
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 21 September 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1089609
Introduction: Gait analysis is one of the most important components of functional outcome evaluation in patients with lower-extremity tumors. Disparities between operated limbs when compared with non-operated limbs and healthy populations based on gait parameters have rarely been studied. In the present study, we attempted to analyze the gait difference and its impacts on daily life.
Methods: The gait parameters of distal femoral tumor-resected patients were collected from PubMed, CNKI, MEDLINE, Embase, Cochrane, and Google Scholar till September 30, 2022, by strictly following the inclusion and exclusion criteria. Differences between gait parameters in the operated and non-operated limbs or healthy limbs of distal femoral tumor patients were analyzed based on stance phase, swing phase, cadence, and velocity. The fixed-effects and random-effects models were used to conduct a meta-analysis.
Results: Six studies were included according to the selection criteria. There were 224 patients in total in these studies. Standard mean differences were calculated for all of our outcomes. Our results showed that there was a minimal difference in the standard mean difference of gait parameters between operated and non-operated limbs and healthy limbs.
Conclusion: Distal femoral tumor resections have been associated with deficient muscle function and strength and impaired gait parameters. Minimal differences in the gait parameters highlighted the advantage of distal femoral resection when replaced with a prosthesis.
The distal femur is a common site for primary bone neoplasm, and metastatic neoplasm lesions are common at the proximal femur (1). The appropriate option for reconstruction of the lower limb after resection of the femur or tibia is controversial (2). Options include the use of autografts (3), allografts (4), custom-made mega prostheses (5), and modular endoprostheses. The functional outcome for patients treated with distal femoral replacement prosthesis generally is thought to be acceptable. Before the introduction of this prosthesis, amputation was the preferred choice of treatment for these lesions (2). These custom-made prostheses are associated with lower complications but with increased costs/expenses (2). Restoration of gait function after reconstruction has been associated with improved functional outcomes and increased longevity of the reconstruction (6). Only a few studies have been conducted on gait analysis in patients after musculoskeletal tumor resection and reconstruction of the lower limb (7).
Gait analysis, which was founded by Jacquelin Perry, has been widely used in different fields of orthopedics (8). In gait analysis, the gait pattern is analyzed and studied in two different approaches based on non-wearable sensors (9). Gait is a highly valuable function that represents the integration of various physiological systems, including the central and peripheral nervous systems, perceptual system, and musculoskeletal system (10). Non-wearable systems (NWSs) analyze gait in the laboratory; in contrast, wearable systems (WSs) analyze gait data outside the laboratory during the person’s everyday activities (9). Walking movements are controlled by the structures of the lower limb, mainly the ankle and foot joints. The axis of rotation with each joint allows for joints to have a predominant plane of motion, perpendicular to that axis (11). Several studies stated that the gait cycle is significantly changed in walking velocity and stride length in patients undergoing prosthetic replacement of the distal femur, in comparison with the normal population (7, 12). These changes in walking need to be studied, as the quality of life counts are valued more by tumor patients due to their shortened life span (10).
By searching abundant pieces of literature on gait analysis and distal femoral tumor resection, we conducted this meta-analysis to obtain a comprehensive conclusion on gait parameters in distal femoral tumor patients treated with resections. These results will help us to guide and manage distal femoral tumor patients and improve their gait parameters. In our study, we compared gait parameters, mainly temporal–spatial parameters such as the stance phase, cadence, and velocity along with the swing phase in the operated limbs versus the non-operated limbs. The operated limbs were set as the experimental group, and the non-operated limbs and healthy limbs were set as the control group.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to perform this study (Figure 1) (13).
PubMed, CNKI, MEDLINE, Cochrane, Embase, and Google Scholar databases were searched for relevant data till September 30, 2022. Relevant article searching started on October 1, 2021, and three authors of our study used 1-year duration. The reference lists of relevant studies were also hand-searched by two different authors. Keywords used for searching included distal femoral tumor resection, gait analysis, stance phase, swing phase, cadence, and velocity. Also, a manual search of the reference lists of the studies found in the databases was conducted.
