
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 26 April 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1094040
 Wei Zhang1†
Wei Zhang1† Hongyuan Jia2†
Hongyuan Jia2† Xue Chen2
Xue Chen2 Wei Diao1
Wei Diao1 Xuefeng Leng3
Xuefeng Leng3 Bangrong Cao4
Bangrong Cao4 Yi Wang2
Yi Wang2 Zhuzhong Cheng1*
Zhuzhong Cheng1* Qifeng Wang2*
Qifeng Wang2*Objective: To investigate the predicting prognosis and guiding postoperative chemoradiotherapy (POCRT) value of preoperative mean platelet volume (MPV) in patients with locally advanced esophageal squamous cell carcinoma (LA-ESCC).
Methods: We proposed a blood biomarker, MPV, for predicting disease-free survival (DFS) and overall survival (OS) in LA-ESCC patients who underwent surgery (S) alone or S+POCRT. The median cut-off value of MPV was 11.4 fl. We further evaluated whether MPV could guide POCRT in the study and external validation groups. We used multivariable Cox proportional hazard regression analysis, Kaplan–Meier curves, and log-rank tests to ensure the robustness of our findings.
Results: In the developed group, a total of 879 patients were included. MVP was associated with OS and DFS defined by clinicopathological variables and remained an independent prognostic factor in the multivariate analysis (P = 0.001 and P = 0.002, respectively). For patients with high MVP, 5-year OS and 0DFS were significantly improved compared to those with low MPV (P = 0.0011 and P = 0.0018, respectively). Subgroup analysis revealed that POCRT was associated with improved 5-year OS and DFS compared with S alone in the low-MVP group (P < 0.0001 and P = 0.0002, respectively). External validation group analysis (n = 118) showed that POCRT significantly increased 5-year OS and DFS (P = 0.0035 and P = 0.0062, respectively) in patients with low MPV. For patients with high MPV, POCRT group showed similar survival rates compared with S alone in the developed and validation groups.
Conclusions: MPV as a novel biomarker may serve as an independent prognosis factor and contribute to identifying patients most likely to benefit from POCRT for LA-ESCC.
Esophageal cancer is a malignant disease with high morbidity and mortality rates worldwide (1), and half of all attributable deaths and incidences occur in China (2). Clinical treatment mainly depends on the clinical stage and pathological type to guide comprehensive treatment (3). For patients with locally advanced esophageal squamous cell carcinoma (LA-ESCC) (T3-4N0 and T1-4N1-3M0), postoperative adjuvant radiotherapy and chemotherapy are recommended (4–7). However, there are still significant differences in treatment outcomes even when patients receive the same combination of treatment patterns under the same staging conditions (8, 9). These results indicate that the current clinicopathological risk stratification model is not an appropriate guide for precise treatment. Some patients do not benefit from postoperative adjuvant chemoradiotherapy, but experience shortened survival due to side effects. There is a critical need for additional prognostic and/or predictive biomarkers beyond the current staging system, which may be used to better inform prognosis and guide treatment strategies (10, 11).
Over the past few decades, increased interest has been found in establishing novel non-invasive predictive biomarkers from hematological serological parameters for various tumors, such as: carcinoembryonic antigen, C-reactive protein, and platelet-related parameters (12, 13). With the ease obtained from routine blood tests, the platelet-related parameters attracted numbers of considerable attention as prognostic indicators in various cancer, including esophageal cancer. Increase numbers of evidence have revealed that platelet activation plays a vital role in tumor growth, invasion, and metastasis (14, 15). As an indicator of platelet activation, mean platelet volume (MPV), was regarded as closely associated with thromboembolism in patients with ischemic stroke, myocardial infarction, and cerebrovascular thromboembolism (16). Recent research has found that MPV levels were significantly higher in colorectal cancer, ovarian cancer, gastric cancer, hepatocellular carcinoma and papillary thyroid carcinomas than in healthy subjects, indicating increased MPV level may serve as an independent prognostic factor for cancer patients (17).
In esophagus cancer, however, the prognosis value of MPV has not yet been comprehensively investigated. He et al. found that decreased MPV is significantly associated with poor prognosis in esophageal squamous cell carcinoma patients (18). However, the results of a recent meta-analysis included 3 studies indicating that MPV were non-independent prognostic factors for OS in patients with esophageal squamous cell carcinoma (19). Therefore, this study aimed to evaluate the relationship between preoperative MPV and long-term survival in patients with LA-ESCC. Moreover, another further aim was to investigate the guiding treatment value of MPV to identify LA-ESCC patients who are most likely to benefit from postoperative adjuvant chemoradiotherapy by developed and external validation group.
Between January 2008 and December 2017, we retrospectively identified 1,942 patients with LA-ESCC who underwent esophagectomy with curative intent, which served as developed group. The eligibility criteria were: 1. histologically proven thoracic LA-ESCC; 2. curative R0 resection; 3. the surgical technique used was standard McKeown esophagectomy or Ivor-Lewis esophagectomy; 4. pathologically proven stage IIB–IVa ESCC based on the 8th edition American Joint Committee on Cancer (AJCC) staging system;5. age ≥18 years with a Karnofsky performance status (KPS) ≥70; 6. adequate bone marrow, renal, and hepatic functions; 7. routine blood test one week before the operation, including MPV; and 8. fit for postoperative chemoradiation. The exclusion criteria were cervical esophageal tumors, preoperative chemoradiation or chemotherapy, postoperative chemotherapy, or radiotherapy alone, and other pathological types, such as adenocarcinoma and small cell carcinoma. The study protocol was approved by the appropriate institutional ethics review board (SCCHEC-02-2020-015) and that of Cancer Hospital, Chinese Academy of Medical Sciences (ID:14-090/880). For external validation, we selected 172 patients with lymph node-positive or stage III ESCC who were enrolled in a prospective phase III randomized controlled trial (20) from October 2014 through December 2019. A flow chart of patient selection is shown in Figure 1.
