
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 March 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1094480
 Tae Hyun Kim1†
Tae Hyun Kim1† Yoonjin Kwak2†
Yoonjin Kwak2† Changhoon Song3*
Changhoon Song3* Hye Seung Lee2
Hye Seung Lee2 Duck-Woo Kim4
Duck-Woo Kim4 Heung-Kwon Oh4
Heung-Kwon Oh4 Jin Won Kim5
Jin Won Kim5 Keun-Wook Lee5
Keun-Wook Lee5 Sung-Bum Kang4
Sung-Bum Kang4 Jae-Sung Kim3*
Jae-Sung Kim3*Introduction: Glucose transporter-1 (GLUT-1) has been studied as a possible predictor for survival outcomes in locally advanced rectal cancer (LARC).
Methods: We aimed to investigate the prognostic role of GLUT-1 in LARC using the data of 208 patients with clinical T3–4 stage and/or node-positive rectal adenocarcinoma, all of whom underwent neoadjuvant chemoradiotherapy (CRT) and subsequent total mesorectal excision (TME). Both pre-CRT and post-CRT specimens were immunohistologically stained for GLUT-1. Patients were classified into GLUT-1-positive and GLUT-1-negative groups and distant metastasis-free survival (DMFS) and overall survival (OS) was analyzed and compared.
Results: At a median follow-up of 74 months, post-CRT GLUT-1 status showed a significant correlation with worse DMFS (p=0.027, HR 2.26) and OS (p=0.030, HR 2.30). When patients were classified into 4 groups according to yp stage II/III status and post-CRT GLUT-1 positivity [yp stage II & GLUT-1 (-), yp stage II & GLUT-1 (+), yp stage III & GLUT-1 (-), yp stage III & GLUT-1 (+)], the 5-year DMFS rates were 92.3%, 63.9%, 65.4%, and 46.5%, respectively (p=0.013). GLUT-1 (-) groups showed markedly better outcomes for both yp stage II and III patients compared to GLUT-1 (+) groups. A similar tendency was observed for OS.
Discussion: In conclusion, post-CRT GLUT-1 may serve as a prognostic marker in LARC.
The hypoxic tumor microenvironment induces various reactions in tumor cells, including increased glucose utilization, resulting in increased anaerobic glycolysis (1). Consequently, glucose transporters (GLUTs) also exhibit high levels of expression, with GLUT-1 to GLUT-4 playing the most important role in glucose regulation (2). GLUTs have specific locations according to their subtypes, and certain types are only expressed in certain cell lines. GLUT-1, for instance, is mainly expressed in endothelial cells of the blood–brain barrier, but it has also been found to be upregulated in various tumor cells (3–6). Moreover, the expression level of GLUT-1 has been reported to be increased by oncogenes such as Ras and Src and by transcription factors involved in tumorigenesis such as SIX1 (7, 8). In light of these findings, GLUT-1 is currently being studied as a surrogate marker for tumor prognosis. In fact, some prior studies have shown an association between survival outcomes and GLUT-1 in various tumor types, including breast, lung, and pancreatic cancers (9–11).
However, the results of prior studies regarding colorectal cancers are not consistent. GLUT-1 has been shown to be associated with worse disease-specific mortality, overall survival, and tumor regression grade in some studies (12–15); others have reported statistically nonsignificant results (16–19), and one study reported a positive effect of GLUT-1 on overall survival (20). The agreement between studies is further reduced when the scope is narrowed to rectal cancer, where the number of patients per study and the absolute number of studies are both low (13–16, 18, 19). Additionally, for locally advanced rectal cancer (LARC), the majority of the studies only utilized either pre-neoadjuvant chemoradiotherapy (pre-CRT) specimens or post-neoadjuvant chemoradiotherapy (post-CRT) specimens, suggesting the need for a more comprehensive analysis using both sample types.
Based on the pitfalls presented above, this study aimed to investigate the correlation between GLUT-1 and survival outcomes in LARC using pre-CRT and post-CRT samples and to investigate distant metastasis-free survival (DMFS) and overall survival (OS).
A total of 208 patients from a single institution with histologically confirmed rectal adenocarcinoma, clinical T3–4 and/or node-positive or low-lying T2 were recruited between 2004 and 2012. All patients were newly diagnosed and free of metastasis. Patients received neoadjuvant CRT followed by total mesorectal excision (TME) in 6 to 8 weeks. Radiation therapy was delivered in 5.5 weeks (45 Gy to the pelvis in 25 fractions and 5.4 Gy to the primary lesion in 3 fractions). The chemotherapy regimens were one of the three; 5-fluorouracil with leucovorin (FL), capecitabine, or 5-fluorouracil with leucovorin and oxaliplatin (FOLFOX). TME was performed in one of the three ways: low anterior resection, ultralow anterior resection or abdominoperineal resection. Adjuvant chemotherapy was recommended after resection for all patients who were medically fit. The median follow-up time was 74 months (8-164 months). This study was approved by the Seoul National University Bundang Hospital Institutional Review Board. Written informed consent was waived due to the retrospective design of the study, but the patients were anonymized to protect privacy. The waiver of informed consent was authorized by Seoul National University Bundang Hospital Institutional Review Board (B-2109-707-106). All methods of this study were performed in accordance with the relevant guidelines and regulations.
