
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 27 January 2023
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1088534
This article is part of the Research Topic Biomarkers of Immune Checkpoint Inhibitors in Lung Cancer: Dusk or Dawn? View all 8 articles
Cancers harboring serine threonine kinase (STK11) alteration or SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily B, member 1 (SMARCB1) mutation are conventionally considered as treatment-refractory to immune checkpoint inhibitors or chemotherapy, respectively. However in the present report, we demonstrated a case of dedifferentiated non-small cell lung cancer, characterized by STK11 loss (due to promoter loss) mutation co-mutated with SMARCB1 deficiency mutation, has achieved significantly partial response to neo-adjuvant treatment with pembrolizumab and platinum doublet regimen. Our case highlighted that either STK11 loss, or SMARCB1 deficiency mutation, might not be used to select patients for PD-(L)1 blockade therapy or chemotherapy, respectively. SKT11 loss accompanied with SMARCB1 deficiency mutation may benefit from immunotherapy combined with chemotherapy.
Serine threonine kinase (STK11, also shown as liver kinase B1, LKB1) is considered as one of tumor suppressor genes, which encodes for a serine/threonine 11 that activates AMP-activated protein kinase (AMPK), hence being capable of regulating cell response to DNA damage, tumor growth, and energy metabolism (1, 2). There have been increasing studies reported that STK11 alterations be associated with primary resistance to immunotherapy agents including anti-programmed cell death protein 1 (anti-PD-1) and its ligand (anti-PD-L1) drugs, which have been emerged as standard first-line treatment with or without cytotoxic drugs (in the circumstance of PD-L1 expression ≥ 50%) in non-small cell lung cancer in absence of targetable mutations (3–8).
SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily B, member 1 (SMARCB1, also known as integrase interactor 1, INI1) is also a tumor suppressor gene located in chromosome 22q11.2, expressed in the nucleus of almost all normal tissues of the body (9, 10). SMARCB1 is considered to be one of the core subunits of the chromatin remodeling complex SWI/SNF, a subfamily of ATP-dependent chromatin remodeling complexes functioned as involving in chromatin remodeling, dynamic modification of chromatin structure, changing chromatin from dense to open state, thereby controlling gene expression and regulating transcription mechanism proteins (11, 12). Mutation of SMARCB1 was frequently presented in sinonasal carcinoma, gastrointestinal cancer, and pancreatic cancer types, however, rarely reported in lung carcinoma (13, 14). According to the former literature, cancer patients with pathologic SMARCB1 mutations were reported resulting in a worse prognosis independently from the conventional treatment strategies including chemotherapy, radiation therapy or surgery (12, 15, 16).
Herein, we reported a rare case of dedifferentiated lung cancer harboring STK11 loss and SMARCB1 deficiency, due to p.S67X mutations, which presented nearly complete response to neo-adjuvant treatment with pembrolizumab and platinum doublet. Subsequently, he received radical left upper lobectomy, and still being disease free status.