1) English language studies that included patients diagnosed with primary bone tumors located in the distal femur,
2) studies that focused on gait parameter comparisons in distal femoral resection patients, and
3) studies that presented gait analysis performed in a certified laboratory center and studies that compared stance phase, swing phase, cadence, and velocity.
1) Non-English studies and unpublished studies,
2) non-comparative studies of patients undergoing distal femoral tumor resection and studies that lack data on gait analysis,
3) review, case reports, and letters to the editor, and
4) studies that lack adequate clinical data.
In this meta-analysis, the authors stated that there were no restrictions on the date of publication for the inclusion of studies. When necessary, discussions between the reviewers resolved disputes and uncertainties regarding eligibility and viability.
Two authors scanned all the abstracts and titles to evaluate whether the studies assessed the questions raised by our study. Three authors of our study collected the outcomes from the studies. The authors constructed a structured table and then inputted all the data and the related information into a database. The following data were extracted from articles according to the inclusion criteria: the name of the first author, year of publication, study design and protocol, number of patients in each group, patients’ age and gender, stance phase, swing phase, velocity, and cadence.
All the included studies were retrospective, and the included studies focused on similar research issues. The included studies had a low bias and disparity as studies, were similar in the context of inclusion criteria, surgical procedures, and study periods. The Newcastle–Ottawa Scale (NOS) was used for the quality assessment of the meta-analysis (14). In our study, the primary outcome was the stance phase, and the secondary outcomes were the swing phase, cadence, and velocity. Gait parameters were defined as parameters obtained after participants walked in a pathway guided by analytics, and these parameters were used to assess dynamic posture and coordination during movements.
The measured outcomes of our study were the stance phase, swing phase, cadence, and velocity, which were all continuous data. T he software Cochrane Collaboration (ReviewManager 5.2) was used to compute the standardized mean difference (SMD) and 95% confidence intervals (CIs) for all outcomes, as the ReviewManager illustrates a more detailed forest plot in continuous data analysis. Statistical heterogeneity among the included studies was evaluated by the I2 tests (13). Statistically significant heterogeneity was defined as an I2 value >0.5 (13). Heterogeneity in our study was defined as low, moderate, and high based on I2 value (<40%, low; 30%–60%, moderate; 50%–90%, substantial; >75%, high). I2 illustrates the percentage of the total variability in effect estimates among trials that are due to heterogeneity rather than chance (13). A random-effects model was selected for heterogeneous data; otherwise, a fixed-effects model was selected. Funnel plots were used to detect the publication bias, which exhibited the intervention effect from the individual study against the respective standard error. A symmetrical inverted funnel-shaped plot suggested that there was no publication bias, and publication bias was suggested by the asymmetry of the plot.
In the primary article search, 112 relevant articles were retrieved, and 50 were excluded based on the exclusion criteria. The abstracts of the remaining 62 were screened, and 32 were excluded based on the exclusion criteria. After all the reviews of the remaining 30 studies, 15 were excluded due to a lack of outcome (n = 15) and duplication in the study population with other articles (n = 9). In a word, to conduct a meta-analysis, a total of six articles were included in the meta-analysis. The study selection chart is presented in Figure 1. The basic characteristics of the included studies are summarized in Table 1, and the outcomes are summarized in Table 2. There were 224 patients in total in these studies, and all the patients were diagnosed with primary bone tumors. Reconstruction methods used in studies were endoprosthesis, allograft, and mega prosthesis. Gait analyses were conducted at each study in different durations, but all the studies had one thing in common: each study conducted analysis after 6 months of post-operative duration and was conducted in certified laboratory centers.
The stance phase was recorded in all six studies in either the operated limbs or the non-operated limbs. For this outcome, a random-effects model analysis was used. There was a minimal decrease in SMD of the stance phase in the operated limbs than in non-operated limbs of patients undergoing distal femoral resection (SMD = −0.96, 95% CI [−1.71, −0.21], p = 0.002) as shown in Figure 2.
