
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 09 February 2023
Sec. Cancer Genetics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1091635
Background: Renal cell carcinoma (RCC) is the most common renal malignancy, and may metastasize to different sites in the body via hematogenous and lymphomatous routes. The pancreas is a rare metastatic site of metastatic RCC (mRCC) and isolated pancreatic metastasis of RCC (isPMRCC) is even rarer.
Results: The present report describes a case of isPMRCC that recurred 16 years after surgery. The patient responded well to the treatment with pancreaticoduodenectomy and systemic therapy, and no recurrence was recorded after 2 years.
Conclusions: isPMRCC is a distinct subgroup of RCC with unique clinical characteristics that may be explained by its underlying molecular mechanisms. Surgery and systemic therapy confer survival benefits to patients with isPMRCCs, although the recurrence problem has to be paid attention to.
Tumors of the renal and perirenal tissues account for 2% to 3% of all malignancies, and among these, renal cell carcinoma (RCC) accounts for 85% of all renal parenchymal tumors (1). The metastasis of RCC is sporadic, with the metastatic sites including the lung, bone, adrenal gland, liver, kidney, brain, etc. (2) RCC may also metastasize to rare sites, such as the duodenum, and the intestine (3, 4). However, pancreatic metastasis (PM) of RCC is quite rare (5). PM may appear in two clinical manifestations:(1) isolated pancreatic metastasis and (2) in the context of multi-organ metastatic disease. Cases of isolated pancreatic metastasis are extremely rare, accounting for approximately 1% to 3% of diagnoses (6).
The present report describes the case of a patient who developed the isolated pancreatic metastasis of renal cell carcinoma (isPMRCC) at the head of the pancreas 16 years after the curative resection.
A 73-year-old woman was admitted to our hospital with abnormal imaging results and no symptoms. Contrast-enhanced CT scan (Figure 1) revealed a mass in the head of the pancreas, with atrophy of the body and the tail of the pancreas, and the pancreatic duct was dilated, which suggested pancreatic cancer. Magnetic Resonance Cholangiopancreatography (Figure 1) revealed a space-occupying lesion in the head of the pancreas. The physical examination of the abdomen was negative. The past medical history of the patient revealed that she had undergone a right partial nephrectomy for renal cell carcinoma 16 years earlier. At the time of the surgery, the clinical stage of renal cell carcinoma was stage I (T1aN0M0), according to the TNM classification. Pathology revealed a Fuhrman grade 3 clear cell RCC (ccRCC). The patient did not receive adjuvant chemotherapy or targeted therapy later. Rather, they were followed up for abdominal computed tomography (CT) and ultrasonography (US), which revealed no evidence of recurrent disease prior to the present admission at this hospital. A further metastatic workup, including Positron Emission Tomography/Computed Tomography (PET/CT), did not disclose any evidence of extra pancreatic lesions.