A 61-year-old Chinese man was admitted to hospital on June 6th, 2022 with chest distress and slight pain on left chest for approximately three months. He denied smoking or alcohol history, nor any other medical or family disease history. Chest CT on June 6th, 2022 showed a large space-occupying lesion (size as 8.8 × 5.1 cm) in the superior lobe of left lung, which was closed to mediastinum, presented as obstructive pneumonia, accompanied with vein and artery in left superior pulmonary involvement (Figure 1A). In addition, chest CT scan also revealed multiple enlargements of mediastinal lymph nodes (Figure 1B). Besides, there was no other distant metastatic lesion detected according to abdomen CT scans and brain MRI findings. Subsequently, he received CT guided percutaneous lung puncture biopsy, results of which revealed dedifferentiated carcinoma most likely originating from lung (Figure 2A) according to the imaging findings and immunohistochemistry outcomes presented as: TTF-1 (negative), NapsinA (negative), P40 (negative), P63 (negative), CK5/6 (negative), CK(AE1/AE3) (positive), Syn (negative), CgA (negative), CD56 (negative), INI-1 (negative), Ki-67 50% (positive), programmed cell death ligand-1 (PD-L1) negative. Furthermore, via next-generation sequencing (NGS) was performed using tissue sample (tumor cellularity 25%, including 550 cancer-related genes, BIOMED, China) showed STK11 loss, due to the loss of promoter (frequency as 30.67%) and SMARCB1 deficiency, due to p.S67X*10.74% mutations, with a tumor mutational burden (TMB) of 6.85 mutations per megabase, as well as PD-L1 tumor proportion score (22C3) being less than 1%. Based on those, the patient was diagnosed as dedifferentiated lung cancer, with vein and artery in left superior pulmonary involvement, along with metastasis on mediastinal lymph nodes, which was staged as IIIB (cT4N2M0, technically unresectable) according to the criteria of American Joint Committee on Cancer (AJCC) 8th edition. After the discussion by multi-disciplinary team (MDT, including medical oncologist, surgical oncologist, radiation oncologist, and radiologist), regimen of chemotherapy combined with anti-PD-1 agent was adopted as palliative or neo-adjuvant treatment, which depended on the response status during the drug administration. In addition, maintenance therapy with anti-PD-1 agent should also be taken into consideration after disease control if necessary. Exactly, combined treatment with chemotherapy and immunotherapy has already been recommended as standard choice in advanced NSCLC patients in absence of sensitive targetable