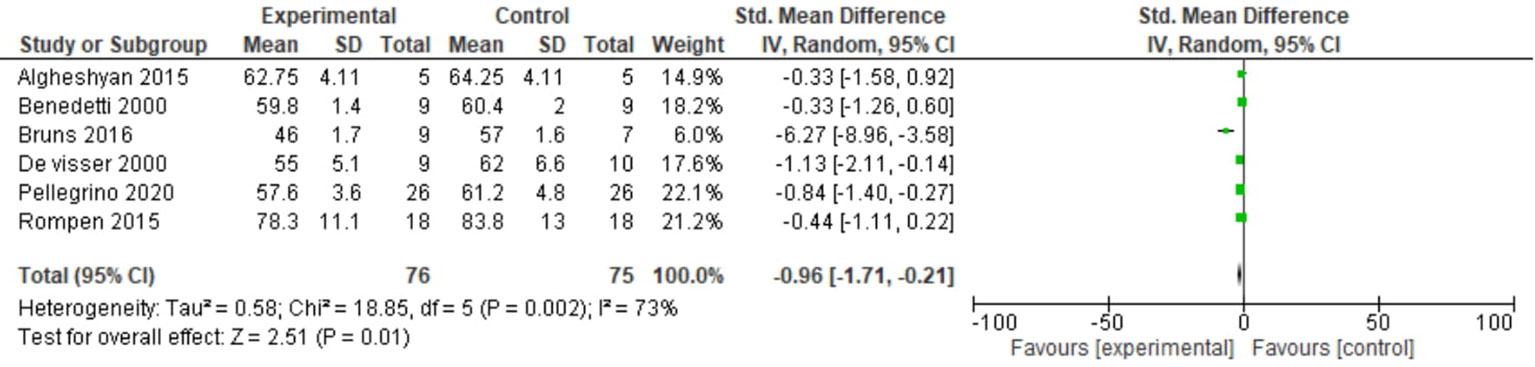
Figure 2 Forest plot of stance phase comparing operated and non-operated limbs of distal femur tumor patients.
Two studies reported the swing phase. For this outcome, a fixed-effects model analysis was used. There was a minimal increase in the swing phase in the operated limbs than non-operated limbs of patients undergoing distal femoral resection (SMD = 1.07, 95% CI [0.62, 1.52], p = 0.46) as shown in Figure 3.
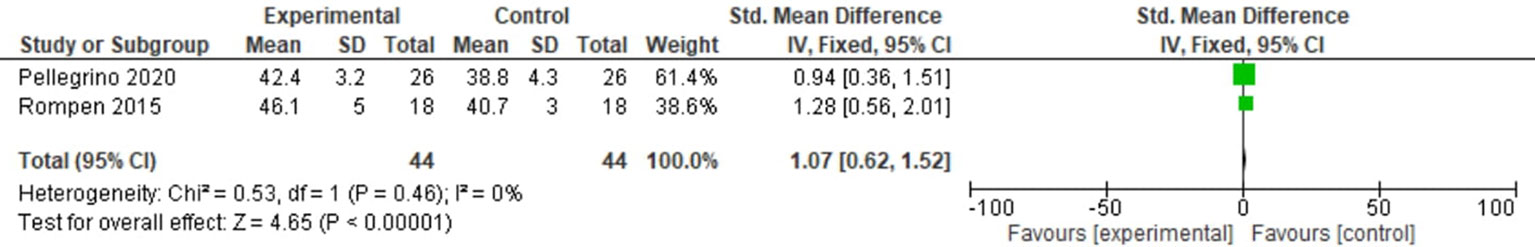
Figure 3 Forest plot of swing phase comparing operated and non-operated limbs of distal femoral tumor patients.