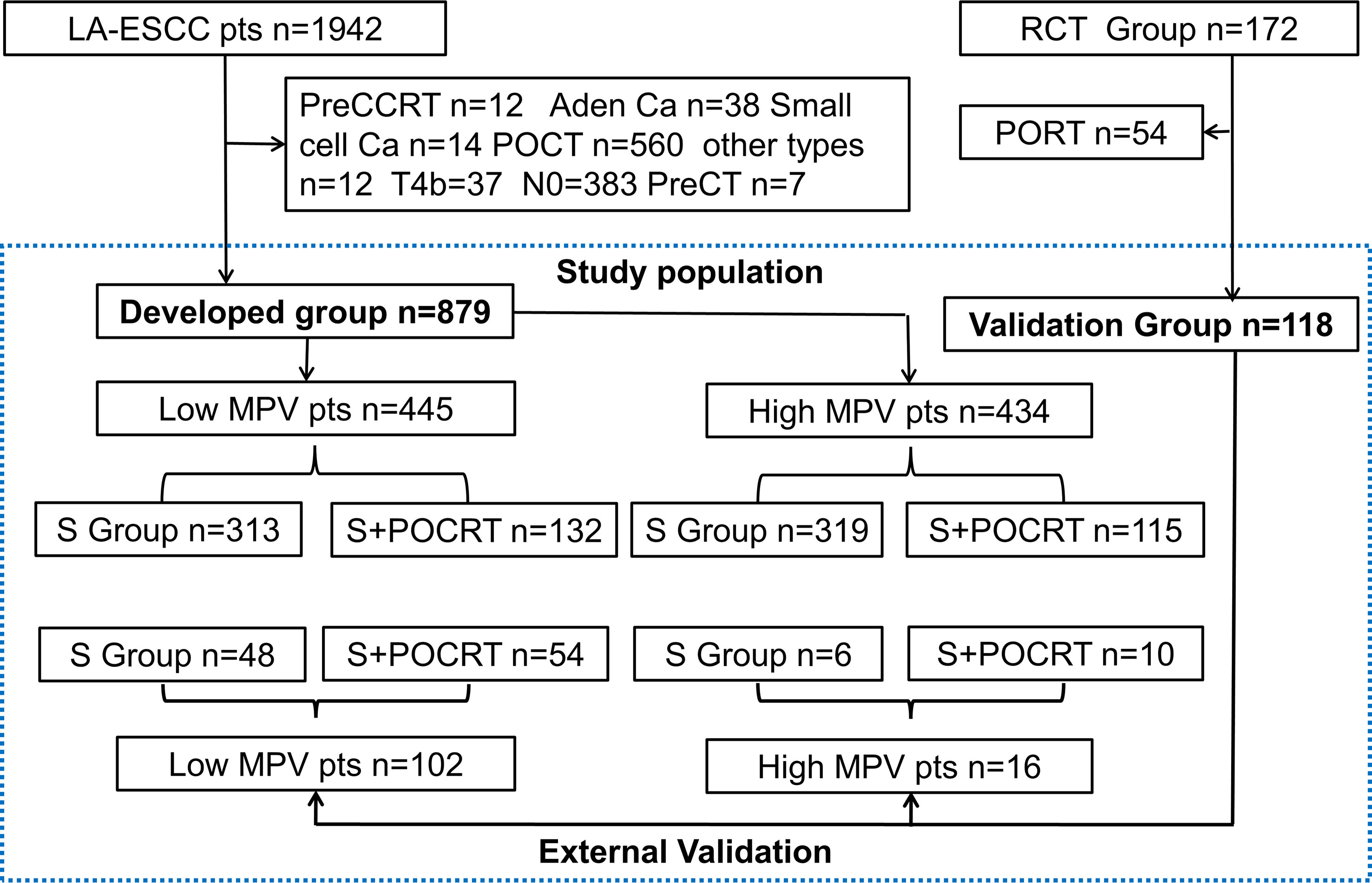
Figure 1 The study flow diagram. LA-ESCC, local advanced esophageal squamous cell carcinoma; preCCRT, preoperative concurrent chemoradiation; Ca, carcinoma; MPV, mean platelet volume; POCRT, postoperative chemoradiotherapy; PORT, postoperative radiotherapy; SA, surgery alone; TNM, Tumor Nodes Metastasis; VATS, Video-assisted thoracoscopic surgery. RCT, randomized clinical trial.
All patients received intravenous and inhalation-based general anesthesia. The surgical techniques used were standard McKeown esophagectomy (n=702) and Ivor-Lewis esophagectomy (n=177). Surgical approaches included minimally invasive esophagectomy (n=439) and open esophagectomy (n=440). Pathological staging of the surgical specimen was based on the 8th edition of the TNM classification for esophageal cancer (21). The validation treatment has been previously described in detail by Ni et al. (20).
Radiotherapy began 4–10 weeks after surgery. Computed tomography was used to identify anatomical landmarks and delineate mediastinal lymph node stations. The clinical target volume (CTV) was defined as the tumor bed and the high-risk lymphatic drainage area. Anastomosis was included in the CTV for patients with upper thoracic tumors and those with an insufficient proximal margin (<3 cm). Radiotherapy involved a total dose of 50–54 Gy delivered to 95% of the planning target volume in 25–30 fractions (five fractions/week for 5–6 weeks).