mutations. The patient thereby was started on pembrolizumab (200 mg on day 1, every 21 days), and carboplatin/nab-paclitaxel (nab-paclitaxel 260 mg/m2 on day 1, carboplatin AUC 5 on day 1, every 21 days) as initial treatment. After three cycles′ treatment, restaging chest CT scans showed significant improvement on the primary lesion, as well as metastatic mediastinal lymph nodes (Figures 1C, D). Repeated brain MRI and abdomen CT scans did not suggest any progression disease on other organs either. Efficacy assessment was evaluated as partial response (PR) according to the criteria of Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Restaging by the above imaging assessment was resulted in down-staging as cT2N2M0 (IIIA). Based on the downstaging obtained, the patient underwent a radical left upper lobectomy on September 6th, 2022. Pathologic findings from the surgery confirmed the diagnosis of dedifferentiated lung carcinoma (Figure 2B). Immunohistochemistry outcomes were similar to the original biopsy with evidence of immune cell infiltration observed. On October 4th, 2022, the patient switched to maintenance therapy with pembrolizumab four weeks after the surgery. The only adverse event observed during the treatment was neutropenia (grade 2 according to Common Terminology Criteria Adverse Events version 5.0), which happened after the first cycle exposure of the combined regimen. At the time of manuscript preparation, the patient continues to receive mono-therapy of pembrolizumab, and still maintained an ongoing disease free status.
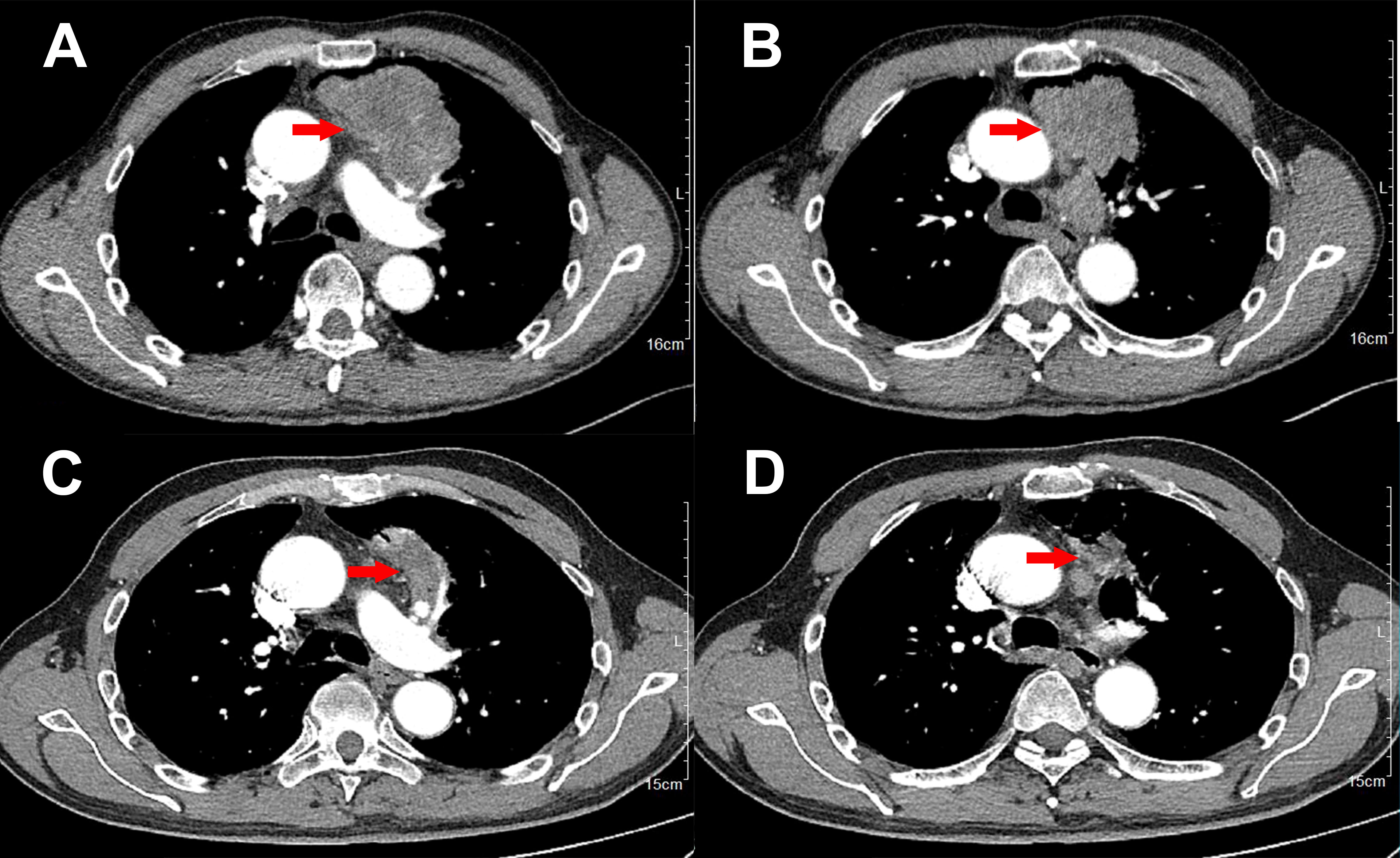
Figure 1 Variations of the primary lesions in lung by chest CT scans during the treatment (red arrowheads). (A, B), June 6th, 2022. (C, D), August 27th, 2022.

Figure 2 Histological findings with hematoxylin and eosin-stained biopsy specimen from biopsy on June 6th, 2022 (A, × 200), and surgery on September 6th, 2022 (B, × 400).
Herein in the present report, we demonstrated a case of dedifferentiated non-small cell lung cancer, characterized by STK11 loss and SMARCB1 deficiency, due to p.S67X mutations, which has been conventionally considered as treatment-refractory, has achieved significantly partial response to neo-adjuvant treatment with pembrolizumab and platinum doublet regimen. To the best of our knowledge, the present case was the first one to report the co-alteration of STK11 loss and SMARCB1 deficiency mutation. Results of the present case highlighted that biomarkers including loss, or SMARCB1 deficiency mutation, might be probably predictive to response to PD-1 blockade therapy.