Two studies reported cadence in our study. For this outcome, a random-effects model analysis was used. There was a minimal decrease in the SMD of cadence in the operated limbs compared with healthy populations in patients undergoing distal femoral resection (SMD= −1.53, 95% CI [−2.77, −0.28], p = 0.02) as shown in Figure 4.
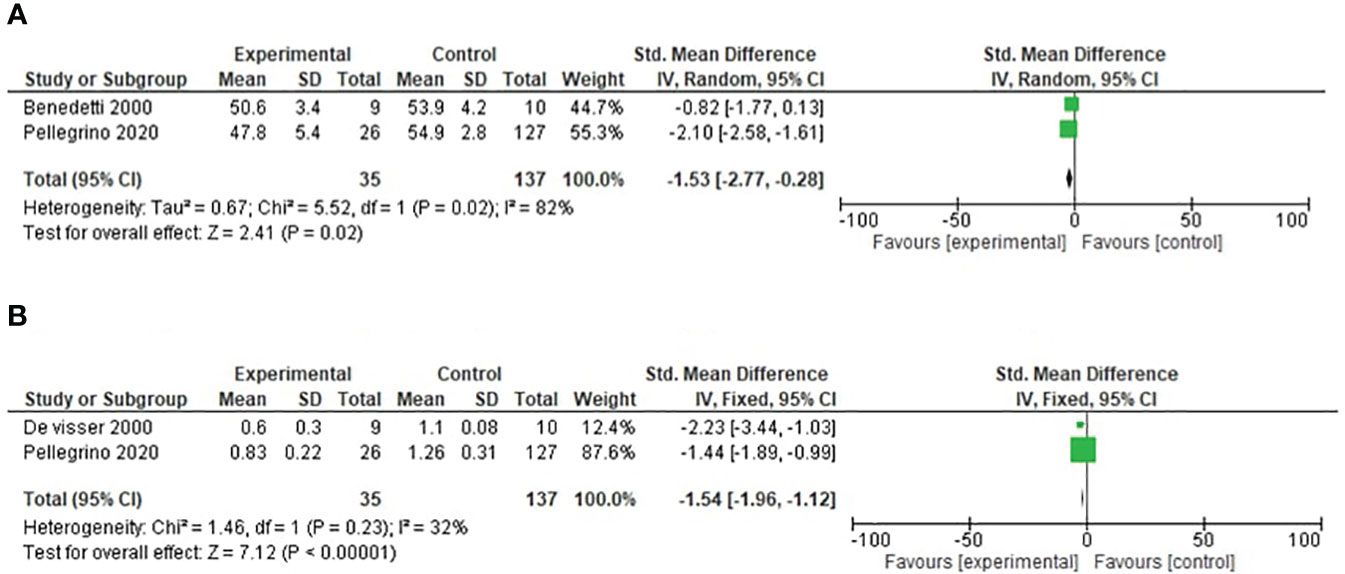
Figure 4 (A) Forest plot of cadence comparing operated and healthy populations in distal femoral tumor patients. (B) Forest plot of velocity comparing operated and healthy populations in distal femoral tumor patients.
Two studies reported velocity in our study. For this outcome, a fixed-effects model analysis was used. There was a minimal decrease in the SMD of velocity in the operated limbs compared with healthy populations in patients undergoing distal femoral resection (SMD = −1.54, 95% CI [−1.96, −1.12], p = 0.34) as shown in Figure 4.
Sensitivity analyses were performed to determine the reliability of the results of the included studies by removing each study in turn. The magnitude and direction of the combined estimates did not change significantly by excluding individual studies, suggesting that the meta-analysis results are reliable and that the results of this meta-analysis are significant and not fluctuating. A sensitivity analysis was performed for the primary outcome stance phase. The statistical values were as follows: when the first study was excluded (SMD = −1.11, 95% CI [−1.97, −0.24], p = 0.001), when the second study was only excluded (SMD = −1.15, 95% CI [−2.06, −0.24], p = 0.001), when the third study was only excluded (SMD = −0.60, 95% CI [−1.00, −0.30], p = 0.67), when the fourth study was excluded (SMD = −0.97, 95% CI [−1.88, −0.07], p = 0.001), when the fifth study was excluded (SMD = −1.12, 95% CI [−2.17, −0.07], p = 0.0009), and when the sixth study was excluded (SMD = −1.19, 95% CI [−2.18, −0.19], p = 0.001). All the sensitivity analysis figures are added in the Supplementary Material.
