
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 08 February 2023
Sec. Cardio-Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1096027
 Kaiyi Chi1,2
Kaiyi Chi1,2 Ruoyun Zhou2,3
Ruoyun Zhou2,3 Zehao Luo2,4
Zehao Luo2,4 Hongjun Zhao2,4
Hongjun Zhao2,4 Yanting Jiang2,4
Yanting Jiang2,4 Baixin He1,2
Baixin He1,2 Yemin Li2,5
Yemin Li2,5 Dongting Chen1,2
Dongting Chen1,2 Manting Feng1,2
Manting Feng1,2 Yinglan Liang2,6
Yinglan Liang2,6 Wenting Yang2,7
Wenting Yang2,7 Ruisi Liu2,7
Ruisi Liu2,7 Dunchen Yao8
Dunchen Yao8 Xiaozhen Lin9*†
Xiaozhen Lin9*† Xiuhong Xu10*†
Xiuhong Xu10*†Objective: The study aimed to evaluate the non-cancer-specific death risk and identify the risk factors affecting the non-cancer-specific survival (NCSS) in patients with primary central nervous system lymphoma (PCNSL).
Methods: This multi-center cohort study included 2497 patients with PCNSL in the Surveillance, Epidemiology and End Results (SEER) database from 2007 to 2016, with a mean follow-up of 4.54 years. The non-cancer-specific death risk in patients with PCNSL and primary central nervous system diffuse large B-cell lymphoma (PCNS-DLBCL) was evaluated using the proportion of deaths, standardized mortality ratio (SMR), and absolute excess risk (AER). Univariate and multivariate competing risk regression models were utilized to identify the risk factors of NCSS.
Results: PCNSL was the most frequent cause of death in PCNSL patients (75.03%). Non-cancer-specific causes constituted a non-negligible portion of death (20.61%). Compared with the general population, PCNSL patients had higher risks of death from cardiovascular disease (CVD) (SMR, 2.55; AER, 77.29), Alzheimer’s disease (SMR, 2.71; AER, 8.79), respiratory disease (SMR, 2.12; AER, 15.63), and other non-cancer-specific diseases (SMR, 4.12; AER, 83.12). Male sex, Black race, earlier year of diagnosis (2007–2011), being unmarried, and a lack of chemotherapy were risk factors for NCSS in patients with PCNSL and PCNS-DLBCL (all P < 0.05).
Conclusion: Non-cancer-specific causes were important competing causes of death in PCNSL patients. More attention is recommended to non-cancer-specific causes of death in the management of PCNSL patients.
Primary central nervous system lymphomas (PCNSL) are highly malignant extranodal lymphomas that originate in and are limited to the central nervous system, including the parenchyma, spinal cord, and meningeal, cranial, and ophthalmic nerves (1). Diffuse large B cell lymphoma (DLBCL), an aggressive histologic subtype, is the most common type in PCNSL (90%) (2). The treatment of PCNSL has dramatically evolved over recent years (3). However, patients with PCNSL still have a poor prognosis, with a 5-year overall survival (OS) rate of 28.6%, which is lower than that of patients with lymphomas originating at other locations (4). The rates of non-cancer-specific causes of death in cancer patients are high, suggesting that determining the risk factors for non-cancer-specific death can improve patient outcomes (5). Likewise, the risk of non-cancer-specific death in PCNSL patients should not be negligible and deserves more attention.
To date, most studies of prognostic risk factors in patients with PCNSL have focused on OS and cancer-specific survival (CSS) (6–8) and ignored non-cancer-specific survival (NCSS). In addition, the analysis of death causes of patients with primary central nervous system diffuse large B-cell lymphoma (PCNS-DLBCL) has not been reported. A previous study evaluated the risk of cardiovascular disease (CVD) death in PCNSL patients with cancer therapy but did not systematically analyze NCSS in PCNSL patients (9). Therefore, further studies are needed to comprehensively analyze the risk of non-cancer-specific death and identify risk factors of NCSS in patients with PCNSL.
Because PCNSL is rare, it is difficult to conduct a large randomized controlled trial (10). National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program contains patient data from 18 registries in the United States (11), which can fulfill the deficiency of limited cases reported in a single center. We conducted a multi-center retrospective study based on SEER to comprehensively evaluate the risk of non-cancer-specific death in patients with PCNSL and identify risk factors of NCSS. These findings may provide better insights and a scientific basis for improving the prognosis and management of patients with PCNSL.
Data were obtained from the SEER database, an authoritative data system that covers about 30% of the population of the United States (12). Institutional review board approval was not required for publicly available information (13, 14). Patients in the SEER database initially diagnosed with PCNSL from 2007 to 2016 were included. Patients were selected if they were diagnosed with a single, primary non-Hodgkin’s lymphoma of the central nervous system from 2007–2016 and subsequently followed up. Patients were excluded if their cause of death, race, or marital status was unknown.