Somatic STK11 mutations were reported presenting in approximately 30% of non-small cell lung cancer patients, especially among smokers (17). In addition, STK11 mutation often occurred with co-mutation with kirsten ratsarcoma viral oncogene homolog (KRAS) and kelch-like ECH-associated protein 1 (KEAP1), which usually resulted in even worse prognosis (18, 19). Most recently, a retrospective study was conducted among USA institutes including the Dana-Farber Cancer Institute/Massachusetts General Hospital, the Memorial Sloan Kettering Cancer Center, and the MD Anderson Cancer Center, to investigate the efficacy prediction role of STK11/KRAS/KEAP1 mutations to immunotherapy efficacy (4). Results of the observational study showed that STK11 and KEAP1 mutations were associated with significantly worse PFS (STK11 hazard ratio (HR) = 2.04, P < 0.0001; KEAP1 HR = 2.05, P < 0.0001) and OS (STK11 HR = 2.09, P < 0.0001; KEAP1 HR = 2.24, P < 0.0001) survival to immunotherapy among KRAS mutant lung adenocarcinoma (4). Besides, another retrospective research also drew the similar conclusion. CLICaP was a retrospective study performed to evaluate a cohort of Hispanic patients diagnosed with metastatic NSCLC from the US and seven Latin American countries treated with immune checkpoint inhibitors (ICIs) alone or in combination as first-line treatment. Results of the study revealed that OS was significantly shorter for patients carrying STK11 mutations (STK11-mutant 14.2 months versus STK11-wild 27.0 months (P = 0.0001)) or KEAP1 (KEAP1-mutant 12.0 months versus KEAP1-wild 24.4 months (P = 0.005)) mutations (3). Although the potential mechanism still remained uncovered, STK11 has been proposed to induce T-cell exhaustion and immune-suppressed or “cold” tumor microenvironment according to relevant literatures (20). Nevertheless, there were studies reported that STK11 mutation may be associated with inferior outcomes by combined platinum-doublet plus immunotherapy even in high PD-L1 expression and high TMB patients (21, 22). However in the present case, the mutant type of STK11 was presented as the loss promoter of STK11 gene, which may lead to the silence expression of STK11 gene, thereby resulted in function deficiency of tumor suppressor. The difference of the alteration types might stand as one of potential reasons for the diverse clinical outcomes. Besides, the missence of SMARCB1 p.S67X*10.74% mutation may also contribute to the favorable outcomes in the present case, prognosis of which was consistent to a recent study as mentioned before (16).
SMARCB1-deficient carcinoma is a recently recognized entity in several organs, especially reported in sinonasal carcinoma (13, 23). Mutation of SMARCB1 deficiency was rarely reported in thoracic neoplasms. It was not until 2018 that Yoshida et al. reported the first case of SMARCB1-deficient pleural squamous cell carcinoma (24). In that case, patient with SMARCB1-deficient carcinoma of the pleura represented primary resistant to cisplatin plus gemcitabine therapy, thereby progressed rapidly and finally resulted in 10 months OS after diagnosis (24). Coincidentally, another recent case also reported the characteristics of poor prognosis and chemotherapy resistance in a Chinese patient with lung cancer harboring SMARCB1 deficiency, of which OS presented as 5 months merely (25). However, there were several studies with limited sample suggested that immunotherapy, including immune checkpoint inhibitors, may present as a promising therapeutic strategy for SMARCB1-deficient tumors, especially in sarcomas, and sinonasal carcinoma (26, 27). Mechanistically, SMARCB1 deficiency favored the de-repression of multiple endogenous retroviral elements (ERVs), resulting in cytosolic double-stranded RNA accumulation which activates cytoplasmic sensors including TLR3 and MDA5, and thereby cell-autonomous IFN-α and IFN-λ responses (27). In addition, an improved anti-cancer innate immune response driven by ERVs de-repression has also been observed in various cancer types (28), and correlated to response to anti-PD-(L)1 treatment (29). Practically in the present case, the favorable outcomes of the patient by immunotherapy also suggested that anti-PD-(L)1 treatment might be potentially effective in non-small cell lung cancer patients harboring SMARCB1 deficiency. Comparing to the former results by those published literatures, we speculated that the emerging mutation of STK11 loss (due to the loss of promoter) may play a role on the different outcomes between the present case and former studies. The co-current mutation of STK11 loss and SMARCB1 deficiency may offset the aggressive outcomes by SMARCB1 deficiency, hence resulted in the sensitivity of chemotherapy, as well as anti-PD-1 inhibitors. Even so, further investigation for potential mechanism by basic experiments should still be needed. In addition, because of the limited sample size in the present case report, a larger sample size in real world status should also be necessary to identify the clinical phenomenon.
