3):314–25. doi: 10.1093/neuonc/noy177
38. Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am (2012) 45(2):257–2. doi: 10.1016/j.otc.2011.12.008
39. Carlson ML, Habermann EB, Wagie AE, Driscoll CL, Van Gompel JJ, Jacob JT, et al. The changing landscape of vestibular schwannoma management in the united states–a shift toward conservatism. Otolaryngol Head Neck Surg (2015) 153(3):440–6. doi: 10.1177/0194599815590105
Keywords: schwannoma, neutrophil-to-lymphocyte ratio, neurofibromatosis type 2, prognostic factor, retreatment
Citation: Takahara K, Tamura R, Kuranari Y, Karatsu K, Akiyama T and Toda M (2023) Prognostic significance of preoperative neutrophil-to-lymphocyte ratio in surgically resected schwannomas. Front. Oncol. 13:1099384. doi: 10.3389/fonc.2023.1099384
Received: 15 November 2022; Accepted: 30 January 2023;
Published: 10 February 2023.
Edited by:
Arianna Rustici, University of Bologna, ItalyReviewed by:
Liemei Guo, Shanghai Jiao Tong University, ChinaGianluca Ferini, Rem Radiotherapy, Italy
Copyright © 2023 Takahara, Tamura, Kuranari, Karatsu, Akiyama and Toda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryota Tamura, bW9sdG9iZWxsby1yLTYxMEBob3RtYWlsLmNvLmpw
 Kento Takahara
Kento Takahara Ryota Tamura
Ryota Tamura Yuki Kuranari2
Yuki Kuranari2 Takenori Akiyama
Takenori Akiyama Masahiro Toda
Masahiro Toda