
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 14 March 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1093469
This article is part of the Research TopicUpdates on Immune Checkpoint Inhibitors in Lung CancerView all 7 articles
Background: Atezolizumab may provide clinical benefits to patients with advanced non-small cell lung cancer (NSCLC). However, the price of atezolizumab is relatively high, and its economic outcomes have remained unclear. In this study, we used two models to examine the cost-effectiveness of initial atezolizumab monotherapy versus chemotherapy for patients with PD-L1 high-expressing EGFR and ALK wild-type advanced NSCLC in the context of the Chinese healthcare system.
Methods: Partitioned Survival model and Markov model were performed to evaluate the cost-effectiveness of first-line single-agent atezolizumab versus platinum-based chemotherapy for patients with advanced NSCLC with PD-L1 high-expressing EGFR and ALK wild-type disease. Clinical outcomes and safety information were obtained from the most recent data from the IMpower110 trial, while cost and utility values were obtained from Chinese hospitals and relevant literature. Total costs, life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were estimated. One-way and probabilistic sensitivity analyses were performed to explore model uncertainty. Scenario analyses were also conducted for the Patient Assistance Program (PAP) and various provinces in China.
Results: In the Partitioned Survival model, the total cost of atezolizumab was $145,038, providing 2.92 LYs and 2.39 QALYs, while the total cost of chemotherapy was $69,803, providing 2.12 LYs and 1.65 QALYs. The ICER for atezolizumab versus chemotherapy was $102,424.83/QALY; in the Markov model, the ICER was $104,806.71/QALY. Atezolizumab was not cost-effective at the WTP threshold of three times China’s per capita gross domestic product (GDP). Sensitivity analysis showed that the cost of atezolizumab, the utility of PFS, and the discount rate had a significant impact on ICER; PAP significantly reduced ICER, but atezolizumab was still not cost-effective in China.
Conclusion: First-line monotherapy with atezolizumab for patients with PD-L1 high-expressing EGFR and ALK wild-type advanced NSCLC was estimated to be less cost-effective than chemotherapy in terms of the Chinese healthcare system; offering PAP increased the likelihood that atezolizumab would be cost-effective. In some areas of China with higher levels of economic development, atezolizumab was likely to be cost-effective. To improve the cost-effectiveness of atezolizumab, drug prices would need to be reduced.
Lung cancer is the leading cause of global mortality among malignant tumors, with 2.2 million new cases and 1.8 million deaths worldwide in 2020, according to WHO/IARC (1). In China, lung cancer was the leading cause of cancer-related deaths in 2016, leading to approximately 828,000 new cases and 657,000 deaths (2). Lung cancer is histologically classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC); NSCLC accounts for about 85% of cases (3). Due to the lack of reliable markers for early diagnosis, approximately 60% or more were diagnosed as stages III and IV (4, 5), with 5-year overall survival rates of 15% to 20% (6). The median overall survival of conventional platinum-based two-drug regimens for patients with advanced NSCLC with wild-type driver genes is less than one year, and there is a need to improve their efficacy (7). In recent years, immune checkpoint inhibitors (ICIs) have made tremendous strides in tumor therapy, Multiple studies have shown the efficacy and safety of ICIs to be superior to conventional chemotherapy (8, 9). The NCCN recommends atezolizumab monotherapy as first-line treatment for advanced NSCLC in 2022, based on clinical evidence (10). The IMpower110 was a phase III, a multicenter, randomized, open-controlled trial conducted at 142 institutions in 19 countries (11). Subjects were randomized to atezolizumab and chemotherapy in a 1:1 ratio for each. Patients were divided into high-expressing and high- or intermediate-expressing groups according to PD-L1 expression, and treatment response was compared separately. On July 12, 2021, IMpower110 updated additional 17-month follow-up data, showing that the OS benefit was maintained in the high-expressing group [hazard ration (HR) = 0.76, 95% confidence interval (CI) = 0.54-1.09, median = 20.2 months vs. 14.7 months] (12). Atezolizumab monotherapy has been approved by the Food and Drug Administration (FDA) as first-line therapy for high PD-L1 metastatic NSCLC. In China, the National Medical Products Administration (NMPA) has approved several atezolizumab-related therapies for NSCLC.