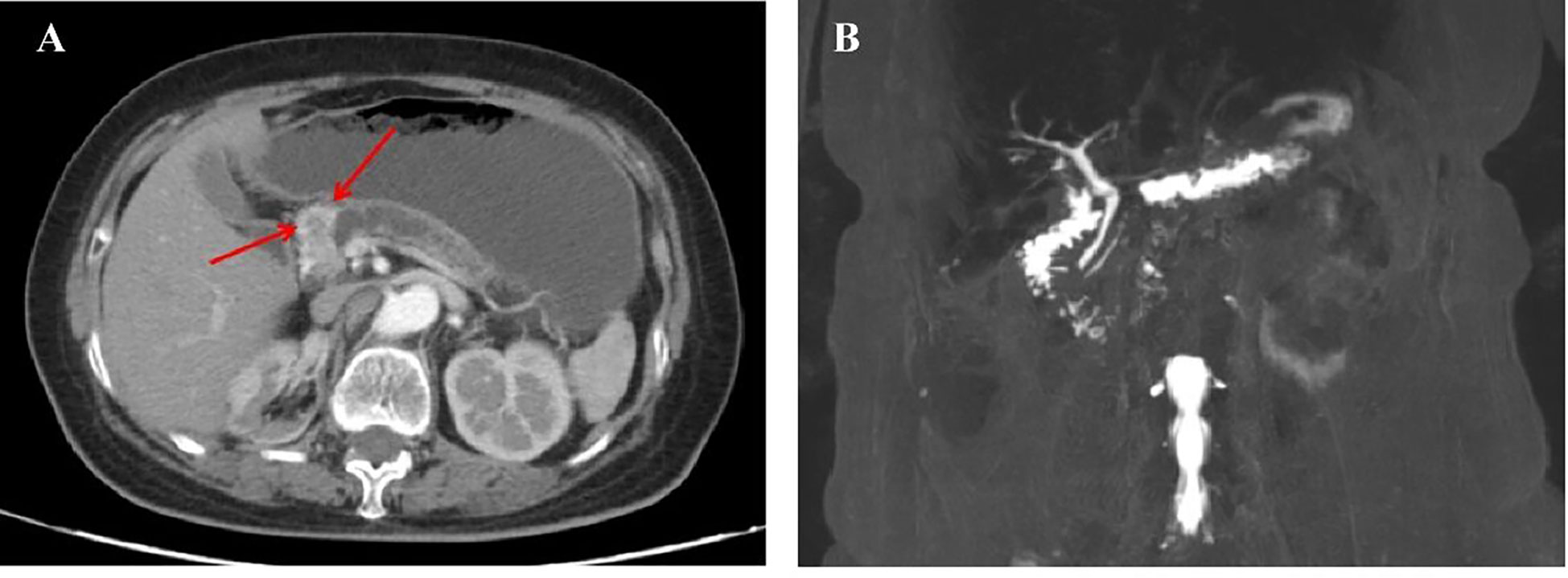
Figure 1 (A) An Abdominal computed tomographic scan and contrast-enhanced scan taken on March 17, 2020, showing a mass in the head of the pancreas, with atrophy of the body and tail of the pancreas, and the pancreatic duct obviously dilated. (B) Magnetic Resonance Cholangiopancreatography imaging examination taken on March 19, 2020, showing a space-occupying lesion in the head of the pancreas, with obvious dilation of the pancreatic duct at the tail of the pancreas.
The possibility of a neuroendocrine tumor was considered, and metastasis from renal cancer could not be excluded. There were indications for surgery according to the NCCN guidelines, and the specific surgical approach was determined based on the intraoperative mass location and intraoperative rapid frozen pathology. Pancreaticoduodenectomy was performed. The surgical specimen demonstrated a 3*2.5*2cm tumor in the head of the pancreas, which was consistent with metastatic renal cell carcinoma and morphology, history, and immunohistochemistry analyses. No tumor involvement was detected in the common bile duct incision margin, pancreatic head stump margin, upper gastric incision margin, and inferior duodenal incision margin. No cancer metastasis was observed in the surrounding lymph nodes, although there was a tumor thrombus in small intratumoral vessels. The microscopic image examination after hematoxylin and eosin staining (Figures 2A–C) revealed renal cell carcinoma within the pancreatic tissue. Histopathological examination (Figures 2D–F) revealed paired box gene 8-positive (PAX-8+), cluster of differentiation 10-positive (CD10+) and creatine kinase-positive (CK+) cells. According to the patient’s medical history, histopathological examination, clinical images, and pathological findings, a final diagnosis of isolated pancreatic metastasis of renal clear cell carcinoma was established. The outcomes of next-generation sequencing (NGS) verified three gene mutations (Table 1) in von Hippel-Lindau (VHL), phosphatase and tensin homolog (PTEN), and lysine-specific histone demethylase 5C (KDM5C), a low tumor mutation burden (TMB) of 6.3 Muts/Mb, and a microsatellite stable (MSS) status. A multidisciplinary team (MDT) discussed the case, and the patient was then treated with pazopanib-targeted therapy after surgery to prevent tumor recurrence. The patient was followed in the outpatient clinic for over 2 years, and no recurrence was reported. The timeline of the case is presented in Figure 3.
