- 1Department of Anesthesiology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Urology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 4Department of Anesthesiology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 5Department of Anesthesiology and Perioperative Medicine, The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States
- 6Department of Anesthesiology, Chi Mei Medical Center, Tainan, Taiwan
- 7School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
Background: This meta-analysis was conducted to compare cancer recurrence and survival rates in patients with bladder cancer receiving surgery under general anesthesia alone (i.e., GA group) or regional anesthesia (RA) with or without GA (i.e., RA ± GA group).
Methods: Literature search on Cochrane library, EMBASE, Google scholar, and Medline databases was performed to identify all relevant studies from inception to April 30, 2022. The primary outcome was cancer recurrence rate, while the secondary outcomes included overall survival rate and cancer-specific survival rate. Subgroup analyses were performed based on study design [(Propensity-score matching (PSM) vs. no-PSM)] and type of surgery [transurethral resection of bladder tumor (TURBT) vs. radical cystectomy].
Results: Ten retrospective studies with a total of 13,218 patients (RA ± GA group n=4,884, GA group n=8,334) were included. There was no difference between RA ± GA group and GA group in age, the proportion of males, severe comorbidities, the proportion of patients receiving chemotherapy, and the pathological findings (all p >0.05). Patients in the RA ± GA group had significantly lower rate of bladder cancer recurrence [odds ratio (OR): 0.74, 95%CI: 0.61 to 0.9, p=0.003, I2 = 24%, six studies] compared to those in the GA group. Subgroup analyses based on study design revealed a consistent finding, while the beneficial effect of RA ± GA on reducing cancer recurrence was only significant in patients receiving TURBT (p=0.02), but not in those undergoing radical cystectomy (p=0.16). There were no significant differences in overall survival rate and cancer-specific survival rate between RA ± GA and GA groups.
Conclusions: For patients receiving surgery for bladder cancer, the application of regional anesthesia with or without general anesthesia is associated with significant decrease in cancer recurrence, especially in patients undergoing TURBT for non-muscle invasive bladder cancer. Because of the limited number of studies included and potential confounding factors, our results should be interpreted carefully.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022328134.
1 Introduction
Bladder cancer is a common cancer with up to four times higher incidence in men than women (1). Cancer recurrence is still the leading cause of death in those with bladder malignancies (2). When indicated, surgery remains as the preferred therapy in non-metastatic bladder cancers (2). Cancer recurrence and survival rate are affected by many kinds of factors, such as tumor-related factors (3, 4), including circulating tumor DNA, tumor diameter, lymph node metastasis and pathological type. It has been suggested that the choice of anesthesia technique during surgery for cancer resection could affect postoperative oncological outcomes including cancer recurrence (5–7). Many mechanisms have been proposed by which anesthetics could induce immunosuppression and promote cancer metastasis (5, 7).
Regional anesthesia (RA) including nerve and neuraxial blocks with epidural or spinal anesthesia has been associated with improved long-term survival, and fewer recurrences in different cancers (8–10). Numerous amounts of retrospective studies were conducted to compare postoperative outcomes of patients receiving general anesthesia (GA) alone or regional anesthesia with or without general anesthesia (RA ± GA) (5, 8–10). The association between RA and favorable oncological outcomes is yet to be determined. Based on current available data, there are not enough evidence to support the use of RA rather than GA to decrease perioperative immunosuppression, angiogenesis, and cancer metastasis (7).
Regional anesthesia and analgesia are commonly used during bladder cancer surgeries. And it has been reported that in patients undergoing surgery for bladder cancer, RA was associated with lower postoperative cancer recurrence (11) and higher survival rates compared to GA alone (12–14). Nevertheless, when compared to GA, RA during bladder cancer surgery was reported to have worse oncological outcomes (15), increased risk of perioperative complications, higher hospital readmission rates, and longer length of hospital stay (16). Therefore, to incorporate the principle of “do no harm”, it is essential to critically investigate the role of RA in postoperative oncological outcomes after bladder cancer surgery and exclude any possible harm related to intraoperative use of RA.
This meta-analysis aims to evaluate the impact of RA, spinal or epidural, on postoperative oncologic outcomes in bladder cancer patients. We hypothesize that RA could decrease postoperative cancer recurrence in bladder cancer patients.
2 Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (17) and registered with the International Prospective Register of Systematic Reviews (CRD42022328134).
2.1 Data source and search strategy
Literature search was conducted for all relevant studies on Cochrane library, EMBASE, Google scholar, and Medline databases. The search covered all studies from inception to April 30, 2022 using the following search terms: (“Bladder cancer” or “Bladder Neoplasm*” or “Bladder Tumor*” or “Transurethral Resection of Bladder Tumor*” or “TURBT” or “Cystectomy”) and (“anesthetic technique” or “epidural” or “spinal” or “neuraxial blockade” or “regional anesthesia” or “regional analgesia” or “Extradural Anesthesia” or “Peridural Anesthesia” or “intrathecal”) and (General anesthesia*) and (“Recurrence” or “metastasis” or “Disease-Free Survival” or “Progression-Free Survival” or “Survival” or “Prognosis” or “mortality”). The search strategy for one of these databases are demonstrated in Supplemental Table 1.
2.2 Eligibility criteria
Studies are considered eligible based on the PICO (population, intervention, comparison, and outcome) format: (P) adults with bladder cancer undergoing a variety of surgeries for bladder cancer treatment, (I) regional anesthesia including spinal and epidural anesthesia with or without administration of general anesthesia (i.e., RA ± GA group), (C) general anesthesia alone with volatile or total intravenous anesthesia and (i.e., GA group) (O) recurrence rate, overall survival rate, and cancer-specific survival rate. References of the retrieved studies were also reviewed for eligibility of being included in the current study.
Only original articles with adult patients (>18years old) who underwent surgery for treatment of bladder cancer were included. Studies presented as letters, abstracts, reviews, and case reports were excluded. Studies that used conscious sedation or peripheral nerve block as the anesthetic technique were excluded. We also excluded studies in which outcomes or anesthesia techniques were not clearly described and studies without a control group. No restriction was placed on gender, study design, study location, language, and sample size during literature search.
2.3 Study selection
Study selecting as well as data collecting, and risk assessment were independently performed by two authors. First, the titles and abstracts of all retrieved articles were screened for eligibility of being incorporated into the present study. Next, the full texts of all potentially eligible studies were screened according to the inclusion and exclusion criteria. All disagreements were settled by consulting a third independent reviewer.
2.4 Data extraction
The following details were extracted from each report: year of publication, first author, characteristics of participants, sample size, type of surgery, study design [Propensity-score matching (PSM) vs. no-PSM], American Society of Anesthesiologists physical status (ASA-PS), proportion of gender, anesthetic techniques, anesthetic agents, recurrence rate, survival-related information, and information regarding histological examination and pathological Tumor-Node-Metastasis (pTNM) Staging if available. Data were extracted and assessed for applicability by two reviewers and discrepancies were settled by a third reviewer. We planned to contact the authors of the included articles if data confirmation was needed in an attempt to access the original data.
2.5 Outcomes and subgroup analysis
Patients were divided into two groups based on anesthesia technique, RA ± GA group and GA alone group. The primary outcome was cancer recurrence rate, while the secondary outcomes included overall survival rate and cancer-specific survival rate. Factors such as age, proportion of male gender, severe comorbidities, chemotherapy treatment, and findings on histological examination as well as pTNM Staging were evaluated for significancy between the 2 groups. The definitions of these factors were according to what was originally presented in included studies. Patients with severe comorbidities were defined as those with ASA-PS ≥ 3, Charlson comorbidity score ≥2, age-adjusted comorbidity index≥5, or patients with cerebral vascular disease. Additionally, a subgroup analysis was performed based on study design [PSM vs. no-PSM] and type of surgery [transurethral resection of bladder tumor (TURBT) vs. radical cystectomy].