Atezolizumab provides clinical benefit but at a relatively high cost. Based on preliminary data from IMpower110, only a few literature studies have examined the cost-effectiveness of first-line atezolizumab monotherapy for advanced NSCLC from a Chinese perspective (13, 14). IMpower110 was updated on July 12, 2021, and no economic studies based on new survival data have been conducted since. Therefore, to explore the cost-effectiveness of first-line single-agent atezolizumab chemotherapy for patients with PD-L1 high-expressing EGFR and ALK wild-type advanced NSCLC, we performed an economic study based on the updated IMpower110 from the perspective of the Chinese healthcare system using the Partitioned Survival model (PartSA model) and Dynamic Markov model (Markov model) were constructed.
Clinical information is derived from the most recent data from the IMpower110 study. Patients aged 18 years and older, histologically diagnosed with stage IV NSCLC (UICC/AICC 7th edition), epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) wild type, and no prior treatment for NSCLC; OS benefit was seen only in patients with high PD-L1 expression (PD-L1 expressing tumor cells 50% or more), so only they were included in the study, 107 patients in the atezolizumab group and 98 patients in the chemotherapy group, respectively Drug doses were as follows: The experimental group received 1,200 mg atezolizumab intravenously every 3 weeks; non-squamous cell carcinoma patients in the control group received cisplatin 75 mg/m2 + pemetrexed 500 mg/m2 or AUC6 carboplatin + pemetrexed 500 mg/m2 for 4 to 6 cycles (calculated as 5 cycles), followed by pemetrexed maintenance therapy; patients with squamous cell carcinoma in the control group received cisplatin 75 mg/m2 + gemcitabine 1,250 mg/m2 or AUC5 carboplatin + gemcitabine 1,000 mg/m2 for 4 to 6 cycles (calculated as 5 cycles), followed by best supportive care. Gemcitabine was used twice per cycle (12, 15).
The mean body surface area was calculated using body data from The Report on Nutrition and Chronic Diseases of Chinese Residents (2010) (16) and the IMpower110 trial, and the drug doses were calculated. The probability of receiving cisplatin and carboplatin was assumed to be equal, since 69.7% of the patients had squamous cell carcinoma and 30.3% had non-squamous cell carcinoma.
Treatment after disease progression was based on the patient’s condition. Secondary therapy was assumed, as shown in Supplementary Table 1, based on drugs whose use exceeded 5% in the IMpower110 study. Nivolumab was used once every 2 weeks.
PartSA and Markov models were constructed using TreeageProHealthcare2022 software. Patients were divided into three states: progression-free survival (PFS), progression disease (PD), and death (Death) (according to the Response Evaluation Criteria in Solid Tumors version 1.1). All simulated patients started in the PFS state, progressed through the Healthy state, and finally ended in the Death state (Figure 1). In the Markov model, the time-dependent transition probabilities between states were calculated with survival curve parameters, and the probability of natural mortality was assumed to be the probability from PFS to death, with a half-cycle correction; in the ParSA model, the proportion of patients in different states was calculated in the area divided by the PFS and OS curves. The treatment cycle was assumed to be 3 weeks, in accordance with the treatment schedule. The horizon of the simulation study was 10 years (170 cycles, during which most patients died).
Digital points were obtained from the OS and PFS curves reported in IMpower110 with GetData Graph Digitizer software, and individual data were reconstructed with Stata software. The median survival times of the reconstructed survival curves were compared with the original KM curves in Supplementary Table 2. As shown in Supplementary Table 3, we parameterized the reconstructed data using four survival distributions (Exponential, Weibull, Loglogistic, and lognormal) and finally evaluated the parametric survival distribution using the Akaike information criterion (AIC) and Bayesian information criterion (BIC). Finally, a lognormal distribution was chosen to fit the OS and PFS of the atezolizumab group and the OS of the chemotherapy group, resulting in the following survival function:
The PFS of the chemotherapy group was fitted with a logistic distribution, and a survival function was calculated:
The distribution parameters of the four survival curves are estimated in Table 1 and the survival curves are in Figure 2.