Platinum drugs were mainly used for chemotherapy during postoperative chemoradiotherapy (POCRT). Cisplatin-based, nedaplatin-based, oxaliplatin-based, and carboplatin-based regimens were used for 92, 30, 30, and 54 patients, respectively. In addition, Tegafur, Gimeracil and Oteracil Porassium Capsules treatment was administered to 41 patients.
The correlation between preoperative MPV values and the clinical pathology of patients with LA-ESCC was analyzed. Patients were divided into four groups according to the median MPV value (low ≤ 11.4 fl v.s. high >11.4 fl) and different treatments (surgery alone (S alone) or S+POCRT). The clinicopathological indicators of different MPV groups (low and high) were compared and analyzed using the Chi-square test or Fisher’s exact test. The univariate analysis included sex, age, KPS score, tumor length, tumor location, tumor differentiation, lymphovascular invasion, nerve invasion, number of lymph node dissections, pathological TNM stage, and POCRT. Multivariate Cox regression analysis was performed for variables with P < 0.1 in the univariate analysis. Overall survival (OS) and disease-free survival (DFS) of patients with low- and high-MVP were compared using the Kaplan–Meier method, and the log-rank test was used for patients that underwent S alone. Kaplan–Meier curves and log-rank tests were used for stratification according to the MPV group to compare the survival difference between the S+POCRT and S alone group. For external validation, we selected patients from a prospective randomized stage III study to evaluate whether POCRT improved OS and DFS compared with S alone in the low MPV group. Statistical analysis was performed using R software V. 3.5.1 (https://www.Rproject.org/). Statistical significance was set at P < 0.05.
