- Department of Ultrasound, Shengjing Hospital of China Medical University, Shenyang, China
Background: PET-CT is the first choice for the imaging diagnosis of intraperitoneal lymphomas. Contrast-enhanced ultrasound (CEUS) is rare in the diagnosis of intraperitoneal nodal lymphoma.
Case summary: A 62-year-old man was admitted for examination with “right upper abdominal pain”. Ultrasound was used to refer to the masses in the hilar region, spleen, and anterior sacral region respectively. The masses were all hypoechoic, and blood flow signals could be detected by CDFI. Laboratory tests of CA125 were within normal limits. CEUS examination was performed on the three masses respectively. The three masses showed different perfusion patterns. Thickened vessels appeared around the mass in the hilar region, a peripheral centrally directed perfusion pattern was observed in the splenic mass, and blood supply vessels appeared in the center of the presacral mass with a significant filling defect. They all showed a contrast pattern with rapid clearance and hypoenhancement compared with the surrounding areas. Ultrasound guided needle biopsy revealed non-Hodgkin’s lymphoma, diffuse large B-cell lymphoma, non-germinal center origin. After biopsy, the patient was treated with R-CHOP regimen for chemotherapy, and the tumor disappeared by routine ultrasound review after 5 cycles of chemotherapy.
Conclusion: To the best of our knowledge, this report is the first to describe the findings of CEUS in intraperitoneal nodal lymphoma. CEUS has various manifestations in intraperitoneal nodal lymphoma. Future studies are still needed to explore the diagnostic features of CEUS in intraperitoneal nodal lymphoma.
Introduction
The incidence of lymphoma continues to rise worldwide. According to Global Cancer Statistics 2018, non-Hodgkin’s lymphoma ranks 13th and 11th among all forms of malignancy in terms of morbidity and mortality (1). The diagnosis of lymphoma depends on pathology, and imaging examination can provide more staging information. The application of contrast-enhanced ultrasound (CEUS) in the diagnosis of lymphoma is rare in medicine. Intraperitoneal lymph node lymphoma is less common than superficial lymph nodes. Up to the present, no studies have reported the diagnosis of intraperitoneal nodal lymphoma by contrast-enhanced ultrasound.
Case presentation
Chief complaints
A 62-year-old male patient was admitted to the hospital because of “Pain in the right upper abdomen”. Through CT upper abdominal plain scan, a mass in the hilar region could be seen clearly. Thus, further examination was recommended.
History of past illness
The patient had no medical history of other blood diseases, no low fever, no night sweats, and no significant weight loss. In 2015, he underwent cerebrovascular stenting for basilar artery stenosis, and recovered well after surgery. Rosuvastatin, Clopidogrel and Aspirin were taken orally to control the disease. In 2016, he underwent cholecystectomy due to gallstones, and no abdominal discomfort occurred after the operation. He had a history of hypertension for more than 20 years, and his blood pressure was well controlled without other chronic diseases at ordinary times.
Personal and family history
The patient smoked for more than 20 years and quit smoking for more than 10 years. Denied any history of alcohol use. No history of drug or food allergy.
Physical examination
There was a palpable mass in the anterior sacral area, which was hard and slightly tender. No tenderness, rebound pain, muscle tension in the rest of the place. All other vital signs were stable, blood pressure was not high, and no positive signs were detected.
Laboratory examinations
The patient’s serum β2 microglobulin was 2.58mg/L at admission, which was higher than the normal value (0.7-1.8mg/L). Urinary β2 microglobulin 0.762 mg/L, higher than the normal value (< 0.24mg/L) thymidine kinase (TK1)0.33pmol/L, still in the normal range. Serum CA125 was 10.58U/mL, and there was no obvious abnormality.
Imaging examinations
Because a hilar mass was showed after scanning the upper abdomen through CT, the patient was scheduled for further examination through contrast-enhanced ultrasound (Figure 1). The ultrasound examination on abdomen was performed using a Resona9 ultrasound system (Mindray Medical International, China) equipped with an SC6-1U (1-6 MHZ) transducer. Conventional ultrasound examination showed a hypoechoic mass in the hilar region, the size of the mass was 4.65x5.33x3.91cm, the boundary was clear, the shape was irregular, and CDFI could detect blood flow signals. Another nearly-circular hypoechoic mass was found in the spleen. The size of the mass was 4.96x4.74x5.19cm, the boundary was clear, and the blood flow signal could be detected by CDFI. Another hypoechoic mass was seen in the anterior sacral area, with a size of 5.84x5.20x4.68cm, located below the bifurcation of the abdominal aorta, with clear boundary and irregular shape. There was no adhesion with the surrounding intestine. Blood flow signals could be detected by CDFI. Further CEUS diagnosis was recommended by the patient’s physician and informed consent was obtained. Depth, gain, and focus are thoroughly adjusted to achieve optimal visualization according to the radiologist’s habits. The timer was activated after a high-dose injection of 1.5 mL of Sonovue (Bracco, Italy) suspension (an ultrasound contrast agent) and 5 mL of saline (Bracco, Italy). CEUS examination was performed on all three masses. After conducting contrast-enhanced ultrasound, it showed that the blood vessels were thickened at the edge of the tumor in the hilar region, which were enhanced earlier than the surrounding tissues. The tumor showed diffusing snowflake enhancement inside, and showed uneven hypoenhancement after reaching the peak (compared with the liver tissue), and was rapid wash-out compared to that of the liver tissue. The splenic mass showed a peripheral enhancement pattern to the center, with uneven hypoenhancement. The enhancement was later than that of the normal spleen tissue, and rapid wash-out compared to normal spleen. There was no significant change in the lesion range after CEUS compared with the two-dimensional ultrasound. In the presacral mass, the central vessels of the mass were enhanced first, and the enhancement began later than the peripheral tissues, with uneven hypoenhancement. And there were obvious filling defects, and the clearance was earlier than that of the peripheral tissues. The boundary between the three masses and surrounding tissues was obvious (Figure 2). The time-intensity curve (TIC) showed that the ascending slope of the lesion was higher than that of the surrounding tissue, although the lesion was delayed hypoperfusion, which also suggested that the lesion area had more blood vessels and less resistance. The clearance time of the lesion was significantly earlier than that of the surrounding tissue, which was consistent with the characteristics of malignancy. The three masses did not have the same pattern of enhancement, and there were obvious filling defect areas in the presacral mass, while the enhancement was more uniform in the hilar region and the splenic mass. Although the enhancement patterns of the three masses were different, they all showed rapid wash-out, which was consistent with the characteristics of malignant masses. Among them, the thickened vessels at the edge of the tumor in the hilar region were consistent with the CEUS features of lymphoma in previous studies (2). The CEUS pattern of splenic mass was basically consistent with the characteristics of nodular splenic infiltration of malignant lymphoma mentioned in the literature. The CEUS pattern of central vessel enhancement first in the presacral mass has not been confirmed in the literature in lymphoma, but considering that the patient had no medical history of other malignant tumors and considering homology with other masses. Finally, the patient was diagnosed lymphoma under the CEUS pattern combined with the patient’s medical history.
