- 1Department of Hepatic Surgery and Liver Transplantation Center, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Guangdong Province Key Laboratory of Liver Disease Research, Guangzhou, China
- 3Department of Anesthesiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 4Surgical Intensive Care Unit, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background: The purpose of this study is to evaluate the effects of chemotherapy and radiotherapy on the prognosis of unresectable HCC patients with portal and/or hepatic vein invasion.
Methods: A retrospective analysis of unresectable HCC patients with portal and/or hepatic vein invasion registered in the Surveillance, Epidemiology, End Results (SEER) database was performed. The propensity score-matching (PSM) method was used to balance differences between groups. Overall survival (OS) and cancer-specific survival (CSS) were the interesting endpoints. OS was calculated from the date of diagnosis to the date of death caused by any cause or the last follow-up. CSS was defined as the interval between the date of diagnosis and date of death due only to HCC or last follow-up. OS and CSS were analyzed by using Kaplan-Meier analysis, Cox proportional hazards model, and Fine-Gray competing-risk model.
Results: A total of 2,614 patients were included. 50.2% patients received chemotherapy or radiotherapy and 7.5% patients received both chemotherapy and radiotherapy. Compared to the untreated group, chemotherapy or radiotherapy (COR) (HR = 0.538, 95% CI 0.495-0.585, p < 0.001) and chemotherapy and radiotherapy (CAR) (HR = 0.371, 95% CI 0.316-0.436, p < 0.001) showed better OS. In the COR group, Cox analysis results showed AFP, tumor size, N stage and M stage were independent risk factor of OS. Competing-risk analysis results showed AFP, tumor size and M stage were independent risk factor of CSS. In the CAR group, AFP and M stage were independent risk factors of OS. Competing-risk analysis results showed M stage were independent risk factor of CSS. Kaplan Meier analysis showed chemotherapy combined with radiotherapy significantly improves OS (10.0 vs. 5.0 months, p < 0.001) and CSS (10.0 vs. 6.0 months, p = 0.006) than monotherapy.
Conclusion: AFP positive and distant metastasis are the main risk factors affecting OS and CSS of unresectable HCC patients with portal and/or hepatic vein invasion. Chemotherapy combined with radiotherapy significantly improves OS and CSS of unresectable HCC patients with portal and/or hepatic vein invasion.
1 Introduction
Hepatocellular carcinoma (HCC) is the most common type of hepatic malignant tumor in the world, with a high mortality rate. Approximately 10% to 40% of HCC patients show macrovascular invasion at the time of initial diagnosis (1). There are two distinct types of macrovascular invasion: portal vein tumor thrombosis (PVTT) and hepatic vein tumor thrombosis (HVTT) (2). It has been reported that the incidence of macrovascular invasion is more than doubled in patients with hepatocellular carcinoma compared with cirrhotic patients without malignancy(24.4% vs 11.4%; p = 0.05) (3). HCC with macrovascular invasion is listed in advanced stage by the American Association for the Study of Liver Diseases/Barcelona-Clinic Liver Cancer (AASLD/BCLC) staging system and treatment recommendations. However, there is no consensus on the diagnosis and treatment criteria for hepatocellular carcinoma combined with PVTT/HVTT, which brings great difficulties to the selection of treatment regimens and the prediction of treatment effects.
In recent years, hepatectomy, local interventional therapy, radiotherapy, molecular targeted therapy and immunotherapy have been widely used, which is of great significance for the quality of life and prolonging survival time of some patients. Currently, Sorafenib or Lenvatinib are recommended as first-line TKI treatment for HCC combined with PVTT (4). Several studies have shown that the combination of TKI and ICI can significantly improve the survival benefits of patients with advanced HCC (5–7), so combination therapy has become the latest trend in the treatment of advanced HCC. These studies have also shown that not all HCC patients can benefit from the combination therapy, one of the reasons is likely to be some patients with primary immune resistance.
Transarterial chemoembolization (TACE) and hepatic artery infusion chemotherapy (HAIC) have become the most commonly used palliative treatments for patients with unresectable HCC and are no longer considered an absolute contraindication for patients with PVTT/HVTT- HCC (8). Moreover, advanced radiotherapy techniques have yielded encouraging results in HCC patients with PVTT/HVTT (9, 10). For all that, current studies on chemotherapy or radiotherapy in patients with concomitant PVTT/HVTT-HCC are limited to single-center, small studies, so our study aimed to use the national-scale SEER database for a retrospective analysis to investigate the effects of chemotherapy and radiotherapy regimens on the survival of patients with PVTT/HVTT- HCC.
2 Materials and methods
2.1 Data source
The SEER registry is a multicentric database for cancer research. This database contains data from 17 areas of the United States, representing roughly 28% of the total U.S. population. Patient demographics, initial cancer location, illness grade/stage, surgery, radiotherapy, chemotherapy, and survival status can all be obtained from the SEER database.
2.2 Patient screening
We use the PICO principle to filter data, P (Patient): We selected patients diagnosed with HCC from 2004 to 2015 in the SEER database according to the International Classification of Diseases for Oncology. The inclusion criteria were as follows (1): a clear histopathological diagnosis of HCC (2); the primary site of the tumor is located in the liver with only one primary tumor (3); tumor invades the portal or hepatic veins and has not been treated surgically (4); follow-up for more than one month (5); age over 18 years old (6); complete survival data. The screening process is shown in Figure 1. I (Intervention): We chose whether to receive radiotherapy or chemotherapy as the intervention (this was recorded as yes or no in the SEER database). C (Comparison): We compared patients who received radiotherapy or chemotherapy alone with those who received radiotherapy combined with chemotherapy. O (Outcome): Overall survival (OS) and cancer-specific survival (CSS) were the interesting endpoints. T (time): The deadline for follow-up is December 31, 2022.