Participant variables included sex (male/female), race (White/Black/others), age at diagnosis (< 60 years/≥ 60 years) (15), year of diagnosis (2007–2011/2012–2016), marital status (married/unmarried), pathological type (DLBCL/others), surgery (yes/no evidence), radiotherapy (yes/no evidence), and chemotherapy (yes/no evidence) (9). Other races included American Indian/Alaska Native and Asian/Pacific Islander. Other pathological types included precursor non-Hodgkin B-cell lymphoma; chronic/small lymphocytic leukemia/lymphoma; mantle-cell lymphoma; lymphoplasmacytic lymphoma; intravascular large B-cell lymphoma; Burkitt lymphoma/leukemia; extranodal margin zone lymphoma (MZL); mucosa-associated lymphoid tissue (MALT) cell lymphoma; follicular lymphoma; plasmacytoma; multiple myeloma/plasma-cell leukemia; non-Hodgkin 1ymphoma, B-cell, not otherwise specified (NOS); peripheral T-cell lymphoma, NOS; anaplastic large cell lymphoma, T-/null-cell lymphoma; adult T-cell leukemia/lymphoma; and non-Hodgkin lymphoma, NOS, unknown lineage (12).
NCSS was defined as the period from the date of diagnosis to death from non-cancer-specific causes (5). Follow-up time was calculated as the period from the date of diagnosis with PCNSL until the date of death or last follow-up on December 31, 2016.
Categorical variables were compared using the χ2 test. Standardized mortality ratios (SMRs) and absolute excess risks (AERs) were estimated for non-cancer-specific causes of death after PCNSL diagnosis and compared with the general population. SMRs refer to the ratio of observed to expected deaths (16, 17). AERs were calculated as AERs = 10,000 ([number observed–number expected]/[person−years at risk]) (18), reflecting the absolute increase in the risk of non-cancer-specific causes in the population. Univariate and multivariate competing risk models were used to analyze the risk factors of NCSS. For fully adjusted for baseline, the inclusion criteria that factors were included in multivariate competing risk models were statistically significant factors according to univariate analysis or factors with potential prognostic effect (19–21). All statistical analyses were performed using SEER*Stat (version 8.4.0), SPSS (version 25.0), and Stata (version 15.0) software, with a P of < 0.05 defined as statistically significant.
A review of the SEER database identified a total of 2497 eligible patients diagnosed with PCNSL from 2007–2016. Evaluation of their demographic characteristics showed that 60.9% of patients were aged ≥ 60 years, and 39.1% of patients were aged < 60 years. The proportion of male patients (53.2%) was higher than that of female patients (46.8%). Of all patients, 78.3% of patients were White, 8.6% of patients were Black, and 13.1% of patients were of other races. A total of 58.2% of patients were unmarried. Additionally, 51.3% of patients were diagnosed in 2007–2011, and 48.7% of patients in 2012–2016. Evaluation of their clinical characteristics showed that 82.8% of these patients had DLBCL, 42.1% of patients underwent surgery, 28.8% of patients received radiotherapy, and 71.6% of patients received chemotherapy (Table 1). The mean follow-up time was 4.54 ± 0.08 years.