Tissue sampling was performed twice: before neoadjuvant CRT using colonoscopic biopsy (pre-CRT) and during surgery using a surgical specimen (post-CRT). The samples were stained for GLUT-1 using immunohistochemistry with an antibody from Abcam (Cambridge, United Kingdom). The histological score (H-score) for each pre-CRT and post-CRT specimen was calculated by multiplying the percentage of GLUT-1 staining in the specimen by the intensity of GLUT-1 staining in the same specimen. The percentage of GLUT-1 staining was scored from 0 to 100, whereas the intensity of GLUT-1 staining was scored from 0 to 3 (0 denoted negative staining and 3 denoted maximum staining, Figure 1). The immunohistochemical evaluation was performed by one dedicated GI pathologist (Y.K). GLUT-1 expression was considered positive when the H-score was equal to or greater than 1 and was considered negative when the H-score was less than 1.
The log-rank test was used to compare survival curves between the GLUT-1-positive and GLUT-1-negative groups. Additionally, factors with p value <0.1 in the univariate analysis using Cox proportional hazards model were used to construct a multivariate Cox proportional hazards model and calculate the hazard ratio for each factor. All variables were transformed into categorical variables using conventional or clinically significant cutoffs. The baseline CEA value was classified as low or high using 5 ng/ml as the cutoff value. ypT stage was grouped as 0-2 or 3-4, whereas ypN stage was grouped as 0 or 1-2. Statistical analysis was performed with R software 4.1.0, and statistical significance was indicated by p<0.05.
The median age of the patients was 60.6 years (range, 49.1-72.1, Table 1). The majority of tumors were diagnosed as clinical T3 stage (n=176, 84.6%), and clinical lymph node involvement was seen in most of the patients (n=171, 82.2%) (Table 1). Nearly 90% of patients received either low anterior resection or ultralow anterior resection, classified as sphincter-preserving surgery (n=186, 89.4%). Following radical surgery, 178 patients (85.6%) received 5-FU–based adjuvant chemotherapy. In terms of pathologic stage, although half of the patients remained in the ypT3 stage (n=109, 52.4%), approximately a quarter of patients were diagnosed as ypT2 stage (n=57, 27.4%), and some showed a complete response (n=33, 15.9%). Similarly, the majority of the patients were diagnosed as ypN0 regarding ypN stage (n=134, 64.7%). The planned radiation dose was 50.4Gy for all patients, and the planned dose was delivered to all but 1 patient who terminated early. Majority of the patients received chemotherapy in either FL (n=91, 43.7%) or capecitabine (n=107, 51.4%). There was no premature termination of chemotherapy.
Regarding GLUT-1 status, the median H-scores for pre-CRT and post-CRT specimens were 0 and 12.5, respectively, indicating an increasing tendency after neoadjuvant therapy (Figure 2). The percentages of positive staining were 38% and 61%, in alignment with the increasing tendency of the H-score.
Kaplan–Meier curves for DMFS/OS were generated based on GLUT-1 expression status and treatment phase (pre-CRT/post-CRT) (Figure 3). The pre-CRT GLUT-1 status did not result in a statistically significant difference for DMFS and OS. However, there was a significant difference between the post-CRT GLUT-1-positive group and the post-GLUT-1-negative group in terms of both DMFS (p=0.018) and OS (p=0.015). The actuarial 5-year DMFS rate of patients was 63.9% for the post-CRT GLUT-1-positive group and 80.6% for the post-CRT GLUT-1-negative group. For 5-year OS, the value was 66.7% for the GLUT-1-positive group and 86.9% for the GLUT-1-negative group. For both DMFS and OS, positive post-CRT GLUT-1 staining was significantly associated with worse prognosis.
