
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 30 January 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1091074
This article is part of the Research TopicCase Reports in Hematological Malignancies : 2022View all 34 articles
Background: Hematological malignancies of the heart (CHMs) are extremely rare, and include leukemia, lymphoma infiltration, and multiple myeloma with extramedullary manifestations. Cardiac lymphoma can be divided into primary cardiac lymphoma (PCL) and secondary cardiac lymphoma (SCL). Compared to PCL, SCL is relatively more common. Histologically, the most frequent SCL is diffuse large B-cell lymphoma (DLBCL). The prognosis of lymphoma in patients with cardiac involvement is extremely poor. CAR T-cell immunotherapy has been recently become a highly effective treatment for relapsed or refractory diffuse large B-cell lymphoma. To date, there are no guidelines that provide a clear consensus on the management of patients with secondary heart or pericardial involvement. We report a case of relapsed/refractory DLBCL that secondarily affected the heart.
Case presentation: A male patient was diagnosed with double-expressor DLBCL based on biopsies of mediastinal and peripancreatic masses and fluorescence in situ hybridization. The patient received first-line chemotherapy and anti-CD19 CAR T cell immunotherapy, but developed heart metastases after 12 months. Considering his physical condition and economic situation of the patient, two cycles of multiline chemotherapies were administered, followed by CAR-NK cell immunotherapy and allogeneic hematopoietic stem cell transplantation (allo-HSCT) at another hospital. After achieving a six-month survival, the patient died of severe pneumonia.
Conclusion: The response of our patient emphasizes the importance of early diagnosis and timely treatment to improve the prognosis of SCL and serves as an important reference for SCL treatment strategies.
Secondary cardiac lymphoma (SCL) is a relatively uncommon cancer that affects the heart and/or pericardium, with mortality rates of approximately 8.5% to 25% (1). It is most common in men, especially in immunosuppressed patients, with a median age of 60 years (2, 3). Among cardiac lymphomas, diffuse large B-cell lymphoma (DLBCL) is the most prevalent histology (80%); other histological subtypes are less common (1, 3, 4). The clinical signs and symptoms of SCL tend to be nonspecific, including the superior vena cava (SVC), dyspnea, constitutional complaints, chest pain and B-symptoms (i.e., weight loss, fatigue, night sweats) and so on, resulting in frequently missed diagnoses (1, 2, 4). As secondary cardiac lymphoma is a rare disease, treatment recommendations are largely derived from retrospective studies and case reports (2, 4). Therapies for cardiac lymphoma include mainly chemotherapy, often combined with radiotherapy, surgery, autologous stem cell transplantation, and T cell therapy with the chimeric antigen receptor (CAR) (1, 5, 6). Chemotherapy with cyclophosphamide, vincristine, doxorubicin, and prednisolone (CHOP) is widely used as first-line treatment, but its overall survival rate (OS) is only 18 months, while patients treated with BACOP (bleomycin + doxorubicin + cyclophosphamide + vincristine + prednisone) have a survival of 49 months. Patients receiving surgical resection have a survival of more than 18 months and those with radiotherapy achieve a survival of 15 months (7). The addition of a monoclonal CD20 antibody (rituximab) to the CHOP protocol has shown potential to increase OS rate (1, 8, 9). Despite the fact that these therapies are associated with the greatest improvement in survival, not every patient receives complete multimodal treatment due to individual factors. Therefore, more effective treatment strategies should be developed to improve the outcomes of patients with cardiac involvement. Here, we report a case of relapsed/refractory DLBCL that secondarily affected the heart and was novelly treated with CAR-NK and allo-HSCT.
A 59-year-old man came to our hospital on 10 November 2020, complaining of persistent pitting edema of the face and neck that had been occurring for one month, along with night sweats and backaches, with no weight loss, low fever, dyspnea, cough, pitting edema of the lower extremities, and other symptoms. Laboratory test results are as follows: hemoglobin 114 g/L, platelet count 454×109/L, LDH 519 IU/L, serum β2 microglobulin 2.59 mg/L. Chest computed tomography (CT) revealed multiple space-occupying lesions in the middle and upper mediastinum. Positron emission tomography CT (PET-CT) revealed abnormally increased fluorodeoxyglucose (FDG) uptake in the middle and upper mediastinum lymph nodes with a maximum lymph node size of 6.3 × 4.3 cm and the maximum standard uptake value (SUV max) was 13.8, the boundary between cardiovascular vessels and masses was not clear (Figures 1A-C, E, F). The pancreatic uncinate showed a round focus with high FDG uptake of approximately 2.0 cm in diameter, the SUVmax was 5.7 (Figures 1D-F). On 18 November 2020, a CT-guided percutaneous pancreas and mediastinum biopsy was performed. Hematoxylin-eosin staining of pancreatic mases (Figure 1G) and mediastinum (Figures 1H, I) showed medium to large abnormal lymphocytes with irregular nucleus. Immunohistochemical staining revealed that tumor cells were positive for CD19 (>95%), CD3, CD20, CD30, BCL-6, PAX-5, c-Myc, Ki-67 (90%+), and was negative for Syn, CgA, CK, CA199, CK20, CK7, CD56, and BCL-2. Both bone marrow cytology and biopsy results revealed no aberrant lymphocyte infiltration. In summary, the patient was diagnosed with DLBCL at stage IVB. The IPI score was 3 and the ECOG performance status was 1. The immunohistochemistry results revealed a non-germinal center B-cell-like lymphoma (non-GCB) subtype. The patient had no family history of hematological malignancies.