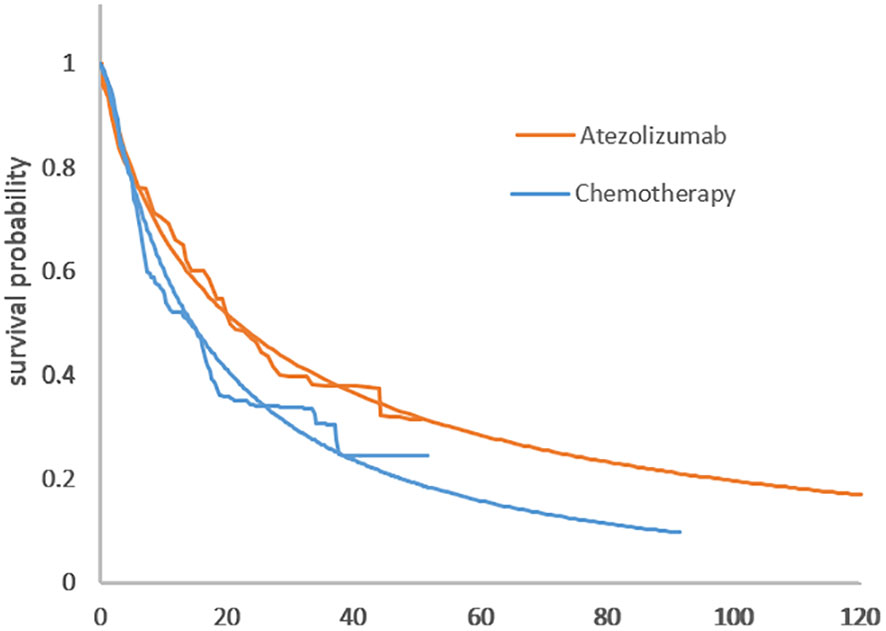
Figure 2 (A) Comparison of the adopted fitting curves and the KM survival curve of IMpower110. (A) PFS curve. (B) Comparison of KM survival curves with the fitting curve employed in IMpower110. (B) OS curve.
Only direct medical costs were considered, including drug acquisition costs, side effect costs, IHC costs, best supportive care costs, and follow-up costs. We used data from a Chinese drug information website (www.yaozh.com), treatment costs from the West China Hospital (WCH) of Sichuan University in 2021, and relevant literature. Drug costs were calculated by multiplying the unit cost of a drug by the number of drugs used; if one drug was not used up in one treatment, it was considered wasted. The health utility values were derived from a 2018 study by Chinese scholars (17) focusing on advanced NSCLC using Euro-QoL-5dimensions (EQ-5D). The model also included the diseconomies of side effects. A discount rate of 5% per annum was used. ICER was calculated as a measure of cost-effectiveness in accordance with the China Pharmacoeconomic Evaluation Guidelines (2020) (18); $34,928.54 (September 2022 exchange rate, $1 = RMB6.955), three times the per capita gross domestic product (GDP) of China in 2021, was used as the willingness-to-pay (WTP). All parameter information is presented in Table 2.
One-way sensitivity analysis (OSA) and probabilistic sensitivity analysis (PSA) were used to test the uncertainty of the model; OSA assumed that the prices of atezolizumab, pembrolizumab, and nivolumab could only be reduced and used ±20% of the base case value or 95% of confidence intervals were used as the basis for the range of uncertainty for each parameter. The results were displayed on a tornado diagram; PSA ran a Monte Carlo simulation with 1,000 iterations to generate a cost-effectiveness acceptability curve and a cost-effectiveness scatterplot to represent the results.
To improve the cost-effectiveness of covered drugs, the Chinese government has adopted a drug price negotiation mechanism, directly reduced the reimbursement price of medical insurance, discounted the entire course of treatment, and implemented a patient assistance program (PAP) (20). In the scenario analysis, it was assumed that half of the patients met the PAP requirements.
In China, there was a large gap between rich and poor in different provinces: the highest per capita GDP for provinces and cities in 2021 was $79,358.73 (Beijing) and the lowest was $17,704.96 (Gansu), a difference of 4.48 times. Assuming a WTP of three times the national per capita GDP may ignore regional differences. In the scenario analysis, we estimated the cost-effectiveness price of atezolizumab when the WTP was set at 1 and 3 times the GDP per capita for each region.
In the PartSA model, the total cost for the atezolizumab group was $145,038, yielding 2.92 LYs and 2.39 QALYs, while the total cost for the chemotherapy group was $69,803, yielding 2.12 LYs and 1.65 QALYs. The ICER for atezolizumab versus chemotherapy was $102,424.83/QALY; in the Markov model, the ICER was $104,806.71/QALY. Atezolizumab was not cost-effective at the WTP threshold of three times per capita GDP; when PAP was available, ICERs for atezolizumab and chemotherapy in the PartSA and Markov models were $49,213.93/QALY and $54,874.23/QALY, respectively. QALY; although PAP reduced costs significantly, atezolizumab was still not cost-effective at the WTP threshold (Table 3).