2.6 Risk of bias assessment and certainty of evidence
The Quality in Prognostic Studies (QUIPS) tool was applied to assess the risk of bias (methodological quality) for the included studies (18–20). Studies were checked by QUIPS tool based on six domains including: study participation, study attrition, prognostic factor measurement, outcome measurement, adjustment for other prognostic factors, and statistical analysis and reporting. Each domain was assigned a low, moderate, or high risk of bias. Studies were defined as having an overall low risk of bias when all the six domains are rated as having low (or low to moderate) risk of bias.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) (21, 22) was adopted and the certainty of the evidence for our primary and secondary outcomes was assigned to four grades (i.e., high, moderate, low, and very low). Certainty of evidence was evaluated by two independent reviewers based on the probability of study limitations, publication bias, effect consistency, imprecision, and indirectness. All doubts about certainty ratings were settled through discussion.
2.7 Data synthesis and analysis
Cochrane Review Manager (RevMan 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for meta-analysis. The pooled results were presented as odds ratios (ORs) and mean difference (MD) with 95% confidence intervals (CIs), respectively. The degree of study heterogeneity was assessed with I2 statistics (23). A substantial level of heterogeneity was defined by an I2 greater than 50%. The outcomes of the analyses were evaluated using a random effects model on the basis that there is heterogeneity across the studies included in the study. We assessed the potential publication bias of the results by visually inspecting a funnel plot when we encountered ten or more trials reporting the same outcome. Sensitivity analysis was performed with a leave-one-out approach to weigh the potential influence of the data from an individual trial on the overall outcome. The level of significance was set at <0.05 for all outcome analyses.
3 Results
3.1 Study selection and characteristics
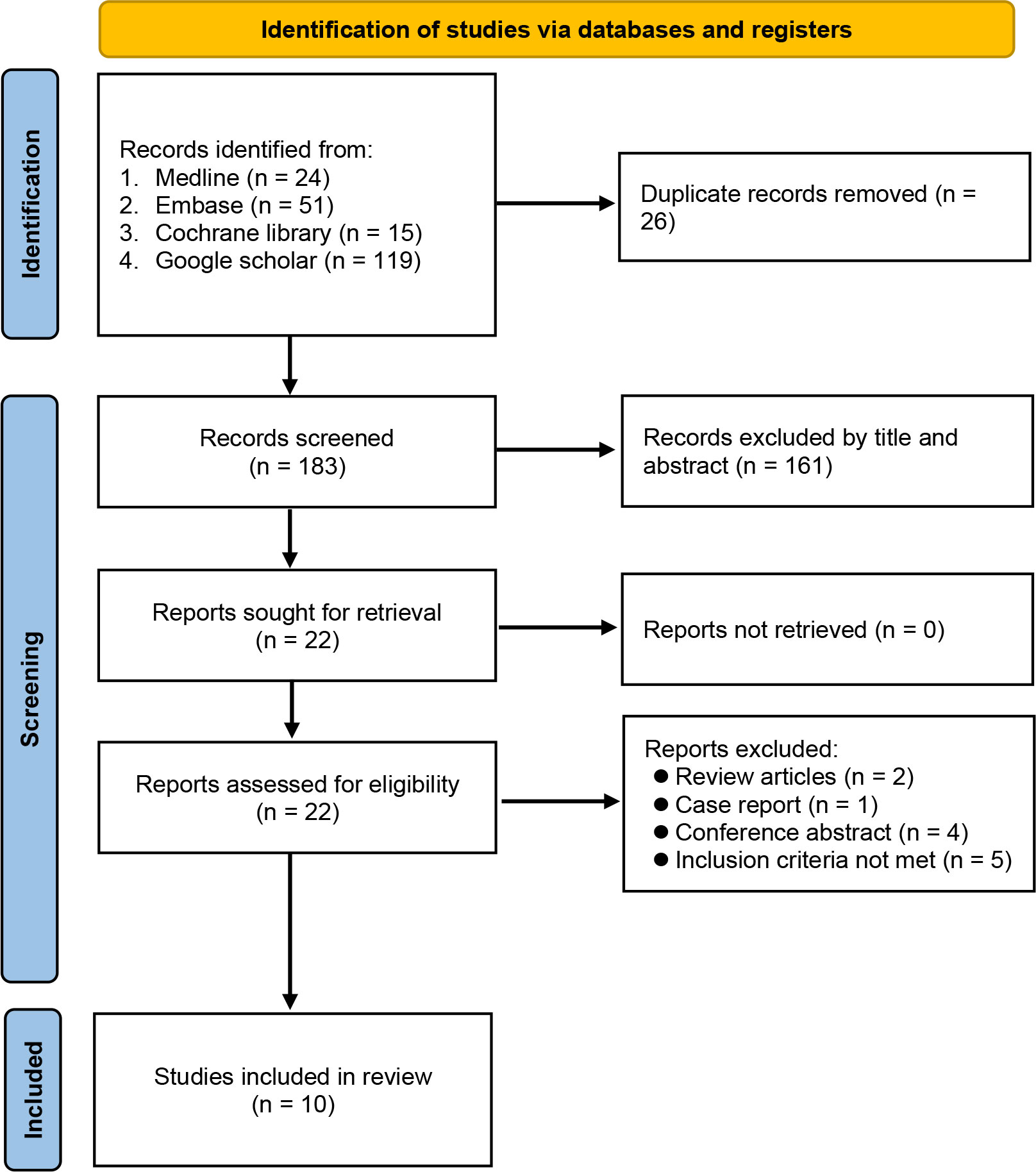
Database searching identified 209 potentially eligible records at initial search from Medline, EMBASE, Cochrane library, and Google scholar. Duplicated records (n=26) and those that did not meet the inclusion criteria by title and abstract (n=161) were excluded. Twenty-two studies with full-texts available were assessed for eligibility. After final evaluation, ten trials were included with a total of 13,218 patients (RA ± GA group n=4,884, GA group n=8,334). The included studies were published from 2016 to 2022 (5, 11–16, 24–26) and according to publication dates, six out of ten studies were published in recent five years (5, 11, 12, 14–16). A flow diagram depicting the process of study selection is demonstrated in Figure 1.