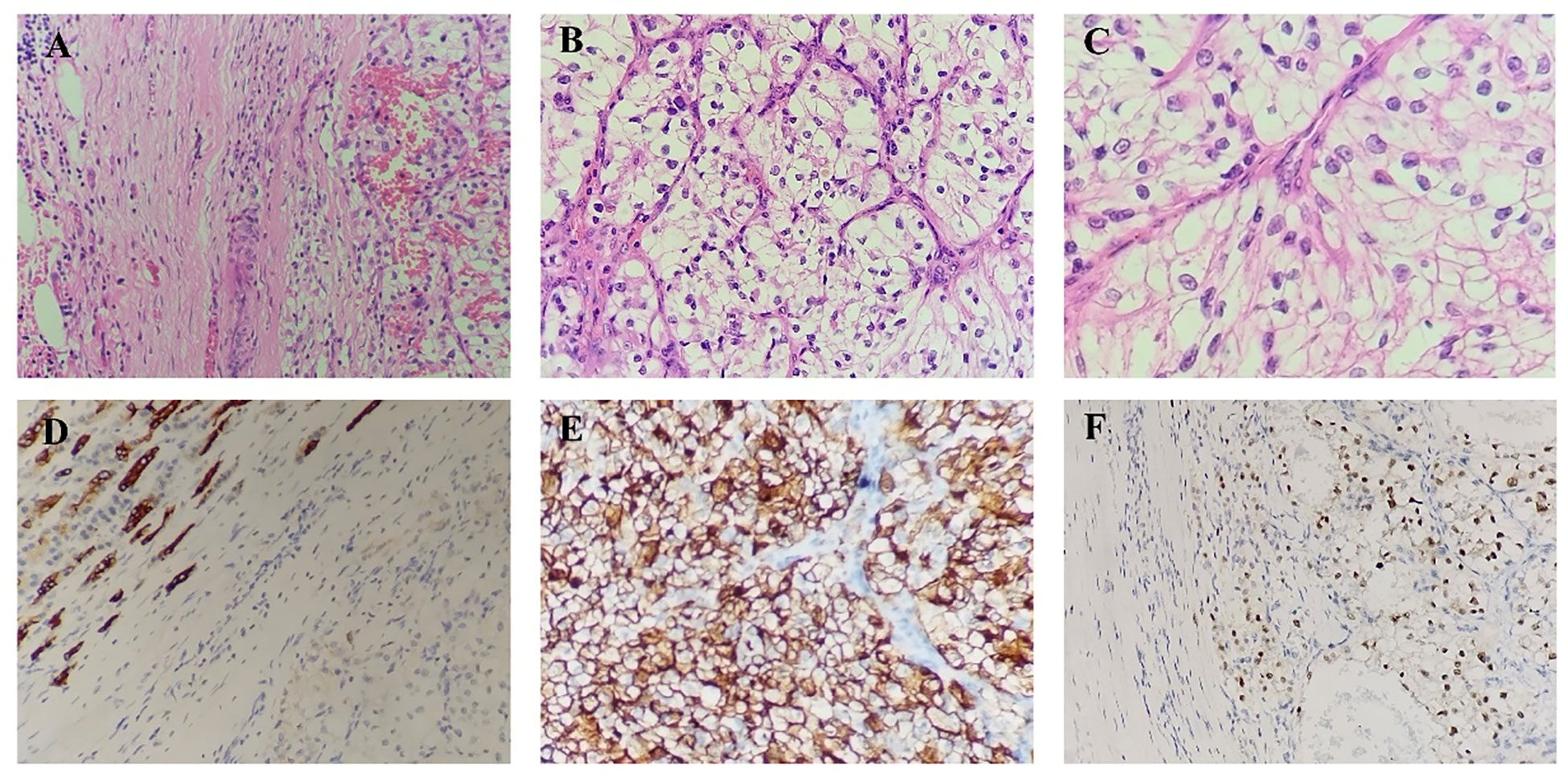
Figure 2 (A–C). Microscopic picture with hematoxylin and eosin staining showing the renal cell carcinoma within the pancreatic tissue. (D). Immunohistochemistry staining of the resected lesion from the pancreas, showing positive CK. (E). Immunohistochemistry staining of the resected lesion from the pancreas, showing positive CD10. (F). Immunohistochemistry staining of the resected lesion from the pancreas, showing positive PAX-8.