The leading cause of death in PCNSL patients was primary cancer, observed in 75.03% of patients who died. Non-cancer-specific causes of death were less common, occurring in 20.61% of patients, with 4.35% of patients dying from other cancers. Evaluation of the non-cancer-specific causes of death showed that 44.88% of patients died of infectious diseases, 25.41% of patients died of CVD, and 2.97% of patients died of Alzheimer’s disease. In patients with PCNS-DLBCL, we found that non-cancer-specific causes of death accounted for 19.81% of the overall death rate (Figure 1).
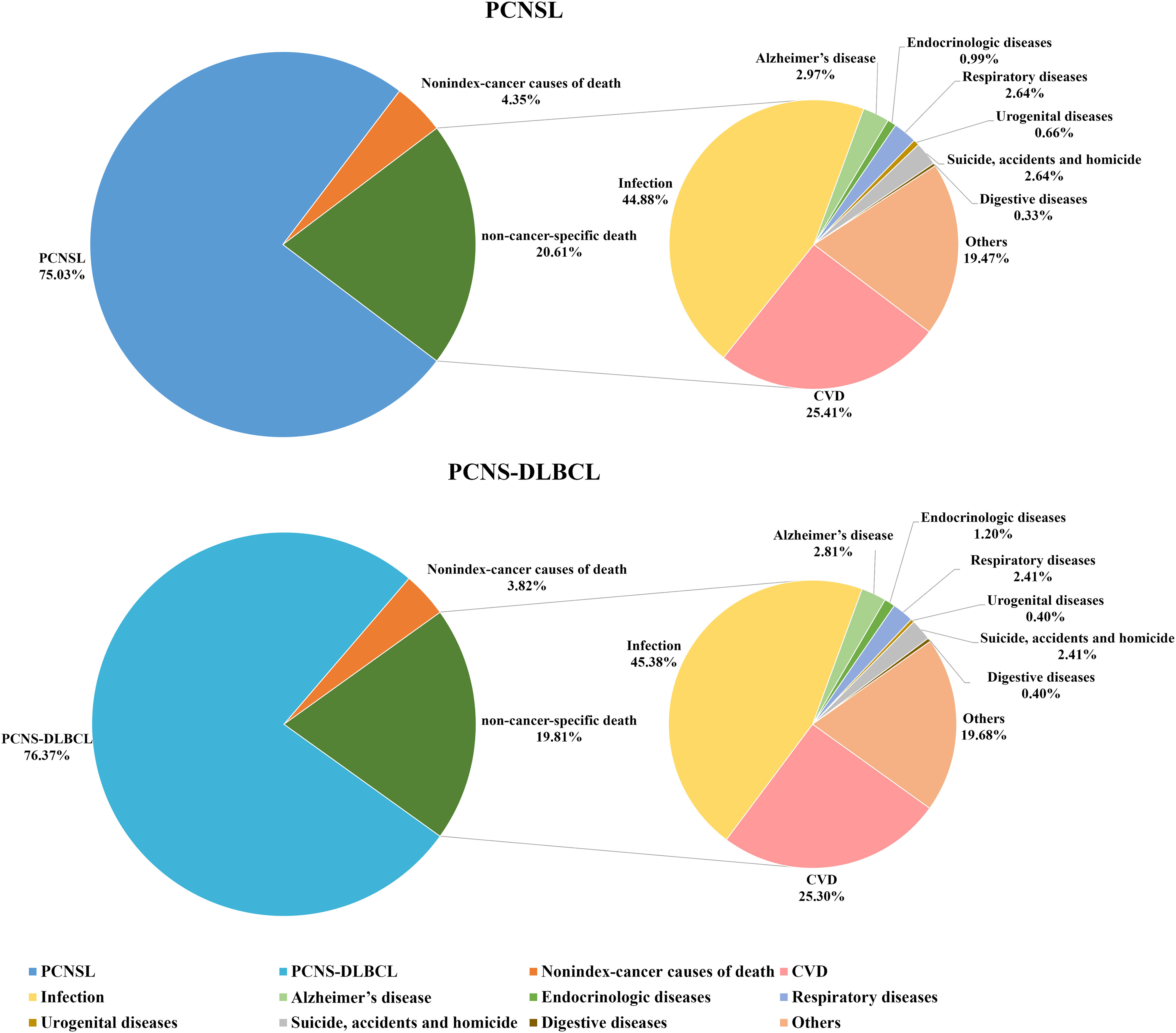
Figure 1 Proportions of deaths in patients with PCNSL and PCNS-DLBCL. PCNSL, primary central nervous system lymphoma; PCNS-DLBCL, primary central nervous system diffuse large B-cell lymphoma.
Compared with the general population, patients with PCNSL had higher risks of death from CVD (SMR, 2.55; 95% CI, 2.00–3.21; AER, 77.29), Alzheimer’s disease (SMR, 2.71; 95% CI, 1.17–5.34; AER, 8.79), respiratory disease (SMR, 2.12; 95% CI, 1.23–3.39; AER, 15.63), and other non-cancer-specific diseases (SMR, 4.12; 95% CI, 3.17–5.27; AER, 83.12). Evaluation of the risks of short-term and long-term mortality from CVD showed that these risks were especially elevated for diseases of the heart (SMR, 2.24; 95% CI, 1.65–2.95; AER, 47.17), hypertension without heart disease (SMR, 5.03; 95% CI, 1.63–11.73; AER, 6.98), and cerebrovascular diseases (SMR, 4.00; 95% CI, 2.41–6.24; AER, 24.82) (Table 2). Compared with the general population, patients with PCNS-DLBCL had higher risks of death from CVD (SMR, 2.24; 95% CI, 1.67–2.93; AER, 65.15), Alzheimer’s disease (SMR, 2.88; 95% CI, 1.16–5.94; AER, 10.37), and other non-cancer-specific diseases (SMR, 3.22; 95% CI, 2.30–4.39; AER, 62.55) (Table 3).
Univariate competitive risk model analyses showed that male sex, Black race, age at diagnosis < 60 years, earlier year of diagnosis (2007–2011), being unmarried, and lack of chemotherapy were related to worse NCSS in patients with PCNSL (Supplementary Figure S1, Supplementary Figure S2 and Table 4). Multivariate competitive risk model analyses further showed that male sex (hazard ratio [HR] = 1.45, 95% confidence interval [CI] = 1.11–1.89), Black race (HR = 2.06, 95% CI = 1.43–2.96), earlier year of diagnosis (2007–2011) (HR = 1.59, 95% CI = 1.20–2.10), being unmarried (HR = 1.70, 95% CI = 1.31–2.20), and lack of chemotherapy (HR = 2.11, 95% CI = 1.60–2.78) were risk factors for NCSS in patients with PCNSL (Table 4). Univariate competitive risk model analyses showed that male sex, Black race, age at diagnosis < 60 years, earlier year of diagnosis (2007–2011), being unmarried, and lack of chemotherapy were related to worse NCSS in patients with PCNS-DLBCL (Supplementary Figure S3, Supplementary Figure S4 and Table 5). Multivariate competitive risk model analyses showed that male sex (HR = 1.49, 95% CI = 1.12–2.00), Black race (HR = 2.19, 95% CI = 1.46–3.29), earlier year of diagnosis (2007–2011) (HR = 1.51, 95% CI = 1.10–2.06), being unmarried (HR = 1.77, 95% CI = 1.33–2.33), and lack of chemotherapy (HR = 2.29, 95% CI = 1.69–3.08) were risk factors for NCSS in patients with PCNS-DLBCL (Table 5).