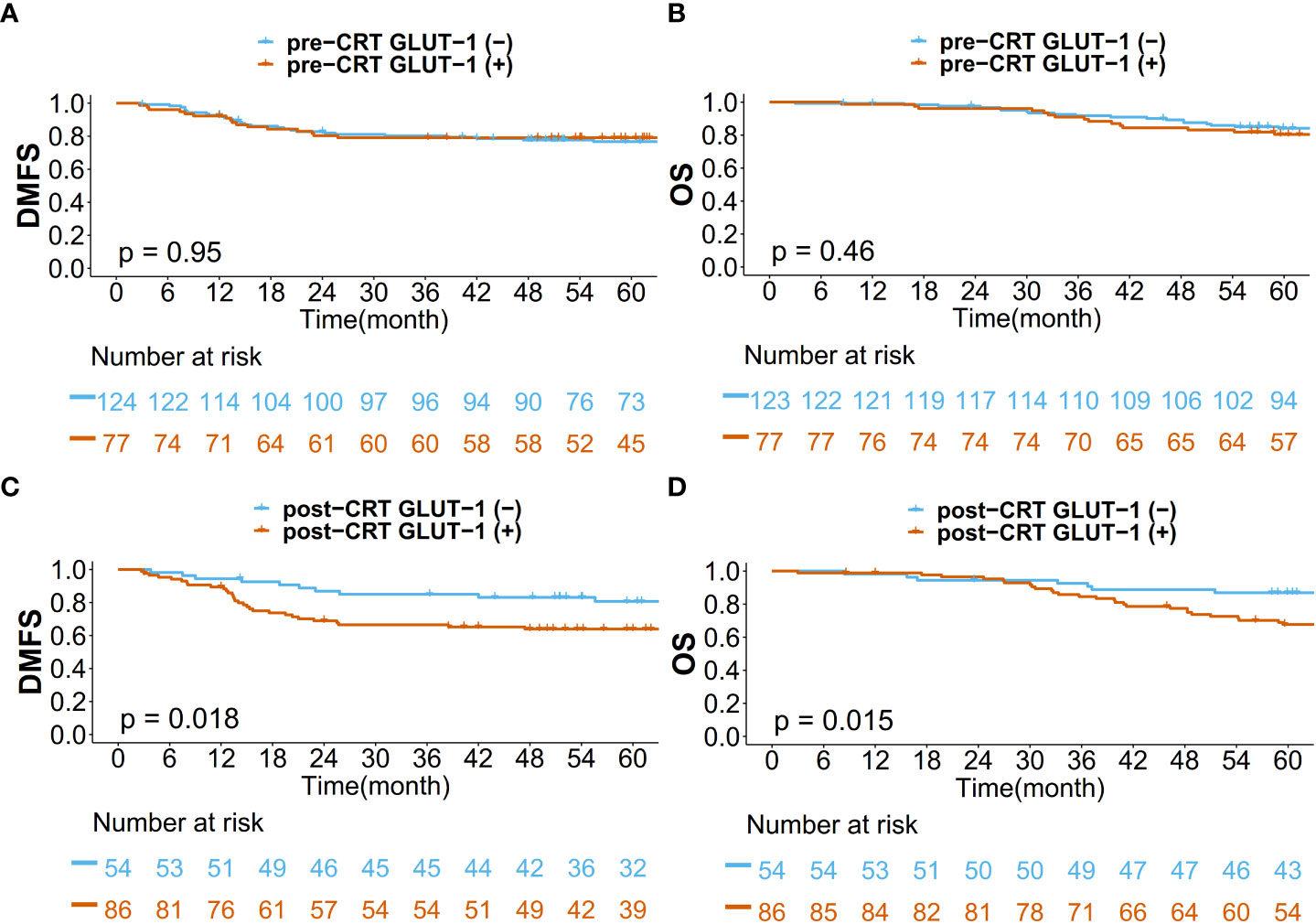
Figure 3 Kaplan–Meier curves for (A) DMFS based on pre-CRT status, (B) OS based on pre-CRT status, (C) DMFS based on post-CRT status, and (D) OS based on post-CRT status.
Univariate Cox proportional hazards analysis was performed using clinical information (Table 2). Age, sex, baseline CEA, ypT stage, ypN stage, pathologic grade, and post-CRT GLUT-1 status were included. Age, sex, and pathologic grade showed nonsignificant p values in the univariate analysis. Multivariate analysis was conducted with the factors with p value <0.1 in the univariate analysis (Table 3). The multivariate analysis revealed that baseline CEA, ypN stage, and post-CRT GLUT-1 status were significant predictors for DMFS, and ypN stage and post-CRT GLUT-1 status were the only predictors that met the statistical criteria for predicting OS. The hazard ratios for post-CRT GLUT-1 status were 2.26 (p=0.027) and 2.30 (p=0.030) for DMFS and OS, respectively; these values were higher than those for baseline CEA for both clinical outcomes.
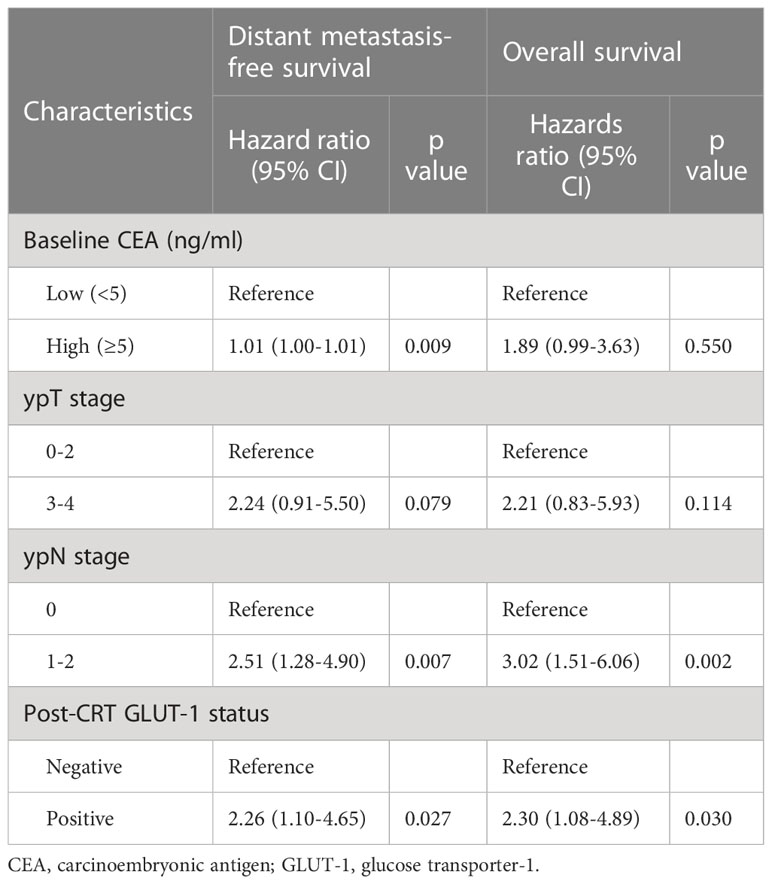
Table 2 Results of univariate Cox analysis for distant metastasis-free survival and overall survival.