The Tornado diagram shows that the crucial parameters impacting ICER were similar in the two models. The cost of atezolizumab, the utility value of PFS, and the discount rate had a pronounced impact on ICER. For a range of parameters, ICER was higher than WTP, making atezolizumab not cost-effective for chemotherapy (Figures 3A, B) In the presence of PAP, the cost of atezolizumab, the discount rate, the cost of pembrolizumab, and the utility value for PFS had a significant impact on ICER. When the price of atezolizumab was reduced to $4,187.92 (PartSA model) or $3936.64 (Markov model), atezolizumab was cost-effective at the WTP threshold (Figures 3C, D).
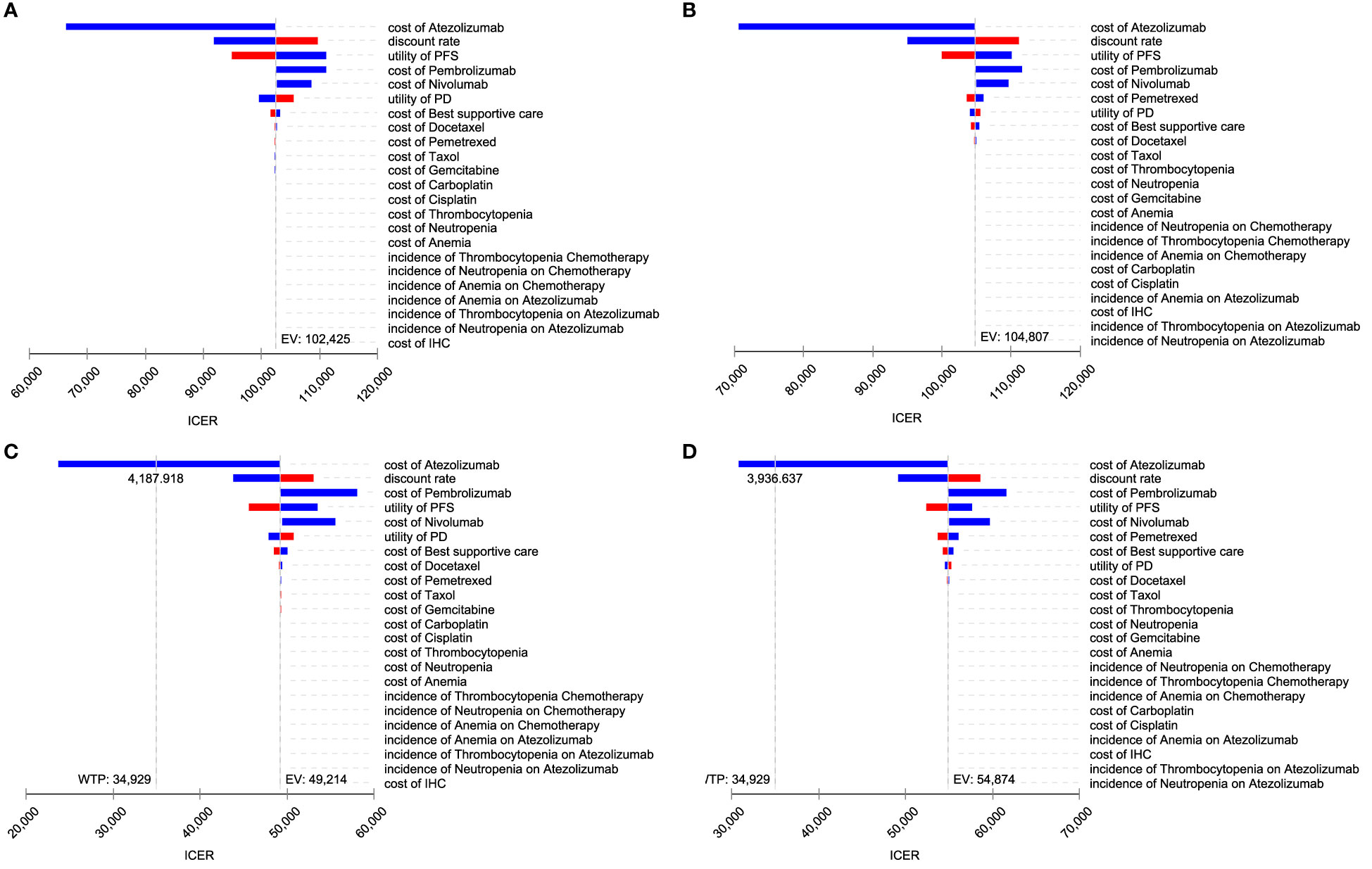
Figure 3 Toronto diagram of one-way sensitivity analysis. (A) PartSA mode. (B) Markov model. (C) PartSA model when PAP is available. (D) Markov model when PAP is available.