The common sites for the metastasis of RCC are the lungs, liver, lymph nodes, and brain (2). The pancreas is one of the rare sites of RCC metastasis, accounting for < 2% of patients with advanced disease (7). Isolated metastasis from RCC is rare, with an incidence of between approximately 1% and 4% for cases with pancreatic origins (7). In terminal systemic disease tumors, pancreatic metastasis rarely occurs, in approximately 2% of surgical cases, with a corresponding poor prognosis (8, 9). However, among patients with multi-organ site mRCC, concomitant PM presents a better prognosis compared to the cases without PM (median OS: 42 months vs. 23 months) (10). Furthermore, isPMRCC is characterized by an unusually favorable prognosis after surgical therapy.
This type of tumor presents low tumor aggressiveness, which results in a long interval between the initial diagnosis and the metastatic disease. The median interval is 7.1-10.0 years (11), and PM from RCC may occur over 36 years after the primary diagnosis. In the case presented here, metastasis to the head of the pancreas was detected during imaging surveillance 16 years after the resection of the primary tumor. This kind of metachronous metastasis is rare. Certain researchers suggest that such patients harbored ccRCC micro metastasis in the tumor microenvironment (TME), which then gradually established at the PM site and released cytokines that promoted angiogenesis and induced immune tolerance (12, 13). Moreover, a chronic state of low-grade inflammation due to long-term endogenous or exogenous factors could induce tumor progression (14). Notably, most of these patients are asymptomatic, and the diagnosis is usually incidentally or established during radiological surveillance (15, 16). Owing to the long interval and atypical clinical symptoms, an early diagnosis becomes challenging. Therefore, patients with a history of RCC require a meticulous long-term follow-up to ensure that recurrence is not missed.
As a distinct case of RCC, isPMRCC has a clinical hallmark of confinement to the pancreas while the other organs are left unaffected for a long duration. This kind of organotropism may be explained based on seed and soil theory, according to which the metastatic process is determined by the interaction between the tumor cells (seed) and the host organism (soil) (17, 18). Research on microRNA has identified that miRNAs regulate the metastatic potential of tumor cells and explain the seed and soil concept biochemically (17, 19). Their interactions are related to genetic changes associated with the activation of proto-oncogenes or inactivation of suppressor genes (20), such as PTEN, and may involve interactions with the tumor microenvironment, the immune system, and altered epigenetic status (21).
Certain possible biochemical mechanisms are reported for the characteristics of RCC. It is reported that the genome of ccRCC is distinctive. In ccRCC, the presence of some mutations is common, as reported in a retrospective review (N=105) (22). The most frequent mutations were detected in VHL (83%), PBRM1(51%), SETD2(35%), BAP1(24%), KDM5C (16%), and TERT (14%). Notably, chromosome 3p deletions and VHL mutation are always at the trunk of the phylogenetic tree, which suggests that these two kinds of mutations are key early events in the development of RCC. Turajlic et al. were the first highlight the genetic changes relevant to the metastatic behaviors of renal cell cancer and also discuss the evolutionary classification of different metastatic potentials (Figure 4) (24). Concerning pancreatic metastasis, gene targeting experiments in mouse kidneys revealed that the inactivation of VHL and PBRM1 resulted in ccRCC with a prominent vascular network and compact nests, which indicates that these alterations probably drive the characteristic histology of PM. However, the inactivation of VHL and BAP1 resulted in further aggressive, inflammatory, and less-vascularized tumors. Singla et al. (25) reported that certain specific genetic changes in the PM cell clones are associated with less aggressive disease, including a low frequency of copy number alterations, a low frequency of BAP1 mutations, and a high frequency of PBRM1 loss. In the case presented here, no specific BAP1 gene mutation was detected, which suggested gradual tumor growth (25).