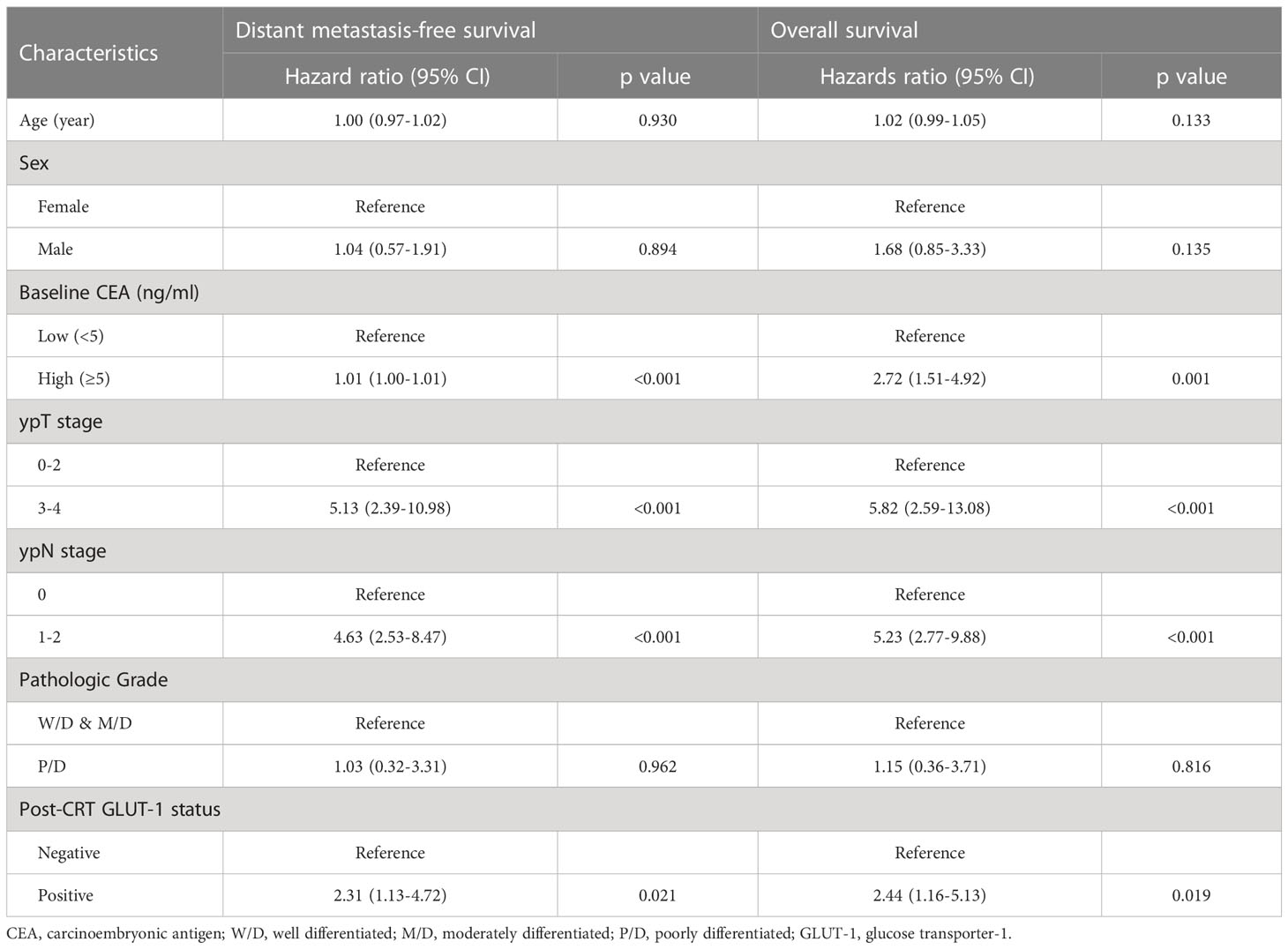
Table 3 Results of multivariate Cox analysis for distant metastasis-free survival and overall survival.