For PSA, the cost-effectiveness scatterplots (Supplementary Figure 1) and cost-effectiveness acceptability curves (Figure 4) indicate that atezolizumab provides more QALYs at a higher cost At the WTP threshold, the probability that atezolizumab is cost-effective is 0% At the WTP threshold of greater than $102,424.83 (PartSA model) or $104,806.71 (Markov model), the probability that atezolizumab monotherapy was more cost-effective than chemotherapy increased.
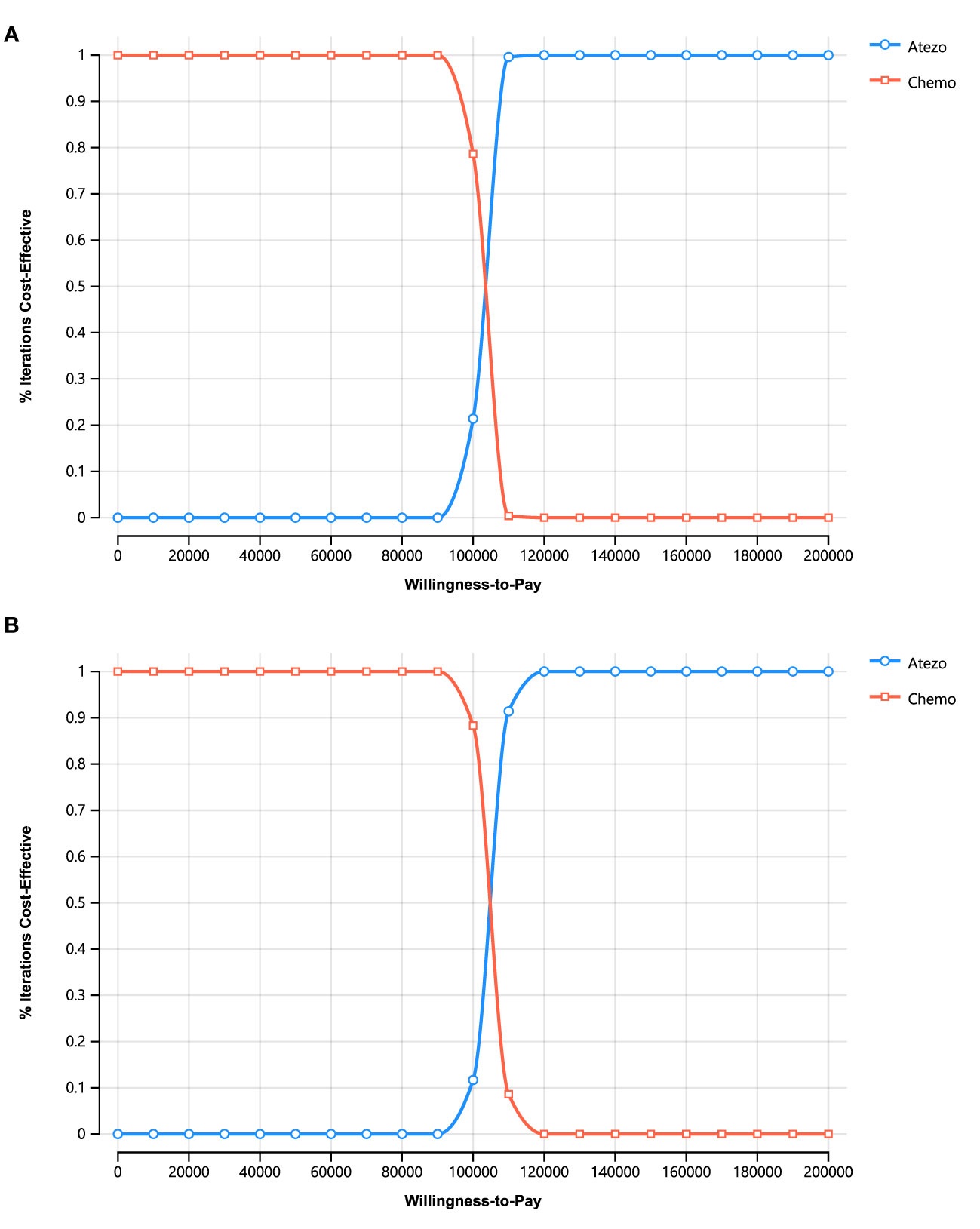
Figure 4 Cost-effectiveness acceptability curves for stochastic analysis. (A) PartSA model. (B) Markov Model.