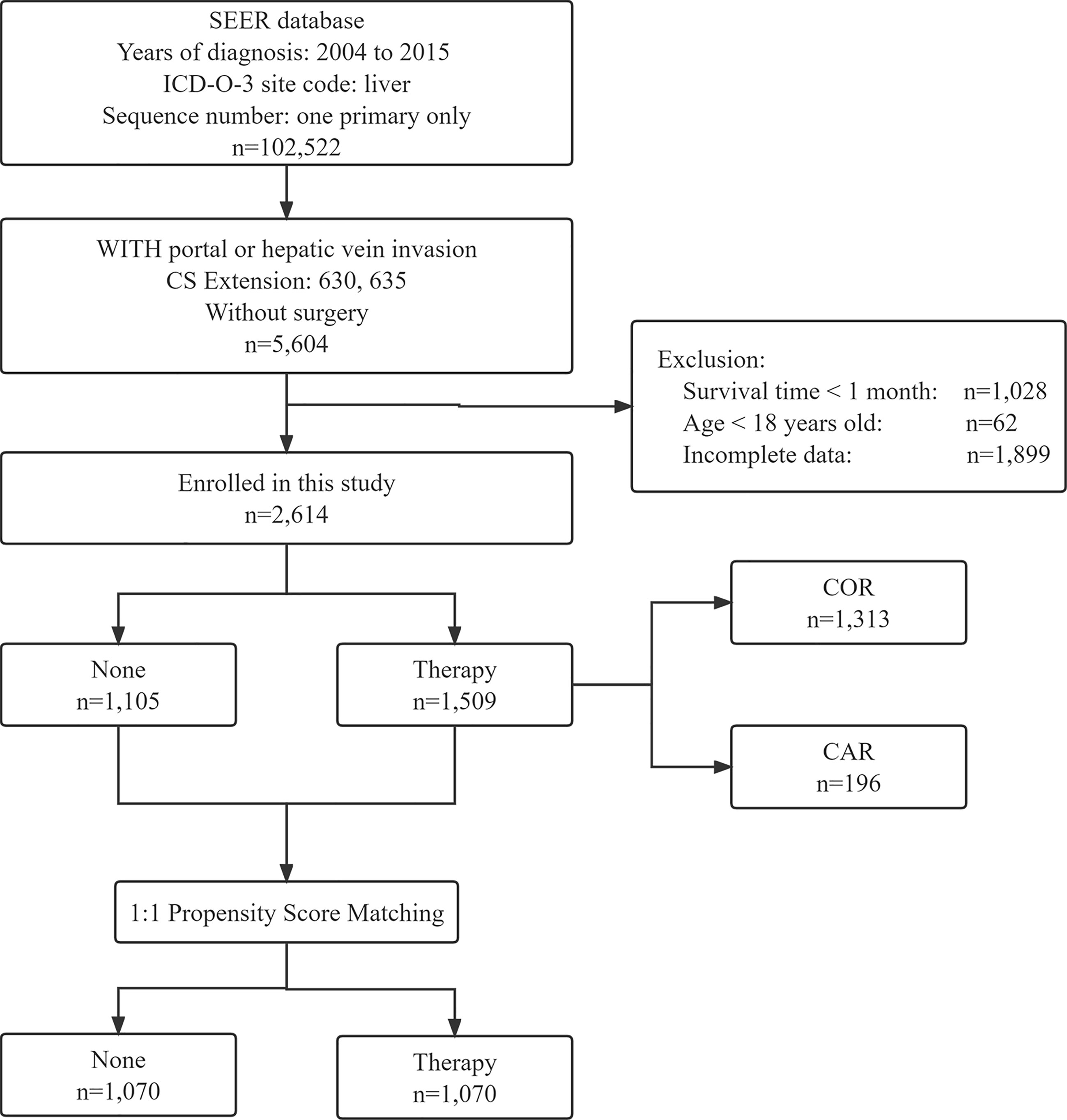
Figure 1 Flow chart showing the inclusion and exclusion process of patients in our study. SEER, Surveillance; Epidemiology, End Results; COR, Chemotherapy or Radiotherapy; CAR, Chemotherapy and Radiotherapy.
2.3 Clinical variables of patients
Demographic variables include age, sex, race, marital status, AFP, tumor size, SEER stage (SEER-specific), N and M stage, TNM, radiotherapy, chemotherapy, SEER classification of causes of death, months of survival, and final status. Since SEER stage, M stage and TNM results are the same, we only show the results of M stage.
2.4 Statistical analysis
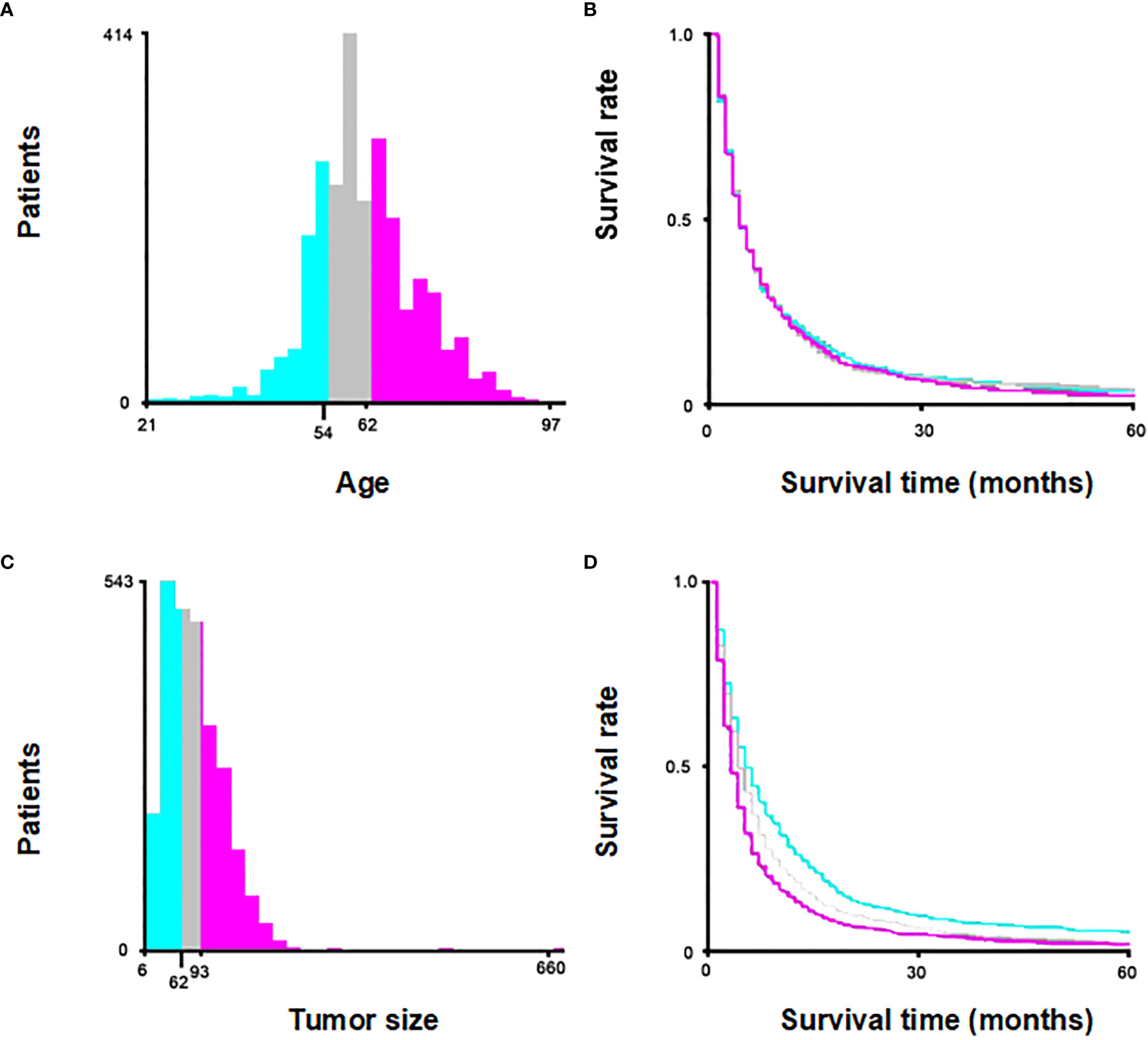
Age and tumor size were categorically divided based on the optimal cut-off value generated by X-tile software version 3.6.1 (Yale University School of Medicine, US) (Figure 2). Categorical variables were expressed as a number (percent, %) and compared by the chi-square test. In order to minimize the effect of confounding factors when comparing between two groups, a 1:1 propensity score-matching (PSM) method was used to match with None and Therapy group. The nearest-neighbor matching algorithm without replacement was applied to ensure suitable matches. The Kaplan-Meier analysis was used to generate cumulative survival curves, and the log-rank test was used to compare the differences. Univariate and multivariate survival analyses were performed using Cox proportional hazards models, and hazard ratios (HRs) were computed with 95% confidence intervals (CIs). Fine-Gray competing-risk model was used to estimate the cumulative incidence of CSS by the “cmprsk” R package. Univariate and multivariate analysis were performed using the Fine-Gray competing-risk model to identify independent risk factors affecting CSS. The statistical analyses were performed using R software version 4.2.0. A p-value < 0.05 indicated statistical significance.