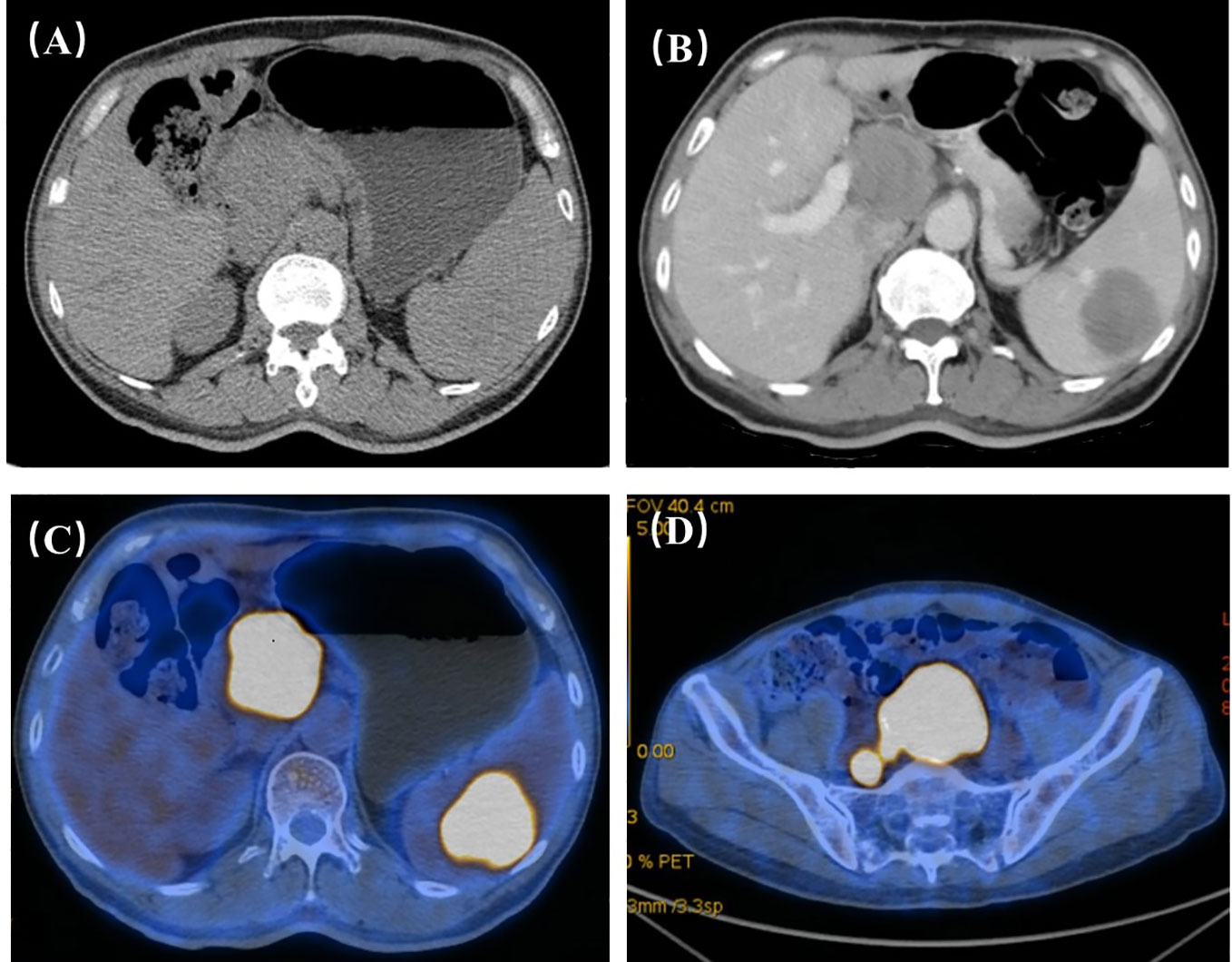
Figure 1 (A) Through plain scan of the upper abdomen, it clearly showed the masses in the hilar region, but the masses in the spleen were not obvious. (B) Through contrast-enhanced CT, it could show the masses in the hilar region and splenic region, and the masses showed progressive and uneven enhancement. The hilar region nodules: plain scan 30HU arterial phase, 40HU portal vein phase, 52HU delayed phase, 65HU; spleen nodules: plain scan 35HU arterial phase, 46HU portal vein phase, 52HU delayed phase, 63HU; upper abdominal enhancement suggested: Further examination is recommended for hilar and splenic masses. (C, D) FDG metabolism of hilar mass, splenic mass and presacral mass on PET-CT increased, and finally PET-CT suggested that the three were homologous and had a high possibility of lymphoma.