In this study, a total of 1,942 patients were identified as LA-ESCC for developed group, and finally, a total of 879 patients (632 for S alone and 247 for S+POCRT) were included for further analysis. After excluding the S plus postoperative radiotherapy group (n = 54), finally, a total of 118 patients (S alone (n = 54) and S+POCRT (n= 64)) were included for further validation analysis (Table S1). Based on the median MPV value of 11.4 fl, the patients were divided into two groups (low MPV, n = 445; high MPV group, n = 434). Figure 1 presented the detailed study flow. Table 1 presented the detailed clinicopathological characteristics, in which 62.9% (553) of patients were men with a median age of 64 (range, 39–85) years and a median follow-up period of 42.5 (range, 24–116) months. The 5-year OS and DFS rates in the overall study cohort were 35.2% and 28.0%, respectively.
Overall, significant associations were found between the MPV and factors such as sex (P < 0.001), lymphovascular invasion (P = 0.027), and lymph node metastasis (P = 0.039), and no significant differences in age (P = 0.292), tumor location (P = 0.568), T stage (P = 0.325) and treatment (P = 0.333) (Table 1).
The Kaplan–Meier curves exhibited that patients with low MPV had a worse OS (P < 0.001, Figure 2A) compared with the high MPV group. For patients with high MPV (n = 434), 5-year OS was significantly improved compared to those with low MPV (n = 445) (40.3% vs. 30.4%, P = 0.001, Figure 2A). Cox multivariate analysis showed that low MPV was associated with worse OS (HR 0.75, 95%CI: 0.64 – 0.89, P = 0.001, Table 2). Compared with the S alone group, the S+POCRT group had significantly better 5-year OS outcomes (44.9% vs. 31.0%, P = 0.0005, Figure 2C). Cox multivariate analysis showed that S+POCRT was associated with improved OS (HR 0.71, 95%CI: 0.58 – 0.86, P = 0.001, Table 2) as independent prognostic factors.
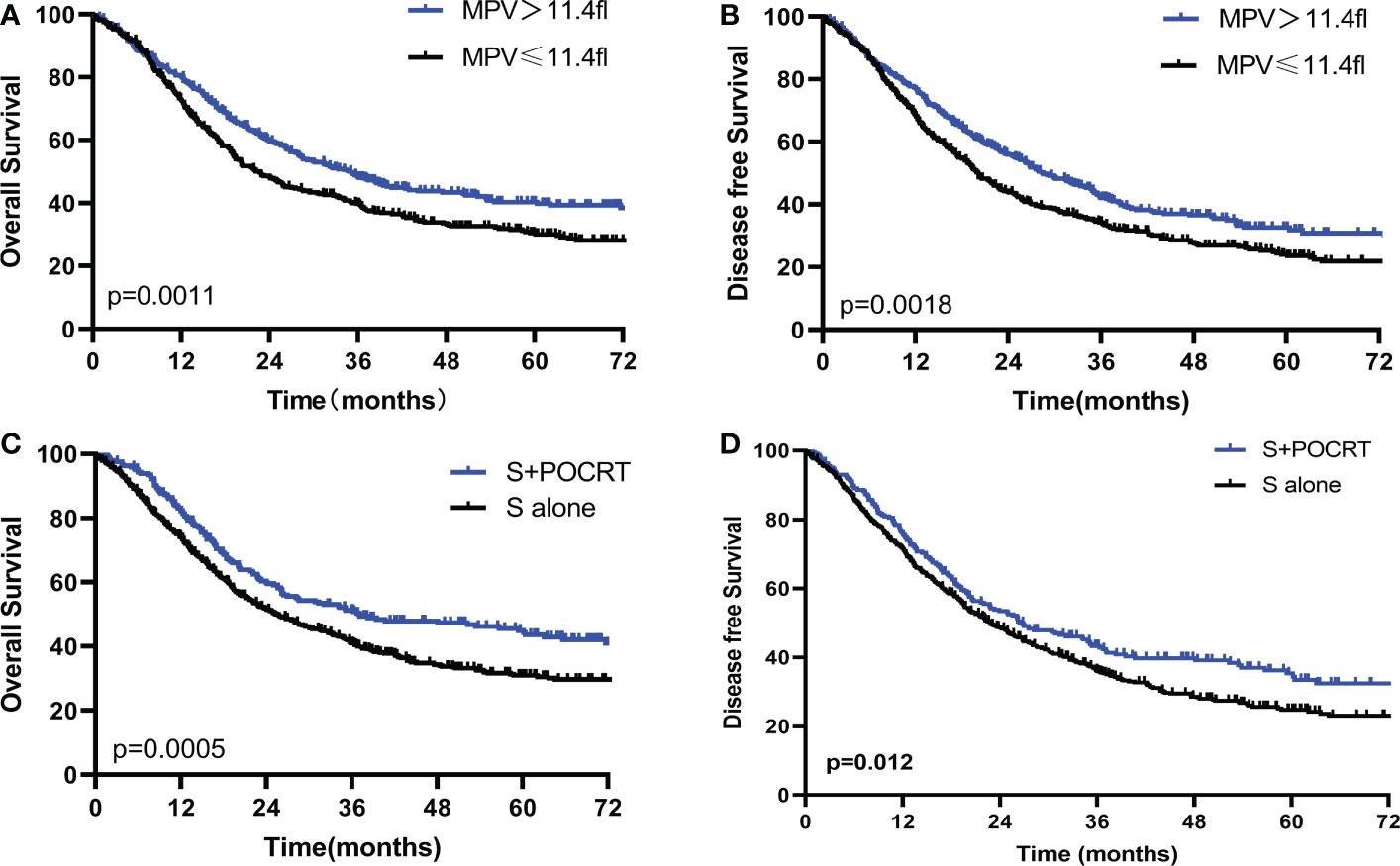
Figure 2 Comparison of overall survival and disease-free survival between high and low MPV (A, B), and POCRT and SA group (C, D) in the developed population. POCRT, postoperative chemoradiotherapy; SA, surgery alone; MPV, mean platelet volume.
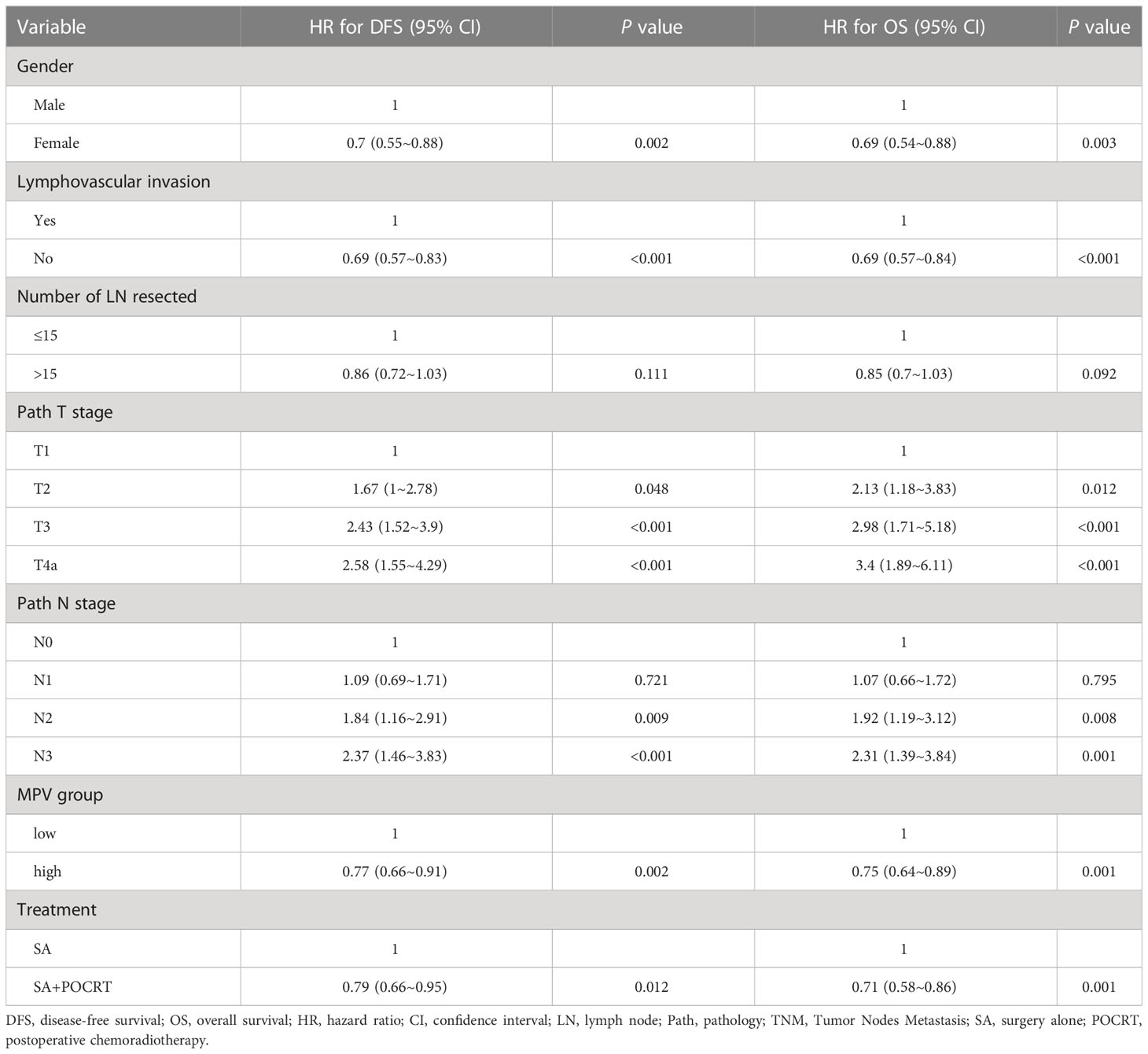
Table 2 Multivariable analysis associations between Mean platelet volume and Overall survival and disease-free survival.