3 Results
3.1 Clinical basic characteristics
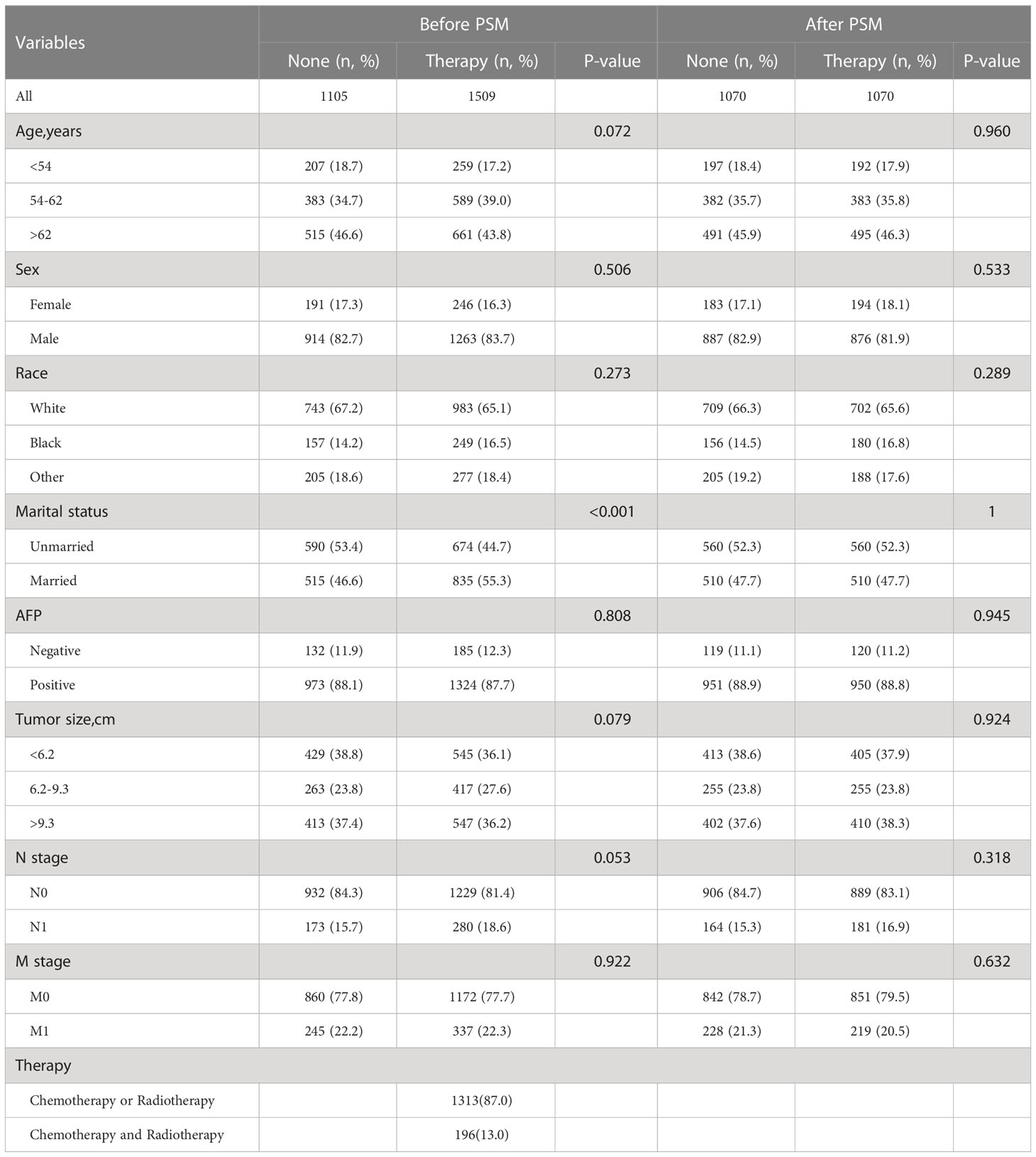
A total of 2,614 patients with HCC were extracted from the SEER database. 1105 were not treated with chemotherapy and/or radiotherapy and 1509 were treated with chemotherapy and/or radiotherapy, of which 1313 patients received chemotherapy or radiation therapy and 196 patients received both chemotherapy and radiotherapy. About 45.0% patients are > 62 years old and predominantly male. Among our subjects, the white population had a significantly higher incidence of HCC than other races. Interestingly, the majority of the unmarried are more inclined to do no treatment at all, while the married opted for chemotherapy or radiotherapy. Most patients were AFP positive and without lymph node and distant metastases. Of the patients under treatment, nearly 90% received chemotherapy or radiotherapy, and very few received both chemotherapy and radiotherapy. More details are shown in Table 1.
After grouping according to whether or not to receive treatment, using PSM for age, sex, race, marital status, AFP, tumor size, N stage, and M stage for 1:1 proximity matching, all variables in the two matched groups were not significantly different after PSM. The Kaplan-Meier analysis showed OS (p < 0.001) and CSS (p < 0.001) of the matched pre- and post-treatment groups were superior to the untreated group (Figure 3).
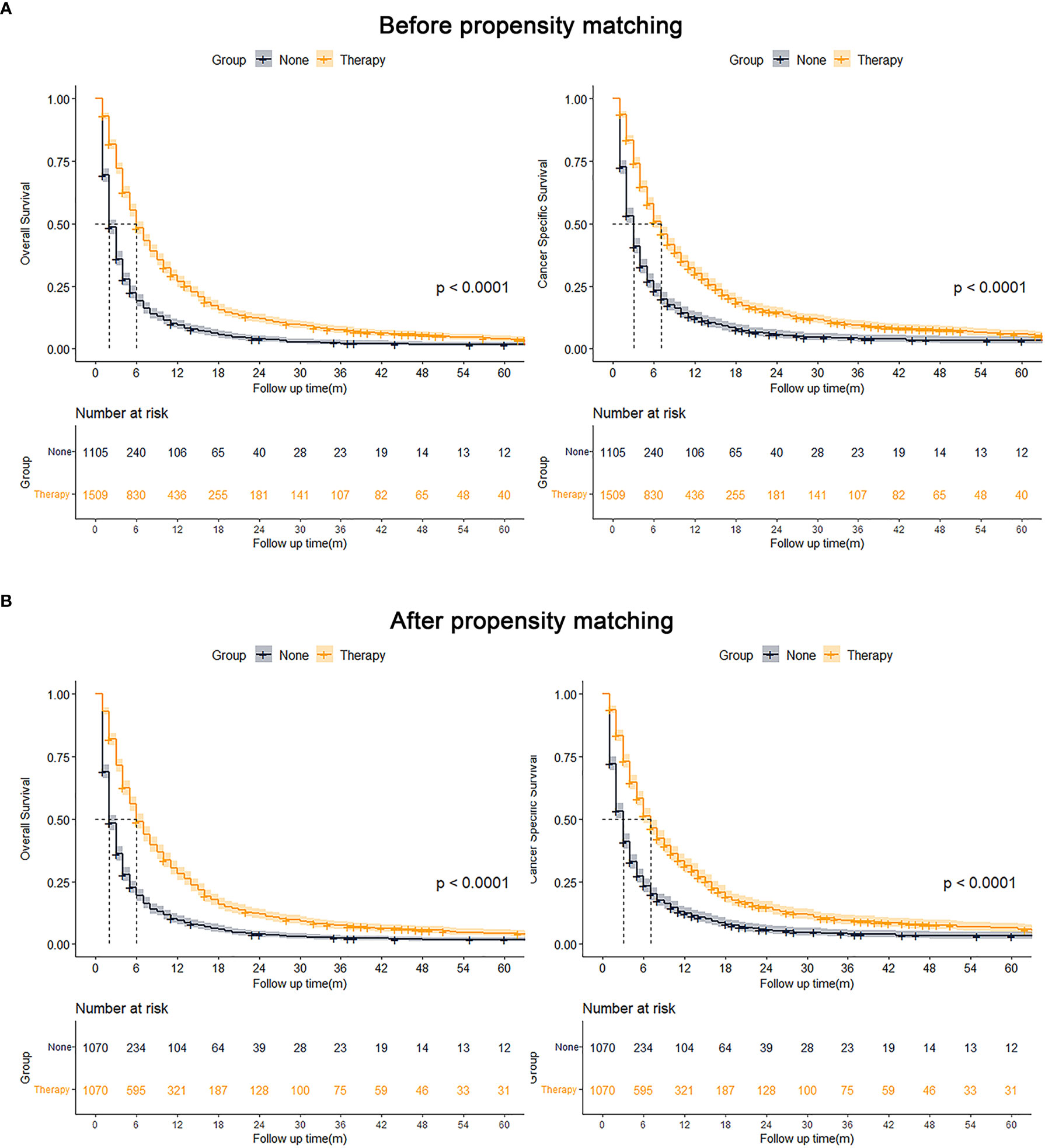
Figure 3 Kaplan-Meier analysis results for OS and CSS before (A, left, OS; right, CSS) and after (B, left, OS; right, CSS) PSM of all patients.