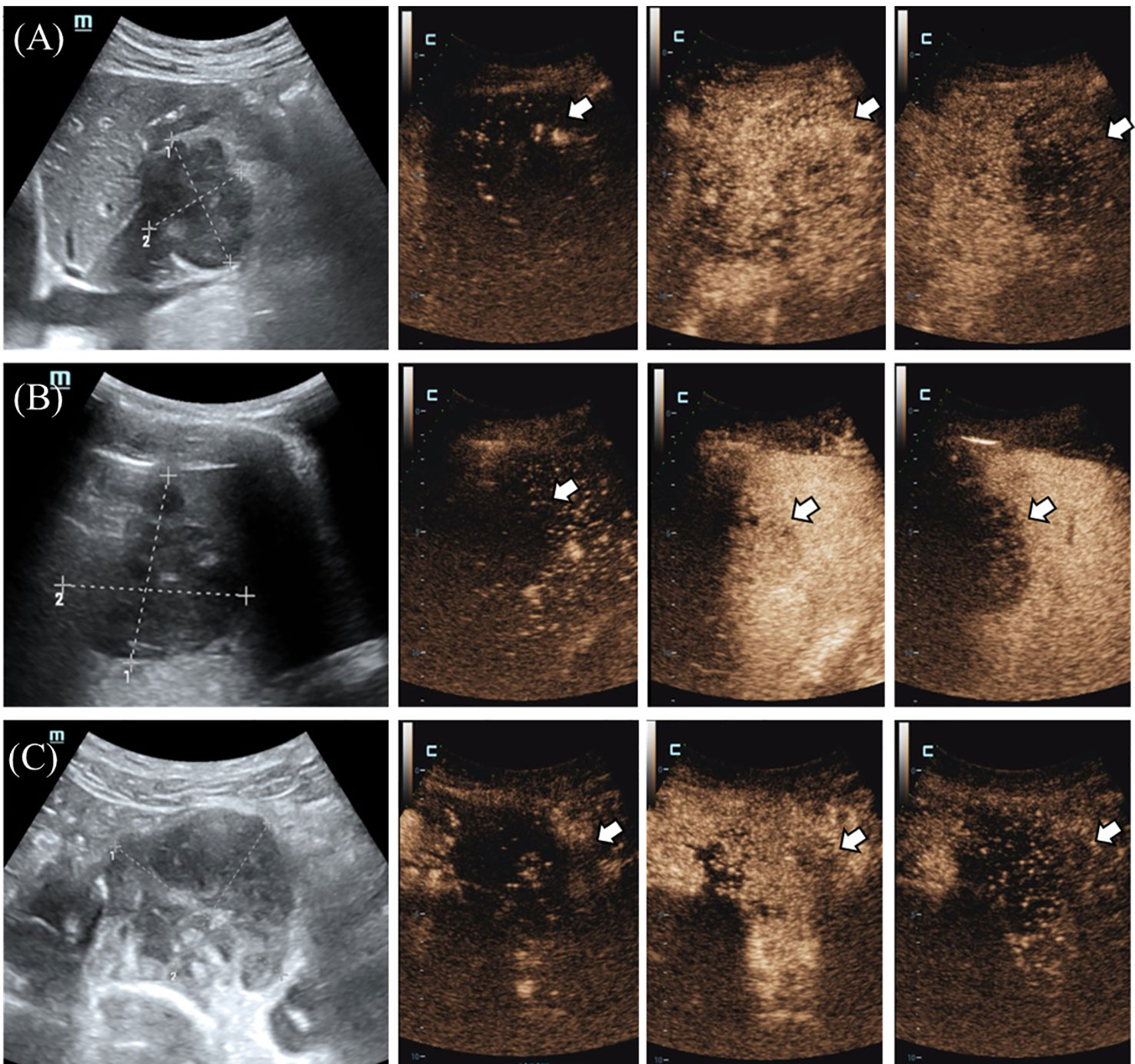
Figure 2 (A) The tumor in the hilar region showed thickened blood vessels, located at the edge of the tumor (indicated by the arrow), and was enhanced and cleared earlier than the surrounding tissue. (B) The splenic mass (shown by the arrow) showed a peripheral to central enhancement pattern with uneven hypoenhancement, which was enhanced later and cleared earlier than normal splenic tissue. (C) In the presacral mass (shown by the arrow), the central vessels of the mass were enhanced first, and the enhancement began later than the peripheral tissues, with uneven hypoenhancement, and there were obvious filling defects, and the clearance was earlier than the peripheral tissues.
Pathological findings and immunohistochemical staining
Puncture biopsy was performed on the patient’s anterior sacral mass, and 16G automatic puncture biopsy needle (Bard, America) was used. The final pathological results suggested non-Hodgkin B-cell lymphoma, diffuse large B-cell lymphoma, and non-germinal center origin. Immunohistochemical results showed that CD20, CD21, CD19 were positive, Ki-67>90%, Bcl-2>90%, Bcl-6>80%.
Results of flow cytometry
Through flow cytometry, the results showed double clonal B mature lymphocytes.
Final diagnosis
The final diagnosis of this case was intranodal type diffuse large B-cell lymphoma with splenic infiltration.
Treatment
The patient was diagnosed with diffuse large B-cell lymphoma and received R-CHOP chemotherapy, namely rituximab, dexamethasone, vincristine, epirubicin, and cyclophosphamide, for a course of 8 cycles. After 4 cycles of chemotherapy, abdominal enhanced CT examination showed that the size of the hilar lesion was about 2.96x2.11cm, the presacral mass was unclear, and the size of the splenic mass was about 2.79x3.04cm. After 5 cycles of chemotherapy, conventional ultrasound scan of the whole abdomen showed no definite lesions.