Despite numbers of literatures suggesting the biological plausibility of SKT11 mutation may lead to primary resistant to anti-PD-(L)1 therapy, the issue is far from settled. An exploratory analysis from the trial KEYNOTE-042 revealed that pembrolizumab monotherapy was associated with improved OS when compared with platinum-doublet regardless of STK11 and KEAP1 mutational status (30). Just be similar to that, we have reported that SKT11 loss co-mutation with SMARCB1 deficiency mutation may also benefit from immunotherapy combined with chemotherapy. Recently, an enhancer of zeste homolog 2 (EZH2) inhibitor tazemetostat, has been approved by Food and Drug Administration (FDA) for the treatment of SMARCB1-deficient epithelioid sarcoma, representing the first molecularly targeted agent for SWI/SNF-deficient malignancies (31). However, the effectiveness of the target agent on NSCLC patients has not been established yet, which need further identification. Therefore, the combined regimen adopted in the present case might prove some therapeutic thoughts for clinical physicians.
In the present report, we demonstrated a case of dedifferentiated non-small cell lung cancer, characterized by STK11 loss and SMARCB1 deficiency mutation that conventionally been considered as treatment-refractory, has achieved significantly partial response to neo-adjuvant treatment with pembrolizumab and platinum doublet regimen. Our case highlighted that either STK11 loss, or SMARCB1 deficiency mutation, might not be used to select patients for PD-(L)1 blockade therapy or chemotherapy, respectively. In addition, awareness and recognition of the co-mutations of STK11 loss and SMARCB1 deficiency mutation may help sharpen focus on effective therapeutic development, and expand our understanding on the potential mechanism, as well as pathological significance of the rare co-mutations.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethical Committee of People′s Hospital of Quzhou. The patients/participants provided their written informed consent to participate in this study.
JC, conceptualization, methodology, software, writing- original draft preparation, software, and validation. JW, data curation, visualization, investigation, supervision, writing-reviewing, and editing. All authors contributed to the article and approved the submitted version.
The study was supported by Key Science and Technology Project of Quzhou (2022K48), Instructional Project of Quzhou (2020057), Instructional Project of Quzhou (2021005), ‘New 115’ Talent Project of Quzhou, and ‘258’ Talent Project of Quzhou.
The authors thank the patient for his participation and agreement to publication of the report. We also thank Dr. Hong Shen for her agreement to publication of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schabath MB, Welsh EA, Fulp WJ, Chen L, Teer JK, Thompson ZJ, et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene (2016) 35:3209–16. doi: 10.1038/onc.2015.375
2. Granado-Martinez P, Garcia-Ortega S, Gonzalez-Sanchez E, McGrail K, Selgas R, Grueso J, et al. STK11 (LKB1) missense somatic mutant isoforms promote tumor growth, motility and inflammation. Commun Biol (2020) 3:366. doi: 10.1038/s42003-020-1092-0
3. Cordeiro de Lima VC, Corassa M, Saldanha E, Freitas H, Arrieta O, Raez L, et al. STK11 and KEAP1 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value among hispanics (STRIKE registry-CLICaP). Lung Cancer (2022) 170:114–21. doi: 10.1016/j.lungcan.2022.06.010