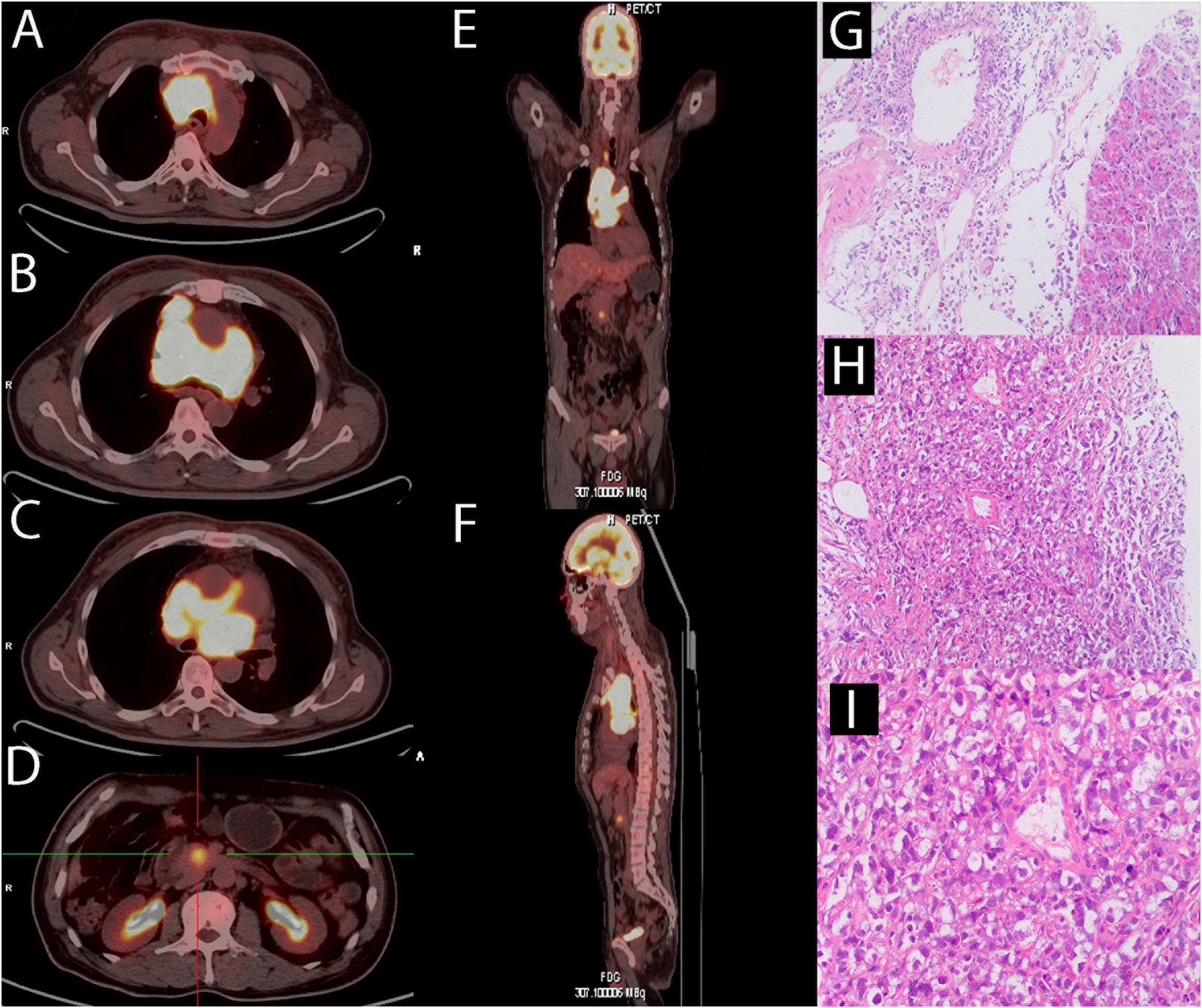
Figure 1 At diagnosis of lymphoma, a PET-CT scan and a biopsy were performed. (A-C) PET/CT revealed multiple enlarged lymph nodes in the middle and upper mediastinum and the maximum plane size was approximately 6.3 cm x 4.3 cm. There were masses showing infiltration around the ascending aorta and pulmonary artery. The SUVmax was 13.8. (D) PET/CT revealed the uncinate process of pancreas infiltration, with a diameter of about 2 cm and the SUVmax was 5.7. (E, F) Overall, there is no significant abnormal radiation uptake outside of the pancreas and mediastinal lymph nodes. (G)The hematoxylin-eosin staining revealed medium to large cells with irregular nucleus in the pancreas (original magnification 10 x). (H, I) The hematoxylin-eosin staining revealed medium to large cells with irregular nucleus in the mediastinum (H:original magnification 10 x; G:original magnification 20 x).
The patient was started on induction chemotherapy with one course of R-CHOP (rituximab 375 mg/m2 on day 0, doxorubicin 30 mg/m2 and cyclophosphamide 750 mg/m2 on day 1, vincristine 2 mg on day 1, and prednisolone 60 mg/m2 on days 1–5) and three courses of R2-CHOP (rituximab 375 mg/m2 on day 0, doxorubicin 30 mg/m2, cyclophosphamide 750 mg/m2, and vincristine 2 mg on day 1, prednisolone 60 mg/m2 on days 1–5, and lenalidomide 25 mg on days 1–21), he achieved partial remission by PET-CT. The patient then received two separate apheresis procedures, including granulocyte colony-stimulating factor (G-CSF)-stimulated autologous hematopoietic stem cell (HSC) collection (CD34+ 4.35×106/kg). Followed by three more courses of R2-CHOP. Unfortunately, although the patient had no obvious symptoms, PET-CT showed progressive disease. To prevent the progression of the disease, he was transferred to another institution for CAR T cell therapy. The patient was given a standard dose of FC (Fludarabine 25mg/m2 on days -7 to -5, cyclophosphamide 250 mg/m2 on days -7 to -5) as conditioning regimen one week before CAR-T cell therapy. On 6 September 2021 (day 0), CD19-targeted CAR-T cells (CAR19) (2.0x107 cells/kg) were infused into the patient. One month after CAR-T cell therapy, a second contrast-enhanced chest CT during follow-up showed that lymph nodes in the middle and upper mediastinum were larger than before. Shortness of breath, palpitations, hypoxemia, and fever ensued. Combined with the history of the disease and PET-CT, he was considered to have a progressive disease. On PET-CT, the largest lymph node was approximately 8.3 cm and the SUVmax was 13, the