Discussion
Diffuse large B-cell lymphoma (DLBCL) is the most prevalent subtype of non-Hodgkin lymphoma. Patients usually presented with progressive lymphadenopathy, extranodal disease, or presented with the both, which require treatments (3). The definitive diagnosis of lymphoma clinically depends on the detailed examination of tumor tissue. In addition to morphological characteristics, the accurate classification of lymphoma also requires specialized tests, including immunohistochemistry, flow cytometry, fluorescence in situ hybridization (FISH) and molecular testing (4, 5). Positron Emission Tomography-Computed Tomography (PET-CT) was used to evaluate organ involvement and clinical staging (6). However, PET-CT is often limited by the high cost of examination and the high radiation dose. In recent years, many studies have focused on more accurate diagnosis of lymphoma by imaging methods, further differentiating from other space-occupying lesions and prognosis evaluation (7–9). Different treatments from the most malignant tumors together with the negative prognosis, the timely diagnosis of lymphoma becomes necessary.
CEUS has the advantages of convenience and easy operation. Contrast-enhanced ultrasound can display the changes of microcirculation perfusion in real time. At present, contrast-enhanced ultrasound has been widely used (10). CEUS is rarely reported in the diagnosis of lymphoma compared with other diseases. Many studies in the literature focus on the CEUS features and differential diagnostic efficacy of superficial lymph nodes (11–13). It is rare to study abdominal lymph nodes.
In this case, under the CEUS mode the distribution of microvessels presented by different masses was different. Thickened blood vessels appeared around the mass in the hepatic portal area, which became the first perfusion area of the tumor. It may be caused by surrounding blood vessels in the invasive growth process of the tumor. The volume of the pre-sacral mass was large, and the rapid growth rate led to the lack of blood supply in the tumor. Due to the above the filling defect inside the mass was found by contrast-enhanced ultrasound. Due to the different growth location and size, the microvascular distribution of each homologous tumor is slightly different.
Superficial lymph nodes are the most common site of lymphomas, especially those in the head and neck. Contrast-enhanced ultrasound can clearly show the distribution of blood vessels in lymph nodes, which makes the diagnosis of superficial lymph nodes more accurate (Table 1). The studies on CEUS diagnosis of superficial lymph nodes are more mature. The study of Shan-Shan Yin et al. suggested that the CEUS findings of lymphoma are significantly different from those of lymph node metastasis and reactive lymph nodes. Through CEUS findings of lymphoma, it can mostly be seen the diffusing and even enhancement, and rarely be seen the non-perfusion filling defect areas. Lymph node metastases usually presented with centripetal enhancement, and non-perfusion filling defect area is more common. Reactive hyperplastic lymph nodes presented with uniform hyperenhancement due to vascular hyperplasia, and lymph node tuberculosis presented with unclear lymph node boundary (11). Much of the literature comes to similar conclusions (14–16). This may be related to angiogenesis and vascular distribution in the lesion. Due to the rapid growth rate of malignant metastases, immature neovascularization and non-vascular necrotic areas are common in metastatic lymph nodes, which hinders the distribution of contrast agent to these areas then leads to perfusion defects. In most lymphomas, blood vessels are highly hyperplastic, which makes the microvesicles of contrast agent easy to flow and rapidly distribute throughout the lesion, resulting in more uniform enhancement (16). Although the features of contrast-enhanced ultrasound in lymphoma have been unanimously agreed in many literatures, there are still some exceptions. In the study, Ming Yu et al. reported 6 cases of lymphoma without perfusion (17). Due to the uncertainty in the qualitative manifestations of contrast-enhanced ultrasound in lymphoma, literature studies have made focus on the quantitative analysis of contrast-enhanced ultrasound. Shan-shan Yin et al. also analyzed the arrival time parameters of contrast media and found that compared with lymphoma, the contrast had arrived earlier in metastatic cancer but spent longer to the center (11). Xiaoyan Niu et al. suggested that quantitative indicators of CEUS were correlated with PET-CT indicators, which had potential diagnostic value for lymphoma (18). CEUS has a high diagnostic value in the diagnosis of benign and malignant superficial lymph nodes, which has been confirmed by studies. However, CEUS still manifested similarities in lymph nodes with different pathologies, and the treatment of lymph node diseases is completely different. Therefore, more studies are devoted to further explore the more accurate diagnosis of lymphoma by CEUS. Liu SR et al. injected subcapsular contrast injection and observed that lymph nodes of lymphoma patients had lymphatic vessel distortion and uneven distribution of contrast agent, which was completely different from reactive proliferative lymph nodes with uniform distribution of contrast agent and lymph node metastasis with local concentration of contrast agent and lymphatic vessel rupture (19). Compared with intravenous contrast medium injection, subcapsular contrast medium injection has a better effect in identifying the types of lymph nodes, it requires higher technical requirements for the operator. Thus, this kind of injection cannot be popularized wider. The prognosis of different subtypes of lymphoma varies greatly. According to the growth pattern and prognosis of lymphoma, it can be divided into aggressive lymphoma and indolent lymphoma. Studies suggest that the contrast-enhanced ultrasonography of indolent lymphoma is more similar to that of reactive proliferative lymph nodes, presenting rapid uniform hyperenhancement (13). The later the stage, the more aggressive and malignant the lymphoma, the richer the blood vessels in the tumor, the higher the blood flow velocity, and the higher the RI. The difference of ΔT and AS between early and advanced lymphomas was statistically significant, suggesting that advanced lymphomas had abundant blood flow at the CEUS quantitative level (20). However, there was no significant difference in contrast-enhanced ultrasound performance between HL and NHL. Further analysis of the various subtypes of lymphoma by contrast-enhanced ultrasound is not available. Studies suggest that contrast-enhanced ultrasound has a high application value in evaluating the response of lymphoma to drugs after chemotherapy, and it can accurately respond to changes in the blood supply of the lesion after chemotherapy. For the lesions that responded to chemotherapy, the area under the curve of CEUS quantitative analysis before and after chemotherapy showed statistically significant difference in perfusion index (PI) (21). in the study of M. Kumagawa et al., peak enhancement (PE) and PI are considered to be the effective indicators for evaluating whether a patient has achieved a complete response after chemotherapy (22). The prediction of lesion response after chemotherapy can quickly make clear the next treatment plan of patients more quickly.