3.2 Survival analysis of all patients
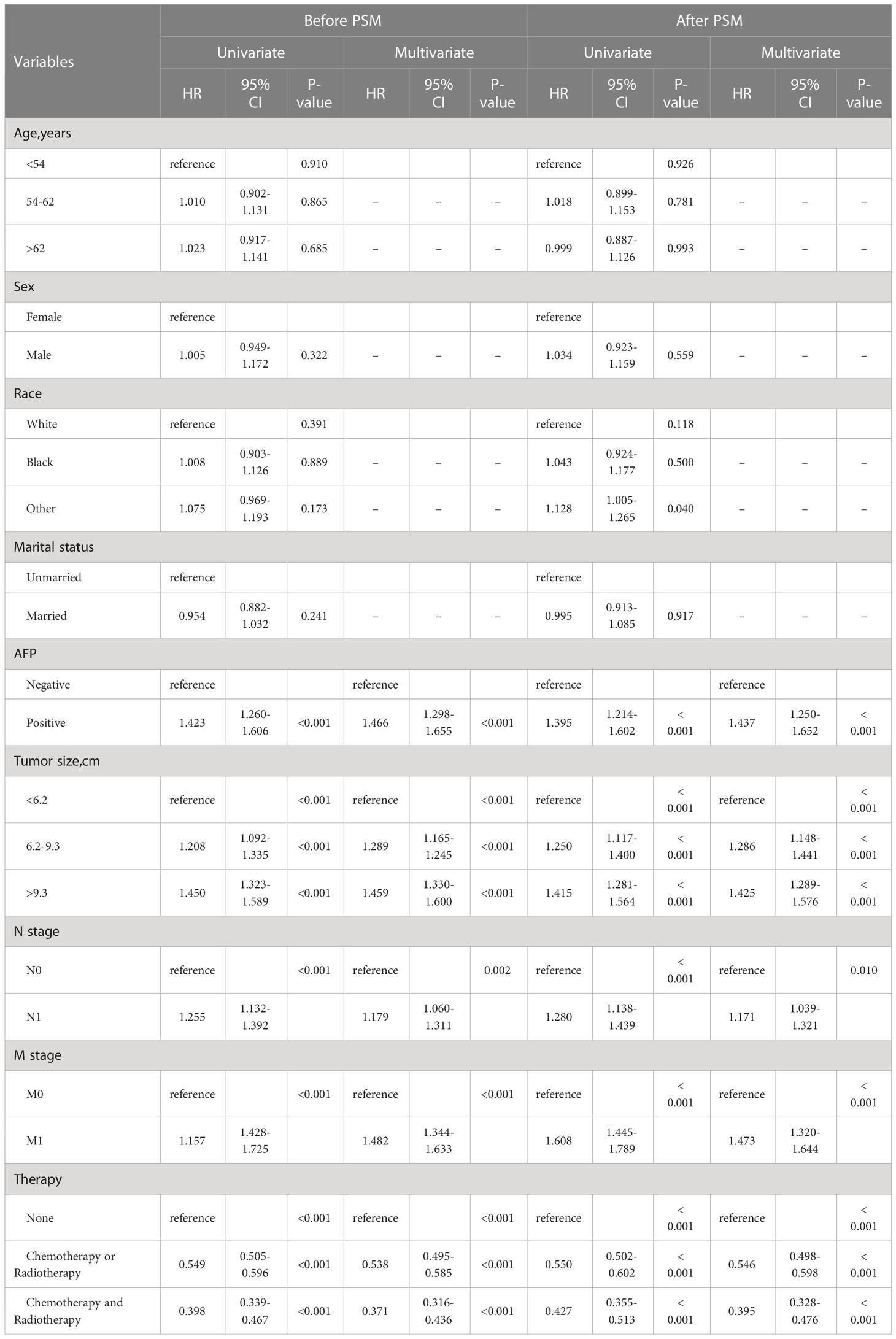
Univariate and multivariate Cox analysis results of all patients before PSM showed AFP, tumor size, N stage, M stage, and treatment were considered as independent predictors of OS. Especially in terms of treatment modality, patients who received COR (HR = 0.538, 95% CI 0.495-0.585, p < 0.001) and CAR (HR = 0.371, 95% CI 0.316-0.436, p < 0.001) showed better OS compared to receive no treatment (Table 2). The same results are also shown after PSM (Table 2).