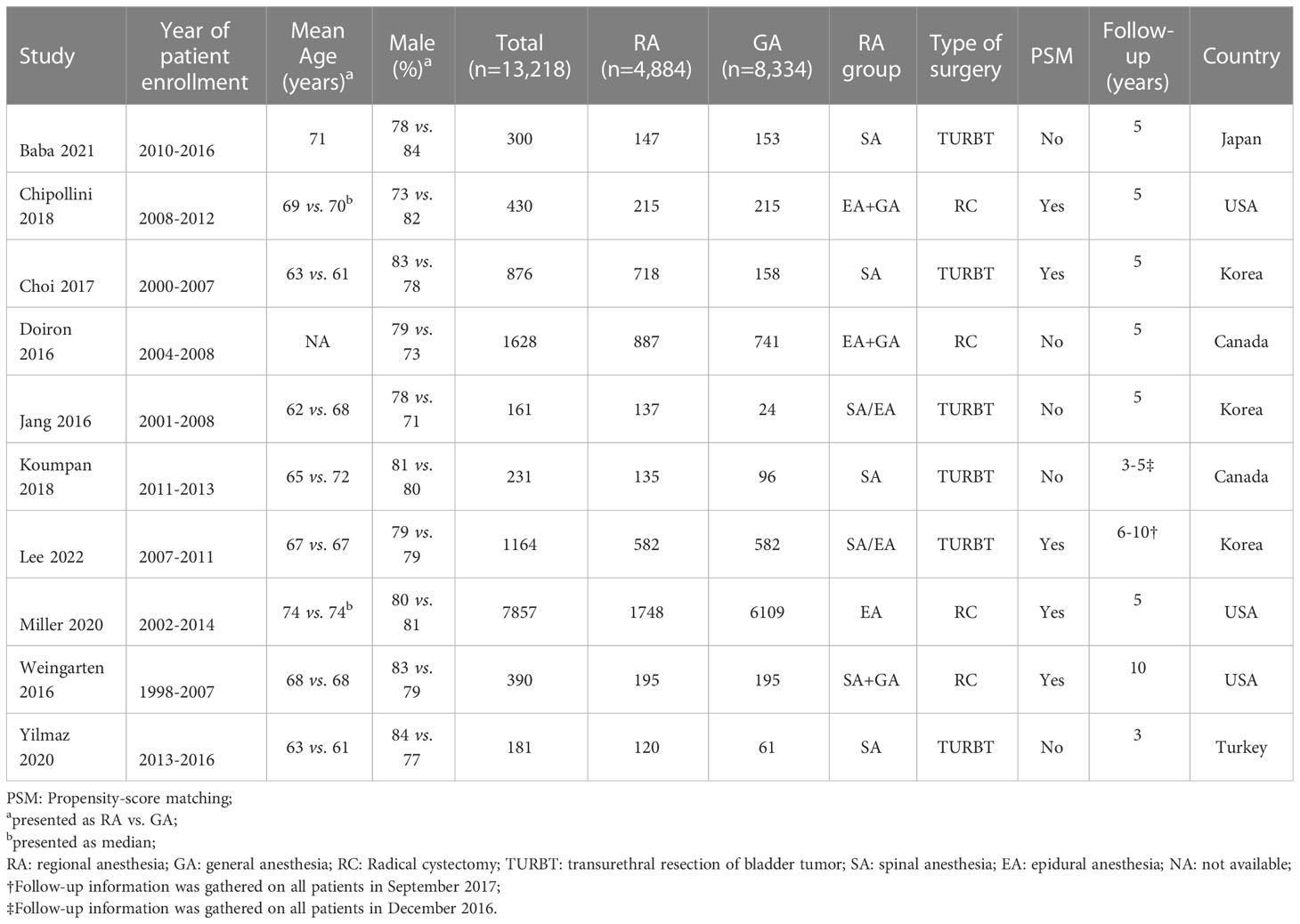
All studies were conducted in a retrospective design and study characteristics are listed in Table 1. Patients age ranged from 62 to 74 years with wide variation in sample size ranging from 161 to 7,857. The proportion of male gender was between 71% and 84%. Participants received TURBT in six studies (5, 11–14, 24), while radical cystectomy (RC) was performed in four other studies (15, 16, 25, 26). RA was provided in several ways, including spinal anesthesia in four studies (11, 12, 14, 24), epidural anesthesia in one study (16), and in two reports patients received a combination of spinal and epidural anesthesia (5, 13). A combination of GA and RA with either epidural or spinal was reported in three other studies (15, 25, 26). The use of a solution of local anesthetics only was used in four studies (12–14, 24), two studies reported the administration of opioids for RA (15, 26) and four manuscripts did not provide details on the type of anesthetics used for RA (5, 11, 16, 25). As for the GA group, five studies used volatile agents for maintenance (12–14, 24, 26), while other studies did not provide the information (5, 11, 15, 16, 25). In terms of statistical analysis, a PSM was utilized in five of the studies (5, 15, 16, 24, 26). Finally, the included 10 studies were conducted in five countries. Three studies were conducted in Korea (5, 13, 24), two in Canada (11, 25), three in USA (15, 16, 26), one in Japan (12), and one in Turkey (14).
3.2 Risk of bias assessment
The risk of bias for all enrolled studies is shown in Figure 2. Regarding study participation, moderate risk of bias was considered in five studies due to differences in patient characteristics (e.g., age or male proportion) (11–13, 15, 25). As for the other five studies, risk of bias was considered as low not only for study participation, but also study attrition, prognostic factor measurement, outcome measurement, adjustment for other prognostic factors, and statistical analysis and reporting (5, 14, 16, 24, 26). Therefore, the overall risk of bias was moderate in the five of the studies (11–13, 15, 25), and low in the other five (5, 14, 16, 24, 26).
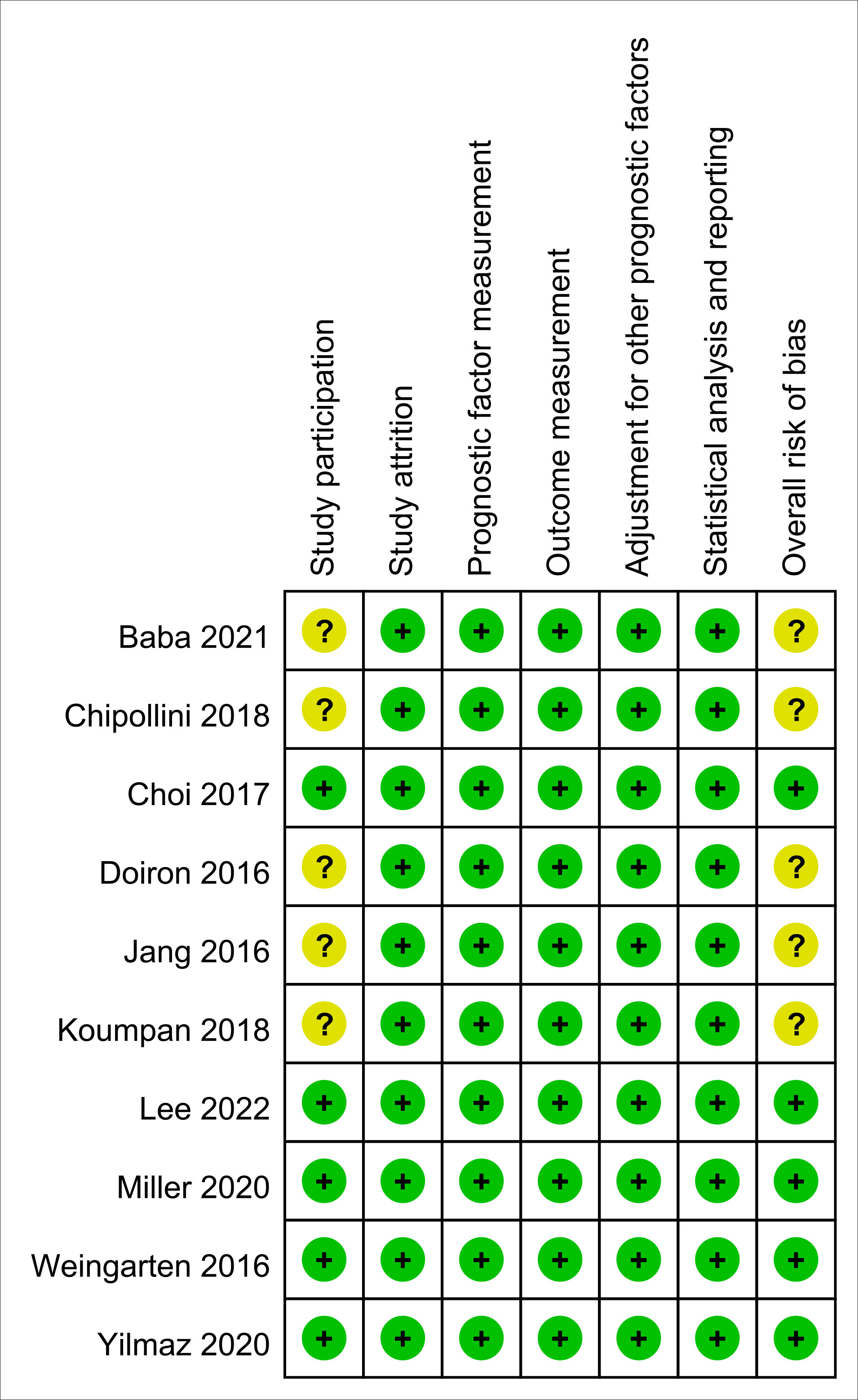
Figure 2 Risk of bias assessment based on Quality in Prognostic Studies (QUIPS) tool. Green: low risk; Yellow: moderate risk.