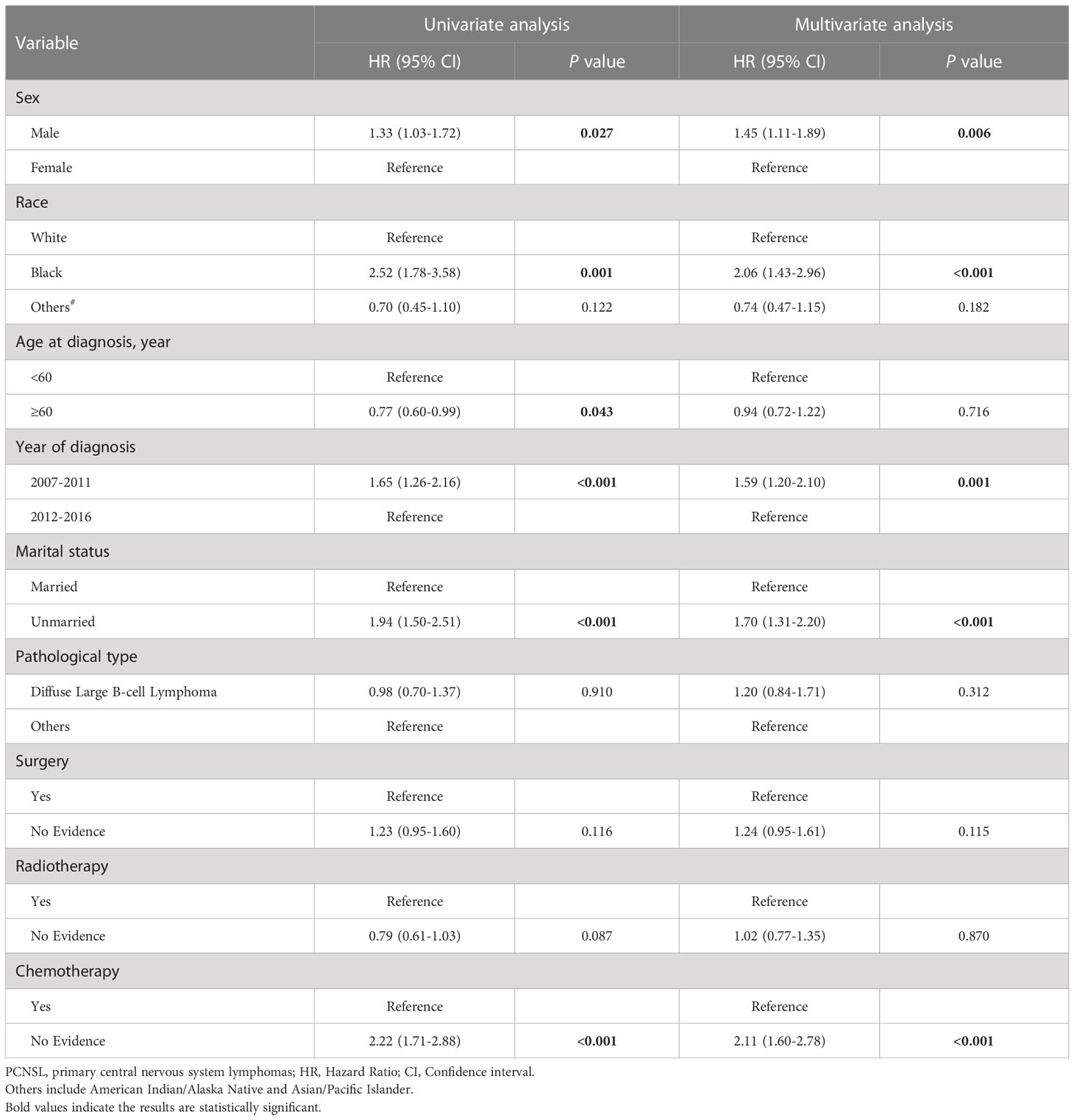
Table 4 Competing risk regression analyses of risk factors for non-cancer-specific survival in PCNSL.