For DFS, patients with low MPV had a worse OS compared with the high MPV group (5-year DFS: 32.6% vs. 24.1%, P =0.001, Figure 2B). Cox multivariate analysis showed that low MVP was associated with worse OS (HR 0.77, 95%CI: 0.66 – 0.91, P = 0.002, Table 2). Compared with the S alone group, the S+POCRT group had significantly better 5-year DFS outcomes (35.3% vs. 24.7%, P = 0.012, Figure 2D). Cox multivariate analysis showed that S+POCRT was associated with improved DFS (HR 0.79, 95%CI: 0.66 – 0.95, P = 0.001, Table 2) as independent prognostic factors.
To further evaluate the value of MPV guidance in POCRT for LA-ESCC, patients were divided into four groups according to treatment methods (S and S+POCRT) and MPV (Table S2). For patients with low MPV, the S+POCRT group had significantly better 5-year OS and DFS outcomes (OS: 46.5%; P < 0.0001, Figure 3A; DFS: 37.8%; P = 0.0002, Figure 3B) than that of the S alone group (OS: 23.0%; DFS: 17.8%). Cox multivariate analysis showed that S+POCRT was associated with improved OS and DFS as independent prognostic factors, independent of pTNM stage (both P < 0.001, Table S3). For patients with high MPV, S+POCRT had similar 5-year OS and DFS outcomes (OS 39.2% vs. 43.0%, P = 0.481; DFS 32.2% vs. 36.6%, P = 0.386, Figures S1A–D), relative to the S alone group. Cox multivariate analysis showed that S+POCRT was not associated with OS and DFS (Table S4; Figures S1A, B) in patients with high MPV.
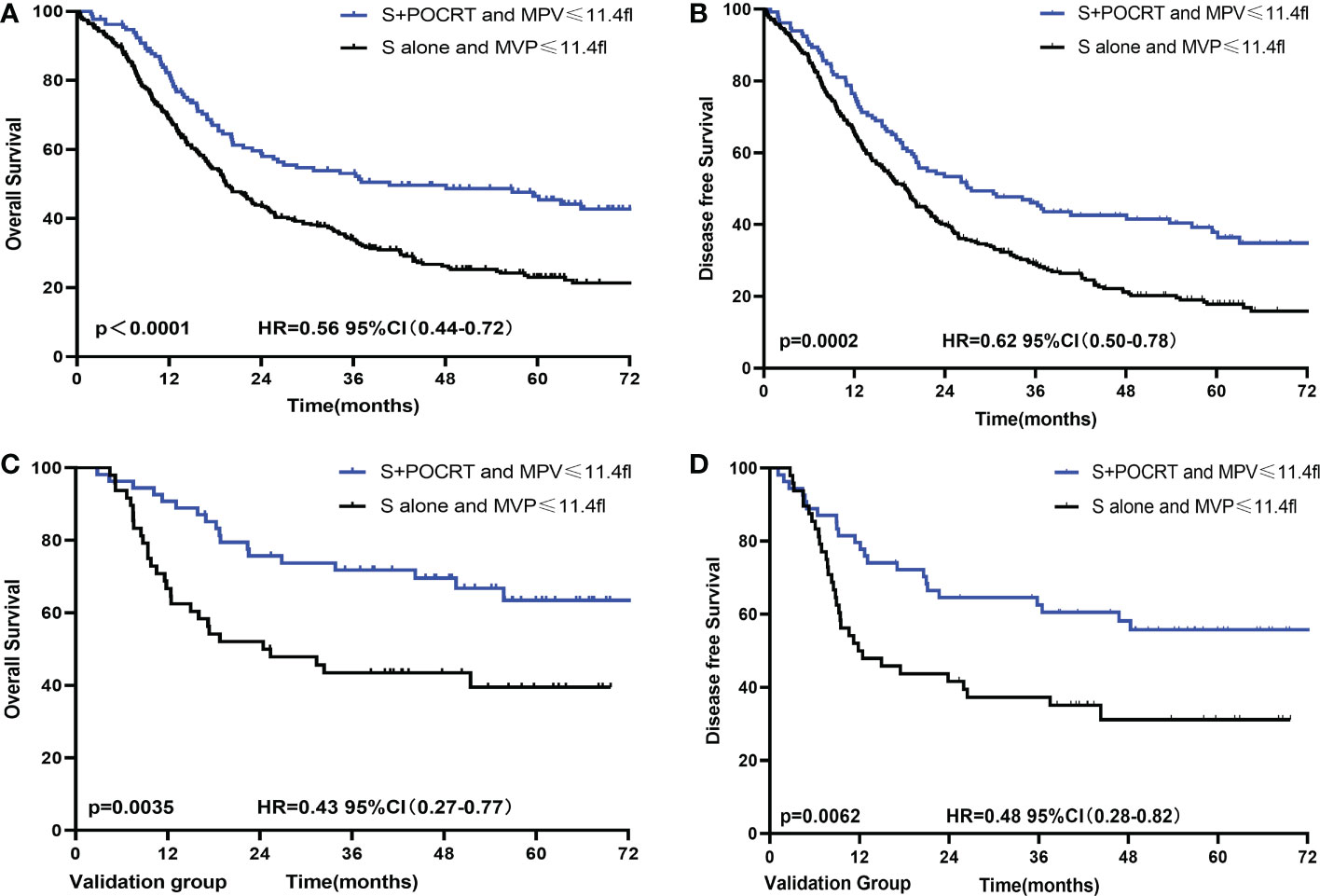
Figure 3 Comparison of overall survival and disease-free survival between POCRT and SA group for low MPV in developed (A, B) and validation (C, D) population. CI, confidence interval; HR, hazard ratio; POCRT, postoperative chemoradiotherapy; SA, surgery alone; MPV, mean platelet volume.