4. Ricciuti B, Arbour KC, Lin JJ, Vajdi A, Vokes N, Hong L, et al. Diminished efficacy of programmed death-(Ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol (2022) 17:399–410. doi: 10.1016/j.jtho.2021.10.013
5. Rosellini P, Amintas S, Caumont C, Veillon R, Galland-Girodet S, Cuguilliere A, et al. Clinical impact of STK11 mutation in advanced-stage non-small cell lung cancer. Eur J Cancer (2022) 172:85–95. doi: 10.1016/j.ejca.2022.05.026
6. Sitthideatphaiboon P, Galan-Cobo A, Negrao MV, Qu X, Poteete A, Zhang F, et al. STK11/LKB1 mutations in NSCLC are associated with KEAP1/NRF2-dependent radiotherapy resistance targetable by glutaminase inhibition. Clin Cancer Res (2021) 27:1720–33. doi: 10.1158/1078-0432.CCR-20-2859
7. Shang X, Li Z, Sun J, Zhao C, Lin J, Wang H. Survival analysis for non-squamous NSCLC patients harbored STK11 or KEAP1 mutation receiving atezolizumab. Lung Cancer (2021) 154:105–12. doi: 10.1016/j.lungcan.2021.02.010
8. An J, Yan M, Yu N, Chennamadhavuni A, Furqan M, Mott SL, et al. Outcomes of patients with stage III non-small cell lung cancer (NSCLC) that harbor a STK11 mutation. Transl Lung Cancer Res (2021) 10:3608–15. doi: 10.21037/tlcr-21-177
9. Agaimy A, Koch M, Lell M, Semrau S, Dudek W, Wachter DL, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol (2014) 38:1274–81. doi: 10.1097/PAS.0000000000000236
10. Wasserman JK, Dickson BC, Perez-Ordonez B, de Almeida JR, Irish JC, Weinreb I. INI1 (SMARCB1)-deficient sinonasal carcinoma: A clinicopathologic report of 2 cases. Head Neck Pathol (2017) 11:256–61. doi: 10.1007/s12105-016-0752-3
11. Lee VH, Tsang RK, Lo AWI, Chan SY, Chung JC, Tong CC, et al. SMARCB1 (INI-1)-Deficient sinonasal carcinoma: A systematic review and pooled analysis of treatment outcomes. Cancers (Basel) (2022) 14. doi: 10.3390/cancers14133285
12. Rickard JA, Burr ML, Williams B, Murugasu A, Fellowes A, John T, et al. SMARCB1/INI1-deficient primary lung carcinoma with hepatic metastasis. Pathology (2022) 54:817–20. doi: 10.1016/j.pathol.2021.11.010
13. Wang R, Wang L, Fang J, Zhong Q, Hou L, Ma H, et al. Clinical diagnosis and treatment analyses on SMARCB1 (Integrase interactor 1)-deficient sinonasal carcinoma: Case series with systematic review of the literature. World Neurosurg (2022) 161:e229–e43. doi: 10.1016/j.wneu.2022.01.114
14. Ahadi MS, Fuchs TL, Clarkson A, Sheen A, Sioson L, Chou A, et al. Switch/sucrose-non-fermentable (SWI/SNF) complex (SMARCA4, SMARCA2, INI1/SMARCB1)-deficient colorectal carcinomas are strongly associated with microsatellite instability: An incidence study in 4508 colorectal carcinomas. Histopathology (2022) 80:906–21. doi: 10.1111/his.14612
15. Kaur R, Mehta J, Borges AM. Role of SMARCA4 (BRG1) and SMARCB1 (INI1) in dedifferentiated endometrial carcinoma with paradoxical aberrant expression of MMR in the well-differentiated component: A case report and review of the literature. Int J Surg Pathol (2021) 29:571–7. doi: 10.1177/1066896920959453
16. Haberecker M, Buhler MM, Mendieta AP, Guggenberger R, Arnold F, Markert E, et al. Molecular and immunophenotypic characterization of SMARCB1 (INI1) - deficient intrathoracic neoplasms. Mod Pathol (2022) 35:1860–9. doi: 10.1038/s41379-022-01133-4
17. Facchinetti F, Bluthgen MV, Tergemina-Clain G, Faivre L, Pignon JP, Planchard D, et al. LKB1/STK11 mutations in non-small cell lung cancer patients: Descriptive analysis and prognostic value. Lung Cancer (2017) 112:62–8. doi: 10.1016/j.lungcan.2017.08.002
18. Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discovery (2018) 8:822–35. doi: 10.1158/2159-8290.CD-18-0099