When patients were classified according to yp stage, approximately a quarter accounted for yp stage II (n=51, 24.5%) and another quarter accounted for yp stage III (n=60, 28.8%). The 5-year actuarial DMFS rates for patients in yp stages 0, I, II, and III were 97.0%, 88.7%, 77.9% and 54.1%, respectively (Figure 4A). For 5-year actuarial OS, the values were 100%, 93.5%, 85.8% and 57.5%, respectively (Figure 4B). For patients in yp stages II and III, patients were classified into 4 groups according to yp stage II/III and post-CRT GLUT-1 status (Figures 4C, D): yp stage II & GLUT-1 (-), yp stage II & GLUT-1 (+), yp stage III & GLUT-1 (-), and yp stage III & GLUT-1 (+). For yp stage II patients, yp stage II & GLUT-1 (-) group showed a more favorable 5-year actuarial survival rates of 92.3% and 91.7% for both DMFS and OS whereas the yp stage II & GLUT-1 (+) group showed a less favorable 5-year actuarial survival rates of 63.9% and 79.0%. This tendency was maintained in the yp stage III patients as well; 5-year actuarial DMFS and OS for yp stage III & GLUT-1(-) group was 65.4% and 72.7% when the values were 46.5% and 43.4% for yp stage III & GLUT-1 (+) group. Taken altogether, yp stage II & GLUT-1 (-) group showed the most favorable outcomes, whereas the yp stage III & GLUT-1 (+) group showed the least favorable 5-year actuarial survival rates. The yp stage II & GLUT-1 (+) and yp stage III & GLUT-1 (-) group together showed intermediate outcomes. The new prognostic grouping based on post-CRT GLUT-1 status was shown to be statistically significant for predicting both distant metastasis and overall survival (p=0.0130 and p=0.0082).
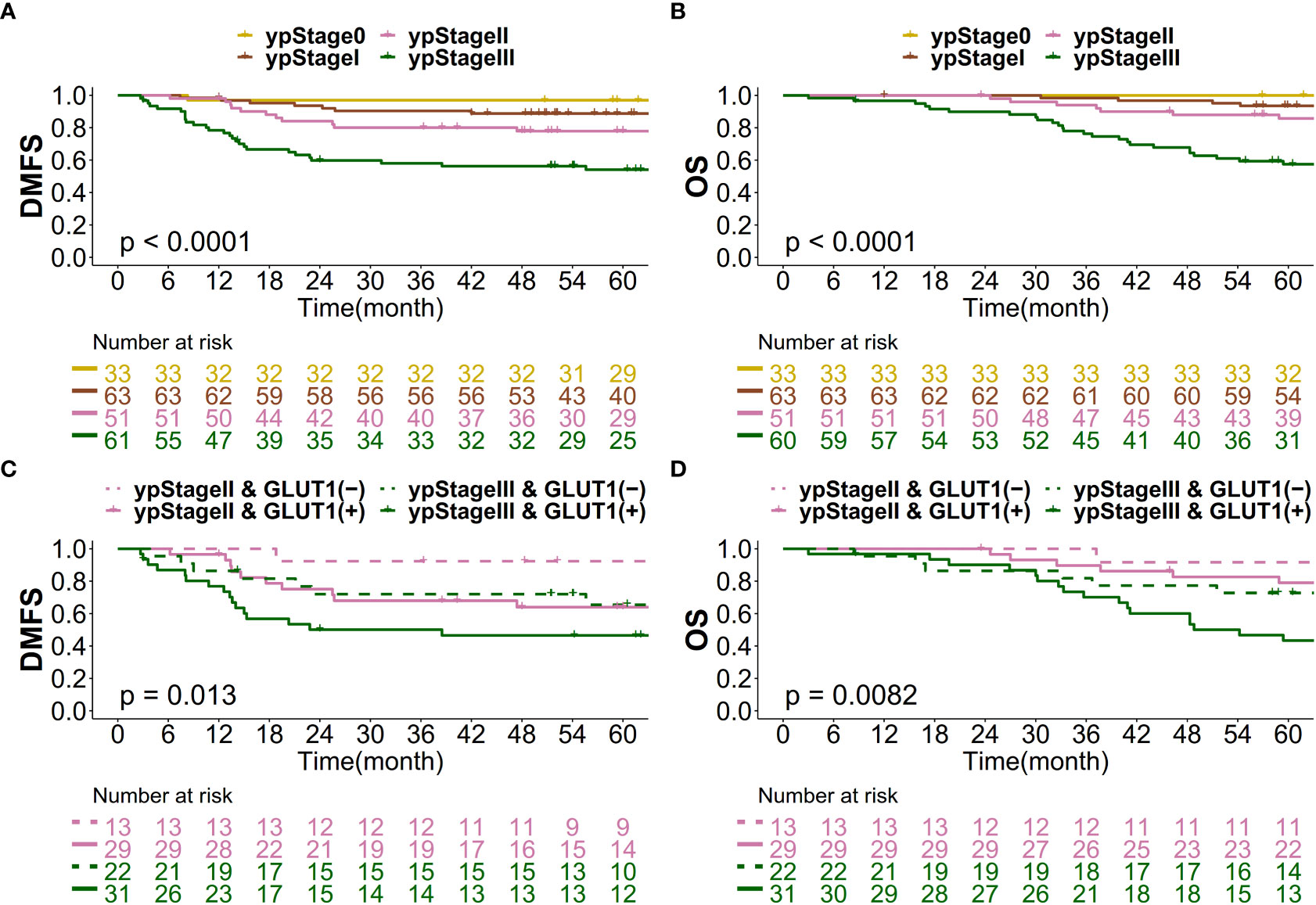
Figure 4 Kaplan–Meier curve for (A) DMFS based on yp stage, (B) OS based on yp stage, (C) DMFS based on the new prognostic grouping, and (D) OS based on the new prognostic grouping.