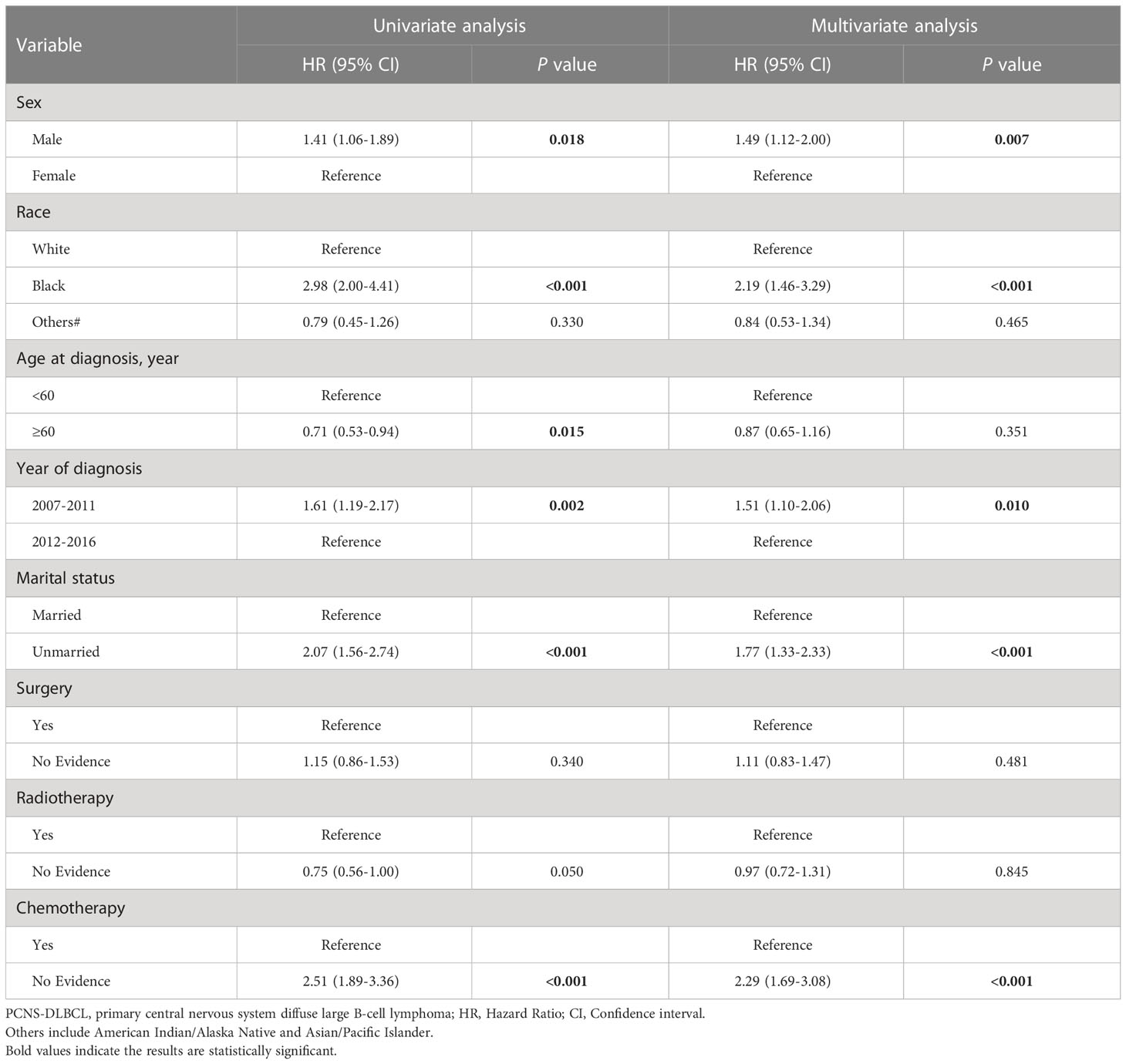
Table 5 Competing risk regression analyses of risk factors for non-cancer-specific survival in PCNS-DLBCL.
This multi-center retrospective cohort study showed that non-cancer-specific causes were an important competing cause of death in PCNSL patients. This study found that 20.61% of patients with PCNSL died of non-cancer-related causes, such as infection, CVD, and Alzheimer’s disease. Similarly, a large population-based study has reported that the risks of non-cancer-related deaths were significantly higher than that of cancer-related deaths after most cancer diagnoses (5). However, that study did not describe conditions related to PCNSL. Our findings were consistent with that earlier report, suggesting that deaths in these patients might be driven by chronic comorbid conditions and acute or iatrogenic infections (5). These results showed that more efforts should be made to manage both cancer- and non-cancer-specific risks of death that contribute to prognosis in these patients.
As important social demographic variables, sex and race were usually considered in previous studies to personalize the treatment of cancer. In line with our results, a recent study showed that sex and race are prognostic factors for OS (22). However, it did not mention the effect on NCSS. In this study, we demonstrated that male and Black race were risk factors for NCSS in PCNSL and PCNS-DLBCL patients. This may be due to gender-biased differences in the X chromosome, hormone levels, and metabolic pathways (23, 24). For race, worse NCSS in those of Black race has been possibly attributed to a higher rate of human immunodeficiency virus (HIV) infection (25), lower economic income, poorer residential environment, and a lack of insurance (26). These factors may result in reduced access to medical resources for diagnosis, treatment, and follow-up, leading to non-cancer-specific death. Understanding the impact of sex and race on NCSS may provide useful information for patients to predict disease progression. Hence, oncologists and neurologists should improve awareness and pay attention to the prevention of non-cancer-specific death of males and those of Black race with PCNSL and further perfect the management strategy.
Marital status is one of the important psychosocial factors but has been ignored in PCNSL (20, 27). In the present study, unmarried PCNSL and PCNS-DLBCL patients had reduced NCSS than married patients, suggesting that marriage plays a beneficial role through social and emotional support (20, 27, 28). Married patients have greater psychological and financial support from their spouses, enabling patients to better relieve mental distress and share finances (29). Additionally, patient adherence to treatment plans and lifestyle habits may depend on the supervision of a partner or spouse during treatment, thus, improving their prognosis (20).