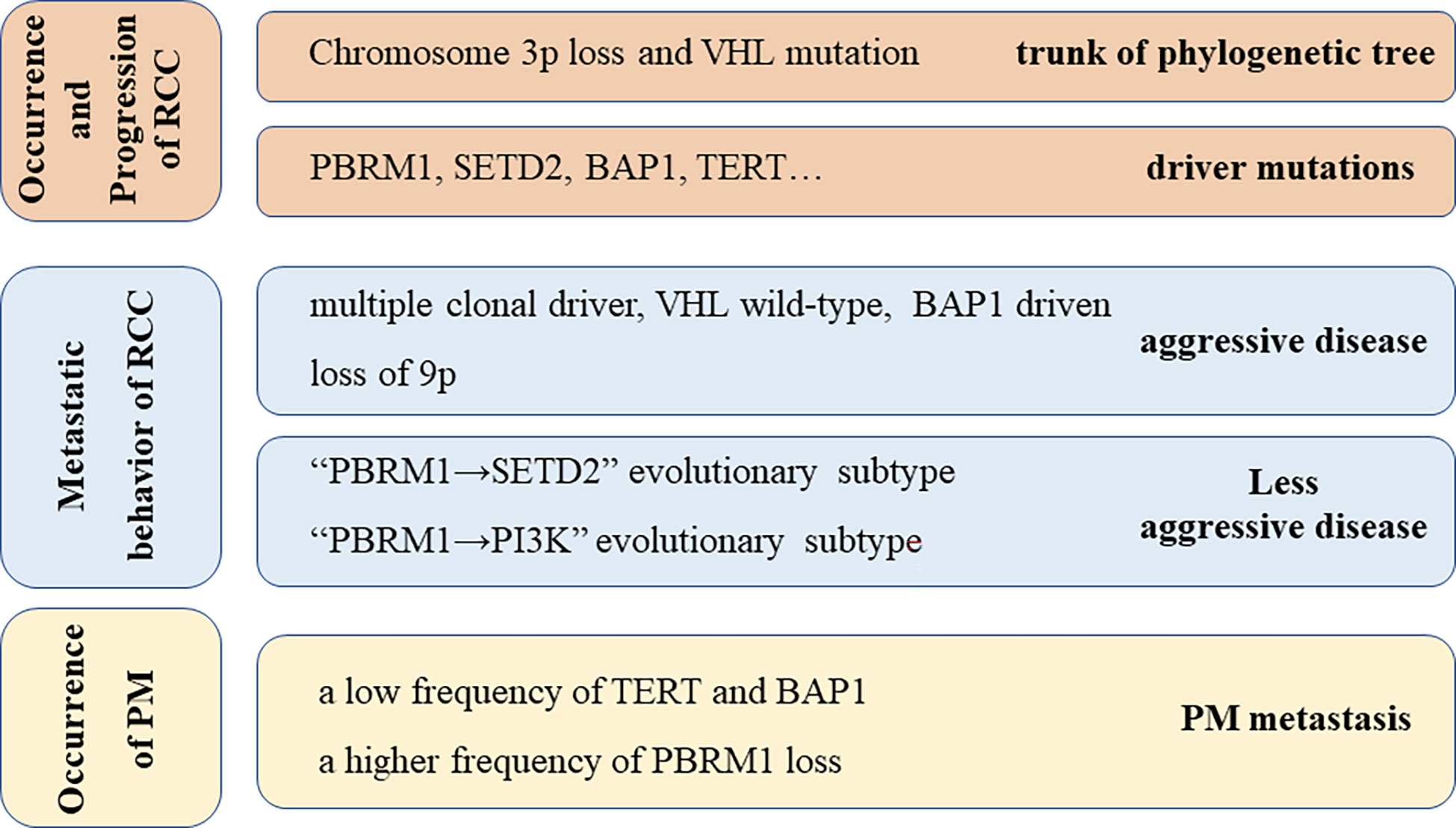
Figure 4 Underlying Genetic alterations possibly responsible for the behavior of RCC and PM metastasis, including genetic alterations responsible for the occurrence and progression of RCC (23), metastatic behavior of RCC (24), and occurrence of PM (25).