The external validation group comprised patients from a prospective clinical trial. These patients were divided into different MPV groups (cutoff value = 11.4 fl, Table 3). For patients with a low MPV, the S+POCRT group had significantly better OS outcomes (5-year OS: 63.4% vs. 39.5%; P = 0.0035; Figure 3C) than did patients with a high MPV. Compared with the S alone group (5-year DFS: 31.2%), the S+POCRT group had better DFS outcomes (5-year DFS: 55.8%; P = 0.0062; Figure 3D). In the subgroup analysis of patients with low MPV, Cox multivariate analysis showed that S+POCRT was associated with OS and DFS as independent prognostic factors (P = 0.014; Table S5). For patients with high MPV, relative to the S alone group, S+POCRT had similar 5-year OS and DFS outcomes (Figures S1C, D).
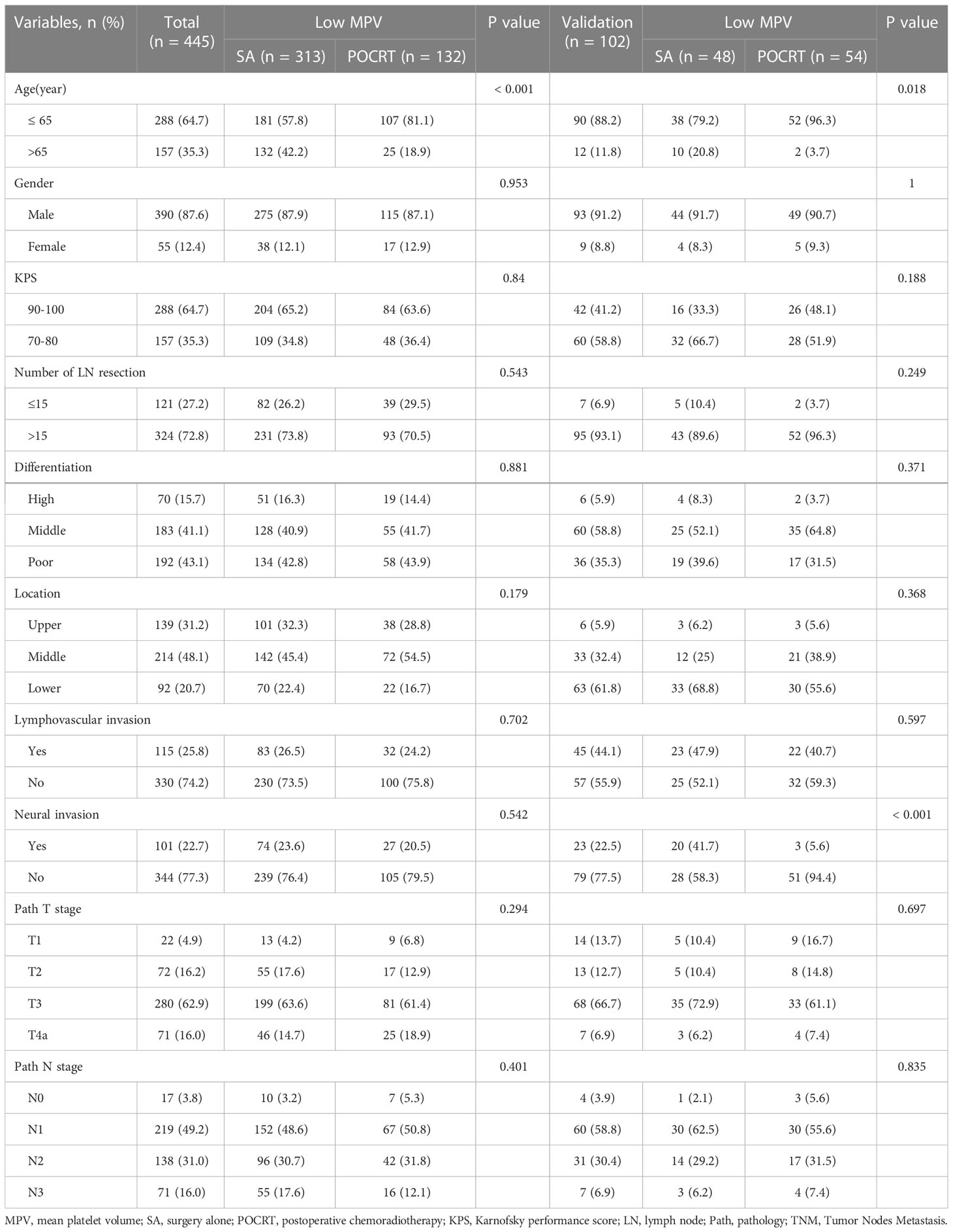
Table 3 Clinicopathological factors in developed and validation group with low mean platelet volume.
Esophagus cancer is a highly lethal malignancy (1). For LA-ESCC, comprehensive treatment is the main method used to improve the therapeutic effect. Clinical stage and pathological type are mainly tools for guiding treatment. Despite receiving similar treatment patterns and having similar staging conditions, patients may still experience significant differences in treatment outcomes. This is largely due to the fact that pathological staging does not consider the tumor microenvironment. Considering the limitation of pathology in guiding treatment, lots of novel non-invasive predictive biomarkers were developed and used to guide treatment strategies. The MPV is an indicator of platelet activation and is considered a novel biomarker for predicting the prognosis of various cancer, which a decreased MPV is significantly associated with a poor prognosis (18). For esophagus cancer, however, the prognosis value of MPV is still unclear. In this study, we found MPV can serve as an independent prognostic factor for LA-ESCC, and patients with low MPV were significantly associated with worse 5-year OS (HR 0.75, 95%CI: 0.64 – 0.89, P = 0.001) and DFS (HR 0.77, 95%CI: 0.66 – 0.91, P = 0.002).