The year of diagnosis is related to NCSS in PCNSL and PCNS-DLBCL patients, with the last 5 years (2012–2016) having a greater NCSS than the first 5 years (2007–2011) of diagnosis. This improvement in NCSS might be attributed to improved PCNSL regimens, resulting in reduced toxicity and, therefore, improved patient prognosis (30). Indeed, 2012 was a time point related to the recommendations of whole brain radiotherapy (WBRT), which was considered a preferred treatment for PCNSL patients with a poor general condition and those who do not respond after systemic high-dose methotrexate (HD-MTX) or relapse in a short time (31). Additionally, first-line treatment with HD-MTX and autologous stem cell transplantation has been shown to be feasible and effective since 2011 (2). Supporting evidence has also been derived from a multi-center retrospective study (9), which concluded that the risks of CVD-associated deaths were 50% lower in PCNSL patients diagnosed in 2010–2015 than in those diagnosed in 2004–2009.
The present study found that chemotherapy could improve NCSS in PCNSL and PCNS-DLBCL patients. This is consistent with a previous study, showing that chemotherapy was related to lower cardiovascular death risk of PCNSL patients (9). HD-MTX is the first-line chemotherapy treatment for PCNSL patients, which also causes neurotoxicity (32). Decreasing toxicity and side effects of chemotherapy regimens for PCNSL over time may contribute to improved NCSS (30). Most physicians use WBRT as part of their usual care for patients with PCNSL to control the high risk of neurotoxicity associated with chemotherapy (33). Rituximab, a monoclonal antibody targeting the B-cell surface antigen CD20, has significantly improved the efficacy and clinical outcome of DLBCL and has been included in the first-line treatment regimen for PCNSL (33, 34). Moreover, high-dose chemotherapy with stem-cell rescue (HDC-ASCT), as a promising consolidative strategy, is also introduced and used among patients with sufficient organ function (35). Our study stressed the important role of chemotherapy in patients with PCNSL and provided a scientific basis for further treatment.
HIV status may have confounding effects on our results, but it is still uncertain and need to be further studied. It should be noted that the pooled prevalence of HIV infection among PCNSL patients was only 6.1% (36), suggesting that the effects of HIV status on our results may be limited. The association among HIV infection and younger age (37, 38), being unmarried (39, 40) as well as chemotherapy (6, 41, 42) is still disputable.
Although DLBCL is an aggressive subtype, the proportion of non-cancer-specific deaths (19.81%) is not low. We, for the first time, studied the prognostic factors of NCSS in PCNS-DLBCL. These findings highlight the importance of non-cancer-specific death risk in PCNS-DLBCL patients. More attention should be paid to minimizing the risk of non-cancer-specific death causes during and after lymphoma treatment and throughout cancer survivorship (36).
The strengths of our study were its multi-center design and long-term follow-up. To our knowledge, this study is the largest to evaluate the risk of NCSS in patients with PCNSL. Long-term follow-up allowed assessments of the short- and long-term risks of non-cancer-specific death. However, the present study had several limitations. First, the SEER database lacks some of the clinical information related to PCNSL prognoses, such as the results of the cerebrospinal fluid examination, serum lactate dehydrogenase levels, International Extranodal Lymphoma Study Group score and Memorial Sloan Kettering Cancer Center score, and Karnofsky performance scores, which might have resulted in statistical bias. Furthermore, the SEER database lacks detailed information on treatment, such as stem cell transplant, the types and doses of chemotherapy drugs, radiotherapy doses, and duration of treatment. The lack of information on HIV status in SEER prevents further study of its impact on the NCSS in PCNSL patients. Despite these limitations, the present study included a large amount of real and reliable data, suggesting that the findings have clinical reference value.
Non-cancer-specific causes were important competing causes of death in PCNSL patients. Male sex, Black race, earlier year of diagnosis (2007–2011), being unmarried, and a lack of chemotherapy were risk factors for NCSS in patients with PCNSL and PCNS-DLBCL. Efforts should be made to manage non-cancer-specific death risks that contribute to prognosis in these patients.
Publicly available datasets were analyzed in this study. This data can be found here: http://seer.cancer.gov.
Institutional review board approval was not required for publicly available information.
KC: study design, data collection, analysis, interpretation of results, figure design, drafting of the manuscript, and review and editing of the manuscript. RZ, ZL, and HZ: analysis, interpretation of results, drafting of the manuscript, and review and editing of the manuscript. YJ, BH, YML, and DC: interpretation of results and drafting of the manuscript writing. MF, YLL, WY, and RL: study design and drafting of the manuscript; DY: data collection and editing of the manuscript. XL and XX: conception, funding acquisition, project administration and supervision, and review and editing of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the President Foundation of Integrated Hospital of Traditional Chinese Medicine, Southern Medical University (no. 1202102004); the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation. (“Climbing Program” Special Funds) (pdjh2023a0425); and the College Students’ Innovation Entrepreneurship Training Plan Program of China (grant nos. 2021C006 and 2021C005).