The funnel plots of the stance phase, cadence, velocity, and swing phase are shown in the figure added in the Supplementary Material. The funnel plot was used for all the outcomes of our study. The findings of the funnel plots stated that there was no evidence of publication bias in all the outcomes.
Gait analysis is one of the most important components of functional outcome assessment in patients with lower-extremity tumors (14). The gait pattern after lower-extremity limb salvage surgery is expected to be abnormal due to the amount of bone and soft tissue resection when compared to joint replacement surgery (7). Most of the reconstructed distal femurs following tumor resection yielded a reduced range of motion as reflected in observed flexion and extension when compared to normal limbs (15). Knee gait parameters are affected in distal femoral resections (14). Resected tumor patients need a high degree of knee extension for the loading affecting the stance phase (16). Knee flexion is lower resulting from weak quadriceps affecting the initial response of the gait cycle ultimately affecting the swing phase (14). Temporal parameters of gait include step time, stride time, stance time cadence, swing time, and single limb support time, and these parameters contribute to a variability domain of gait performance (17, 18). However, in our study, we could not include all these parameters, as we could not find sufficient studies comparing these parameters.
In our study, we observed a minimal decrease in SMD of the stance phase in the operated limbs than the non-operated limbs (SMD = −0.96, 95% CI [−1.71, −0.21], p = 0.002). The stance phase represents 60% of the gait cycle. Biomechanical gait characteristic variations (such as the decrease in gait speed, step length, and width; an increase in the shorter swing phase time; and a reduction of stance phase time) can be observed through kinematic gait analysis (19). These gait characteristic variations are often reported in the elderly, but the main factor related to gait performance is the reduction in muscular strength of the lower limbs, especially the quadriceps muscle (19). In a study conducted by Capanna et al., the patient undergoing distal femoral resection was found to have satisfactory results in vastus lateralis and intermedius excisions and then quadriceps excision (20). Several of the studies had similar results of reduced stance phase duration, justifying our result (21, 22). The duration of the stance phase is expected to be longer in the non-operated limbs, as this presumably is consistent with taking over part of the loading function of the operated limbs (23). The non-operated limbs had to provide support, which lasted long enough to allow the swing, ultimately leading to increased swing duration (23). Even in our study, there was an increase in the swing phase of SMD of 1.07 in operated patients.
Secondary parameters also had minimum differences in our studies, which also correlates with some of the literature (14). Velocity is the single most important component of the gait cycle, as it represents the efficacy of this means of ambulation (24). The results highlighted a reduced mean velocity in operated patients when compared with the healthy participants. This finding is consistent with the study that reported velocity after limb salvage surgery is between 64% and 88% of healthy individuals, whereas this can be justified by decreased strength due to extensive soft tissues and bone resections (21). Cadence defined as a stride per minute varies according to age. In our study, cadence in both groups was also similar. Cadence (steps/min) 54.9, velocity (m/s) 1.26, stance phase 60, and swing phase 40 are the normal limits stated in a study conducted by Pelligrono et al. (21). The result of our study showed that forest plots only had minimal reductions when compared to normal individuals, signifying that resection of the tumor did not affect a large amount in the gait parameters. The authors could not find a study stating that a rehabilitation program improves gait parameters; there was one study conducted on Parkinson’s patients where the author found improved gait parameters after 10 weeks of rehabilitation (25). All the reported studies did not mention any rehabilitation program. Each study performed gait analysis after 1 to 2 years of surgery and followed the same procedure capturing motion through Vicon cameras.