Decreased MPV was found associated with poor prognosis in LA-ESCC. However, the cause of decreased MPV in LA-ESCC remains unclear (15, 22). The main cause of low MPV, an important indicator of platelet function, may be chronic inflammation which caused the excessive platelet consumption (17). Consistent with our findings, Shen et al. (23) also found that a lower MPV was associated with poor prognosis in esophageal cancer, although they used a different cutoff value, which may be attributable to differences in pathological staging and number of patients. Both our study and Shen’s study found a significant correlation between low MPV and lymphovascular infiltration and lymph node metastasis. Different MPVs have different prognostic values for different types of cancer (17). For gastric cancer, Shen et al. reported that increased MPV was associated with poor prognosis (23), whereas high MPV in blood tumors, renal cancer, hepatocellular carcinoma, and lung cancer is associated with advanced-stage or an unfavorable disease-like thrombotic state (24, 25).
With significantly improved overall survival, postoperative adjuvant radiotherapy and chemotherapy are the recommended treatment strategy for LA-ESCC (20, 26, 27). However, the treatment results under the pathological staging guide are controversial (5, 7, 8). A meta-analysis including 11 articles and a total of 2,047 ESCC patients found that the 3-year OS between adjuvant chemotherapy (n = 887) and S alone (n = 1160) groups) was not significantly different (risk ratio = 0.89, 95%CI, 0.72 -1.09; P= 0.25) (9). In this study, we found that compared with the S alone group, the S+POCRT group had significantly improved 5-year OS outcomes (HR 0.71, 95%CI: 0.58 – 0.86, P = 0.001) and DFS (HR 0.79, 95%CI: 0.66 – 0.95, P = 0.001). Therefore, standard adjuvant treatment remains controversial. Individualized and accurate identification of patients who can benefit from adjuvant radiotherapy and chemotherapy is important for improving the curative effect.
Another important finding of this study is that the MPV level can be used as a guide for selecting treatment for LA-ESCC patients. Patients with low MPV may experience improved 5-year OS and DFS outcomes with POCRT, while patients with high MPV may have similar 5-year OS (P = 0.481) and DFS outcomes (P = 0.386) as the S alone group but underwent additional side effects of POCRT. A similar result was found in a limited prospective clinical trial. Therefore, LA-ESCC patients with high MPV which indicates low risk for recurrence and metastasis, avoid choosing the postoperative radiotherapy therapeutic schedule. A small side effects treatment scheme, such as the Nivolumab which has proven curative effects by the checkmate 577 study, may be an optimal treatment option (28). For patients who did not achieve pathological complete response after receiving neoadjuvant chemoradiation, postoperative adjuvant immunotherapy can significantly improve disease-free progression.
This study has some limitations. First, it was a retrospective study. A large sample size and multicentric prospective study is needed to carry out to investigate the prognostic significance and POCRT guiding value of MPV in LA-ESCC. Second, the optimal cut-off value in this study was determine by median MPV value (11.4 fl) in developed group and 11.4 fl was directly used as the cut-off value in the external validation group, which may lead to inaccuracy grouping. A more accurate methods are needed to determine the optimal cut-off value in the further studies. Third, this study cannot represent early esophageal cancer, nor can it guide preoperative radiotherapy and chemotherapy, which are all aspects that must be studied in the future.
In conclusion, MPV can serve as an independent prognostic factor for LA-ESCC. Patients with low MPV were significantly associated with poor prognoses. Additionally, MPV level can contribute to treatment selection for LA-ESCC patients, which patients with low MPV may benefit from POCRT, resulting in an improved 5-year survival rate. Although MPV is a non-specific marker, this noninvasive, convenient, and inexpensive biomarker may complement the present pTNM staging in guiding treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The Sichuan Cancer Hospital ethics committee. The patients/participants provided their written informed consent to participate in this study.
In this paper, WZ and HJ conceived and designed the study. WZ and HJ performed data collection, manuscript preparation. XC performed manuscript preparation. WD performed manuscript improved. XL, BC and YW performed statistical analysis, and data interpretation. ZC and QW guaranteed the integrity of the entire study and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Department of Sichuan Province (Grant Nos. 2023YF0488, 2023YFQ0055, 2023NSFSC1719, 2019YFS0378, and 2018SZ0210).