The findings of our study indicate that post-CRT GLUT-1 status is significantly associated with DMFS and OS in patients with LARC. Furthermore, post-CRT GLUT-1 status has shown the possibility of serving as a prognostic subgroup together with ypN stage.
Of particular interest is the increase in GLUT-1 H-scores after CRT in our study. One possible hypothesis is that GLUT-1 is mainly located at the central hypoxic region of the tumor, and the peripheral normoxic region with low GLUT-1 levels was eradicated during the treatment. Considering that H-score was calculated by multiplying the percentage of GLUT-1 staining in the specimen by the intensity of GLUT-1 staining in the same specimen, the increase in percentage of GLUT-1 staining due to eradication of peripheral normoxic region may have resulted in higher H-score in the post-CRT specimen, although it is not possible to confirm such phenomenon directly from current study. The central distribution of GLUT-1 after CRT in rectal cancer has already been reported in one prior study (21). This hypothesis is further supported by the results of another study that further showed that GLUT-1 immunohistochemistry staining conducted on the superficial part of the tumor had no prognostic capability, whereas GLUT-1 staining in a deep part of the tumor could indicate OS (13).
The main mechanism behind the strong association between GLUT-1 and survival outcomes is thought to be tumor hypoxia. Various studies have suggested worse clinical outcomes in hypoxic tumors using markers such as HIF1-a, CA-9 and hemoglobin levels in LARC (16, 19, 22). Since tumor hypoxia plays a key role in both early and late tumor metastasis by promoting invasion, migration, angiogenesis, and adaptation to the metastasis site, it is likely to decrease OS by promoting distant metastasis (23). This is in alignment with the fact that distant metastasis is the primary cause of treatment failure in LARC patients and with the findings of a prior study where GLUT-1 was reported to be associated with distant metastasis but not regional recurrence (13, 24).
Regarding the prognostic subgroup, the large difference in survival in patients with the same yp stage is worth noting. Such a phenomenon has also been observed in our prior study, where yp stage II and III rectal cancer patients were classified into good response (GR) and poor response (PR) groups according to the Dworak tumor regression grade (25). In the abovementioned study, the yp stage II & GR group showed similar survival results as the yp stage 0-I group, while the yp stage III & GR group showed similar survival results as the yp stage II group. This, together with the subgroup analysis of our study, implies the heterogeneity of yp stage II and III rectal cancer patients and thus calls for a more customized treatment according to each patient’s prognosis.
Meanwhile, circulating tumor DNA (ctDNA) is an emerging biomarker that has gained popularity nowadays. ctDNA can be a prognostic and predictive biomarker in gastrointestinal cancers (26–28). Recently, Kotani et al. have reported results from GALAXY, which is an observational arm of the ongoing CIRCULATE-Japan study (UMIN000039205) that analyzed preoperative and postoperative ctDNA in patients with stage II-IV resectable colorectal cancer (28). In the multivariate analysis for recurrence in patients with pathologic stage II-III, ctDNA positivity (at 4 weeks after surgery) was the most significant prognostic factor for recurrence (HR 10.82, 95% CI 7.07-16.6, p<0.001). All other clinicopathologic factors including pathologic N stage, MSI, BRAF, and RAS status were not significant. Furthermore, postsurgical ctDNA positivity was predictive of response to adjuvant chemotherapy (HR 6.59, 95% CI 3.53–12.3, p<0.0001). A collaborative study from Memorial Sloan Kettering Cancer Center and University of South Florida have reported that monitoring ctDNA may lead to a faster response assessment compared with traditional radiologic assessment in patients with anal cancer after definitive chemoradiotherapy (29). The median time to molecular ctDNA remission (30 days) was significantly shorter than the median time to complete clinical response (136 days). However, in rectal cancer, it is difficult to conclude as to the prognostic value of ctDNA as most studies are small, the statistical assumptions are dubious and the follow-up period is rather short in some of the included studies. In our current study, we showed the added prognostic value of post-CRT GLUT-1 by immunohistochemistry over conventional staging system. This method is affordable and can be applied to the real clinical practice right away.