masses fused in the pulmonary gaps and their boundaries were unclear, the Deauville score was 5. He had pneumonia and refractory bilateral pleural effusion; bilateral drainage was performed as a salvage procedure. Cytology, biochemistry, and flow cytometric analysis of the pleural fluid did not show any infiltration of abnormal lymphocytes. As a result of antibiotic therapy, pleural chest drainage, and supportive therapies, the patient’s symptoms improved. R-GemOx (gemcitabine 1000 mg/m2 and oxaliplatin100 mg/m2 on day 1, and rituximab 375mg/m2 on day 0) was initiated on 13 November 2021. Three weeks later, the patient’s symptoms began to worsen, and the pleural fluid was sent for a circulating tumor DNA (ctDNA) test, which revealed somatic hypermutation (MYC, SOCS1, IGLL5, BTG1, DTX1, PIM1) and high-frequency mutation (TET2, IL4R, ACTG1, B2M, SGK1, HIST1H1E), indicating malignant pleural effusion. Transthoracic echocardiography left ventricular contrast echocardiography (LVO) and myocardial contrast echocardiography (MCE) detected heart metastases (Figures 2A-F). All clinical evidence indicated that the patient’s dyspnea and refractory bilateral pleural effusion were due to cardiac metastases; the possibility of thrombi was excluded. The patient was then treated with one cycle of PD-1+BR (sintilimab 200 mg on day 0, rituximab 375 mg/m2 on day 1, bendamustine 90 mg/m2 on days 2–3). After that, he was eager to undergo a CAR-T clinical trial for the second time. Unfortunately, at this time his T cell counts were very low. He was then treated with CAR-NK immunotherapy. He was administered the FCM regimen (fludarabine 30 mg/m2 on days -5, -4, -3, cyclophosphamide 300 mg/m2 on day -5, melphalan100 mg/m2 on day -4) regimen five days before CAR-NK. On 31 January 2021 (day 0), CAR-NK cells (5.6x106 cells/kg) were infused into the patient. On+7d he experienced grade 2 cytokine release syndrome (CRS) and was treated with tocilizumab 8 mg/kg, after which his symptoms improved. One month after CAR-NK immunotherapy, patient white blood cells and lymphocytes did not graft and the copy numbers of the CAR-NK transgene decreased. He experienced a transient reduction in pleural effusion and relief of dyspnea, chest CT showed that the cardiac mass was smaller than before which indicated stable disease. We then informed the patient and his family about his condition and treatment plan, and both signed consent forms for allogeneic hematopoietic stem cell transplantation. He received FBM (fludarabine 30mg/m2 on days -7 to -2d, busulfan 3.2 mg/kg on days -7, -6, and