The authors thank the SEER database and the National Cancer Institute in the US.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1096027/full#supplementary-material
1. Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol (2019) 21(3):296–305. doi: 10.1093/neuonc/noy192
2. Batchelor TT. Primary central nervous system lymphoma: A curable disease. Hematol Oncol (2019) 37 Suppl 1:15–8. doi: 10.1002/hon.2598
3. Rees GPDWDJJ. Guidelines on the diagnosis and management of primary CNS and intra-ocular lymphoma (PCNSL) (2011). Available at: www.bnos.org.uk.
4. Deng X, Xu X, Lin D, Zhang X, Yu L, Sheng H, et al. Real-world impact of surgical excision on overall survival in primary central nervous system lymphoma. Front Oncol (2020) 10:131. doi: 10.3389/fonc.2020.00131
5. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol (2017) 28(2):400–7. doi: 10.1093/annonc/mdw604
6. Deng X, Yang X, Yang C, Chen K, Ren J, Zeng J, et al. Socioeconomic deprivation and survival outcomes in primary central nervous system lymphomas. Front Oncol (2022) 12:929585. doi: 10.3389/fonc.2022.929585
7. Chen C, Sun P, Sun XQ, Chen SY, Hang Y, Wang Y, et al. Primary treatment and recent survival trends in patients with primary diffuse large b-cell lymphoma of central nervous system, 1995-2016: A population-based SEER analysis. Hematological Oncol (2021) 1–9. doi: 10.1002/hon.2918
8. Yang C, Ren X, Cui Y, Jiang H, Yu K, Li M, et al. Nomograms for predicting cancer-specific survival in patients with primary central nervous system lymphoma: A population-based analysis. Ann Trans Med (2021) 9(13):1055. doi: 10.21037/atm-21-753
9. Guan T, Qiu Z, Su M, Yang J, Tang Y, Jiang Y, et al. Cardiovascular death risk in primary central nervous system lymphoma patients treated with chemotherapy: A registry-based cohort study. Front Oncol (2021) 11:641955. doi: 10.3389/fonc.2021.641955
10. Oliveira GH, Al-Kindi SG, Hoimes C, Park SJ. Characteristics and survival of malignant cardiac tumors: A 40-year analysis of >500 patients. Circulation (2015) 132(25):2395–402. doi: 10.1161/CIRCULATIONAHA.115.016418
11. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, epidemiology, and end results (SEER) database. JAMA surgery (2018) 153(6):588–9. doi: 10.1001/jamasurg.2018.0501
12. National Cancer Institute. About the SEER program 1–9. Available at: https://seer.cancer.gov/about/.
13. Stoltzfus KC, Zhang Y, Sturgeon K, Sinoway LI, Trifiletti DM, Chinchilli VM, et al. Fatal heart disease among cancer patients. Nat Commun (2020) 11(1):2011. doi: 10.1038/s41467-020-15639-5
14. Qiu Z, Tang Y, Jiang Y, Su M, Wang X, Xu X, et al. Cardiovascular outcomes in the patients with primary central nervous system lymphoma: A multi-registry based cohort study of 4,038 cases. Front Oncol (2021) 11:691038. doi: 10.3389/fonc.2021.691038
15. Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic scoring system for primary CNS lymphomas: The international extranodal lymphoma study group experience. J Clin Oncol (2003) 21(2):266–72. doi: 10.1200/JCO.2003.09.139
16. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J (2019) 40(48):3889–97. doi: 10.1093/eurheartj/ehz766
17. Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the united states. Jama (2020) 324(24):2521–35. doi: 10.1001/jama.2020.23130
18. Dores GM, Curtis RE, Dalal NH, Linet MS, Morton LM. Cause-specific mortality following initial chemotherapy in a population-based cohort of patients with classical Hodgkin lymphoma, 2000-2016. J Clin Oncol (2020) 38(35):4149–62. doi: 10.1200/JCO.20.00264
19. Paterson DI, Wiebe N, Cheung WY, Mackey JR, Pituskin E, Reiman A, et al. Incident cardiovascular disease among adults with cancer: A population-based cohort study. JACC CardioOncology (2022) 4(1):85–94. doi: 10.1016/j.jaccao.2022.01.100
20. Guan T, Wang Y, Li F, Chen D, Wei Q, Wang K, et al. Association of marital status with cardiovascular outcome in patients with breast cancer. J Thorac disease (2022) 14(4):841–50. doi: 10.21037/jtd-21-1261
21. Weberpals J, Jansen L, Müller OJ, Brenner H. Long-term heart-specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: a registry-based cohort study. Eur Heart J (2018) 39(43):3896–903. doi: 10.1093/eurheartj/ehy167
22. Tang D, Chen Y, Shi Y, Tao H, Tao S, Zhang Q, et al. Epidemiologic characteristics, prognostic factors, and treatment outcomes in primary central nervous system lymphoma: A SEER-based study. Front Oncol (2022) 12:817043. doi: 10.3389/fonc.2022.817043