19. Wohlhieter CA, Richards AL, Uddin F, Hulton CH, Quintanal-Villalonga A, Martin A, et al. Concurrent mutations in STK11 and KEAP1 promote ferroptosis protection and SCD1 dependence in lung cancer. Cell Rep (2020) 33:108444. doi: 10.1016/j.celrep.2020.108444
20. Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun (2016) 7:10501. doi: 10.1038/ncomms10501
21. Hasegawa T, Yanagitani N, Ninomiya H, Sakamoto H, Tozuka T, Yoshida H, et al. Association between the efficacy of pembrolizumab and low STK11/LKB1 expression in high-PD-L1-expressing non-small-cell lung cancer. In Vivo (2020) 34:2997–3003. doi: 10.21873/invivo.12131
22. Arbour K, Shen R, Plodkowski A, Rizvi H, Ni A, Long N, et al. MA19.09 concurrent mutations in STK11 and KEAP1 is associated with resistance to PD-(L)1 blockade in patients with NSCLC despite high TMB. J Thorac Oncol (2018) 13:S424. doi: 10.1016/j.jtho.2018.08.480
23. Ng JKM, Chan JYK, Li JJX, Tang K, Yeung DCM, Chan ABW. SMARCB1 (INI1)-deficient sinonasal carcinoma with yolk sac differentiation showing Co-loss of SMARCA4 immunostaining - a case report and literature review. Head Neck Pathol (2022) 16:934–41. doi: 10.1007/s12105-022-01423-y
24. Yoshida K, Fujiwara Y, Goto Y, Kohno T, Yoshida A, Tsuta K, et al. The first case of SMARCB1 (INI1) - deficient squamous cell carcinoma of the pleura: a case report. BMC Cancer (2018) 18:398. doi: 10.1186/s12885-018-4321-x
25. Zhou YH, Qin S, Yan JX, Ji J, Lan T, Liu Y. INI1 (SMARCB1) deletion of lung cancer: report of a case. Zhonghua Bing Li Xue Za Zhi (2022) 51:902–4. doi: 10.3760/cma.j.cn112151-20220207-00076
26. Yang H, Zhou L, Zhong G, Li X, Wang Y. SMARCB1 (INI-1)-Deficient sinonasal carcinoma: A case report and literature review. Ear Nose Throat J (2022), 1455613221082622. doi: 10.1177/01455613221082622
27. Ngo C, Postel-Vinay S. Immunotherapy for SMARCB1-deficient sarcomas: Current evidence and future developments. Biomedicines (2022) 10. doi: 10.3390/biomedicines10030650
28. Roulois D, Loo Yau H, Singhania R, Wang Y, Danesh A, Shen SY, et al. DNA-Demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell (2015) 162:961–73. doi: 10.1016/j.cell.2015.07.056
29. Solovyov A, Vabret N, Arora KS, Snyder A, Funt SA, Bajorin DF, et al. Global cancer transcriptome quantifies repeat element polarization between immunotherapy responsive and T cell suppressive classes. Cell Rep (2018) 23:512–21. doi: 10.1016/j.celrep.2018.03.042
30. Cho BC, Lopes G, Kowalski DM, Kasahara K, Wu Y-L, Castro G, et al. Abstract CT084: Relationship between STK11 and KEAP1 mutational status and efficacy in KEYNOTE-042: pembrolizumab monotherapy versus platinum-based chemotherapy as first-line therapy for PD-L1-positive advanced NSCLC. Cancer Res (2020) 80:CT084–CT. doi: 10.1158/1538-7445.AM2020-CT084
Keywords: STK11, SMARCB1, lung cancer, pembrolizumab, efficacy
Citation: Chen J and Wang J (2023) STK11 loss and SMARCB1 deficiency mutation in a dedifferentiated lung cancer patient present response to neo-adjuvant treatment with pembrolizumab and platinum doublet: A case report. Front. Oncol. 13:1088534. doi: 10.3389/fonc.2023.1088534
Received: 03 November 2022; Accepted: 16 January 2023;
Published: 27 January 2023.
Edited by:
Zichao Luo, National University of Singapore, SingaporeReviewed by:
Miguel Sotelo, Hospital María Auxiliadora, PeruCopyright © 2023 Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junhui Wang, d2FuZ2p1bmh1aTc1MjZAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.