A few shortcomings and limitations of this meta-analysis should be illustrated. First, the lack of comprehensive and verified data from original studies made it difficult to adjust estimates by age, menopause, lifestyle, smoking, race, and so on, while more accurate and reliable analyses required this type of adjustment. Second, we could not post other important gait parameters such as stride time, double limb support, and step time. Third, we could not add other studies other than retrospective studies such as randomized controlled trials (RCTs) and prospective studies, which could have more clarification on this rare topic. Fourth, there were only minimal studies, so it is difficult to obtain a statistically significant result.
However, our meta-analysis also has some beneficial points. First, a systematic review of the association of gait analysis in patients with distal femoral tumors was statistically more powerful than any single study. All the studies provided significant gait data, which signified the advantage of prosthesis insertion in distal femoral resection in preserving satisfactory gait functions. Second, all included retrospective studies were of high quality and met our inclusion criteria. Third, even though the included studies were few and still produced statistically significant results, our study highlighted the importance of gait variables and the need for further studies elaborating on their impact on rehabilitation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conception/design: NB, WZ, and HD. Provision of study material: NB and XF. Collection and/or assembly of data: NB and DY. Data analysis and interpretation: XF and DY. Manuscript writing: NB. Final approval of manuscript: WZ and HD. I attest to the fact that all the authors listed on the title page have read the manuscript, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to Frontiers in Oncology - Surgical Oncology.
This study was funded by the Fundamental Research Funds for the Central Universities (2019SCUH). The funding source has no role in the design and preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1089609/full#supplementary-material
1. Agarwal MG, Nayak P. Management of skeletal metastases: An orthopaedic surgeon’s guide. Indian J Orthop (2015) 49(1):83–100. doi: 10.4103/0019-5413.143915
2. Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br (2006) 88(6):790–5. doi: 10.1302/0301-620X.88B6.17519
3. Smith WS, Struhl S. Replantation of an autoclaved autogenous segment of bone for treatment of chondrosarcoma. Long-term follow up. J Bone Joint Surg Am (1988) 70(1):70–5. doi: 10.2106/00004623-198870010-00011
4. Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ, et al. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res (2002) 397):106–13. doi: 10.1097/00003086-200204000-00015
5. Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS, et al. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br (1996) 78(1):5–13. doi: 10.1302/0301-620X.78B1.0780005
6. Taylor SJ, Walker PS. Forces and moments telemetered from two distal femoral replacements during various activities. J Biomech (2001) 34(7):839–48. doi: 10.1016/S0021-9290(01)00042-2
7. Singh VA, Heng CW, Yasin NF. Gait analysis in patients with wide resection and endoprosthesis replacement around the knee. Indian J Orthop (2018) 52(1):65–72. doi: 10.4103/ortho.IJOrtho_188_17
8. Baker R. The history of gait analysis before the advent of modern computers. Gait Posture (2007) 26(3):331–42. doi: 10.1016/j.gaitpost.2006.10.014
9. Muro-de-la-Herran A, Garcia-Zapirain B, Mendez-Zorrilla A. Gait analysis methods: an overview of wearable and non-wearable systems, highlighting clinical applications. Sensors (Basel) (2014) 14(2):3362–94. doi: 10.3390/s140203362