3.3 Survival analysis of patients receiving treatment
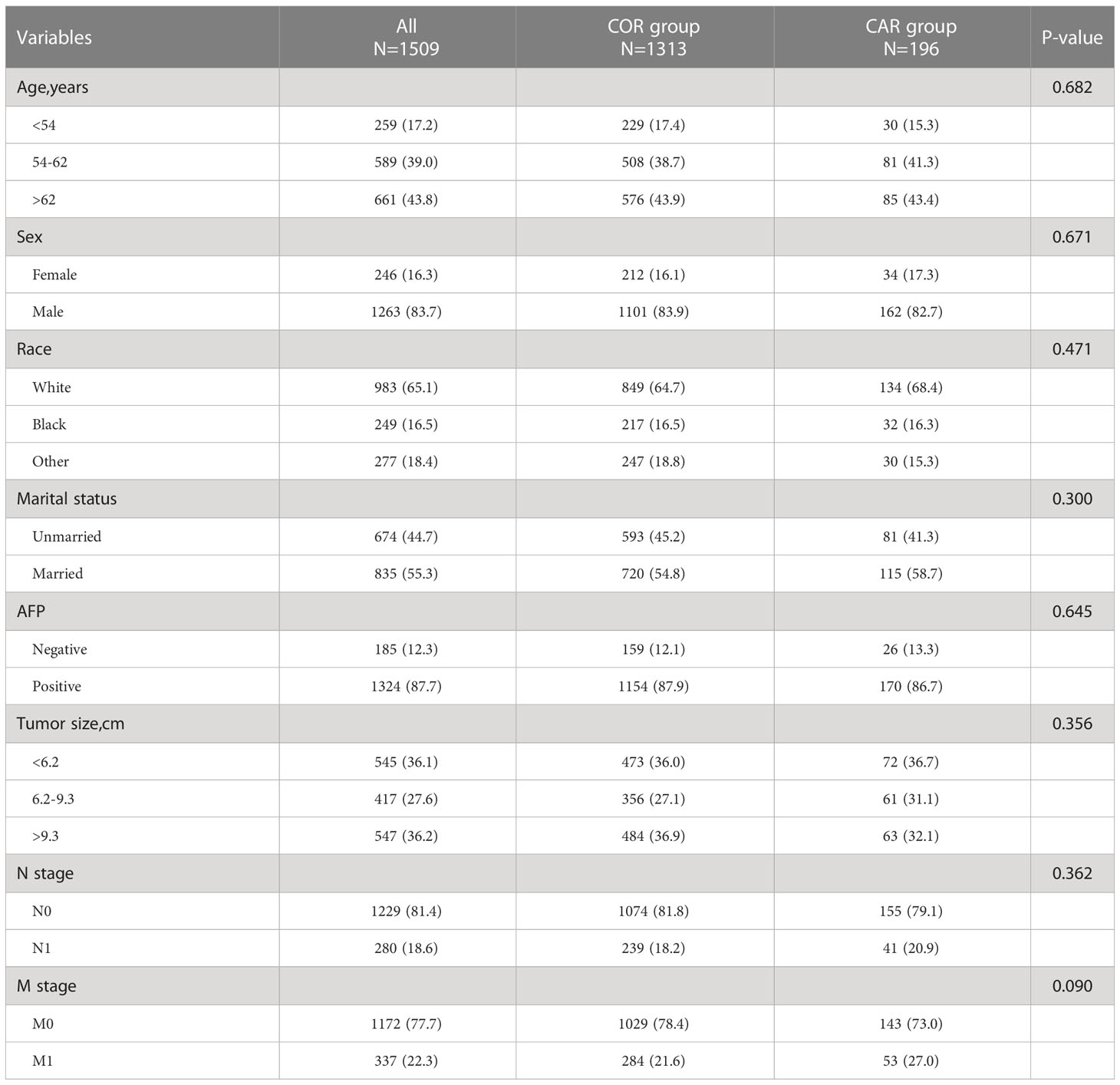
We further explored the impact of different treatment strategies (COR and CAR) on patient survival. There were no significant differences between the COR and CAR groups, so PSM matching was not needed (Table 3). Kaplan-Meier analysis showed CAR had better OS (10 vs. 5 months, p <0.001) (Figure 4A) and CSS (10 vs. 6 months, p <0.001) (Figure 4B) than the COR. The 1-, 3-, and 5-year cumulative cancer-specific death risks were 62.9%, 89.6%, and 93.4% in the CAR group, compared with 75.3%, 93.5%, and 96.4% in the COR group. (Figure 4).
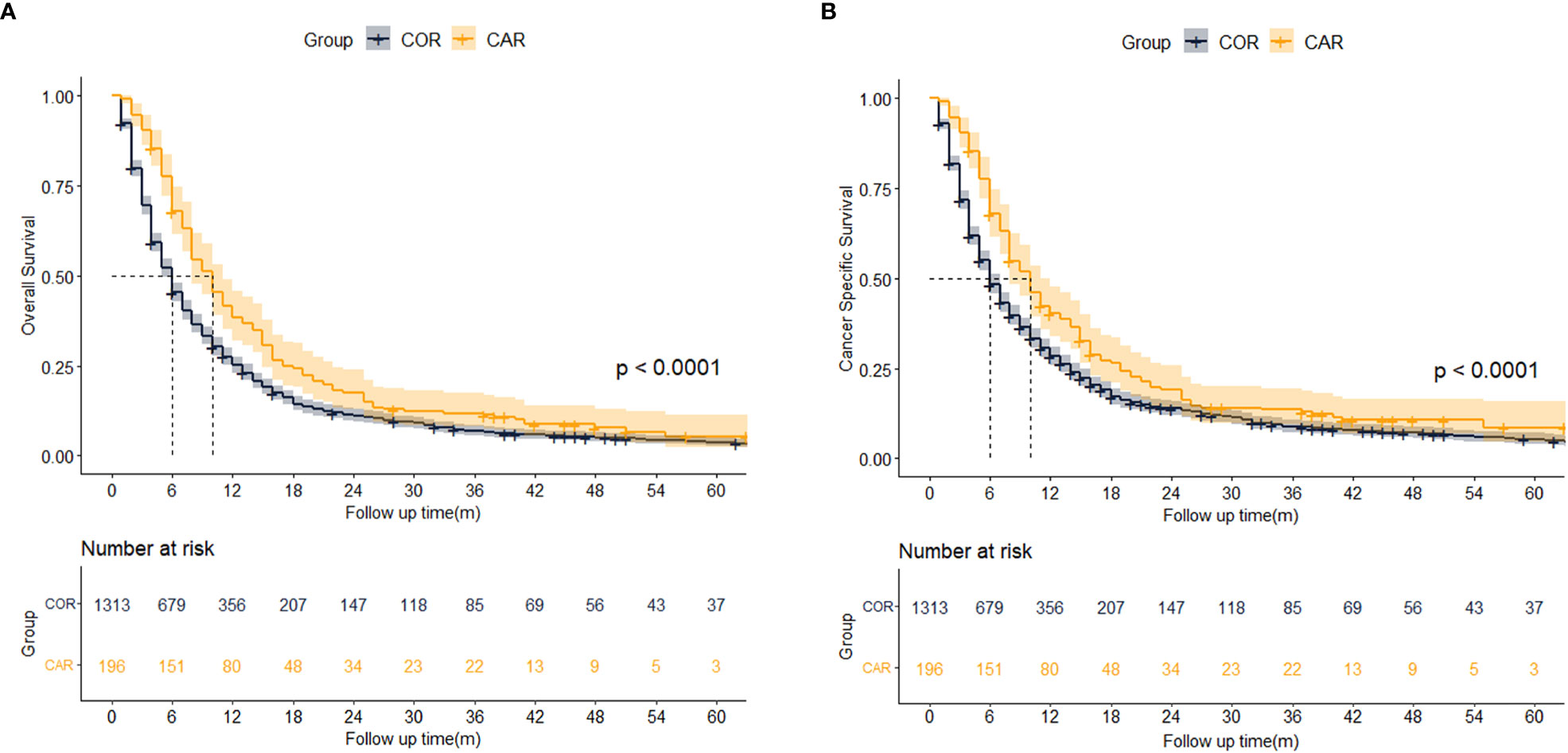
Figure 4 Kaplan-Meier analysis results for OS (A) and CSS (B) between COR and CAR. COR (Blue): Chemotherapy or Radiotherapy; CAR (Yellow): Chemotherapy and Radiotherapy.
Univariate and multivariate Cox analysis results showed AFP (HR = 1.454, 95% CI 1.241-1.74, p < 0.001), N stage (HR = 1.195, 95% CI 1.041-1.372, p = 0.012) M stage (HR = 1.797, 95% CI 1.577-2.048, p < 0.001) were independent risk factors for OS. Compared with tumor size < 6.2 cm, 6.2-9.3 cm (HR = 1.265, 95% CI 1.110-1.442, p < 0.001) and > 9.3 cm (HR = 1.528, 95% CI 1.351-1.729, p < 0.001) had worse OS. Patients who received CAR had better OS than COR. (HR = 0.657, 95% CI 0.561-0.769, p < 0.001) (Table 4). Univariate and multivariate competing-risk analysis results showed AFP (HR = 1.445, 95% CI 1.243-1.680, p < 0.001), M stage (HR = 1.623, 95% CI 1.404-1.877, p < 0.001) were independent risk factors for CSS. Compared with tumor size < 6.2 cm, tumor size > 9.3 cm (HR = 1.522, 95% CI 1.340-1.729, p < 0.001) had worse CSS. Patients who received CAR had better CSS than COR. (HR = 0.786, 95% CI 0.688-0.897, p < 0.001) (Table 4).