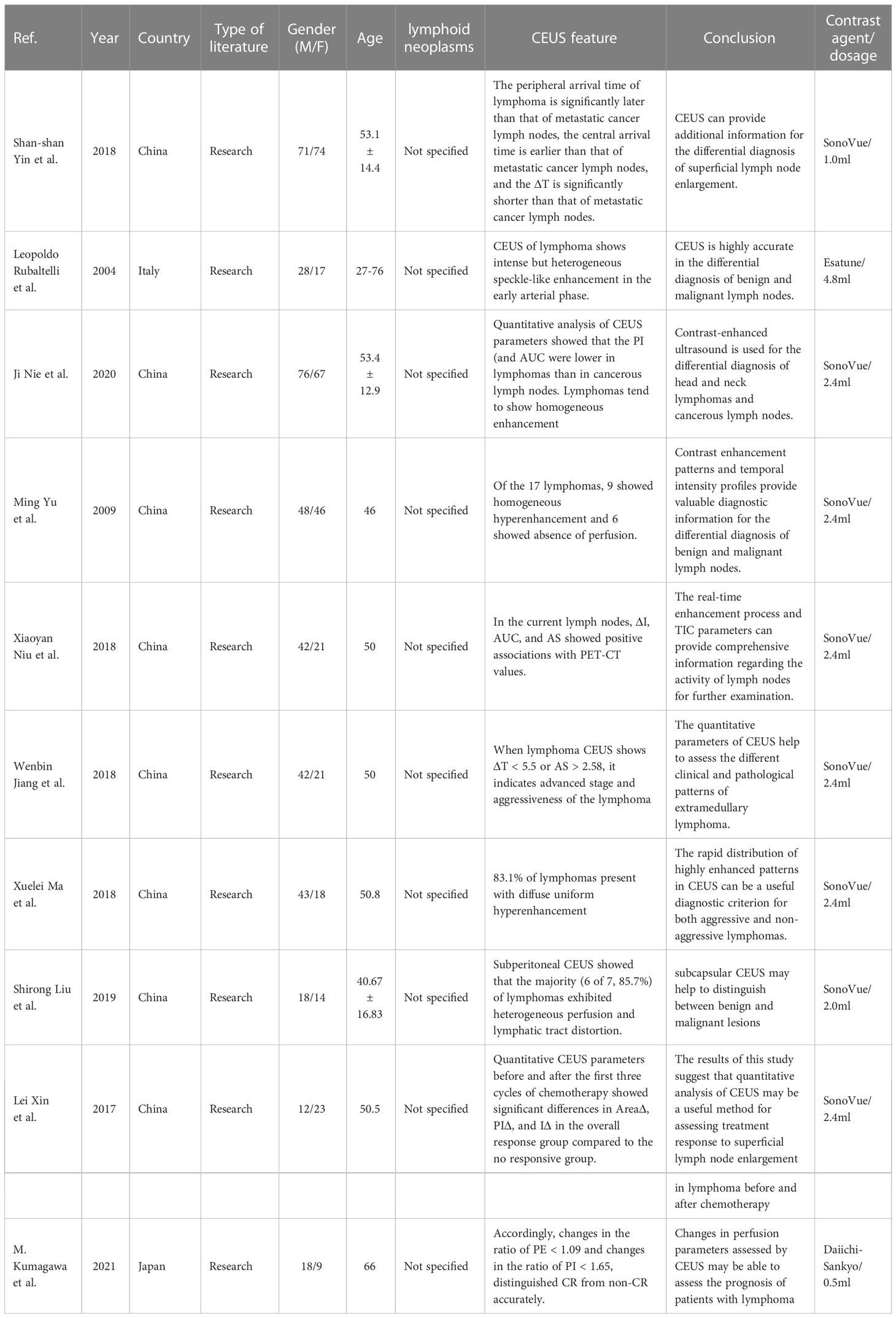
Table 1 Contrast-enhanced ultrasound characteristics of superficial lymph node lymphoma reported in the literature.