3.3 Results of syntheses
3.3.1 Comparison of baseline characteristics and pathological classification between combined RA ± GA group and GA group
There was no difference between RA ± GA group and GA group in age (MD=0.55 years, 95% CI: -0.7 to 1.79, p=0.39) (Figure 3A), the proportion of male gender (OR=0.94, 95% CI: 0.74 to 1.2, p=0.63) (Figure 3B), severe comorbidities (OR=1.03, 95% CI: 0.75 to 1.42, p=0.86) (Figure 3C), and the rate of patients receiving chemotherapy (OR=1.01, 95% CI:0.71 to 1.43, p=0.96) (Figure 3D). The pathological classification between both groups is demonstrated in Figure 4, which showed no difference in tumor-related parameters. Nevertheless, it appeared that the proportion of patients with muscle invasive bladder cancer (i.e., pathological staging T≥2) was higher in the GA group (i.e., 62.6%, 4777/7632) than that in the RA ± GA group (i.e., 54.7%, 2203/4030) without statistical significance.
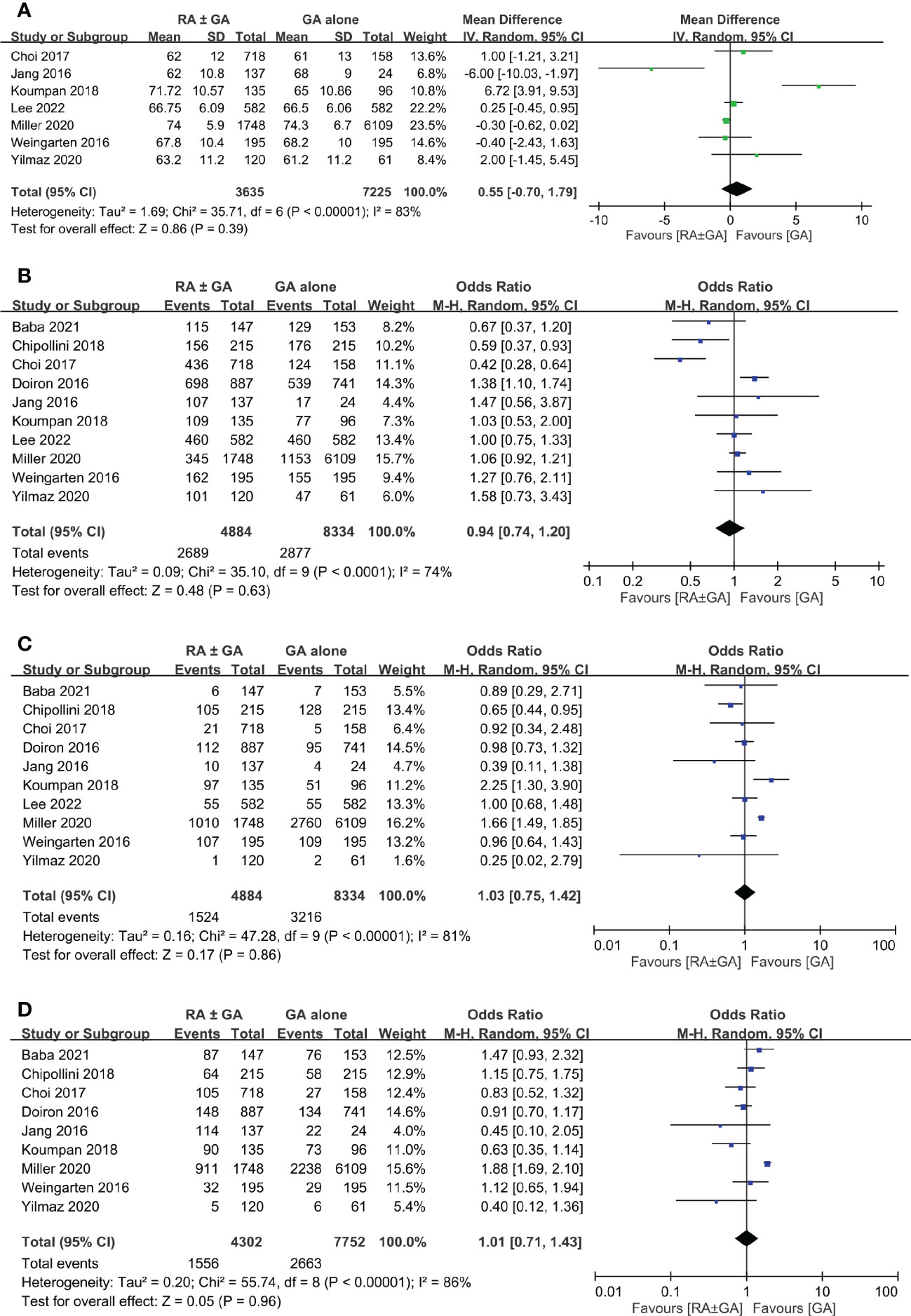
Figure 3 Forest plot showing comparison of (A) age; (B) male gender; (C) severe comorbidities; and (D) chemotherapy treatment between RA ± GA group and GA alone group. RA, regional anesthesia; GA, general anesthesia.
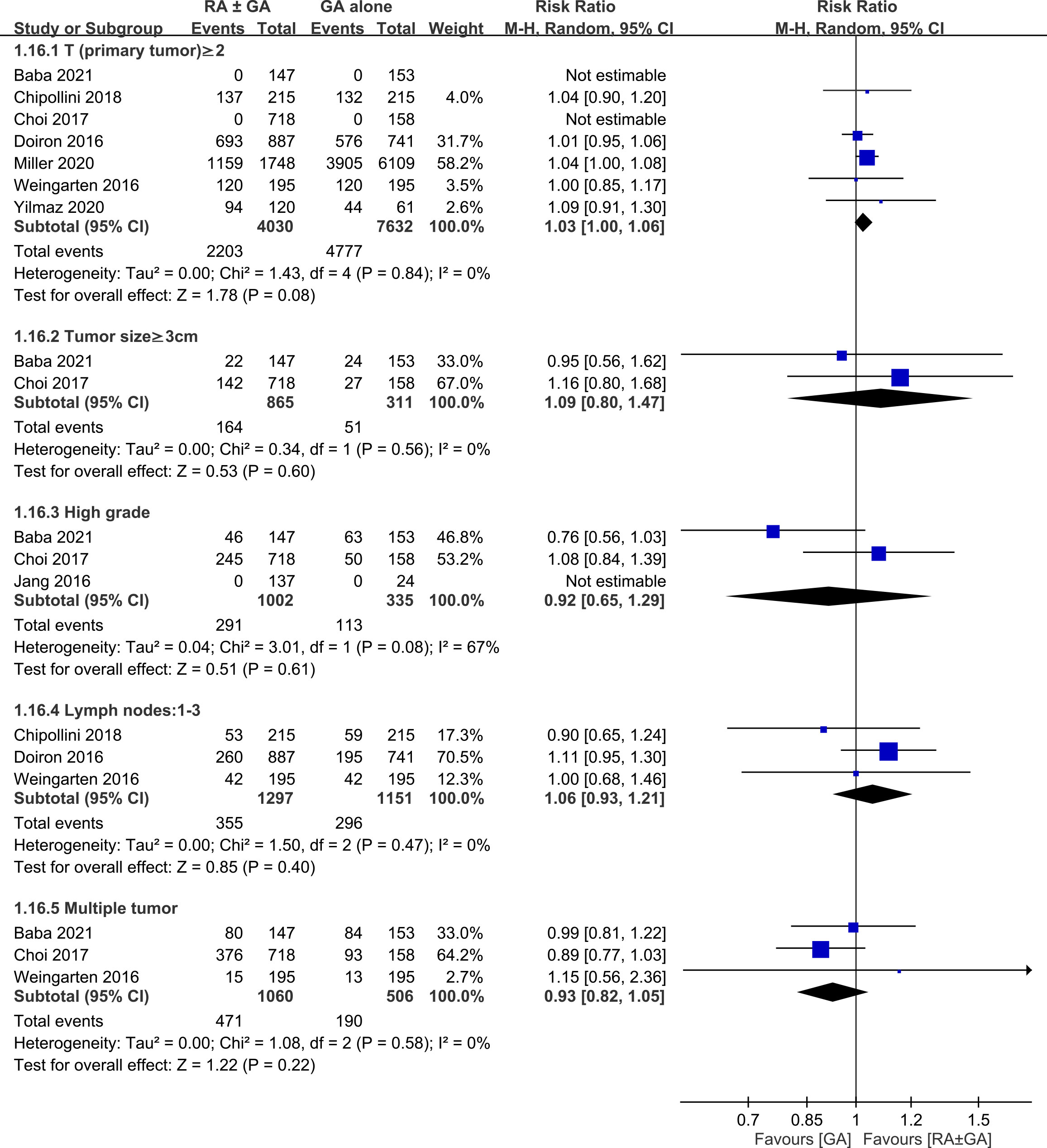
Figure 4 Forest plot showing comparison of pathological findings between RA ± GA group and GA alone group. RA, regional anesthesia; GA, general anesthesia; CI, confidence interval.