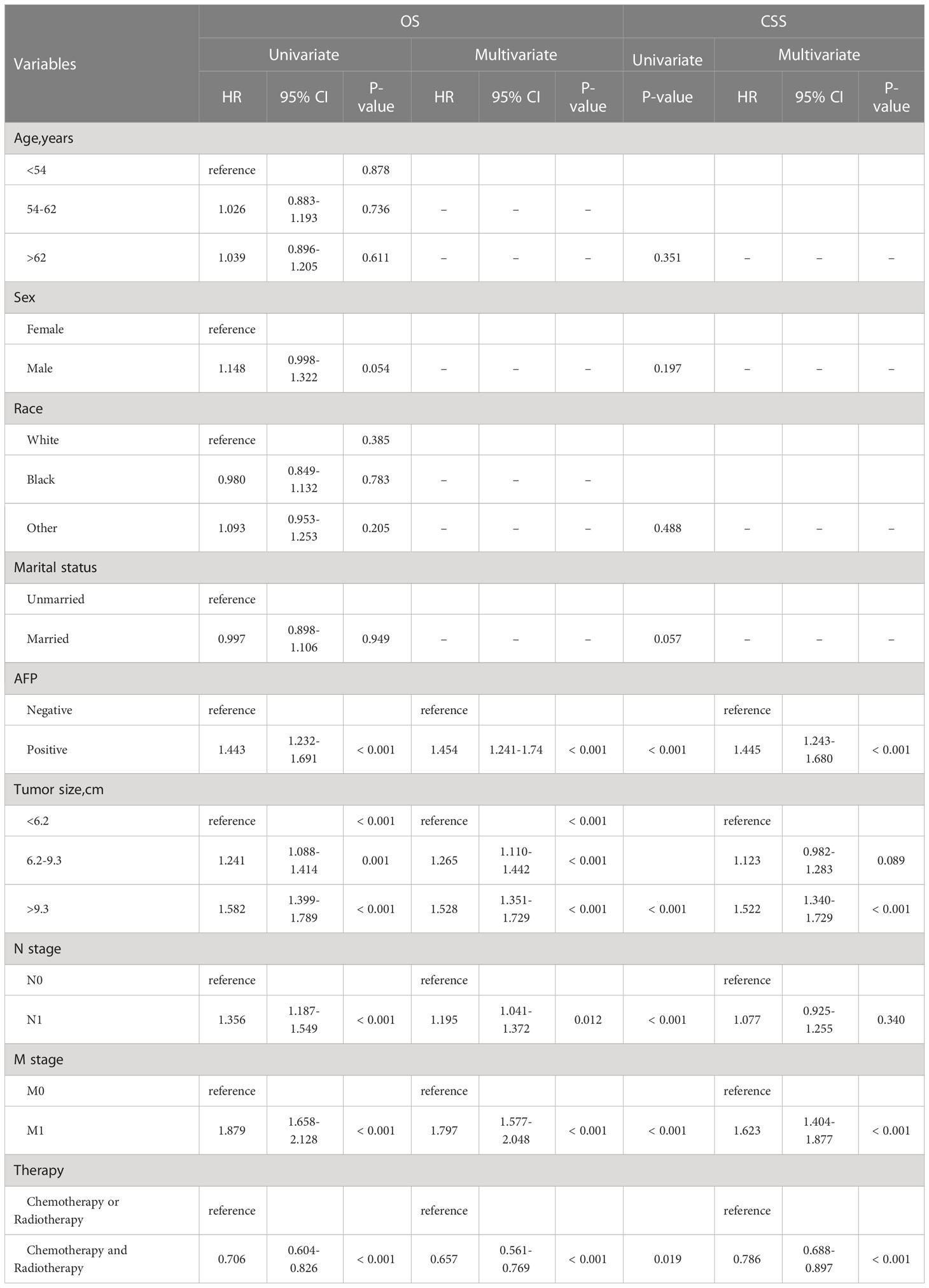
Table 4 Univariate and multivariate Cox analysis of OS and Competing-risk analysis for all eligible patients.
3.4 Survival analysis of different treatment strategies
To identify the effect of various factors on different treatment groups, univariate and multivariate analyses of OS and CSS were performed in each group. In the COR group, univariate and multivariate Cox analysis results showed AFP (HR = 1.416, 95% CI 1.195-1.679, p < 0.001), tumor size (6.2-9.3 cm: HR = 1.258, 95% CI 1.093-1.448, p = 0.001; > 9.3 cm HR = 1.602, 95% CI 1.405-1.827, p < 0.001), N stage (HR = 1.240, 95% CI 1.069-1.440, p = 0.005), M stage (HR = 1.786, 95% CI 1.550-2.058, p < 0.001) were independent risk factor of OS. Univariate and multivariate competing-risk analysis results showed AFP (HR = 1.420, 95% CI 1.205-1.670, p < 0.001), tumor size > 9.3 cm (HR = 1.520, 95% CI 1.330-1.740, p < 0.001), M stage (HR = 1.580, 95% CI 1.347-1.850, p = 0.005) were independent risk factor of CSS (Table S1). In the CAR group, AFP (HR = 1.765, 95% CI 1.132-2.752, p = 0.012) and M stage (HR = 1.693, 95% CI 1.205-2.379, p = 0.002) were independent risk factors of OS. Univariate and multivariate competing-risk analysis results showed M stage (HR = 2.038, 95% CI 1.486-2.790, p < 0.001) were independent risk factors of CSS (Table S2).
When the two groups of patients were stratified by different variables, Kaplan-Meier analysis showed age > 62 (10.0 vs. 5.0 months, p<0.001), male (9.0 vs. 5.0 months, p<0.001), the white (10.0 vs. 7.0 months, p=0.017), other race (11.0 vs. 3.0 months, p = 0.003), married (9.0 vs. 9.0 months, p<0.001), AFP positive (9.0 vs. 5.0 months, p <0.001), tumor size > 9.3 cm (8.0 vs. 4.0 months, p<0.001)), N1 (9.0 vs. 4.0 months, p<0.001), N1 (10.0 vs. 6.0 months, p=0.006),M0 (11.0 vs. 6.0 months, p = 0.002), and M1 (6.0 vs. 6.0 months, p = 0.001) had better OS in the CAR group, while no significant differences were seen within the other groups (Figure 5A). The same result is also showed in CSS except for the white (Figure 5B).

Figure 5 Median survival time of OS (A) and CSS (B) between COR and CAR in different subgroups. COR (Blue): Chemotherapy or Radiotherapy; CAR (Yellow): Chemotherapy and Radiotherapy.
4 Discussion
Macrovascular invasion has been recognized as one of the most important adverse prognostic factors affecting the long-term survival of patients with HCC (11). And most patients have already lost the chance of radical surgery by the time of diagnosis. Chemotherapy and radiotherapy techniques are now increasingly used for patients with unresectable PVTT/HVTT-HCC. However, there is a lack of multicenter, large-scale studies on the optimal choice of chemotherapy combined with radiotherapy versus mono-therapy, which makes it difficult to make clinical treatment decisions.
Traditionally, HVTT-HCC and PVTT-HCC have been treated with approximately the same strategy. Untreated patients with concomitant macrovascular invasion have a survival rate of just 2-4 months (12). In this study, when analysing all patients, both before and after PSM matching, it was found that patients who did not receive therapy had an OS of 2 months and a CSS of 3 months, while those who received chemotherapy and/or radiotherapy had an OS of 6 months and a CSS of 7 months, showing that chemotherapy and radiotherapy can significantly improve patients’ survival.