In the PartSA model, the cost-effectiveness price of atezolizumab nationwide was $2,347.72 and $2,955.19 when WTP was 1x and 3x per capita GDP, respectively. In Beijing, they were $2,734.08 and $4,114.29; in Gansu, they were $2,197.94 and $2,505.87. With PAP, the national cost-effectiveness prices for atezolizumab were $3,327.03 and $4,187.92; in Gansu, they were $3,114.78 and $3,551.14 dollars. In Beijing, the original price of atezolizumab was cost-effective versus chemotherapy when the WTP was three times the GDP per capita (Table 4).
Breakthroughs in tumor immunotherapy, for which the Nobel Prize in Physiology or Medicine was awarded, offer hope for the survival of patients with malignant tumors. As immune checkpoint inhibitors become the new standard of care for various tumor therapies, the very high cost of these drugs poses a significant challenge to the healthcare system. The introduction of lung cancer immunotherapy was accompanied by improved survival rates and increased expenditures (21). Therefore, increased pharmaceutical spending is in direct competition with other health and social spending, and therefore, an econometric study should be conducted that carefully balances the awarding of innovation with ensuring affordability. This study is the first cost-effectiveness evaluation comparing atezolizumab monotherapy to platinum-based chemotherapy, based on the most recent data from IMpower110. The PartSA and Markov models show that first-line atezolizumab monotherapy for advanced NSCLC is not cost-effective versus chemotherapy. When PAP became available, ICER decreased significantly, but was still not cost-effective OSA showed that the cost of atezolizumab, PFS utility, and discount rate were the most influential factors; when the WTP threshold exceeded $102,424.83 (PartSA model) or above $104,806.71 (Markov model), the probability that atezolizumab monotherapy was more cost-effective than chemotherapy increased.
Several econometric studies based on preliminary data from the IMpower110 study (13, 14, 22) are shown in Table 5; The WTP in the United States is much higher than the WTP in China, indicating that atezolizumab is likely to be cost-effective from a U.S. perspective. In addition, atezolizumab was rated as cost-effective in areas with a high level of economic development, such as Beijing, China. A study by Shen Li et al. (23) focused on atezolizumab plus chemotherapy in 2022 from the perspective of the US healthcare system, with an ICER of $130,804.59/QALY. Atezolizumab combination therapy as first-line treatment was similar to atezolizumab monotherapy. Although atezolizumab is not cost-effective at this time, its clinical benefits are significant: in the IMpower110 trial, the duration of response (DoR) was up to 38.9 months, the longest of any current immunologic agent, validating its long-tailing effect. If remission is achieved with atezolizumab, there is the potential for long-term benefit. The cost of atezolizumab was the most influential parameter for cost-effectiveness. The study showed that atezolizumab becomes cost-effective when the cost of atezolizumab falls below $2308.47. In 2015, the Chinese government initiated national healthcare negotiations to discuss drug prices for expensive drugs such as cancer drugs and orphan drugs, expanding reimbursement coverage and lowering drug prices. The government agreed to expand reimbursement coverage and reduce drug prices. However, imported immunotherapy drugs such as atezolizumab and pembrolizumab will no longer be eligible for national healthcare reimbursement in China, and their use in clinical practice will be limited. This study provides evidence of the fair price of atezolizumab in China and will help policy-making departments in their economic strategies. Patient assistance programs are also an effective means of improving the economics of atezolizumab, reducing ICERs by about 50%; Guoqiang Liu’s study (14) also mentions patient assistance programs, which can reduce ICERs by about 60%-65%. Another advantage of patient assistance programs is that they can target patients who are truly in financial need rather than a general decline in drug prices.