3.3.2 Primary outcome: Postoperative cancer recurrence rate
Six studies involving 3,391 patients provided the information regarding postoperative recurrence rate. Overall, the meta-analysis demonstrated a lower recurrence rate in the RA ± GA group compared to that in the GA group (OR:0.74, 95% CI: 0.61 to 0.9, p=0.003, I2 = 24%) (Figure 5A).
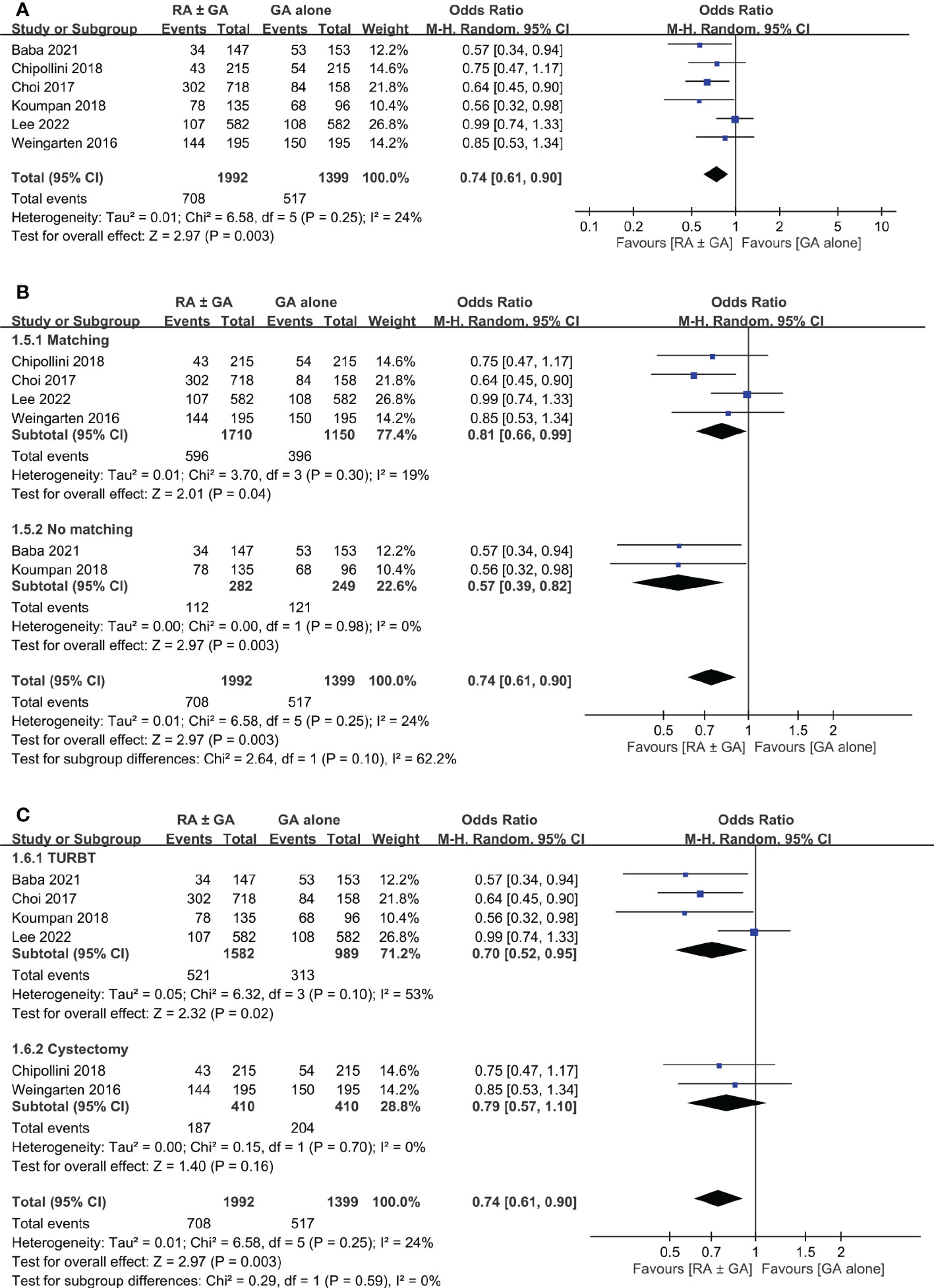
Figure 5 Forest plot showing (A) overall recurrence rate in the RA ± GA group compared to the GA alone group; (B) subgroup analysis of cancer recurrence rate based on study design; and (C) subgroup analysis of cancer recurrence rate based on type of surgery. RA, regional anesthesia; GA, general anesthesia; Matching, used propensity-score matching; No matching, Did not use propensity-score matching; TURBT, transurethral resection of bladder tumor; RC, radical cystectomy.
Subgroup analysis was conducted based on statistical analysis (PSM or not), and type of surgery (TURBT or radical cystectomy). Using PSM had no significant effect on the difference in cancer recurrence rate between the two groups (Figure 5B). However, the type of surgical intervention significantly influenced recurrence rate in RA ± GA group (Figure 5C). Specifically, the recurrence rate was lower in RA ± GA group undergoing TURBT (OR=0.7, 95% CI: 0.52 to 0.95, p=0.02, I2 = 53%), but not in those undergoing radical cystectomy (OR=0.79, 95% CI: 0.57 to 1.1, p=0.16, I2 = 0%) (Figure 5C).
3.3.3 Secondary outcomes: Overall survival rate and cancer-specific survival rate
Postoperative overall survival rate was extracted from five studies. There was no significant difference in this outcome between RA ± GA group and GA alone group (OR=1.28, 95% CI: 0.9 to 1.81, p=0.17, I2 = 46%) (Figure 6A). Three studies comprised details on postoperative cancer-specific survival rate, which again was not impacted by the type of anesthetic techniques (OR=0.83 95% CI: 0.62 to 1.09, p=0.18, I2 = 54%) (Figure 6B). In addition, one study investigating the impact of epidural anesthesia use on survival outcomes in 7857 patients undergoing radical cystectomy also reported that the use of RA was not associated with decreased overall survival rate (hazard ratio (HR): 0.99, 0.95-1.04, p=0.73) or cancer-specific survival rate (HR: 0.96, 0.90-1.02, p=0.20) (16).