melphalan100 mg/m2 on day -2). Graft-versus-host disease (GVHD) prophylaxis consisted of anti-thymocyte globulin 2 mg/kg on day -1, cyclophosphamide 30 mg/kg on days +3 and +4, dexamethasone 40 mg/kg on days +3 and +4, ruxolitinib 5 mg twice a day from on days -1 to +50, 2.5 mg twice a day on days +51 to +100, and 2.5 mg once a day on days +101 to +180, myfortic 720 mg twice a day on days +5 to +34, ciclosporin 15 mg/kg/d (adjusted according to blood concentration), methotrexate 5 mg/m2 on days +3 and +6d. On 31 March 2022 (day 0), the patient underwent haploid allogeneic hematopoietic stem cell transplantation (CD34+ 6.35×106/kg), the donor was his son. Leukocytes and platelet engraftment occurred on day +16. After allo-HSCT, the patient’s condition continued to remain stable. 100% donor chimerism was achieved. On 30 April 2022, chest CT showed no new lesions and his pleural effusion decreased significantly. The timeline of clinical treatment and the state of the disease are shown in Figure 3A. However, during this period, he experienced severe fungal pneumonia. Despite multiple antifungal, antibacterial, and antiviral treatments, the patient eventually died from septic shock on 18 June 2022.
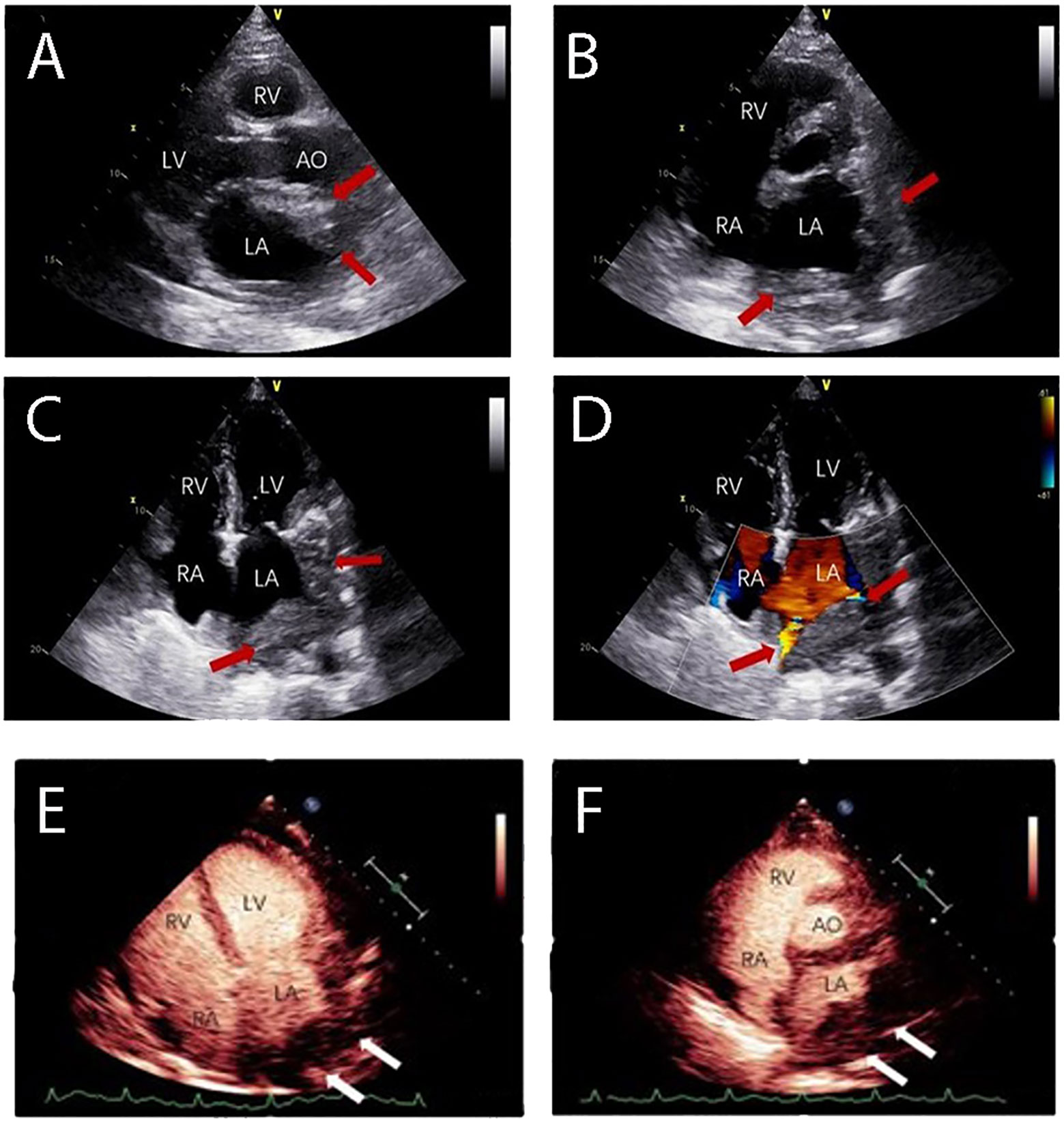
Figure 2 The patient experienced dyspnea and had a refractory bilateral pleural effusion after receiving multi-line chemotherapy. Transthoracic echocardiography showed the heart infiltrated by lymphoma. (A) Long axis section of left ventricle showing a slight hypoechoic mass in the anterior wall of the left atrium. (B) Lateral wall of left atrium and left atrial appendage were infiltrated. (C, D) Four-chamber view showed pulmonary vein infiltration. Cardiac ventricular opacification (CVO): (E, F) An irregular mass was present at the entrance of pulmonary veins in the left atrium with rapid irregular perfusion and complete enhancement. (LV: Left Ventricle; RA: Right Atrium; RV: Right Ventricle; LA: Left Atrium).
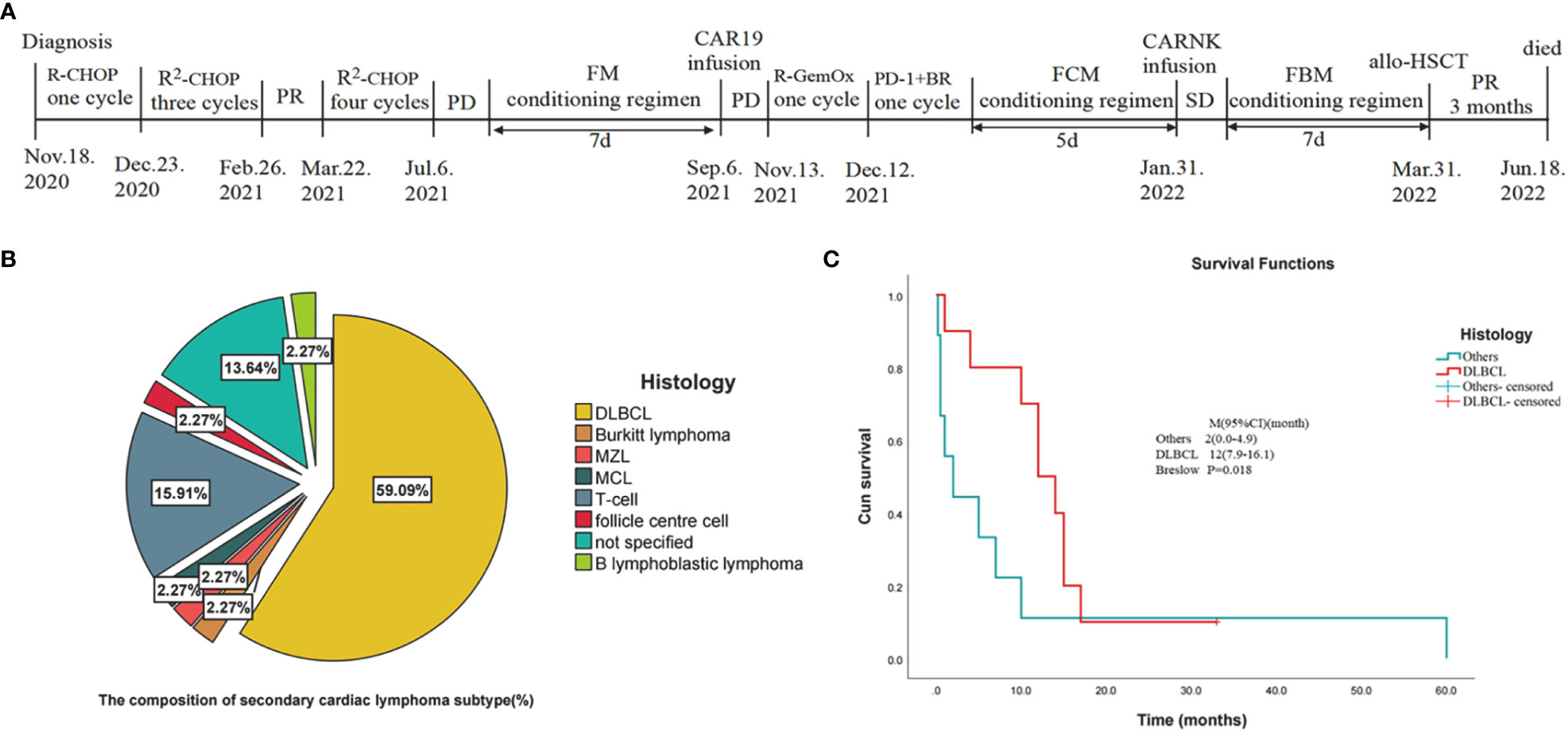
Figure 3 Statistical analysis of the data in the literature review (10–48) and the timeline of disease status and corresponding treatment regimens of our patient. (A) Timeline of disease status and corresponding treatment regimens. (B) Pie chart of tissue type composition of secondary cardiac lymphoma. (DLBCL, diffuse large B-cell lymphoma; MZL, marginal zone lymphoma; MCL, mantle cell lymphoma; T-cell: T cell lymphoma). (C) Kaplan-Meier survival curves for secondary cardiac lymphomas comparing DLBCL with other cancers included in the statistics. (CI, confidence interval).