10. Liu MA, DuMontier C, Murillo A. Gait speed, grip strength, and clinical outcomes in older patients with hematologic Malignancies. Blood (2019) 134(4):374–82. doi: 10.1182/blood.2019000758
11. Dugan SA, Bhat KP. Biomechanics and analysis of running gait. Phys Med Rehabil Clin N Am (2005) 16(3):603–21. doi: 10.1016/j.pmr.2005.02.007
12. Carty CP, Bennett MB, Dickinson IC, Steadman P. Assessment of kinematic and kinetic patterns following limb salvage procedures for bone sarcoma. Gait Posture (2009) 30(4):547–51. doi: 10.1016/j.gaitpost.2009.08.234
13. He X, Gao Z, Xu H, Zhang Z, Fu P. A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J Orthop Surg Res (2017) 12(1):5. doi: 10.1186/s13018-016-0500-0
14. Kim S, Ryu C, Jung ST. Differences in kinematic and kinetic patterns according to the bone tumor location after endoprosthetic knee replacement following bone tumor resection: A comparative gait analysis between distal femur and proximal tibia. J Clin Med (2021) 10(18). doi: 10.3390/jcm10184100
15. AlGheshyan F, Eltoukhy M, Zakaria K, Temple HT, Asfour S. Comparison of gait parameters in distal femoral replacement using a metallic endoprosthesis versus allograft reconstruction. J Orthop (2015) 12(Suppl 1):S25–30. doi: 10.1016/j.jor.2015.01.022
16. de Visser E, Pauwels J, Duysens JE, Mulder T, Veth RP. Gait adaptations during walking under visual and cognitive constraints: a study of patients recovering from limb-saving surgery of the lower limb. Am J Phys Med Rehabil (1998) 77(6):503–9. doi: 10.1097/00002060-199811000-00010
17. Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture (2011) 34(1):111–8. doi: 10.1016/j.gaitpost.2011.03.024
18. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry (2007) 78(9):929–35. doi: 10.1136/jnnp.2006.106914
19. Spinoso DH, Bellei NC, Marques NR, Navega MT. Quadriceps muscle weakness influences the gait pattern in women with knee osteoarthritis. Adv Rheumatol (2018) 58(1):26. doi: 10.1186/s42358-018-0027-7
20. Capanna R, Ruggieri P, Biagini R. The effect of quadriceps excision on functional results after distal femoral resection and prosthetic replacement of bone tumors. Clin Orthop Relat Res (1990) 1991(267):186–96. doi: 10.1097/00003086-199106000-00030
21. Pellegrino P, Conti A, Pautasso A, Boffano M. Gait analysis: Comparative evaluation of conventional total knee replacement and modular distal femoral megaprosthesis. Knee (2020) 27(5):1567–76. doi: 10.1016/j.knee.2020.08.004
22. Rompen JC, Ham SJ, Halbertsma JP, van Horn JR. Gait and function in patients with a femoral endoprosthesis after tumor resection: 18 patients evaluated 12 years after surgery. Acta Orthop Scand (2002) 73(4):439–46. doi: 10.1080/00016470216319
23. De Visser E, Mulder T, Schreuder HW, Veth RP, Duysens J. Gait and electromyographic analysis of patients recovering after limb-saving surgery. Clin Biomech (Bristol Avon) (2000) 15(8):592–9. doi: 10.1016/S0268-0033(00)00021-8
24. Wonsetler EC, Bowden MG. A systematic review of mechanisms of gait speed change post-stroke. Part 1: spatiotemporal parameters and asymmetry ratios. Top Stroke Rehabil (2017) 24(6):435–46. doi: 10.1080/10749357.2017.1285746
Keywords: distal femur tumor, gait analysis, stance phase, swing phase, cadence, velocity
Citation: Banskota N, Fang X, Yuan D, Zhang W and Duan H (2023) Comparative study of gait parameters of patients undergoing distal femoral resections with non-operated and healthy limbs: a meta-analysis study. Front. Oncol. 13:1089609. doi: 10.3389/fonc.2023.1089609
Received: 04 November 2022; Accepted: 29 August 2023;
Published: 21 September 2023.
Edited by:
Leo R. Quinlan, University of Galway, IrelandReviewed by:
José Manuel Pinto Silva Casanova, University of Coimbra, PortugalCopyright © 2023 Banskota, Fang, Yuan, Zhang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenli Zhang, endsYm94QDE2My5jb20=; Hong Duan, ZHVhbmhvbmcxOTcwQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.