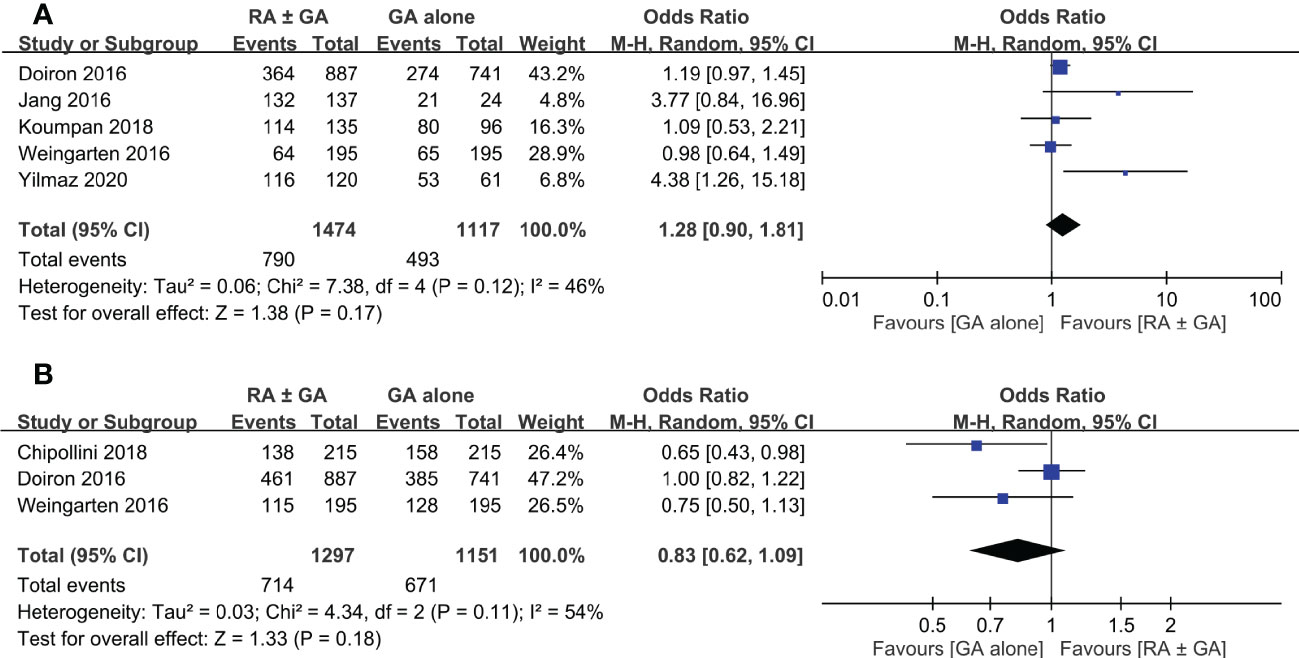
Figure 6 Forest plot showing (A) overall survival rate and (B) cancer-specific survival rate in the RA ± GA group compared to the GA alone group. RA, regional anesthesia; GA, general anesthesia.
3.4 Sensitivity analysis and evidence of GRADE
Sensitivity analysis demonstrated consistent findings on cancer-related outcomes. The quality of evidence according to the GRADE system is presented in Supplementary Table 2. The level of evidence for recurrence rate was graded as low, while the levels of evidence for both overall survival rate and cancer-specific survival rate were graded as very low. The level of evidence was downgraded due to inconsistency or imprecision.
4 Discussion
The purpose of this present meta-analysis was to evaluate the impact of neuraxial regional anesthesia on postoperative bladder cancer recurrence, overall survival, and cancer-specific survival. Based on QUIPS tool, risk of bias for included studies were low to moderate. When compared to GA alone, the use of RA ± GA was associated with significantly lower rate of cancer recurrence (p=0.003). Based on study design, the RA ± GA group had significantly lower recurrence rate in studies with (p=0.003) or without (p=0.04) adopting PSM. However, the beneficial effect of RA was only significant in patients receiving TURBT (p=0.02, I2 = 53%), but not in those undergoing radical cystectomy (p=0.16).
The choice of anesthetics was reported to influence the course of cancer progression (7, 27–29). Previous studies and meta-analysis suggested that when compared to volatile anesthesia, total intravenous anesthesia with propofol significantly improved recurrence-free survival and overall survival (30–33). The use of GA and perioperative opioids as well as even pain itself could significantly suppress the immune response and influence the proliferation of cancer cells, recurrence, and survival in numerous proposed mechanisms (7, 27–29, 34–38). Nonetheless, it has been theorized that RA could have a positive impact on postoperative oncological outcomes by attenuating the perioperative opioid,GA and surgical stress related immunosuppressive effects (5, 7, 39, 40). Several reviews and meta-analysis were conducted in the past to evaluate the effect of RA on oncological outcomes in patients undergoing cancer resection surgery (35, 39, 41–46). Still, no meta-analysis is available to investigate the impact of RA in bladder cancer solely. A previous meta-analysis had investigated the impact of RA on cancer recurrence where 30% of the included studies were in bladder cancer (41). When several cancers discussed collectively, meta-analysis results neither supported nor strongly advised against the use of RA intraoperatively (35, 41, 44, 46). To the best of our knowledge, this is the first meta-analysis for the influence of RA on postoperative oncological outcomes exclusively in bladder cancer.
Despite our current results, two recent studies suggested that RA for bladder cancer surgery was significantly associated with worse cancer and non-cancer related outcomes (15, 16). For example, one study reported that epidural anesthesia using sufentanil was associated with worse recurrence and disease-free survival (15). While epidural use in another study increased the risk of perioperative complications, hospital readmission and the duration of hospitalization, without improvement in the disease specific survival (16). These findings may not only question the benefit of RA but also alert to the potential harmful effects from using RA for bladder cancer surgery. Compared with patients undergoing TURBT who require minimal postoperative pain control with opioids, it is conceivable that the use of regional anesthesia or analgesia cannot completely obliterate the use of opioids because of the extent of surgery in those receiving radical cystectomy (47). Taking into account the finding of a dose-dependent opioid suppression of the human cellular immune system from a previous study (48), the use of opioids in patients undergoing radical cystectomy may partly explain the lack of significant beneficial impact of RA on their long-term prognosis. Moreover, in contrast to patients subjected to TURBT, those receiving radical cystectomy have a more progressive disease that may overshadow the benefits of RA. In addition, local anesthetics have shown anti-metastatic effects by several mechanisms that involves anti-angiogenic, inhibiting cell migration and invasion (29). Therefore, choosing opioid based epidural (15) or spinal analgesia (26) instead of local anesthetics, may for sure reduce the amount of intravenous opioids but it clearly does not satisfy the concept of using RA to achieve an opioid sparing anesthesia. Adverting to a very important point that we must be mindful of in future research covering the effect of anesthesia technique on postoperative cancer outcomes, which is the type of drug used for RA during cancer resection surgery.