Cardiac hematological malignancies (CHM), which comprise leukemic, lymphoma infiltration, and extramedullary manifestations of multiple myeloma, are rarer than any other malignant cardiac tumors (3). Primary and secondary cardiac lymphomas are of two varieties (10). Primary cardiac lymphomas, which comprise approximately 0.5% of all lymphomas and approximately 1.3% of all primary cardiac malignancies, are lymphomas that primarily affect the heart and/or pericardium (49). Secondary cardiac lymphomas, which are 20–40 times more prevalent than initial cardiac lymphomas, account for approximately 5%–20% of disseminated diseases (1, 50). Approximately all cardiac lymphomas have B-cell origins, and DLBCL are the most common type (80%) (1). In a retrospective investigation, DLBCL (58%), T-cell lymphoma (16%), Burkitt lymphoma (9%), and small lymphocytic lymphoma (6%) were the most prevalent histologic subtypes of NHL with biopsy-proven cardiac involvement (4). Here, we reviewed 44 patients with secondary cardiac lymphoma published on PubMed and CNKI between 1976 and 2022 (Table 1). Patients with SCL had an average age of 55 years. SCL is more common in men than in women (64.3% vs 35.7%). DLBCL was the most prevalent NHL subtype in patients with morphologic evidence of cardiac involvement (Figure 3B), accounting for 59.09% of cases, followed by T-cell lymphoma (16%) and Burkitt’s lymphoma (2.27%). There is a chance of direct, lymphatic, or hematogenous heart metastases (51).
Since cardiac lymphoma often does not present symptoms or abnormal behavior, its diagnosis is often delayed (51, 52). Depending on the location, size, growth rate, degree of invasion, and friability of the tumor, patients with cardiac involvement present with a variety of symptoms (10, 53). Based on our review of the literature, 44 cases of secondary cardiac lymphoma were located (Table 1). More specifically, in decreasing order of frequency, the right atrium, right ventricle, left atrium, and left ventricle were affected more frequently. The patient may have symptoms of right heart failure or superior vena cava syndrome (SVC) if a tumor is found in the right atrium or right ventricle (10). However, shortness of breath is often seen if a large mass occurs in the left atrial or left ventricular region (10). The most common clinical symptom described is dyspnea (64%), followed by constitutional problems (26%) and chest pain (24%) (11, 54). In our review (Table 1), the results are the same. In this case, our patient presented dyspnea, left facial swelling, and tachycardia. This was associated with lymphoma that affected the superior vena cava (SVC) and the pulmonary vein, causing limited venous inflow, which is called SVC syndrome (5–8%) (1). As a result, he was diagnosed with secondary cardiac lymphoma.
When regard to detecting cardiac involvement, ECG and chest radiographs are generally insensitive or nonspecific (51, 55, 56). A transthoracic echocardiogram is the first non-invasive method used to examine the heart and pericardium; however, the small acoustic window of this technology remains a major drawback (53). A study that included PCL patients found that transesophageal echocardiography (TEE) had higher sensitivity than transthoracic echocardiogram (TTE) for the diagnosis of lymphomatous involvement (97% vs 75.9%) (51, 57). With a high degree of spatial and temporal resolution, cardiac masses can be diagnosed by computed tomography (CT) based on their distribution, shape, and size (1). Early detection and treatment of cardiac abnormalities, as well as tracking of chemotherapy response, are now possible thanks to PET/CT (1, 51). The optimum imaging technique to determine whether the malignancy has affected the heart is CMRI (1, 51). Cardiovascular tumors show hypointense in T1-weighted sequences, while appearing hyperintense in T2-weighted sequences (11, 51). If there is a possibility of cardiac involvement, magnetic resonance imaging should be chosen despite the fact that these various imaging modalities should be considered complementary rather than competitive (53, 54).
The prognosis for cardiac lymphoma tends to be extremely poor (1). These neoplasms are often not recognized until postmortem due to their nonspecific symptoms (1). Gordon et al. found that patients with primary cardiac B-cell NHL had better outcomes than those with secondary cardiac involvement (2 months versus 6 months) (4). We found that DLBCL secondary cardiac lymphomas had a better median survival time (MST) compared to non-DLBCL secondary cardiac lymphomas (12 vs 2 months) (Figure 3C). It is important to note that patients who are immunocompromised, have extracardiac disease, left ventricular involvement, and do not have an arrhythmia are the most important adverse prognostic factors (58). Treatment guidelines are not available (58); thus, the treatment of cardiac lymphoma is varied. The medical literature presents a variety of treatment options, including chemotherapy, radiotherapy, surgery, and even autologous stem cell transplantation (1). However, it should be noted that chemotherapy is the most effective treatment and, in many cases, is used only for palliative purposes (2). The main chemotherapeutic regimen for cardiac lymphoma is CHOP (1). Adding the monoclonal CD20 antibody rituximab and other monoclonal therapies has been shown to increase overall survival rates (1). Cardiac lymphoma is also sensitive to radiotherapy (59). Radiation is, however, restricted to cardiac masses that progress despite chemotherapy due to cardiovascular side effects (2). Yang et al. suggested that the overall response rate to secondary cardiac lymphoma was 63.2% and the median survival time was 18 months. Petrich et al. reported that the overall response rate of primary cardiac lymphoma to chemotherapy was 79% and complete remission was 59% (57). Similar results have been reported for secondary lymphoma (57). The management of patients with R/R DLBCL has improved significantly in the last year (60). Various novel antibodies, ADCs, specific small-molecule inhibitors, as well as CAR-T cells, have been approved for the treatment of affected patients (61, 62). The outlook for cardiac lymphoma remains poor (1).
Lymphoma metastases to the heart are rare and are associated with a poor prognosis. We report a case of secondary cardiac lymphoma and analyze published case reports. Unfortunately, our patient was unable to undergo an endomyocardial biopsy due to his poor physical condition. Despite the rapid changes in currently available treatment options, the prognosis for cardiac lymphoma remains poor. Various treatments appeared to have improved our patient, but he unfortunately died of severe pneumonia in the end. Therefore, early diagnosis and timely treatment are still significant for improving survival. And our experience has shown that early allo-HSCT has an irreplaceable role in the survival benefit of patients. When an individual has a history of recurrent pleural effusions, dyspnea, and lymphoma, cardiac infiltration should be considered. To improve patient survival, additional treatment options should be explored.