We would like to thank the Zefen Xiao, the corresponding author of reference 20, for providing the data of patients in external validation group. We would like to thank Editage for assistance in English language writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1094040/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Rice TW, Chen LQ, Hofstetter WL, Smithers BM, Rusch VW, Wijnhoven BP, et al. Worldwide esophageal cancer collaboration: pathologic staging data. Dis Esophagus (2016) 29(7):724–33. doi: 10.1111/dote.12520
4. Wang Q, Lang J, Li T, Peng L, Dai W, Jiang Y, et al. Postoperative adjuvant chemotherapy versus chemoradiotherapy for node-positive esophageal squamous cell carcinoma: a propensity score-matched analysis. Radiat Oncol (2020) 15(1):119. doi: 10.1186/s13014-020-01557-9
5. Wang Q, Peng L, Li T, Dai W, Jiang Y, Xie T, et al. Postoperative chemotherapy for thoracic pathological T3n0m0 esophageal squamous cell carcinoma. Ann Surg Oncol (2020) 27(5):1488–95. doi: 10.1245/s10434-019-08112-1
6. Yang J, Zhang W, Xiao Z, Wang Q, Zhou Z, Zhang H, et al. The impact of postoperative conformal radiotherapy after radical surgery on survival and recurrence in pathologic T3n0m0 esophageal carcinoma: a propensity score-matched analysis. J Thorac Oncol (2017) 12(7):1143–51. doi: 10.1016/j.jtho.2017.03.024
7. Speicher PJ, Englum BR, Ganapathi AM, Mulvihill MS, Hartwig MG, Onaitis MW, et al. Adjuvant chemotherapy is associated with improved survival after esophagectomy without induction therapy for node-positive adenocarcinoma. J Thorac Oncol (2015) 10(1):181–8. doi: 10.1097/jto.0000000000000384
8. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
9. Zhang SS, Yang H, Xie X, Luo KJ, Wen J, Bella AE, et al. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus (2014) 27(6):574–84. doi: 10.1111/dote.12073
10. Wang Q, Cao B, Chen J, Li C, Tan L, Zhang W, et al. Tumor compactness based on ct to predict prognosis after multimodal treatment for esophageal squamous cell carcinoma. Sci Rep (2019) 9(1):10497. doi: 10.1038/s41598-019-46899-x
11. Zhang W, Zhu H, Liu X, Wang Q, Zhang X, He J, et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann Thorac Surg (2014) 98(2):513–9. doi: 10.1016/j.athoracsur.2014.03.015
12. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
13. Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, et al. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol (2012) 19(13):4168–77. doi: 10.1245/s10434-012-2498-9
14. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood (2015) 126(5):582–8. doi: 10.1182/blood-2014-08-531582
15. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol (2018) 11(1):125. doi: 10.1186/s13045-018-0669-2
16. Hirahara N, Matsubara T, Kawahara D, Mizota Y, Ishibashi S, Tajima Y. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Clin Oncol (2016) 21(5):909–19. doi: 10.1007/s10147-016-0986-9
17. Pyo JS, Sohn JH, Kang G. Diagnostic and prognostic roles of the mean platelet volume in malignant tumors: a systematic review and meta-analysis. Platelets (2016) 27(8):722–8. doi: 10.3109/09537104.2016.1169265
18. Sun SY, Zhao BQ, Wang J, Mo ZX, Zhao YN, Wang Y, et al. The clinical implications of mean platelet volume and mean platelet Volume/Platelet count ratio in locally advanced esophageal squamous cell carcinoma. Dis Esophagus (2017) 31(2):1–6. doi: 10.1093/dote/dox125
19. Ishibashi Y, Tsujimoto H, Sugasawa H, Kouzu K, Itazaki Y, Sugihara T, et al. Prognostic value of platelet-related measures for overall survival in esophageal squamous cell carcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol (2021) 164:103427. doi: 10.1016/j.critrevonc.2021.103427
20. Ni W, Yu S, Xiao Z, Zhou Z, Chen D, Feng Q, et al. Postoperative adjuvant therapy versus surgery alone for stage iib-iii esophageal squamous cell carcinoma: a phase iii randomized controlled trial. Oncologist (2021) 26(12):e2151–e60. doi: 10.1002/onco.13914
21. Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(4):304–17. doi: 10.3322/caac.21399
22. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des (2011) 17(1):47–58. doi: 10.2174/138161211795049804
23. Shen XM, Xia YY, Lian L, Zhou C, Li XL, Han SG, et al. Mean platelet volume provides beneficial diagnostic and prognostic information for patients with resectable gastric cancer. Oncol Lett (2016) 12(4):2501–6. doi: 10.3892/ol.2016.4913
24. Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet Volume/Platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer (2014) 83(1):97–101. doi: 10.1016/j.lungcan.2013.08.020
25. Zhang F, Chen Z, Wang P, Hu X, Gao Y, He J. Combination of platelet count and mean platelet volume (Cop-mpv) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol (2016) 37(7):9323–31. doi: 10.1007/s13277-015-4774-3
26. Bédard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer (2001) 91(12):2423–30. doi: 10.1002/1097-0142(20010615)91:12<2423::AID-CNCR1277>3.0.CO;2-1
27. Adelstein DJ, Rice TW, Rybicki LA, Saxton JP, Videtic GM, Murthy SC, et al. Mature results from a phase ii trial of postoperative concurrent chemoradiotherapy for poor prognosis cancer of the esophagus and gastroesophageal junction. J Thorac Oncol (2009) 4(10):1264–9. doi: 10.1097/JTO.0b013e3181b26f8e
Keywords: esophageal cancer, prognosis, mean platelet volume, postoperative chemoradiation, guiding
Citation: Zhang W, Jia H, Chen X, Diao W, Leng X, Cao B, Wang Y, Cheng Z and Wang Q (2023) Prognostic significance and postoperative chemoradiotherapy guiding value of mean platelet volume for locally advanced esophageal squamous cell carcinoma patients. Front. Oncol. 13:1094040. doi: 10.3389/fonc.2023.1094040
Received: 09 November 2022; Accepted: 03 April 2023;
Published: 26 April 2023.
Edited by:
Chi Lin, University of Nebraska Medical Center, United StatesReviewed by:
Hong Zhu, Sichuan University, ChinaCopyright © 2023 Zhang, Jia, Chen, Diao, Leng, Cao, Wang, Cheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuzhong Cheng, emh1emhvbmdjaGVuZ0B5ZWFoLm5ldA==; Qifeng Wang, bGl0dGxlY2FuY2VyQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.