Our study has some limitations. First, there is a possibility of inappropriate representation of GLUT-1 status in pre-CRT specimens derived from biopsy. This is a limitation that has been mentioned in a prior study (15). To overcome this issue, the lowest possible cutoff margin was used to avoid precluding any sign of positive GLUT-1 status. Second, this is a retrospective study conducted in a single institution. Although the data structure was designed to make up for the shortcomings of prior studies, a prospective trial with a larger number of patients is required for a more definitive conclusion.
Nonetheless, the results of this study may aid in the utilization of GLUT-1 as a prognostic marker in various ways. Our classification of favorable, intermediate and unfavorable groups according to yp stage II, III and post-CRT GLUT-1 status may serve as a possible grouping system for future trials to determine the optimal chemotherapy regimen in these patients. In addition, we suggest that a more prudent approach might be needed for patients who receive short course radiation therapy and show high levels of GLUT-1 simultaneously. One prior study suggested the possibility of a reduced tumor cell killing in tumor regions with hypoxia when delivering radiation therapy with a hypofractionated schedule (30). Based on this possibility, extra measures such as a higher chemotherapy dose or a more potent chemotherapy regimen might be necessary for patients who receive a short course of radiation therapy but show a high level of GLUT-1.
The utility of GLUT-1 can further be expanded to disease treatment with novel GLUT-1 targeting agents. Combining drugs such as resveratrol or STF-31 that specifically bind to GLUT-1 and conventional cytotoxic agents might facilitate better treatment outcomes in high-risk patients whose tumors exhibit high levels of hypoxia (31).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YK and HL provided the data. CS, J-SK and YK were involved in the study design and obtained the raw data. TK and CS conducted statistical analyses. TK & YK drafted the paper, and JS-K and CS reviewed and revised the manuscript. All authors read and approved the final manuscript. CS and JS-K designed the study. All authors contributed to the article and approved the submitted version.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (#2020R1C1C1014192).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sørensen BS, Horsman MR. Tumor hypoxia: Impact on radiation therapy and molecular pathways. Front Oncol (2020) 10:562. doi: 10.3389/fonc.2020.00562
2. Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol - Endocrinol Metab (2010) 298:141–5. doi: 10.1152/ajpendo.00712.2009
3. Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, et al. Vitamin c crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest (1997) 100:2842–8. doi: 10.1172/JCI119832
4. Brown RS, Wahl RL. Overexpression of glut-1 glucose transporter in human breast cancer an immunohistochemical study. Cancer (1993) 72:2979–85. doi: 10.1002/1097-0142(19931115)72:10<2979::AID-CNCR2820721020>3.0.CO;2-X
5. Effert P, Beniers AJ, Tamimi Y, Handt S, Jakse G. Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res (2004) 24:3057–63.
6. Davis-Yadley AH, Abbott AM, Pimiento JM, Chen DT, Malafa MP. Increased expression of the GLUT-1 gene is associated with worse overall survival in resected pancreatic adenocarcinoma. Pancreas (2016) 45:974. doi: 10.1097/MPA.0000000000000580
7. Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science (1987) 235:1492–5. doi: 10.1126/science.3103217
8. Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional regulation of the warburg effect in cancer by SIX1. Cancer Cell (2018) 33:368–385.e7. doi: 10.1016/j.ccell.2018.01.010
9. Zhang B, Xie Z, Li B. The clinicopathologic impacts and prognostic significance of GLUT1 expression in patients with lung cancer: A meta-analysis. Gene (2019) 689:76–83. doi: 10.1016/J.GENE.2018.12.006
10. Achalandabaso Boira M, Di Martino M, Gordillo C, Adrados M, Martín-Pérez E. GLUT-1 as a predictor of worse prognosis in pancreatic adenocarcinoma: Immunohistochemistry study showing the correlation between expression and survival. BMC Cancer (2020) 20:1–9. doi: 10.1186/s12885-020-07409-9
11. Jang SM, Han H, Jang KS, Jun YJ, Jang SH, Min KW, et al. The glycolytic phenotype is correlated with aggressiveness and poor prognosis in invasive ductal carcinomas. J Breast Cancer (2012) 15:172–80. doi: 10.4048/jbc.2012.15.2.172
12. Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, et al. GLUT1 glucose transporter expression in colorectal carcinoma a marker for poor prognosis BACKGROUND. malignant cells exhibit increased glycolytic metabolism, and in. Cancer (1998) 83(1):34–40. doi: 10.1002/(SICI)1097-0142(19980701)83:1
13. Cooper R, Sarioğlu S, Sökmen S, Füzün M, Küpelioğlu A, Valentine H, et al. Glucose transporter-1 (GLUT-1): A potential marker of prognosis in rectal carcinoma? Br J Cancer 2003 895 (2003) 89:870–6. doi: 10.1038/sj.bjc.6601202
14. Brophy S, Sheehan KM, McNamara DA, Deasy J, Bouchier-Hayes DJ, Kay EW. GLUT-1 expression and response to chemoradiotherapy in rectal cancer. Int J Cancer (2009) 125:2778–82. doi: 10.1002/IJC.24693
15. Shim BY, Jung JH, Lee KM, Kim HJ, Hong SH, Kim SH, et al. Glucose transporter 1 (GLUT1) of anaerobic glycolysis as predictive and prognostic values in neoadjuvant chemoradiotherapy and laparoscopic surgery for locally advanced rectal cancer. Int J Colorectal Dis (2013) 28:375–83. doi: 10.1007/S00384-012-1542-3
16. Havelund BM, Sørensen FB, Lindebjerg J, Spindler KLG, Jakobsen A. Pretreatment HIF-1α and GLUT-1 expressions do not correlate with outcome after preoperative chemoradiotherapy in rectal cancer. Anticancer Res (2011) 31:1559–65.