CEUS is relatively rare in the diagnosis of lymphomas in extranodal organs (Table 2). Through CEUS, it can well reflect the blood supply of the lesion and the distribution of microvessels, and has a good diagnostic efficiency for the differentiation of lymphoma from benign nodules of spleen (23–25). Lymphoma of the spleen is more common in secondary lymphoma invasion and formation, but primary lymphoma is rare to be seen. The findings of primary splenic lymphoma are similar to those of secondary CEUS, with early isoenhancement and early regression (26, 27). Other malignant tumors of the spleen are rare to be seen, and the differentiation of splenic lymphoma from other malignant tumors has not been reported. Christian Gorg et al. believe that CEUS has no added value in the diagnosis of splenic involvement in lymphoma and cannot improve the diagnostic accuracy of conventional ultrasound for splenic involvement (28). CEUS has been used to identify space-occupying lesions of the liver. Intrahepatic lymphoma, as an uncommon intrahepatic malignant tumor, is less common than other malignant tumors. Similar to the spleen, liver lymphomas secondary to intra nodal lymphomas is usually to be seen, primary liver lymphomas are rare. Primary liver lymphoma is often associated with HBV and HCV virus infections. HBV infection-related MALT was more common in primary liver lymphoma (29). More studies are needed to determine whether viral infection affects the progression of liver lymphoma. Color Doppler ultrasound often shows multiple vascular channels in malignant liver lymphoma, which is called “vascular penetration sign”. Contrast-enhanced blood vessels were first observed in the arterial phase of CEUS, and there were still angiograms in the Kupffer phase. This feature is significantly different from the typical contrast-enhanced ultrasound findings of other liver malignant tumors. However, liver malignant lymphoma also has rich blood supply CEUS findings that are not similar to other malignant tumors and cannot be differentiated from other diseases (30, 31). C. Trenker et al. also concluded that the differential diagnosis of hepatic malignant lymphoma from other malignant tumors could not be completed by CEUS in their study of CEUS findings in 38 cases of lymphoma (32). The renal lymphoma was mainly nodular type. It presented mostly hypoenhancement or isoenhancement in the arterial phase and hypoenhancement in the parenchymal phase through CEUS. Contrast-enhanced ultrasound has certain value in the differential diagnosis of renal lymphoma and benign nodules (33). The findings of CEUS in lymphoma with extranodal organ invasion are relatively uniform, and there is little difference between different extranodal organs. There are also extranodal organs with specificity through CEUS, and the literature studies are mainly based on case reports. One case serially reported the contrast-enhanced ultrasound findings of 6 cases of intrapulmonary lymphoma. 83% of the lymphomas were mainly supplied by pulmonary artery, which was not consistent with the findings of other pulmonary malignancies (34). There is a correlation between thyroid lymphoma and nodular Hashimoto’s thyroiditis. Lulu Yang et al. found that the combination of CEUS features and quantitative indicators has a good diagnostic efficacy in differentiating thyroid lymphoma from nodular Hashimoto’s thyroiditis (35), However, more studies are needed to confirm whether it can be widely used in clinical practice. Primary testicular lymphoma is rare, and it is difficult to distinguish lymphoma from other testicular lesions by conventional two-dimensional ultrasound or color Doppler. Literature studies have suggested that testicular lymphoma is mostly characterized by rapid high-enhancement contrast-enhanced ultrasound, which is more extensive than gray-scale ultrasound. An 80% increase in blood flow shows a straight vessel pattern with nonbranched increased vascular hyperplasia (36, 37). Most of the studies related to the diagnosis of extranodal organ lymphoma by CEUS are limited to the differentiation of other space-occupying lesions from benign or malignant. However, it seems that it is still difficult to distinguish lymphoma from other malignant tumors by CEUS. Contrast-enhanced ultrasound does not appear to contribute to the diagnosis of extranodal lymphoma subtypes.
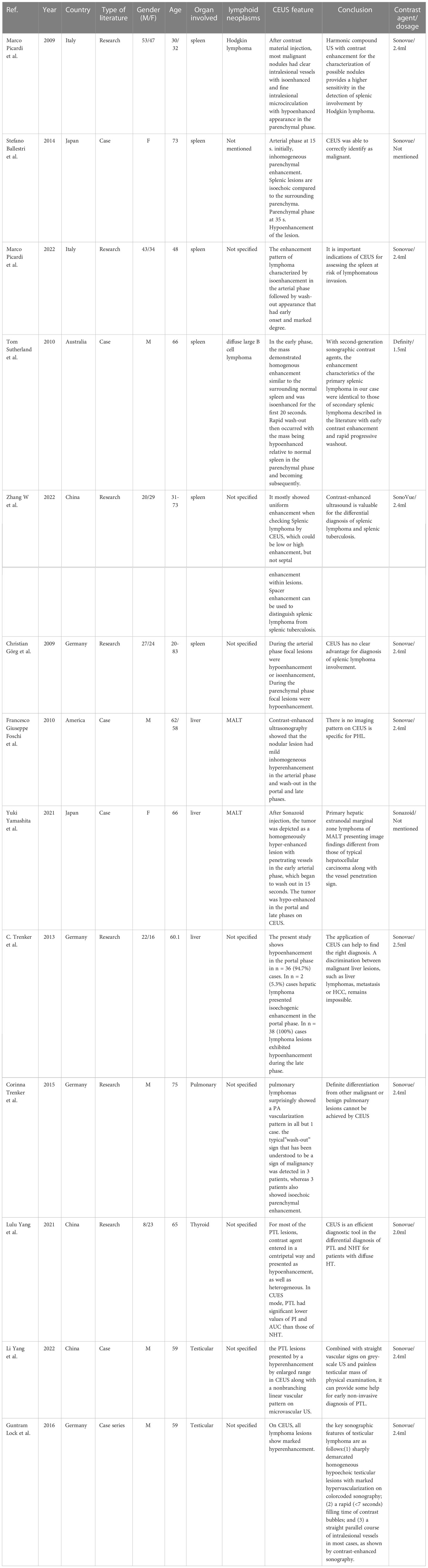
Table 2 Contrast-enhanced ultrasound characteristics of lymphoma involving extranodal organs reported in the literature.
The value of CEUS in the diagnosis and prognostic assessment of superficial intra nodal lymphoma has been validated. However, its diagnostic value for lymphoma with extranodal organ invasion seems to need further research and verification.