23. Kim SY, Song HK, Lee SK, Kim SG, Woo HG, Yang J, et al. Sex-biased molecular signature for overall survival of liver cancer patients. Biomolecules Ther (2020) 28(6):491–502. doi: 10.4062/biomolther.2020.157
24. Roetzer T, Furtner J, Gesperger J, Seebrecht L, Bandke D, Brada M, et al. Sex-specific differences in primary CNS lymphoma. Cancers (Basel) (2020) 12(6):1593. doi: 10.3390/cancers12061593
25. Pulido JS, Vierkant RA, Olson JE, Abrey L, Schiff D, O'Neill BP. Racial differences in primary central nervous system lymphoma incidence and survival rates. Neuro Oncol (2009) 11(3):318–22. doi: 10.1215/15228517-2008-103
26. Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer control J Moffitt Cancer Center (2009) 16(1):53–6. doi: 10.1177/107327480901600108
27. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol (2013) 31(31):3869–76. doi: 10.1200/JCO.2013.49.6489
28. Fournier S, Muller O, Ludman AJ, Lauriers N, Eeckhout E. Influence of socioeconomic factors on delays, management and outcome amongst patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Swiss Med weekly (2013) 143:w13817. doi: 10.4414/smw.2013.13817
29. Li K, Wang F, Wang J, Fan C, Sun J. Marital status independently predicts survival of patients with upper urinary tract urothelial carcinoma: A population-based study. J Cancer Res Ther (2021) 17(7):1709–17. doi: 10.4103/jcrt.jcrt_1713_21
30. Holdhoff M, Mrugala MM, Grommes C, Kaley TJ, Swinnen LJ, Perez-Heydrich C, et al. Challenges in the treatment of newly diagnosed and recurrent primary central nervous system lymphoma. J Natl Compr Cancer Network JNCCN (2020) 18(11):1571–8. doi: 10.6004/jnccn.2020.7667
31. NCCN. The NCCN central nervous system cancers clinical practice guidelines in oncology (version 1.2012.69-71.)[EB/OL]. Available at: http://www.nccn.org [Accessed September 25, 2013].
32. Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood (2022) 140(9):971–9. doi: 10.1182/blood.2020008377
33. Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol (2017) 35(21):2410–8. doi: 10.1200/JCO.2017.72.7602
34. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-b-cell lymphoma. N Engl J Med (2002) 346(4):235–42. doi: 10.1056/NEJMoa011795
35. Ferreri AJ, Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood (2016) 127(13):1642–9. doi: 10.1182/blood-2015-10-636340
36. Franca RA, Travaglino A, Varricchio S, Russo D, Picardi M, Pane F, et al. HIV Prevalence in primary central nervous system lymphoma: A systematic review and meta-analysis. Pathology Res practice (2020) 216(11):153192. doi: 10.1016/j.prp.2020.153192
37. Qiao YC, Xu Y, Jiang DX, Wang X, Wang F, Yang J, et al. Epidemiological analyses of regional and age differences of HIV/AIDS prevalence in China, 2004-2016. Int J Infect Dis IJID Off Publ Int Soc Infect Diseases (2019) 81:215–20. doi: 10.1016/j.ijid.2019.02.016
38. Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV Infection and older americans: The public health perspective. Am J Public Health (2012) 102(8):1516–26. doi: 10.2105/AJPH.2012.300844
39. Shisana O, Zungu-Dirwayi N, Toefy Y, Simbayi LC, Malik S, Zuma K. Marital status and risk of HIV infection in south Africa. South Afr Med J = Suid-Afrikaanse tydskrif vir geneeskunde (2004) 94(7):537–43.
40. Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: An analysis of survey and clinical data. Lancet (London England) (2008) 371(9631):2183–91. doi: 10.1016/S0140-6736(08)60953-8
41. Moulignier A, Lamirel C, Picard H, Lebrette MG, Amiel C, Hamidi M, et al. Long-term AIDS-related PCNSL outcomes with HD-MTX and combined antiretroviral therapy. Neurology (2017) 89(8):796–804. doi: 10.1212/WNL.0000000000004265
Keywords: primary central nervous system lymphoma, primary central nervous system diffuse large B-cell lymphoma, non-cancer-specific survival, risk factors, SEER
Citation: Chi K, Zhou R, Luo Z, Zhao H, Jiang Y, He B, Li Y, Chen D, Feng M, Liang Y, Yang W, Liu R, Yao D, Lin X and Xu X (2023) Non-cancer-specific survival in patients with primary central nervous system lymphoma: A multi-center cohort study. Front. Oncol. 13:1096027. doi: 10.3389/fonc.2023.1096027
Received: 21 November 2022; Accepted: 26 January 2023;
Published: 08 February 2023.
Edited by:
Nadine Norton, Mayo Clinic Florida, United StatesReviewed by:
Tilman Bostel, Johannes Gutenberg University Mainz, GermanyCopyright © 2023 Chi, Zhou, Luo, Zhao, Jiang, He, Li, Chen, Feng, Liang, Yang, Liu, Yao, Lin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhen Lin, bGlueHowMjFAMTYzLmNvbQ==; Xiuhong Xu, ZWlsZWVueHVhZEAxNjMuY29t
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.