Despite the death outcome, the encouragement and optimism of the patient’s family (particularly his son) inspired him to confront the disease. Throughout the treatment procedure, he showed a determined will to survive. He was willing to attempt a variety of treatments that may have been more effective but also riskier. With regard to the disease and treatment, he and his family expressed gratitude to our hospital and doctors.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YY wrote the manuscript and analyzed the data. MS, YY, and ZL revised the manuscript and processed images. YL, MS, YY, and ZL diagnosed and treated the patient. YZ operated cardiac ultrasound and elaborated the picture of cardiac ultrasound. MS funded the research. All authors contributed to the article and approved the submitted version.
The present study was supported by the National Natural Science Foundation of China (No. 81760035).
The authors would like to acknowledge all who contributed to case diagnosis, therapy, and decision-making. Acknowledgment is given to the hospital of First Affiliated Hospital of Kunming Medical University, Kunming, China and especially for the department of Hematology and cardiology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jeudy J, Burke AP, Frazier AA. Cardiac lymphoma. Radiol Clin North Am (2016) 54(4):689–710. doi: 10.1016/j.rcl.2016.03.006
2. Bonelli A, Paris S, Bisegna S, Milesi G, Gavazzi E, Giubbini R, et al. Cardiac lymphoma with early response to chemotherapy: A case report and review of the literature. J Nucl Cardiol (2021) 29(6):3044–56. doi: 10.1007/s12350-021-02570-5
3. Voigt P, Wienbeck S, Weber M-A, Oyama-Manabe N, Beimler M, Schob S, et al. Cardiac hematological malignancies: Typical growth patterns, imaging features, and clinical outcome. Angiology (2018) 69(2):170–6. doi: 10.1177/0003319717713581
4. Gordon MJ, Danilova O, Spurgeon S, Danilov AV. Cardiac non-hodgkin's lymphoma: Clinical characteristics and trends in survival. Eur J Haematol (2016) 97(5):445–52. doi: 10.1111/ejh.12751
5. Ikeda H, Nakamura S, Nishimaki H, Masuda K, Takeo T, Kasai K, et al. Primary lymphoma of the heart: Case report and literature review. Pathol Int (2004) 54(3):187–95. doi: 10.1111/j.1440-1827.2003.01606.x
6. Miller DV, Firchau DJ, McClure RF, Kurtin PJ, Feldman AL. Epstein-Barr Virus-associated diffuse Large b-cell lymphoma arising on cardiac prostheses. Am J Surg Pathol (2010) 34(3):377–84. doi: 10.1097/PAS.0b013e3181ce9128
7. Hsueh S-C, Chung M-T, Fang R, Hsiung M-C, Young M-S, Lu H-F. Primary cardiac lymphoma. J Chin Med Assoc (2006) 69(4):169–74. doi: 10.1016/S1726-4901(09)70200-X
8. Morillas P, Quiles J, Nuñez D, Senent C, Martínez S, Fernández JA, et al. Complete regression of cardiac non-Hodgkin lymphoma. Int J Cardiol (2006) 106(3):426–7. doi: 10.1016/j.ijcard.2005.01.020
9. Takenaka S, Mitsudo K, Inoue K, Goto T, Kadota K, Fujii S, et al. Successful treatment of primary cardiac lymphoma with atrioventricular nodal block. Int Heart J (2005) 46(5):927–31. doi: 10.1536/ihj.46.927
10. Kondo S, Osanai H, Sakamoto Y, Uno H, Tagahara K, Hosono H, et al. Secondary cardiac lymphoma presenting as sick sinus syndrome and atrial fibrillation which required leadless pacemaker implantation. Intern Med (2021) 60(3):431–4. doi: 10.2169/internalmedicine.5573-20
11. Lucchini E, Merlo M, Ballerini M, Porcari A, Sinagra G, Pagnan L, et al. Case report: Cardiac involvement by lymphoma: Rare but heterogeneous condition with challenging behaviors. Front Oncol (2021) 11:665736. doi: 10.3389/fonc.2021.665736
12. Opherk JP, Lotz J, Galanski M. [Cardiac infiltration as complication of cutaneous T-lymphoblastic non-hodgkin's lymphoma]. Rofo (2007) 179(2):175–6. doi: 10.1055/s-2006-927233
13. Khalid K, Faza N, Lakkis NM, Tabbaa R. Cardiac involvement by burkitt lymphoma in a 49-Year-Old man. Tex Heart Inst J (2020) 47(3):210–2. doi: 10.14503/THIJ-17-6448
14. Baek Y-S, Shin S-H, Yi H-G, Kim D-H, Woo S-I, Park K-S, et al. Cardiac involvement in Cd56 negative primary pancreatic extranodal Nk/T-cell lymphoma, nasal type, presenting with ventricular tachycardia during the early stages of chemotherapy. Intern Med (2014) 53(20):2333–6. doi: 10.2169/internalmedicine.53.2764
15. Sanna P, Bertoni F, Zucca E, Roggero E, Passega Sidler E, Fiori G, et al. Cardiac involvement in hiv-related non-hodgkin's lymphoma: A case report and short review of the literature. Ann Hematol (1998) 77(1-2):75–8. doi: 10.1007/s002770050416
16. Magagnoli M, Nozza A, Romano Lutman F, Santoro A. Cardiac involvement in non-hodgkin's lymphoma. Br J Haematol (2002) 119(4):887. doi: 10.1046/j.1365-2141.2002.03868.x
17. Khan NUA, Ahmed S, Wagner P, Rumley RL, Movahed A. Cardiac involvement in non-hodgkin's lymphoma: With and without hiv infection. Int J Cardiovasc Imaging (2004) 20(6):477–81. doi: 10.1007/s10554-004-2783-1
18. Nagayama H, Sueyoshi E, Hayashida T, Sakamoto I, Taguchi J, Kudo T, et al. Cardiac involvement of adult T-cell Leukemia/Lymphoma. Can J Cardiol (2012) 28(4):516.e9–516.e10. doi: 10.1016/j.cjca.2011.11.013
19. Baeßler B, Rudolph V, Friedrichs N, Maintz D, Bunck AC. Case of myocardial relapse of a T-cell lymphoma after hematopoietic stem cell transplantation demonstrated by cardiovascular magnetic resonance and endomyocardial biopsy. Circulation (2014) 130(6):e44–e7. doi: 10.1161/CIRCULATIONAHA.114.010065
20. Kawasaki H, Shigeno K, Ohnishi K, Tsuchida T, Miura K, Kato T, et al. A case of primary cutaneous natural Killer/T-cell lymphoma, nasal type, directly invading to the heart. Leuk Lymphoma (2008) 49(5):1008–11. doi: 10.1080/10428190801947518
21. Wiernik PH, Sutherland JC, Stechmiller BK, Wolff J. Clinically significant cardiac infiltration in acute leukemia, lymphocytic lymphoma, and plasma cell myeloma. Med Pediatr Oncol (1976) 2(1):75–85. doi: 10.1002/mpo.2950020109
22. Liang R, Yu CM, Au WY, Choy CK, Kwong YL. Diagnosis in oncology. case 2: Secondary lymphoma of the heart manifesting as intracavitary masses. J Clin Oncol (2000) 18(9):1998–9.