Conclusion
CEUS findings of this case of intraperitoneal nodular lymphoma showed hypoperfusion in the early stage of enhancement compared with the delayed perfusion of surrounding tissues, and rapid clearance. The pattern of lesion initiation enhancement is varied. To the best of our knowledge, this report is the first to describe CEUS findings of intraperitoneal nodal lymphoma. CEUS is rarely used in the diagnosis of lymphoma compared with other diseases. The value of CEUS for intraperitoneal nodal lymphoma still needs to be confirmed by subsequent studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Paper writing, patient information collection, literature review; XW: Paper writing, information collection; YH: Writing instruction, implementation of intervention, information collection. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Kong J, Fu JJ, Yang W, Sun Y, Wang S, Bai J, et al. Contrast-enhanced ultrasound features of mediastinal lymphomas and thymic epithelial tumors. J Clin ultrasound JCU. (2020) 48(1):19–28. doi: 10.1002/jcu.22782
3. Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet (London England) (2012) 380(9844):848–57. doi: 10.1016/S0140-6736(12)60605-9
4. Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, et al. The Epstein-Barr virus and the pathogenesis of lymphoma. J pathology. (2015) 235(2):312–22. doi: 10.1002/path.4459
5. Li S, Young KH, Medeiros LJ. Diffuse large b-cell lymphoma. Pathology. (2018) 50(1):74–87. doi: 10.1016/j.pathol.2017.09.006
6. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol Off J Am Soc Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
7. Shen J, Xue L, Zhong Y, Wu YL, Zhang W, Yu TF. Feasibility of using dynamic contrast-enhanced MRI for differentiating thymic carcinoma from thymic lymphoma based on semi-quantitative and quantitative models. Clin radiology. (2020) 75(7):560.e19–.e25. doi: 10.1016/j.crad.2020.02.010
8. Fu F, Sun X, Li Y, Liu Y, Shan Y, Ji N, et al. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict chemotherapeutic responses and survival in primary central-nervous-system lymphoma. Eur radiology. (2021) 31(4):1863–71. doi: 10.1007/s00330-020-07296-5
9. Tanaka T, Akiyoshi H, Nishida H, Mie K, Lin LS, Iimori Y, et al. Contrast-enhanced computed tomography findings of canine primary renal tumors including renal cell carcinoma, lymphoma, and hemangiosarcoma. PloS One (2019) 14(11):e0225211. doi: 10.1371/journal.pone.0225211
10. Yusuf GT, Fang C, Huang DY, Sellars ME, Deganello A, Sidhu PS. Endocavitary contrast enhanced ultrasound (CEUS): a novel problem solving technique. Insights into imaging. (2018) 9(3):303–11. doi: 10.1007/s13244-018-0601-x
11. Yin SS, Cui QL, Fan ZH, Yang W, Yan K. Diagnostic value of arrival time parametric imaging using contrast-enhanced ultrasonography in superficial enlarged lymph nodes. J ultrasound Med Off J Am Institute Ultrasound Med (2019) 38(5):1287–98. doi: 10.1002/jum.14809
12. Spiesecke P, Neumann K, Wakonig K, Lerchbaumer MH. Contrast-enhanced ultrasound (CEUS) in characterization of inconclusive cervical lymph nodes: a meta-analysis and systematic review. Sci Rep (2022) 12(1):7804. doi: 10.1038/s41598-022-11542-9
13. Ma X, Ling W, Xia F, Zhang Y, Zhu C, He J. Application of contrast-enhanced ultrasound (CEUS) in lymphomatous lymph nodes: A comparison between PET/CT and contrast-enhanced CT. Contrast media Mol imaging. (2019) 2019:5709698. doi: 10.1155/2019/5709698
14. Rubaltelli L, Khadivi Y, Tregnaghi A, Stramare R, Ferro F, Borsato S, et al. Evaluation of lymph node perfusion using continuous mode harmonic ultrasonography with a second-generation contrast agent. J ultrasound Med Off J Am Institute Ultrasound Med (2004) 23(6):829–36. doi: 10.7863/jum.2004.23.6.829
15. Stramare R, Scagliori E, Mannucci M, Beltrame V, Rubaltelli L. The role of contrast-enhanced gray-scale ultrasonography in the differential diagnosis of superficial lymph nodes. Ultrasound quarterly. (2010) 26(1):45–51. doi: 10.1097/RUQ.0b013e3181cf4469
16. Nie J, Ling W, Yang Q, Jin H, Ou X, Ma X. The value of CEUS in distinguishing cancerous lymph nodes from the primary lymphoma of the head and neck. Front Oncol (2020) 10:473. doi: 10.3389/fonc.2020.00473
17. Yu M, Liu Q, Song HP, Han ZH, Su HL, He GB, et al. Clinical application of contrast-enhanced ultrasonography in diagnosis of superficial lymphadenopathy. J ultrasound Med Off J Am Institute Ultrasound Med (2010) 29(5):735–40. doi: 10.7863/jum.2010.29.5.735
18. Niu X, Jiang W, Zhang X, Ding Z, Xue H, Wang Z, et al. Comparison of contrast-enhanced ultrasound and positron emission Tomography/Computed tomography (PET/CT) in lymphoma. Med Sci monitor Int Med J Exp Clin Res (2018) 24:5558–65. doi: 10.12659/MSM.908849
19. Liu SR, Liu C, Jing HM, Miao LY, Cui LG, Qian LX, et al. Subcapsular injection of ultrasonic contrast agent distinguishes between benign and malignant lymph node lesions exhibiting homogeneous enhancement in intravenous contrast-enhanced ultrasound images. Ultrasound Med Biol (2020) 46(3):582–8. doi: 10.1016/j.ultrasmedbio.2019.12.004