At present, chemotherapy modalities mainly include traditional systemic chemotherapy and hepatic artery infusion chemotherapy (HAIC). Traditional systemic chemotherapy is limited in clinical application due to severe systemic adverse reactions, while HAIC is widely used because it can carry anti-cancer drugs directly to the tumor site and reduce systemic adverse reactions. HAIC was first proposed by Japanese researchers and recommended by Japanese guidelines as the standard treatment for PVTT-HCC. Most of these HAIC-based studies focused on patients with PVTT-HCC, and 5-fluorouracil and systemic interferon or cisplatin were reported to be the most effective combination chemotherapy for PVTT-HCC with a median survival time of approximately 7 months (13, 14). The results of a phase III clinical study conducted by Lyu et al. showed that the objective response rate of the FOLFOX⁃HAIC protocol for the treatment of advanced HCC with high tumor burden (52.3% of patients had portal vein tumor thrombus) reached 31.5% (RECIST criteria), and 12.3% of the patients were successfully converted (15). Wu et al. investigated the effect of TACE combined HAIC versus TACE alone on patient survival and showed that the TACE combined HAIC had a longer median OS than the TACE group (P < 0.05) (16). Ahn YE et al. compared the effect of sorafenib and HAIC on survival in patients with PVTT and found no significant difference in OS (6.4 vs. 10.0 months, P = 0.139), but HAIC had a longer time to tumor progression (TTP) (6.2 vs. 2.1 months, P = 0.006) and higher disease control rate (DCR) (76% vs 37%, P = 0.001) (17).
Conventional radiotherapy is not suitable for HCC because the liver is a radiation-sensitive viscus and the lack of precise irradiation of the target area usually involves the surrounding normal liver tissue, leading to an increased incidence of radiation liver disease. However, with the advent of new radiation technologies such as 3-Dimensional Conformal Radiation Therapy(3D-CRT), Stereotactic Body Radiation Therapy (SBRT), and proton beam therapy (PBT), the clinical application of external radiotherapy for liver cancer is becoming more widespread. Previous studies reported that the OS of patients with PVTT/HVTT-HCC could be appropriately prolonged from 6 to 18 months regardless of radiotherapy modality (18–21). A retrospective study found that PBT improved local control and survival with PVTT-HCC, with a median progression-free survival time (PFS) of 2.3 years. Two of the patients lived 4.3 and 6.4 years, respectively, without relapse and severe adverse reactions (22). Shui et al. reported 70 patients with PVTT-HCC treated with SBRT. The median follow-up period was 9.5 months (range 1.0-21.0 months). Median survival time was 10.0 months (95%CI, 7.7-12.3 months) and overall survival rate (OS) at 6 and 12 months was 67.3% and 40.0%, Patients who respond well to radiation generally have better survival (23). The results of this study also found that either chemotherapy or radiotherapy prolonged patients’ OS from 2 to 6 months and CSS from 3 to 7 months, which is consistent with the results of previous studies.
For the treatment strategy of chemotherapy combined with radiotherapy, previous single-center, small sample size studies have found that HAIC combined with radiotherapy was effective in extending the OS of patients with PVTT from 7.5 months to 12 months (24–26). In a study with only a small sample size, the treatment efficiency of HVTT could be increased from 37% to 79% when HAIC was combined with radiotherapy (27). A Japanese study showed that surgical resection was safe and long-term beneficial for patients with PVTT after downstaging treatment though chemotherapy combined radiotherapy (28). In our study, chemotherapy combined with radiotherapy was found to be effective in prolonging patients’ OS (10 vs. 5 months, P < 0.001) and CSS (10 vs. 6 months, P < 0.001) compared to mono-therapy. It can be seen that this combined therapy strategy can better prolong the OS and CSS of patients and benefit them.
Next, we explored the prognostic factors affecting patients with concomitant PVTT/HVTT. In the survival analysis of all patients, we found that AFP, tumor size, N and M stage, and therapy strategy were independently associated with patient OS. To further explore the impact of different treatment strategies (COR and CAR) on patient survival. We used Cox proportional hazards model and competing-risk model to analyze OS and CSS in the whole treatment group and in each group, and found that AFP and distant metastasis were always the main independent factors affecting OS and CSS regardless of therapy strategy, which is consistent with previous findings (19, 25, 29, 30).
In 2017, based on the CheckMate 040 study, the FDA approved the PD-1 inhibitor (Nivolumab) for advanced HCC patients who did not respond to sorafenib therapy, marking the arrival of the era of HCC immunotherapy (31). Subsequently, the combination of PD-L1 inhibitor (atezolizumab) and vascular endothelial growth factor (VEGF) inhibitor (bevacizumab) proved to be the first drug with significantly better efficacy than sorafenib in more than a decade, ushering in a new era of combination therapy for HCC immunotherapy (5). At present, the main program of combined therapy with ICI is CTLA-4 inhibitor combined with PD-1/PD-L1 inhibitor. The current second-line regimen of nabuliumab in combination with CTLA-4 inhibitor (ipilimumab) is the best option, based on results from Cohort 4 in CheckMate 040. The combination regimen with the highest dose of ipilimumab showed the best efficacy, but also a higher incidence of adverse reactions (32).
The latest results of KEYNOTE-524, a phase I single-arm trial, showed that the mOS, mPFS and ORR of Lenvatinib combined with Pembrolizumab for advanced HCC were 22 months, 8.6 months and 36.0%, this combination regimen has been granted by the US FDA as a breakthrough therapy for the first-line treatment of unresectable HCC (33). In recent years, a number of ICI-based combination therapies have achieved good results in clinical trials, benefiting more patients with end-stage HCC, especially those with PVTT/HVTT.
Obviously, our study also has several limitations. First of all, this study is a retrospective study, and selection bias caused by incomplete data is inevitable. Although we adopted PSM to avoid selection bias, potential confounding factors cannot be ruled out. Second, because the SEER database only records yes or no for chemotherapy and radiotherapy, the lack of detailed information on chemotherapy and radiotherapy makes it difficult to conduct further studies, which also bodes well for more precise studies of the impact of each treatment on patient benefit. Undeniably, with the development of targeted and immunotherapy in recent years, the treatment of HCC has undergone earth-changing changes, greatly improving the survival of patients with advanced HCC. Unfortunately, the SEER database did not record information of targeted or immunotherapy for patients from 2004 to 2015. So we put it up in limitation; Finally, portal vein tumor thrombus and hepatic vein tumor thrombus were uniformly recorded in the SEER database as macrovascular invasion, which could not be studied separately. Even so, we demonstrated the benefit of radiotherapy combined with chemotherapy for long-term survival in PVTT/HVTT-HCC patients using a country-scale database.