A controversial aspect of cost-effectiveness evaluation was the choice of a simulation model (24–26). Economic models have uncertainties, and sensitivity analyses were used to address parameter uncertainties, but Partitioned Survival and Markov models were often used, where structural uncertainties due to alternative models were generally not addressed. In the traditional Markov model, additional assumptions were often made, such as whether persons in PFS and PD states were allowed to transition to a mortality state; in the Partitioned Survival model, survival data reported in clinical trials were used to avoid estimating transition probabilities. In a study by McEwan et al. (27), they found that the PartSA model most accurately reproduced observed survival outcomes. However, Coly et al. (28) found that the PartSA model has an inherent bias in favor of treatment induced by disease progression. Rui and Goeree et al. (24, 29) believe that results such as ICER obtained with the PartSA and Markov models are nearly identical. Smare and Williams et al. (25, 26) pointed out that the prediction results of the two models differ. Although they ultimately reached the same conclusion, this study showed that there are differences between the two models based on empirical evidence. Therefore, the use of multiple models for economic evaluation may be a way to reach robust conclusions. This analysis had several limitations worth considering. First, only patients with PD-L1 expression ≥50% in the IMpower110 trial were included because they were the most efficient, but the characteristics of all advanced NSCLC patients cannot be generalized. Second, the utility values were obtained from the literature and may not be representative of real patients. Third, the support and follow-up costs in this study were based on price estimates for relevant treatment and laboratory items in Chinese hospitals, while the second line of treatment was derived from clinical trials. Because actual patient situations and treatment costs may differ, a sensitivity analysis was performed, but the costs in this part of the study do not affect the conclusions. Fourth, to make the model easier to understand and compute, several assumptions were made to simulate and simplify real-world situations, including parametric fitting and extrapolation of survival curves, the assumption that patients have three states (PFS, PD, and death), and in the Markov model, the probability that a patient moves from the PFS state to the death state is natural death.
First-line monotherapy with atezolizumab for patients with PD-L1 high-expressing EGFR and ALK wild-type advanced NSCLC was estimated to be less cost-effective than chemotherapy in terms of the Chinese healthcare system; offering PAP increased the likelihood that atezolizumab would be cost-effective. In some areas of China with higher levels of economic development, atezolizumab was likely to be cost-effective. To improve the cost-effectiveness of atezolizumab, drug prices would need to be reduced.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
CZ: Literature search, study selection, analysis of the two model, writing -review & editing. YL: Analysis of the two model, writing - original draft. JT: Writing-review & editing. PT: Formulation or evolution of overarching research aims and writing-review & editing. WL: Ideas, formulation or evolution of overarching research aims, supervision and project administration. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1093469/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center (2022) 2(1):1–9. doi: 10.1016/j.jncc.2022.02.002
3. Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clinic Proc (2008) 83(3):355–67. doi: 10.4065/83.3.355
4. Rodak O, Peris-Díaz MD, Olbromski M, Podhorska-Okołów M, Dzięgiel P. Current landscape of non-small cell lung cancer: Epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers (2021) 13(18):4705. doi: 10.3390/cancers13184705
5. Meza R, Meernik C, Jeon J, Cote ML. Lung cancer incidence trends by gender, race and histology in the united states, 1973-2010. PloS One (2015) 10(3):e0121323. doi: 10.1371/journal.pone.0121323
6. Mulherkar R, Grewal AS, Berman AT. Emerging role of immunotherapy in locally advanced non-small cell lung cancer. Clin Adv IN Hematol Oncol (2020) 18(4):212–7.
7. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-Small-Cell lung cancer: Final results of a phase iii trial. J Clin Oncol (2012) 30(17):2055–62. doi: 10.1200/Jco.2011.39.5848
8. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of fda-approved immune checkpoint inhibitors per nccn guidelines with the level of evidence. Cancers (Basel) (2020) 12(3). doi: 10.3390/cancers12030738
9. Mei Z, Bin W, Zhaoyan C, Ting X. Literature analysis of pd-1 inhibitors in elderly patients with advanced non-small cell lung cancer. Herald Med (2021) 40(03):330–5.
10. NCCN guidelines for non-small cell lung Cancer,Version 3.2022: National comprehensive cancer Network(NCCN) (2022). Available at: https://www.nccn.org/.
11. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of pd-L1-Selected patients with nsclc. N Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346
12. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from Impower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected nsclc. J Thorac Oncol (2021) 16(11):1872–82. doi: 10.1016/j.jtho.2021.06.019
13. Cheng S, Pei R, Li J, Li B, Tang L, Yin T, et al. Atezolizumab compared to chemotherapy for first-line treatment in non-small cell lung cancer with high pd-L1 expression: A cost-effectiveness analysis from us and Chinese perspectives. Ann Transl Med (2021) 9(18):1481. doi: 10.21037/atm-21-4294
14. Liu G, Kang S, Wang X, Shang F. Cost-effectiveness analysis of atezolizumab versus chemotherapy as first-line treatment for metastatic non-Small-Cell lung cancer with different pd-L1 expression status. Front Oncol (2021) 11:669195. doi: 10.3389/fonc.2021.669195
15. 2021 CSCO guidelines for non-Small-Cell lung cancer: Chinese society of clinical Oncology(CSCO) (2021). Available at: http://www.csco.org.cn.