Bladder cancers are staged based on the degree of muscle invasion (2, 49). Most bladder cancers are non-muscle invasive; therefore they can be resected for staging and histological identification by TURBT (49). Studies suggested that TURBT under RA was associated with lower cancer recurrence and better survival rate (11–13, 24). However, RA use for radical cystectomy has no significant impact on cancer recurrence, cancer-specific survival, or overall survival rates (25, 26). Our results were consistent with the findings in previous studies and the association between type of bladder surgery and the beneficial effect from RA in TURBT but not in radical cystectomy could be easily explained by disease severity, stage of cancer and invasiveness at the time of operation (50). Moreover, non-muscle invasive bladder cancer could progress to muscle-invasive cancer (51), requiring longer operation time and much complicated surgical resection with radical cystectomy, which is commonly followed by urinary diversion and neobladder reconstruction. The unfavorable effects of prolonged anesthesia on postoperative non-cancer related outcomes are not discussed in this research. However, for advanced bladder cancer requiring radical cystectomy, it is reasonable to expect higher morbidities and postoperative complications. Future reviews or clinical trials that aim to study the impact of RA or any other anesthesia technique on postoperative bladder cancer recurrence and survival rates, must consider dividing the patients in a more precise way based on cancer invasiveness or type of surgery instead of collectively placing all patients under the same heading, as bladder cancer patients.
This meta-analysis was based on retrospective studies; therefore, it is subject to several limitations. First, although our findings from meta-analysis of observational studies may be useful for identifying associations and generating hypotheses, definite causality cannot be established. Compared with observational studies, randomized controlled trials are more reliable for establishing causality due to a better control for confounding factors to minimize bias. Second, in an effort to limit possible influence by confounding factors that could affect cancer recurrence, we accounted for age, male gender, severe comorbidities, and treatment with chemotherapy. In addition, we minimized the heterogeneity in this meta-analysis by including studies focusing on bladder cancer only. However, other factors could still highly influence our results such as the difference in pathological findings (e.g., pTNM staging), surgical techniques, and the use of different medications and anesthesia techniques during regional or general anesthesia (e.g., total intravenous anesthesia or inhalation anesthesia). As shown in Figure 4, despite the lack of statistical significance, the higher proportion of patients with muscle invasive bladder cancer in the GA group than in the RA ± GA group may impair the robustness of our primary outcome. Third, since laparoscopic surgeries are being performed more frequently nowadays, postoperative outcomes after radical cystectomy could also be influenced by adopting laparoscopic or traditional open surgery. Finally, RA provides sufficient anesthesia and pain control during and after the operation and it should be conducted whenever needed. The association between RA and cancer outcomes is still controversial and needs further clinical investigation and carefully planned prospective studies. Based on our search, our results highlighted the crucial need for carefully planned research in the future to increase the level of evidence supporting the beneficial effect of RA in bladder cancer surgery.
In conclusion, this meta-analysis indicates that regional anesthesia with or without general anesthesia is associated with significantly lower recurrence rate in patients with bladder cancer undergoing transurethral resection of bladder tumor. Nevertheless, in light of the limited number of studies included in this review as well as potential confounding factors (e.g., pathological findings), the evidence is not strong enough to support definite conclusions. Until data from prospective randomized controlled trials validate or refute the findings of our research, the use of either GA or RA for bladder cancer is indicated based on patient selection or anesthesiologist’s preference.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: K-CH and S-CW. Methodology: AI, K-JY, Y-fH, and S-CW. Validation: AI, JC, S-CW, and Y-fT. Formal analysis: K-CH, AI, and Y-fT. Investigation: AI, K-JY, Y-fH, and S-CW. Data curation: AI, K-JY, Y-fH, S-CW, and JPC. Writing—original draft preparation: AI, JC, and K-CH. Writing—review and editing: AI and JC. Visualization: AI and K-CH. Supervision: S-CW, JC, and K-CH. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Chi Mei Medical Center, Tainan, Taiwan, grant number CMOR11203.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1097637/full#supplementary-material
References
1. Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, et al. Gender and bladder cancer: A collaborative review of etiology, biology, and outcomes. Eur Urol. (2016) 69:300–10. doi: 10.1016/j.eururo.2015.08.037
2. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: A review. JAMA (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
3. Chen G, Peng J, Xiao Q, Wu HX, Wu X, Wang F, et al. Postoperative circulating tumor DNA as markers of recurrence risk in stages II to III colorectal cancer. J Hematol Oncol (2021) 14:80. doi: 10.1186/s13045-021-01089-z
4. Luo X, Wu A. Analysis of risk factors for postoperative recurrence of thyroid cancer. J BUON. (2019) 24:813–8.
5. Lee SW, Tae BS, Choi YJ, Yoon SM, Lee YS, Kim JH, et al. A comparison of the anesthetic methods for recurrence rates of bladder cancer after transurethral resection of bladder tumors using national health insurance claims data of south Korea. J Clin Med (2022) 11(4):1143. doi: 10.3390/jcm11041143
6. Kim R, Kawai A, Wakisaka M, Kin T. Current status and prospects of anesthesia and breast cancer: Does anesthetic technique affect recurrence and survival rates in breast cancer surgery? Front Oncol (2022) 12:795864. doi: 10.3389/fonc.2022.795864
7. Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev (2017) 36:159–77. doi: 10.1007/s10555-016-9647-8
8. Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology (2008) 109:180–7. doi: 10.1097/ALN.0b013e31817f5b73
9. Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology (2006) 105:660–4. doi: 10.1097/00000542-200610000-00008
10. Holler JP, Ahlbrandt J, Burkhardt E, Gruss M, Rohrig R, Knapheide J, et al. Peridural analgesia may affect long-term survival in patients with colorectal cancer after surgery (PACO-RAS-Study): an analysis of a cancer registry. Ann Surg (2013) 258:989–93. doi: 10.1097/SLA.0b013e3182915f61
11. Koumpan Y, Jaeger M, Mizubuti GB, Tanzola R, Jain K, Hosier G, et al. Spinal anesthesia is associated with lower recurrence rates after resection of nonmuscle invasive bladder cancer. J Urol. (2018) 199:940–6. doi: 10.1016/j.juro.2017.11.064
12. Baba Y, Kikuchi E, Shigeta K, Ogihara K, Matsushima M, Nishimoto Y, et al. Effects of transurethral resection under general anesthesia on tumor recurrence in non-muscle invasive bladder cancer. Int J Clin Oncol (2021) 26:2094–103. doi: 10.1007/s10147-021-02000-z
13. Jang D, Lim CS, Shin YS, Ko YK, Park SI, Song SH, et al. A comparison of regional and general anesthesia effects on 5 year survival and cancer recurrence after transurethral resection of the bladder tumor: a retrospective analysis. BMC Anesthesiol. (2016) 16:16. doi: 10.1186/s12871-016-0181-6
14. Yilmaz G. The effect of anesthesia choice on survival in patients undergoing surgery for bladder cancer: A retrospective analysis. Abant Med J (2020) 9(1):1–7. doi: 10.5505/abantmedj.2020.05902
15. Chipollini J, Alford B, Boulware DC, Forget P, Gilbert SM, Lockhart JL, et al. Epidural anesthesia and cancer outcomes in bladder cancer patients: is it the technique or the medication? a matched-cohort analysis from a tertiary referral center. BMC Anesthesiol. (2018) 18:157. doi: 10.1186/s12871-018-0622-5
16. Miller BL, Abel EJ, Allen G, Schumacher JR, Jarrard D, Downs T, et al. Trends in epidural anesthesia use at the time of radical cystectomy and its association with perioperative and survival outcomes: a population-based analysis. Am J Clin Exp Urol. (2020) 8:28–37.
17. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
18. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
19. Lamberink HJ, Otte WM, Geerts AT, Pavlovic M, Ramos-Lizana J, Marson AG, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol (2017) 16:523–31. doi: 10.1016/S1474-4422(17)30114-X
20. Hayden JA, Wilson MN, Riley RD, Iles R, Pincus T, Ogilvie R. Individual recovery expectations and prognosis of outcomes in non-specific low back pain: prognostic factor review. Cochrane Database Syst Rev (2019) 2019(11):CD011284. doi: 10.1002/14651858.CD011284.pub2
21. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, et al. What is “quality of evidence” and why is it important to clinicians? BMJ (2008) 336:995–8. doi: 10.1136/bmj.39490.551019.BE
22. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Choi WJ, Baek S, Joo EY, Yoon SH, Kim E, Hong B, et al. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget (2017) 8:87667–74. doi: 10.18632/oncotarget.21034
25. Doiron RC, Jaeger M, Booth CM, Wei X, Siemens DR. Is there a measurable association of epidural use at cystectomy and postoperative outcomes? a population-based study. Can Urological Assoc J (2016) 10:321. doi: 10.5489/cuaj.3856
26. Weingarten TN, Taccolini AM, Ahle ST, Dietz KR, Dowd SS, Frank I, et al. Perioperative management and oncological outcomes following radical cystectomy for bladder cancer: a matched retrospective cohort study. Can J Anaesth. (2016) 63:584–95. doi: 10.1007/s12630-016-0599-9
27. Buddeberg BS, Seeberger MD. Anesthesia and oncology: Friend or foe? Front Oncol (2022) 12:802210. doi: 10.3389/fonc.2022.802210
28. Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med (2018) 16:8. doi: 10.1186/s12967-018-1389-7
29. Cata JP, Guerra C, Soto G, Ramirez MF. Anesthesia options and the recurrence of cancer: What we know so far? Local Reg Anesth (2020) 13:57–72. doi: 10.2147/LRA.S240567
30. Pfail JL, Katims AB, Gul Z, Rosenzweig SJ, Razdan S, Nathaniel S, et al. Can anesthetics affect bladder cancer recurrence? total intravenous versus volatile anesthesia in patients undergoing robot-assisted radical cystectomy: A single institution retrospective analysis. Urol Oncol (2021) 39:233.e1–e8. doi: 10.1016/j.urolonc.2020.08.024
31. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: A retrospective analysis. Anesthesiology (2016) 124:69–79. doi: 10.1097/ALN.0000000000000936
32. Jin Z, Li R, Liu J, Lin J. Long-term prognosis after cancer surgery with inhalational anesthesia and total intravenous anesthesia: a systematic review and meta-analysis. Int J Physiol Pathophysiol Pharmacol (2019) 11:83–94.
33. Yap A, Lopez-Olivo MA, Dubowitz J, Hiller J, Riedel B. Global onco-anesthesia research collaboration g. anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. (2019) 66:546–61. doi: 10.1007/s12630-019-01330-x
34. Novy DM, Nelson DV, Koyyalagunta D, Cata JP, Gupta P, Gupta K. Pain, opioid therapy, and survival: a needed discussion. Pain (2020) 161:496–501. doi: 10.1097/j.pain.0000000000001736
35. Lusty AJ, Hosier GW, Koti M, Chenard S, Mizubuti GB, Jaeger M, et al. Anesthetic technique and oncological outcomes in urology: A clinical practice review. Urol Oncol (2019) 37:845–52. doi: 10.1016/j.urolonc.2019.08.004
36. Missair A, Cata JP, Votta-Velis G, Johnson M, Borgeat A, Tiouririne M, et al. Impact of perioperative pain management on cancer recurrence: an ASRA/ESRA special article. Reg Anesth Pain Med (2019) 44:13–28. doi: 10.1136/rapm-2018-000001
37. Dubowitz J, Hiller J, Riedel B. Anesthetic technique and cancer surgery outcomes. Curr Opin Anaesthesiol. (2021) 34:317–25. doi: 10.1097/ACO.0000000000001002
38. Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis. (2018) 35:347–58. doi: 10.1007/s10585-017-9862-x
39. Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manage (2015) 5:387–95. doi: 10.2217/pmt.15.30
40. Cata JP. Outcomes of regional anesthesia in cancer patients. Curr Opin Anaesthesiol. (2018) 31:593–600. doi: 10.1097/ACO.0000000000000636
41. Ang E, Ng KT, Lee ZX, Ti LK, Chaw SH, Wang CY. Effect of regional anaesthesia only versus general anaesthesia on cancer recurrence rate: A systematic review and meta-analysis with trial sequential analysis. J Clin Anesth (2020) 67:110023. doi: 10.1016/j.jclinane.2020.110023
42. Perez-Gonzalez O, Cuellar-Guzman LF, Navarrete-Pacheco M, Ortiz-Martinez JJ, Williams WH, Cata JP. Impact of regional anesthesia on gastroesophageal cancer surgery outcomes: A systematic review of the literature. Anesth Analg. (2018) 127:753–8. doi: 10.1213/ANE.0000000000003602
43. Perez-Gonzalez O, Cuellar-Guzman LF, Soliz J, Cata JP. Impact of regional anesthesia on recurrence, metastasis, and immune response in breast cancer surgery: A systematic review of the literature. Reg Anesth Pain Med (2017) 42:751–6. doi: 10.1097/AAP.0000000000000662
44. Grandhi RK, Lee S, Abd-Elsayed A. The relationship between regional anesthesia and cancer: A metaanalysis. Ochsner J (2017) 17:345–61.
45. Sun Y, Li T, Gan TJ. The effects of perioperative regional anesthesia and analgesia on cancer recurrence and survival after oncology surgery: A systematic review and meta-analysis. Reg Anesth Pain Med (2015) 40:589–98. doi: 10.1097/AAP.0000000000000273
46. Pei L, Tan G, Wang L, Guo W, Xiao B, Gao X, et al. Comparison of combined general-epidural anesthesia with general anesthesia effects on survival and cancer recurrence: a meta-analysis of retrospective and prospective studies. PloS One (2014) 9:e114667. doi: 10.1371/journal.pone.0114667
47. Karadeniz MS, Mammadov O, Çiftci H, Usta SA, Pembeci K. Comparing the effects of combined General/Epidural anaesthesia and general anaesthesia on serum cytokine levels in radical cystectomy. Turkish J anaesthesiol reanimation. (2017) 45:203–9. doi: 10.5152/TJAR.2017.13285
48. Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, et al. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology (1995) 83:500–8. doi: 10.1097/00000542-199509000-00008
49. DeGeorge KC, Holt HR, Hodges SC. Bladder cancer: Diagnosis and treatment. Am Fam Physician. (2017) 96:507–14.
50. Sun M, Abdollah F, Bianchi M, Trinh QD, Shariat SF, Jeldres C, et al. Conditional survival of patients with urothelial carcinoma of the urinary bladder treated with radical cystectomy. Eur J Cancer. (2012) 48:1503–11. doi: 10.1016/j.ejca.2011.11.024
51. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. (2006) 49:466–5. doi: 10.1016/j.eururo.2005.12.031
Keywords: regional anesthesia, general anesthesia, bladder cancer, meta-analysis, cancer recurrence, survival rate
Citation: Illias AM, Yu K-J, Wu S-C, Cata JP, Tsai Y-f and Hung K-C (2023) Association of regional anesthesia with oncological outcomes in patients receiving surgery for bladder cancer: A meta-analysis of observational studies. Front. Oncol. 13:1097637. doi: 10.3389/fonc.2023.1097637
Received: 14 November 2022; Accepted: 09 February 2023;
Published: 22 February 2023.
Edited by:
Gaetano Ciancio, University of Miami, United StatesReviewed by:
Georges Mjaess, Université libre de Bruxelles, BelgiumGiuseppe Simone, Hospital Physiotherapy Institutes (IRCCS), Italy
Copyright © 2023 Illias, Yu, Wu, Cata, Tsai and Hung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuo-Chuan Hung, ZWQxMDI2MDVAZ21haWwuY29t
 Amina M. Illias1,2
Amina M. Illias1,2 Shao-Chun Wu
Shao-Chun Wu Juan P. Cata
Juan P. Cata Yung-fong Tsai
Yung-fong Tsai Kuo-Chuan Hung
Kuo-Chuan Hung