17. Jun YJ, Jang SM, Han HL, Lee KH, Jang KS, Paik SS. Clinicopathologic signifcance of GULT1 expression and its correlation with apaf-1 in colorectal adenocarcinomas. World J Gastroenterol (2011) 17:1866–73. doi: 10.3748/wjg.v17.i14.1866
18. Lee-Kong SA, Ruby JA, Chessin DB, Pucciarelli S, Shia J, Riedel ER, et al. Hypoxia-related proteins in patients with rectal cancer undergoing neoadjuvant combined modality therapy. Dis Colon Rectum (2012) 55:990–5. doi: 10.1097/DCR.0b013e31825bd80c
19. Korkeila EA, Sundström J, Pyrhönen S, Syrjänen K. Carbonic anhydrase IX, hypoxia-inducible factor-1α, ezrin and glucose transporter-1 as predictors of disease outcome in rectal cancer: Multivariate cox survival models following data reduction by principal component analysis of the clinicopathological pre. Anticancer Res (2011) 31:4529–35.
20. Lastraioli E, Bencini L, Bianchini E, Romoli MR, Crociani O, Giommoni E, et al. hERG1 channels and glut-1 as independent prognostic indicators of worse outcome in stage I and II colorectal cancer: A pilot study. Transl Oncol (2012) 5:105–12. doi: 10.1593/tlo.11250
21. Saigusa S, Toiyama Y, Tanaka K, Okugawa Y, Fujikawa H, Matsushita K, et al. Prognostic significance of glucose transporter-1 (GLUT1) gene expression in rectal cancer after preoperative chemoradiotherapy. Surg Today (2012) 42:460–9. doi: 10.1007/s00595-011-0027-2
22. Kang BH, Song C, Kang SB, Lee KW, Lee HS, Kim JS. Nomogram for predicting the pathological tumor response from pre-treatment clinical characteristics in rectal cancer. Anticancer Res (2020) 40:2171–7. doi: 10.21873/anticanres.14177
23. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science (2016) 352:175–80. doi: 10.1126/science.aaf4405
24. Van Gijn W, Marijnen CAM, Nagtegaal ID, Kranenbarg EMK, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol (2011) 12:575–82. doi: 10.1016/S1470-2045(11)70097-3
25. Song C, Chung J-H, Kang S-B, Kim D-W, Oh H-K, Lee HS, et al. Impact of tumor regression grade as a major prognostic factor in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: A proposal for a modified staging system. Cancers (2018) 10:319. doi: 10.3390/CANCERS10090319
26. Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor dna analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol (2019) 5:1710–7. doi: 10.1001/jamaoncol.2019.3616
27. Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages i to III colorectal cancer. JAMA Oncol (2019) 5:1124–31. doi: 10.1001/jamaoncol.2019.0528
28. Kotani D, Oki E, Nakamura Y, Yukami H, Mishima S, Bando H, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med (2023) 29(1):127–34. doi: 10.1038/s41591-022-02115-4
29. Alvarez J, Cercek A, Mohan N, Cuaron JJ, Zinovoy M, Reyngold M, et al. (ctDNA) for response assessment in patients with anal cancer treated with definitive chemoradiation. J Clin Oncol (2023) 41(4_suppl):1. doi: 10.1200/JCO.2023.41.4_suppl.1
30. Carlson DJ, Keall PJ, Loo BW, Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol (2011) 79:1188–95. doi: 10.1016/J.IJROBP.2010.10.007
Keywords: Glucose transporter-1 (GLUT-1), neoadjuvant chemoradiation (CRT), rectal cancer (RC), survival, metastasis
Citation: Kim TH, Kwak Y, Song C, Lee HS, Kim D-W, Oh H-K, Kim JW, Lee K-W, Kang S-B and Kim J-S (2023) GLUT-1 may predict metastases and death in patients with locally advanced rectal cancer. Front. Oncol. 13:1094480. doi: 10.3389/fonc.2023.1094480
Received: 10 November 2022; Accepted: 20 February 2023;
Published: 09 March 2023.
Edited by:
Chi Lin, University of Nebraska Medical Center, United StatesReviewed by:
Martin Leu, University Medical Center Göttingen, GermanyCopyright © 2023 Kim, Kwak, Song, Lee, Kim, Oh, Kim, Lee, Kang and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changhoon Song, c29uZ2NAc251Ymgub3Jn; Jae-Sung Kim, anNraW1Ac251Ymgub3Jn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.