16. Binn L. The report on the status of nutrition and chronic diseases of Chinese residents 2020. Beijing: The state council information office of the people’s pepublic of China, (2020).
17. Shen Y, Wu B, Wang X, Zhu J. Health state utilities in patients with advanced non-Small-Cell lung cancer in China. J Comp Eff Res (2018) 7(5):443–52. doi: 10.2217/cer-2017-0069
19. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol (2017) 13(5):e195–203. doi: 10.1111/ajco.12477
20. Ping-ping Z. Studying on the negotiation practice of medical insurance drugs based on targeted drugs. Chin Health Service Manage (2016) 33(04):275–8.
21. Youn B, Wilson IB, Mor V, Trikalinos NA, Dahabreh IJ. Population-level changes in outcomes and Medicare cost following the introduction of new cancer therapies. Health Serv Res (2021) 56(3):486–96. doi: 10.1111/1475-6773.13624
22. Peng Y, Zeng XH, Peng LB, Liu Q, Yi LD, Luo X, et al. First-line atezolizumab for metastatic nsclc with high pd-L1 expression: A united states-based cost-effectiveness analysis. Adv Ther (2021) 38(5):2447–57. doi: 10.1007/s12325-021-01734-6
23. Lin S, Luo SH, Zhong LX, Lai SB, Zeng DY, Rao X, et al. Cost-effectiveness of atezolizumab plus chemotherapy for advanced non-Small-Cell lung cancer. Int J Clin Pharm-Net (2020) 42(4):1175–83. doi: 10.1007/s11096-020-01076-3
24. Rui M, Wang Y, Fei Z, Zhang X, Shang Y, Li H. Will the Markov model and partitioned survival model lead to different results? a review of recent economic evidence of cancer treatments. Expert Rev Pharmacoecon Outcomes Res (2021) 21(3):373–80. doi: 10.1080/14737167.2021.1893167
25. Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: A comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Making (2017) 37(4):427–39. doi: 10.1177/0272989X16670617
26. Smare C, Lakhdari K, Doan J, Posnett J, Johal S. Evaluating partitioned survival and Markov decision-analytic modeling approaches for use in cost-effectiveness analysis: Estimating and comparing survival outcomes. Pharmacoeconomics (2020) 38(1):97–108. doi: 10.1007/s40273-019-00845-x
27. McEwan P, Gordon J, Ward T, Penrod JR, Yuan Y. Prm83 - empirical assessment of the impact of model choice (Markov state transition versus partitioned survival) in modelling small-cell lung cancer. Value Health (2016) 19(7):A372.
28. Coyle D, Coyle K. Prm74 - the inherent bias from using partitioned survival models in economic evaluation. Value Health (2014) 17(3):A194.
29. Goeree R, Villeneuve J, Goeree J, Penrod JR, Orsini L, Tahami Monfared AA. Economic evaluation of nivolumab for the treatment of second-line advanced squamous nsclc in Canada: A comparison of modeling approaches to estimate and extrapolate survival outcomes. J Med economics (2016) 19(6):630–44. doi: 10.3111/13696998.2016.1151432
Keywords: atezolizumab, non-small-cell lung cancer, partitioned survival model, Markov model, cost-effectiveness
Citation: Zhang C, Liu Y, Tan J, Tian P and Li W (2023) Cost-effectiveness evaluation based on two models of first-line atezolizumab monotherapy and chemotherapy for advanced non-small cell lung cancer with high-PDL1 expression. Front. Oncol. 13:1093469. doi: 10.3389/fonc.2023.1093469
Received: 09 November 2022; Accepted: 28 February 2023;
Published: 14 March 2023.
Edited by:
Liyun Shi, Nanjing University of Chinese Medicine, ChinaReviewed by:
Ritesh Rathore, Roger Williams Medical Center, United StatesCopyright © 2023 Zhang, Liu, Tan, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panwen Tian, MjMxODU1NzYxQHFxLmNvbQ==; Weimin Li, d2VpbWkwMDNAc2N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.