23. Ban-Hoefen M, Zeglin MA, Bisognano JD. Diffuse Large b cell lymphoma presenting as a cardiac mass and odynophagia. Cardiol J (2008) 15(5):471–4.
24. Gurunathan S, Ahmed A, Aqel N, Vamvakidou A, Karogiannis N, Senior R. Giant lymphomatous cardiac mass: In vivo imaging and histological findings. Int J Cardiol (2016) 202:81–3. doi: 10.1016/j.ijcard.2015.08.151
25. Lal KS, Tariq RZ, Okwuosa T. Haemodynamic instability secondary to cardiac involvement by lymphoma. BMJ Case Rep (2016) 2016:bcr2016215775. doi: 10.1136/bcr-2016-215775
26. Rogowitz E, Babiker HM, Krishnadasan R, Jokerst C, Miller TP, Bookman M. Heart of lymphoma: Primary mediastinal Large b-cell lymphoma with endomyocardial involvement. Case Rep Oncol Med (2013) 2013:814291. doi: 10.1155/2013/814291
27. Tsubokura M, Asada N, Odawara J, Aoki T, Yamakura M, Takeuchi M, et al. Identification of cardiac metastasis of primary gastric diffuse Large b-cell lymphoma. Br J Haematol (2007) 137(3):179. doi: 10.1111/j.1365-2141.2007.06511.x
28. Sibbing D, Barthel P, Abbrederis K, Dennig K, Gaa J. Intracardiac right ventricular metastatic tumor of malignant T-cell lymphoma. Int J Cardiol (2007) 117(2):e84–e5. doi: 10.1016/j.ijcard.2006.11.191
29. Cohen Y, Ne D, Libster D, Gillonb D, Polliack A. Large B-cell lymphoma manifesting as an invasive cardiac mass: Sustained local remission after combination of methotrexate and rituximab. Leuk Lymphoma (2002) 43(7):1485–7. doi: 10.1080/1042819022386699
30. Hafeez I, Alai MS, Iqbal K, Aslam K, Lone A, Bhat IA, et al. Lymphoma presenting as severe left ventricular systolic dysfunction: A case report. Oman Med J (2014) 29(3):223–5. doi: 10.5001/omj.2014.55
31. Kanesvaran R, Tao M, Huat ITB, Weng DTS, Eng DNC, Thye LS. Malignant arrhythmia: A case report of nasal Nk/T-cell lymphoma with cardiac involvement. Acta Oncol (2009) 48(4):637–9. doi: 10.1080/02841860902740923
32. Morgan R, Murdock R, Leng P. A man in his 50s presenting with rapid-onset dyspnea and obstructive shock. Chest (2018) 154(6):e153–e6. doi: 10.1016/j.chest.2018.07.048
33. Kumar A, Puvvada S. Mantle cell lymphoma presenting as cardiac tamponade. Blood (2015) 126(10):1255. doi: 10.1182/blood-2015-03-626051
34. Harris S, Waddell J. Non-hodgkin's lymphoma: An unusual cause of myocardial infarction and hypercalcaemia. Clin Oncol (R Coll Radiol) (1993) 5(3):189–90. doi: 10.1016/S0936-6555(05)80328-0
35. Weijs LE, Arsos G, Baarslag HJ, Wittebol S, de Klerk JM. Pericardial involvement in a non-Hodgkin lymphoma patient: Coregistered fdg-pet and ct imaging. Eur Heart J (2007) 28(22):2698. doi: 10.1093/eurheartj/ehm218
36. Manabe M, Yoshii Y, Mukai S, Sakamoto E, Kanashima H, Nakao T, et al. Precursor b-lymphoblastic lymphoma involving an intracardiac mass and myocardial infiltration: A case report. Intern Med (2012) 51(3):315–9. doi: 10.2169/internalmedicine.51.6075
37. Yamamoto E, Tomita N, Ishigatsubo Y. Primary diffuse Large b-cell lymphoma of the testis relapsing in the right atrium. Br J Haematol (2015) 168(4):469. doi: 10.1111/bjh.13212
38. Zlotchenko G, Futuri S, Dillon E, Machnicki S. A rare case of lymphoma involving the tricuspid valve. J Cardiovasc Comput Tomogr (2013) 7(3):207–9. doi: 10.1016/j.jcct.2013.05.007
39. Panareo S, Urso L, Santi I, Rigolin GM, Cuneo A, Cittanti C, et al. Right atrium mass assessed with 18f-fdg Pet/Ct scan turns out to be an uncommon relapse of testicular diffuse Large b-cell lymphoma: A case report. Diagn (Basel) (2020) 10(11):987. doi: 10.3390/diagnostics10110987
40. Johnston ID, Popple AW. Right ventricular outflow tract obstruction secondary to small intestinal lymphoma. Br Heart J (1980) 43(5):593–6. doi: 10.1136/hrt.43.5.593
41. Donnelly MS, Weinberg DS, Skarin AT, Levine HD. Sick sinus syndrome with seroconstrictive pericarditis in malignant lymphoma involving the heart: A case report. Med Pediatr Oncol (1981) 9(3):273–7. doi: 10.1002/mpo.2950090311
42. Cove M, Johnston R, Cowan R, Haji-Michael P. Symptomatic, obstructive cardiac metastasis can respond to chemotherapy. Am J Med (2009) 122(5):e5–6. doi: 10.1016/j.amjmed.2008.12.013
43. Yoshihara M, Itamura H, Fukushima N, Itoh M, Furukawa K, Nagatomo D, et al. Therapeutic management in cardiac lymphoma. Leuk Lymphoma (2014) 55(5):1215–7. doi: 10.3109/10428194.2013.828355