5 Conclusion
It is recommended that surgically unresectable advanced PVTT/HVTT-HCC be treated with radiotherapy combined with chemotherapy. Positive AFP and distant metastasis are risk factors affecting patients’ long-term survival, and it is suggestive to actively treat these patients to prolong the survival of patients.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XQ, JC, and HC contributed to the conception and design of the study. JY, CX, RL, JX, JZha, XS, and TL collected the details of the patients and extracted and analyzed the data. XQ, JC, and HC drafted the manuscript. JZhe, YZ, and YY contributed with a critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China. (Grant/AwardNumber:81900597); Natural Science Foundation of Guangdong Province, China. (Grant/AwardNumber:2021A1515012136); National Natural Science Foundation of China. (Grant/AwardNumber:81802897); Natural Science Foundation of Guangdong Province, China. (Grant/AwardNumber:2021A1515011156); National Natural Science Foundation of China. (Grant/AwardNumber:82100693); National Natural Science Foundation of China. (Grant/AwardNumber:81901943); National Natural Science Foundation of China. (Grant/AwardNumber:81770648); National Natural Science Foundation of China. (Grant/AwardNumber:81970567); Natural Science Foundation of Guangdong Province, China. (Grant/AwardNumber:2021A1515010571); Natural Science Foundation of Guangdong Province, China. (Grant/AwardNumber:2018A030313043).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1098686/full#supplementary-material
References
1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet (2012) 379(9822):1245–55. doi: 10.1016/S0140-6736(11)61347-0
2. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology (2017) 66(2):510–7. doi: 10.1002/hep.29225
3. Zanetto A, Rodriguez-Kastro KI, Germani G, Ferrarese A, Cillo U, Burra P, et al. Mortality in liver transplant recipients with portal vein thrombosis - an updated meta-analysis. Transpl Int (2018) 31(12):1318–29. doi: 10.1111/tri.13353
4. Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis (2018) 38(3):242–51. doi: 10.1055/s-0038-1666805
5. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
6. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
7. Stefanini B, Ielasi L, Chen R, Abbati C, Tonnini M, Tovoli F, et al. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther (2023) 23(3):279–91. doi: 10.1080/14737140.2023.2181162
8. Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). (2017) 19(8):659–66. doi: 10.1016/j.hpb.2017.04.016
9. Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer (2005) 103(11):2419–26. doi: 10.1002/cncr.21043
10. Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-pacific patients with hepatocellular carcinoma. J Clin Oncol (2018) 36(19):1913–21. doi: 10.1200/JCO.2017.76.0892
11. Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery (2005) 137(4):403–10. doi: 10.1016/j.surg.2004.12.012
12. Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol (2006) 12(47):7561–7. doi: 10.3748/wjg.v12.i47.7561
13. Itamoto T, Nakahara H, Tashiro H, Haruta N, Asahara T, Naito A, et al. Hepatic arterial infusion of 5-fluorouracil and cisplatin for unresectable or recurrent hepatocellular carcinoma with tumor thrombus of the portal vein. J Surg Oncol (2002) 80(3):143–8. doi: 10.1002/jso.10116
14. Ikeda M, Okusaka T, Furuse J, Mitsunaga S, Ueno H, Yamaura H, et al. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol (2013) 72(2):463–70. doi: 10.1007/s00280-013-2222-x
15. Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol (2022) 40(5):468–80. doi: 10.1200/JCO.21.01963
16. Wu Z, Gao J, Zhuang W, Yang J, Guo W. Efficacy and safety of transcatheter arterial chemoembolization plus hepatic arterial infusion chemotherapy in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis in the main trunk. J Cancer Res Ther (2022) 18(2):345–51.
17. Ahn YE, Suh SJ, Yim HJ, Seo YS, Yoon EL, Kim TH, et al. Comparison of sorafenib versus hepatic arterial infusion chemotherapy-based treatment for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Gut Liver. (2021) 15(2):284–94. doi: 10.5009/gnl19367
18. Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol (2014) 14:84. doi: 10.1186/1471-230X-14-84
19. Su K, Gu T, Xu K, Wang J, Liao H, Li X, et al. Gamma knife radiosurgery versus transcatheter arterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Hepatol Int (2022) 16(4):858–67. doi: 10.1007/s12072-022-10339-2
20. Lu XJ, Dong J, Ji LJ, Xiao LX, Ling CQ, Zhou J. Tolerability and efficacy of gamma knife radiosurgery on hepatocellular carcinoma with portal vein tumor thrombosis. Oncotarget (2016) 7(3):3614–22. doi: 10.18632/oncotarget.6118
21. Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PloS One (2013) 8(5):e63864. doi: 10.1371/journal.pone.0063864
22. Hata M, Tokuuye K, Sugahara S, Kagei K, Igaki H, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer (2005) 104(4):794–801. doi: 10.1002/cncr.21237
23. Shui Y, Yu W, Ren X, Guo Y, Xu J, Ma T, et al. Stereotactic body radiotherapy based treatment for hepatocellular carcinoma with extensive portal vein tumor thrombosis. Radiat Oncol (2018) 13(1):188. doi: 10.1186/s13014-018-1136-5
24. Naruto K, Kawaoka T, Kodama K, Ogawa Y, Amioka K, Yoshikawa Y, et al. Efficacy and safety of chemoradiation therapy using one-shot cisplatin via hepatic arterial infusion for advanced hepatocellular carcinoma with major macrovascular invasion: a single-arm retrospective cohort study. BMC Gastroenterol (2022) 22(1):275. doi: 10.1186/s12876-022-02359-x
25. Kosaka Y, Kimura T, Kawaoka T, Ogawa Y, Amioka K, Naruto K, et al. Hepatic arterial infusion chemotherapy combined with radiation therapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk or bilobar of the portal vein. Liver Cancer. (2021) 10(2):151–60. doi: 10.1159/000513706
26. Fujino H, Kimura T, Aikata H, Miyaki D, Kawaoka T, Kan H, et al. Role of 3-d conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Hepatol Res (2015) 45(6):607–17. doi: 10.1111/hepr.12392
27. Murakami E, Aikata H, Miyaki D, Nagaoki Y, Katamura Y, Kawaoka T, et al. Hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma in combination with or without three-dimensional conformal radiotherapy to venous tumor thrombosis in hepatic vein or inferior vena cava. Hepatol Res (2012) 42(5):442–53. doi: 10.1111/j.1872-034X.2011.00943.x
28. Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg (2017) 44:223–8. doi: 10.1016/j.ijsu.2017.06.082
29. Zhang XP, Gao YZ, Chen ZH, Chen MS, Li LQ, Wen TF, et al. An Eastern hepatobiliary surgery Hospital/Portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology (2019) 69(5):2076–90. doi: 10.1002/hep.30490
30. Wong TC, Lee VH, Law AL, Pang HH, Lam KO, Lau V, et al. Prospective study of stereotactic body radiation therapy for hepatocellular carcinoma on waitlist for liver transplant. Hepatology (2021) 74(5):2580–94. doi: 10.1002/hep.31992
31. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
32. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
Keywords: hepatocellular carcinoma, chemotherapy, radiotherapy, surveillance, epidemiology, end results database, prognosis
Citation: Qiu X, Cai J, Chen H, Yao J, Xiao C, Li R, Xiao J, Zhang J, Sui X, Lu T, Zheng J, Zhang Y and Yang Y (2023) Chemotherapy combined with radiotherapy can benefit more unresectable HCC patients with portal and/or hepatic vein invasion: a retrospective analysis of the SEER database. Front. Oncol. 13:1098686. doi: 10.3389/fonc.2023.1098686
Received: 15 November 2022; Accepted: 15 May 2023;
Published: 20 June 2023.
Edited by:
Liliana Chemello, University of Padua, ItalyReviewed by:
Antonio Giovanni Solimando, University of Bari Aldo Moro, ItalyLinda Beenet, University of California, Los Angeles, United States
Katerina Malagari, National and Kapodistrian University of Athens, Greece
Korah Pushpamangalam Kuruvilla, University of Colorado Anschutz Medical Campus, United States
Copyright © 2023 Qiu, Cai, Chen, Yao, Xiao, Li, Xiao, Zhang, Sui, Lu, Zheng, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zheng, emhlbmdqNjdAbWFpbDIuc3lzdS5lZHUuY24=; Yingcai Zhang, emhhbmd5YzNAbWFpbC5zeXN1LmVkdS5jbg==; Yang Yang, eXlzeXN1QDE2My5jb20=
†These authors have contributed equally to this work
 Xiaotong Qiu
Xiaotong Qiu Jianye Cai
Jianye Cai Haitian Chen
Haitian Chen Jia Yao
Jia Yao Cuicui Xiao2,3
Cuicui Xiao2,3 Rong Li
Rong Li Xin Sui
Xin Sui Tongyu Lu
Tongyu Lu Jun Zheng
Jun Zheng