In the present case, genetic testing was performed, and the resulting profiling data revealed gene mutations in VHL, PTEN, and KDM5C. VHL mutation is a dominant feature in ccRCC. PTEN is recognized as a dormant tumor suppressor, and a loss of PTEN expression may result in the initiation of tumorigenesis (26). Mutations in both VHL and PTEN are reported to be significantly associated with a poor prognosis (27, 28). In contrast, mutations in KDM5C play a functional role in the epigenetic phenomena (29). KDM5C is a chromatin modifier gene that encodes demethylases that target trimethylated lysine 4 on histone H3. Chromosomal dysfunction may lead to disease development. A study reported that tumors with a mutation in KDM5C are statistically associated with a higher stage (30). In another study, tumors with KDM5C mutation presented heterochromatin and genomic rearrangement, which resulted in a poor prognosis (29). In addition, studies have demonstrated that a mutation in this gene is associated with a higher risk of cancer recurrence and death among patients with small renal masses (30, 31). One main reason for this is that this mutation is related to the distribution of TAMs, which reportedly support tumor cell growth and metastasis through a series of pathways (32). Therefore, a metastatic event could be reasonably suspected in an RCC patient with a KDM5C mutation. Therefore, it was postulated that the occurrence, proliferation, and metastatic behavior of ccRCC could be explained based on the related genome alteration.
Pancreatoduodenectomy was performed in the present case, and no recurrence was detected during follow-up for 2 years after surgery. Pancreatectomy for metastasis is not common, although RCC is the most common primary source (33). A survival benefit is associated with this subset of patients when they undergo surgery (complete oncological resection is associated with a 5-year survival rate of 75%) (10), particularly if RCC is the primary source (34). However, it has been reported that after the treatment of isPMRCC, 41.5% of patients present recurrence (125 patients among 301 patients relapsed after a 30-month interval) (10). The longest reported interval for recurrence was 10 years. Therefore, long-term monitoring is recommended. Since mRCC has a poor outcome overall, targeted therapy (pazopanib) has also been used after surgical treatment to improve the patient’s survival, and the patient exhibited a good response. Pazopanib is a novel TKI that inhibits the vascular endothelial growth factor receptor, platelet-derived growth factor receptor, c-Kit, and other targets. Currently, pazopanib has been approved in the EU, the USA, and other nations for the treatment of advanced RCC (35). In addition, several prospective clinical trials and retrospective studies support the use of pazopanib as a standard or alternative first-line treatment for advanced or metastatic RCC (35–37).
isPMRCC is a distinct subgroup of RCC with unique clinical characteristics that may be explained based on underlying molecular mechanisms. Surgery and systemic therapy present survival benefit in patients with isPMRCCs, although the recurrence problem should be paid attention to.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL, KG collected the data; YL, KG, and SZ wrote the manuscript. All authors read, provided feedback, and approved the final version.
This study was funded by the National Natural Science Foundation of China (81372660), the Zhejiang Province Public Welfare Technology Application Research Project (2017C33200), the Medical Science Research Fund of Zhejiang Province, China (2013KYA157), and the Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liu SZ, Guo LW, Cao XQ, Chen Q, Zhang SK, Zhang M, et al. [Estimation on the incidence and mortality of kidney cancer in China, in 2014]. (2018) 39(10):1346–50. Zhonghua Liu Xing Bing Xue Za Zhi.
2. Flanigan RC, Campbell SC, Clark JI, Picken MM. Metastatic renal cell carcinoma. Curr Treat Options Oncol (2003) 4(5):385–90. doi: 10.1007/s11864-003-0039-2
3. Eo WK, Kim GY, Cho SI. A case of multiple intussusceptions in the small intestine caused by metastatic renal cell carcinoma. Cancer Res Treat (2008) 40(2):97–9. doi: 10.4143/crt.2008.40.2.97
4. Franssen B, Chan C, Ramírez-Del Val A, Llamas F, López-Tello A. [Renal cell carcinoma metastatic to the duodenum and vater ampulla: report of two cases]. Rev Gastroenterol Mex (2011) 76(4):375–9.
5. Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol (2012) 23(4):973–80. doi: 10.1093/annonc/mdr362
6. Gohji K, Matsumoto O, Kamidono S. Solitary pancreatic metastasis from renal cell carcinoma. Hinyokika Kiyo (1990) 36(6):677–81.
7. Tolia BM, Whitmore WF Jr. Solitary metastasis from renal cell carcinoma. J Urol (1975) 114(6):836–8. doi: 10.1016/S0022-5347(17)67155-6
8. Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature. Virchows Arch (2004) 444(6):527–35. doi: 10.1007/s00428-004-0987-3
9. Ito T, Takada R, Omoto S, Tsuda M, Masuda D, Kato H, et al. Analysis of prognostic factors in pancreatic metastases: A multicenter retrospective analysis. Pancreas (2018) 47(8):1033–9. doi: 10.1097/MPA.0000000000001132
10. Sellner F, Thalhammer S, Klimpfinger M. Isolated pancreatic metastases of renal cell cancer: Genetics and epigenetics of an unusual tumour entity. Cancers (Basel) (2022) 14(6):1539. doi: 10.3390/cancers14061539
11. Vickers MM, Al-Harbi H, Choueiri TK, Kollmannsberger C, North S, MacKenzie M, et al. Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy: results from the international metastatic renal cell carcinoma database consortium. Clin Genitourin Cancer (2013) 11(3):311–5. doi: 10.1016/j.clgc.2013.04.012
12. Cao H, Sun Z, Wu J, Hao C, Wang W. Metastatic clear cell renal cell carcinoma to pancreas and distant organs 24 years after radical nephrectomy: A case report and literature review. Front Surg (2022) 9:894272. doi: 10.3389/fsurg.2022.894272
13. Mennitto A, Huber V, Ratta R, Sepe P, de Braud F, Procopio G, et al. Angiogenesis and immunity in renal carcinoma: Can we turn an unhappy relationship into a happy marriage? J Clin Med (2020) 9(4):930. doi: 10.3390/jcm9040930
14. Salminen A. Activation of immunosuppressive network in the aging process. Ageing Res Rev (2020) 57:100998. doi: 10.1016/j.arr.2019.100998
15. Khiella M, Maximus MA, Fayek SA. Renal cell carcinoma metastases to the pancreas and the thyroid gland: A case report and review of the literature. Cureus (2018) 10(12):e3667. doi: 10.7759/cureus.3667
16. Gutiérrez Zurimendi G, Llarena Ibarguren R, Lecumberri Castaños D, Fernández Pereda R, Urresola Olabarrieta A, García De Casasola-Rodríguez G, et al. [Pancreatic metastasis of primary kidney cancer: A presentation of a serie of clinical cases and revision of the literature.]. Arch Esp Urol (2020) 73(2):147–54.