20. Jiang W, Xue H, Wang Q, Zhang X, Wang Z, Zhao C. Value of contrast-enhanced ultrasound and PET/CT in assessment of extramedullary lymphoma. Eur J Radiol. (2018) 99:88–93. doi: 10.1016/j.ejrad.2017.12.001
21. Xin L, Yan Z, Zhang X, Zang Y, Ding Z, Xue H, et al. Parameters for contrast-enhanced ultrasound (CEUS) of enlarged superficial lymph nodes for the evaluation of therapeutic response in lymphoma: A preliminary study. Med Sci monitor Int Med J Exp Clin Res (2017) 23:5430–8. doi: 10.12659/MSM.907293
22. Kumagawa M, Matsumoto N, Miura K, Ogawa M, Takahashi H, Hatta Y, et al. Correlation between alterations in blood flow of malignant lymphomas after induction chemotherapies and clinical outcomes: a pilot study utilising contrast-enhanced ultrasonography for early interim evaluation of lymphoma treatment. Clin radiology. (2021) 76(7):550.e9–.e17. doi: 10.1016/j.crad.2021.02.007
23. Picardi M, Soricelli A, Pane F, Zeppa P, Nicolai E, De Laurentiis M, et al. Contrast-enhanced harmonic compound US of the spleen to increase staging accuracy in patients with Hodgkin lymphoma: a prospective study. Radiology. (2009) 251(2):574–82. doi: 10.1148/radiol.2512081293
24. Picardi M, Giordano C, Trastulli F, Leone A, Della Pepa R, Pugliese N, et al. Sulfur exafluoride contrast-enhanced ultrasound showing early wash-out of marked degree identifies lymphoma invasion of spleen with excellent diagnostic accuracy: A monocentric study of 260 splenic nodules. Cancers (2022) 14(8):1927. doi: 10.3390/cancers14081927
25. Zhang W, Yang G, Zhang X, Ni T. The role of contrast-enhanced ultrasound in differentiating splenic tuberculosis from splenic lymphoma. Front Oncol (2022) 12:891815. doi: 10.3389/fonc.2022.891815
26. Sutherland T, Temple F, Hennessy O, Lee WK. Contrast-enhanced ultrasound features of primary splenic lymphoma. J Clin ultrasound JCU. (2010) 38(6):317–9. doi: 10.1002/jcu.20699
27. Ballestri S, Lonardo A, Romagnoli D, Losi L, Loria P. Primary lymphoma of the spleen mimicking simple benign cysts: contrast-enhanced ultrasonography and other imaging findings. J Med ultrasonics (2001). (2015) 42(2):251–5. doi: 10.1007/s10396-014-0579-z
28. Görg C, Faoro C, Bert T, Tebbe J, Neesse A, Wilhelm C. Contrast enhanced ultrasound of splenic lymphoma involvement. Eur J radiology. (2011) 80(2):169–74. doi: 10.1016/j.ejrad.2009.11.012
29. Yamashita Y, Joshita S, Kobayashi H, Wakabayashi SI, Sugiura A, Yamazaki T, et al. Primary hepatic extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in a patient with chronic hepatitis b virus infection: Case report and summary of the literature. Medicina (Kaunas Lithuania) (2021) 57(3):280. doi: 10.3390/medicina57030280
30. Foschi FG, Dall'Aglio AC, Marano G, Lanzi A, Savini P, Piscaglia F, et al. Role of contrast-enhanced ultrasonography in primary hepatic lymphoma. J ultrasound Med Off J Am Institute Ultrasound Med (2010) 29(9):1353–6. doi: 10.7863/jum.2010.29.9.1353
31. Sawatzki M, Meyenberger C, Brand S, Semela D. Contrast-enhanced ultrasound (CEUS) has excellent diagnostic accuracy in differentiating focal liver lesions: results from a Swiss tertiary gastroenterological centre. Swiss Med weekly (2019) 149:w20087. doi: 10.4414/smw.2019.20087
32. Trenker C, Kunsch S, Michl P, Wissniowski TT, Goerg K, Goerg C. Contrast-enhanced ultrasound (CEUS) in hepatic lymphoma: retrospective evaluation in 38 cases. Ultraschall der Med (Stuttgart Germany 1980). (2014) 35(2):142–8. doi: 10.1055/s-0033-1350179
33. Trenker C, Neesse A, Görg C. Sonographic patterns of renal lymphoma in b-mode imaging and in contrast-enhanced ultrasound (CEUS)–a retrospective evaluation. Eur J radiology. (2015) 84(5):807–10. doi: 10.1016/j.ejrad.2014.12.027
34. Trenker C, Wilhelm C, Neesse A, Rexin P, Görg C. Contrast-enhanced ultrasound in pulmonary lymphoma: A small pilot study. J ultrasound Med Off J Am Institute Ultrasound Med (2018) 37(12):2943–7. doi: 10.1002/jum.14651
35. Yang L, Zhao H, He Y, Zhu X, Yue C, Luo Y, et al. Contrast-enhanced ultrasound in the differential diagnosis of primary thyroid lymphoma and nodular hashimoto's thyroiditis in a background of heterogeneous parenchyma. Front Oncol (2020) 10:597975. doi: 10.3389/fonc.2020.597975
36. Yang L, Tao Y, Weixin Z, Meiling B, Jing H. Contrast-enhanced and microvascular ultrasound imaging features of testicular lymphoma: report of five cases and review literature. BMC urology. (2022) 22(1):6. doi: 10.1186/s12894-022-00957-1
Keywords: non-Hodgkin’s lymphoma, ultrasound, contrast-enhanced ultrasound (CEUS), time-intensity curve (TIC), Positron Emission Tomography- Computed Tomography (PET- CT)
Citation: Zhang YQ, Wang XY and Huang Y (2023) The findings on the CEUS of diffuse large B cell lymphoma in abdomen: A case report and literature review. Front. Oncol. 13:1093196. doi: 10.3389/fonc.2023.1093196
Received: 08 November 2022; Accepted: 20 January 2023;
Published: 02 February 2023.
Edited by:
Antonio Bottari, Università degli Studi di Messina, ItalyCopyright © 2023 Zhang, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Huang, aHVhbmd5aW5nNzEyQDE2My5jb20=
 Yu-Qing Zhang
Yu-Qing Zhang Ying Huang
Ying Huang