44. Lin Y-S, Chu P-H, Kuo M-C, Jung S-M, Lim K-E, Kuo C-T, et al. Use of a b-type natriuretic peptide in evaluating the treatment response of a relapsed lymphoma with cardiac involvement. Int J Hematol (2006) 83(1):44–6. doi: 10.1532/IJH97.A10502
45. Takaya T, Takeuchi Y, Nakajima H, Nishiki-Kosaka S, Hata K, Kijima Y, et al. Usefulness of transesophageal echocardiographic observation during chemotherapy for cardiac metastasis of non-Hodgkin lymphoma complicated with left ventricular diastolic collapse. J Cardiol (2009) 53(3):447–52. doi: 10.1016/j.jjcc.2008.08.009
46. Chemello D, Raupp-da-Rosa P, Teló G, Clausell N. Ventricular tachycardia associated with non-hodgkin's lymphoma. Arq Bras Cardiol (2011) 97(6):e132–e4. doi: 10.1590/S0066-782X2011001500017
47. Sen Z, Yunxiang C, Guihua Y, Wenhao D. Echocardiographic follow-up of a case of secondary atrial septal lymphoma during chemotherapy. Chin J Misdiagn (2019) 14(05):232–3.
48. Liqun W, Yaqi M, Jie L, Lu S, Xin S, Aitao G. Clinicopathological analysis of 4 cases of cardiac lymphoma. Chin J Diagn Pathol (2019) 26(12):793–6.
49. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol (2015) 10(9):1240–2. doi: 10.1097/JTO.0000000000000663
50. Yang C-C, Tsai H-W, Lai S-T, Wu HC, Lo C-Y, Chang Y. Mediastinal diffuse Large b-cell lymphoma invading the left atrium mimicking coronary artery disease with a mural thrombus. J Chin Med Assoc (2012) 75(11):606–9. doi: 10.1016/j.jcma.2012.08.009
51. Al-Mehisen R, Al-Mohaissen M, Yousef H. Cardiac involvement in disseminated diffuse Large b-cell lymphoma, successful management with chemotherapy dose reduction guided by cardiac imaging: A case report and review of literature. World J Clin cases (2019) 7(2):191–202. doi: 10.12998/wjcc.v7.i2.191
52. Poterucha TJ, Kochav J, O'Connor DS, Rosner GF. Cardiac tumors: Clinical presentation, diagnosis, and management. Curr Treat Options Oncol (2019) 20(8):66. doi: 10.1007/s11864-019-0662-1
53. Ito K, Nishimura Y, Tanaka H, Tejima T. Epicardial pacemaker implantation for sick sinus syndrome in a patient with supra vena cava obstructed by a primary cardiac lymphoma. J Cardiol cases (2020) 21(6):234–7. doi: 10.1016/j.jccase.2020.03.007
54. Ryu SJ, Choi BW, Choe KO. Ct and Mr findings of primary cardiac lymphoma: Report upon 2 cases and review. Yonsei Med J (2001) 42(4):451–6. doi: 10.3349/ymj.2001.42.4.451
55. Faganello G, Belham M, Thaman R, Blundell J, Eller T, Wilde P. A case of primary cardiac lymphoma: Analysis of the role of echocardiography in early diagnosis. Echocardiography (2007) 24(8):889–92. doi: 10.1111/j.1540-8175.2007.00472.x
56. McDonnell PJ, Mann RB, Bulkley BH. Involvement of the heart by malignant lymphoma: A clinicopathologic study. Cancer (1982) 49(5):944–51. doi: 10.1002/1097-0142(19820301)49:5<944::AID-CNCR2820490519>3.0.CO;2-C
57. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: An analysis of presentation, treatment, and outcome patterns. Cancer (2011) 117(3):581–9. doi: 10.1002/cncr.25444
58. O'Mahony D, Peikarz RL, Bandettini WP, Arai AE, Wilson WH, Bates SE. Cardiac involvement with lymphoma: A review of the literature. Clin Lymphoma Myeloma (2008) 8(4):249–52. doi: 10.3816/CLM.2008.n.034
59. Reynen K, Köckeritz U, Strasser RH. Metastases to the heart. Ann Oncol (2004) 15(3):375–81. doi: 10.1093/annonc/mdh086
60. Qian Y, Wen T, Li Z. Clinical analysis of 65 patients with secondary lymphoma of heart. Cancer Res On Prev Treat (2004) 031(007):424–425,432. Huaihua, China.
61. Sehn LH, Salles G. Diffuse Large b-cell lymphoma. N Engl J Med (2021) 384(9):842–58. doi: 10.1056/NEJMra2027612
Keywords: cardiac hematological malignancy, secondary cardiac lymphoma, B-cell lymphoma, CAR T-cell immunotherapy, case report
Citation: Yang Y, Li Z, Li Y, Zhao Y and Shi M (2023) Relapsed/refractory diffuse large B cell lymphoma with cardiac involvement: A case report and literature review. Front. Oncol. 13:1091074. doi: 10.3389/fonc.2023.1091074
Received: 06 November 2022; Accepted: 13 January 2023;
Published: 30 January 2023.
Edited by:
Arpad Szallasi, Semmelweis University, HungaryReviewed by:
Breno Moreno De Gusmão, Beneficência Portuguesa de São Paulo, BrazilCopyright © 2023 Yang, Li, Li, Zhao and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxia Shi, c2hteGlhMjAwMkBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.