17. Sellner F. Isolated pancreatic metastases from renal cell carcinoma: An outcome of a special metastatic pathway or of specific tumor cell selection? Clin Exp Metastasis (2018) 35(3):91–102. doi: 10.1007/s10585-018-9910-1
18. Sellner F, Thalhammer S, Klimpfinger M. Tumour evolution and seed and soil mechanism in pancreatic metastases of renal cell carcinoma. Cancers (Basel) (2021) 13(6):1342. doi: 10.3390/cancers13061342
19. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell (2004) 116(2):281–97. doi: 10.1016/S0092-8674(04)00045-5
20. Marta GN, Garicochea B, Carvalho AL, Real JM, Kowalski LP. MicroRNAs, cancer and ionizing radiation: Where are we? Rev Assoc Med Bras (1992)61(3):275–81. doi: 10.1590/1806-9282.61.03.275
21. Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell (2013) 155(4):750–64. doi: 10.1016/j.cell.2013.10.029
22. Carlo MI, Manley B, Patil S, Woo KM, Coskey DT, Redzematovic A, et al. Genomic alterations and outcomes with VEGF-targeted therapy in patients with clear cell renal cell carcinoma. Kidney Cancer (2017) 1(1):49–56. doi: 10.3233/KCA-160003
23. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med (2012) 366(10):883–92. doi: 10.1056/NEJMoa1113205
24. Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell (2018) 173(3):581–594.e12. doi: 10.1016/j.cell.2018.03.057
25. Singla N, Xie Z, Zhang Z, Gao M, Yousuf Q, Onabolu O, et al. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight (2020) 5(7):e134564. doi: 10.1172/jci.insight.134564
26. Berger AH, Pandolfi PP. Haplo-insufficiency: A driving force in cancer. J Pathol (2011) 223(2):137–46. doi: 10.1002/path.2800
27. Schraml P, Struckmann K, Hatz F, Sonnet S, Kully C, Gasser T, et al. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol (2002) 196(2):186–93. doi: 10.1002/path.1034
28. Tang L, Li X, Gao Y, Chen L, Gu L, Chen J, et al. Phosphatase and tensin homolog (PTEN) expression on oncologic outcome in renal cell carcinoma: A systematic review and meta-analysis. PLos One (2017) 12(7):e0179437. doi: 10.1371/journal.pone.0179437
29. de Cubas AA, Rathmell WK. Epigenetic modifiers: activities in renal cell carcinoma. Nat Rev Urol (2018) 15(10):599–614. doi: 10.1038/s41585-018-0052-7
30. Hakimi AA, Chen YB, Wren J, Gonen M, Abdel-Wahab O, Heguy A, et al. Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol (2013) 63(5):848–54. doi: 10.1016/j.eururo.2012.09.005
31. Stein J, Majores M, Rohde M, Lim S, Schneider S, Krappe E, et al. KDM5C is overexpressed in prostate cancer and is a prognostic marker for prostate-specific antigen-relapse following radical prostatectomy. Am J Pathol (2014) 184(9):2430–7. doi: 10.1016/j.ajpath.2014.05.022
32. Chakiryan NH, Hajiran A, Kim Y, Aydin AM, Zemp L, Katende E, et al. Correlating immune cell infiltration patterns with recurrent somatic mutations in advanced clear cell renal cell carcinoma. Eur Urol Focus (2021). doi: 10.1097/JU.0000000000002071.08
33. Huang Q, Zhou H, Liu C, Jin K, Fan K, Cheng H, et al. Surgical resection for metastatic tumors in the pancreas: A single-center experience and systematic review. Ann Surg Oncol (2019) 26(6):1649–56. doi: 10.1245/s10434-019-07258-2
34. Jaen-Torrejimeno I, Rojas-Holguín A, López-Guerra D, Ramia JM, Blanco-Fernández G, et al. Pancreatic resection for metastatic renal cell carcinoma. a systematic review. HPB (Oxford) (2020) 22(4):479–86. doi: 10.1016/j.hpb.2019.10.017
35. Zivi A, Cerbone L, Recine F, Sternberg CN. Safety and tolerability of pazopanib in the treatment of renal cell carcinoma. Expert Opin Drug Saf (2012) 11(5):851–9. doi: 10.1517/14740338.2012.712108
36. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med (2013) 369(8):722–31. doi: 10.1056/NEJMoa1303989
Keywords: renal cell carcinoma (RCC), late recurrence, genetics, VHL, Pten, KDM5C, isolated pancreatic metastasis
Citation: Lou Y, Guo K and Zheng S (2023) Case Report: Pancreatic metastasis of renal cell carcinoma 16 years after nephrectomy. Front. Oncol. 13:1091635. doi: 10.3389/fonc.2023.1091635
Received: 07 November 2022; Accepted: 25 January 2023;
Published: 09 February 2023.
Edited by:
Demitrios Vynios, University of Patras, GreeceReviewed by:
Franz Sellner, Kaiser Franz Josef Hospital, AustriaCopyright © 2023 Lou, Guo and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Zheng, dHp0cmVlQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.