
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 07 February 2023
Sec. Cancer Genetics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1088265
This article is part of the Research TopicTumor Organoid Models and Epigenetic Regulation in Cancer ResearchView all 5 articles
Background: The most typical thyroid gland malignant lesion is papillary thyroid cancer (PTC). In many nations, the prevalence of thyroid cancer (TC) is rising, particularly papillary thyroid microcarcinoma (PTMC). Microwave ablation (MWA) has been gradually carried out in some patients with benign thyroid nodules, some low-risk PTMC, and metastatic lymph nodes in the neck. The role and safety of MWA remain controversial topics. So we conducted this study to provide the latest evidence of MWA for PTMC compared with surgery.
Methods: Patients’ postoperative outcomes (duration of hospital stay and hospitalization expenditures), intraoperative outcomes (surgery time, blood loss, and incision size), and follow-up outcomes were all examined (complication rate, recurrence rate, and lymph node metastasis). The effectiveness and safety of MWA versus surgery for PTMC patients were compared using the weighted mean difference (WMD) and odds ratio (OR).
Results: In total, we included 7 articles (7 trial comparisons) which contained 1, 567 PTMC patients. The results showed that MWA had significant advantages in operative time (WMD = -53.47, 95% CI: -67.62 to -39.32), postoperative hospital stay (WMD =-4.59, 95% CI: -6.40 to -2.77), hospitalization costs (WMD= -70.06, 95% CI: -90.93 to -49.19), blood loss (WMD =-28.07, 95% CI: -33.77 to -22.38), incisions size (WMD =-59.69, 95% CI: -67.79 to -51.59), and complication rates (OR = 0.28; 95% CI: 0.18 to 0.42) compared with surgery. It also showed that recurrence rates and risk of lymph node metastasis are similar to surgery.
Conclusions: For PTMC patients, MWA could be an efficient, safe, and affordable therapy.
Clinically, thyroid nodules are typical. About 7–15% of thyroid nodules are cancerous, while the majority are benign (1). The most typical thyroid gland malignant lesion is papillary thyroid cancer (PTC) (2). The development of papillary thyroid microcarcinoma (PTMC) within the thyroid gland is responsible for around 50% of the rise in the incidence of thyroid cancer that has been observed in numerous nations. The associated mortality rate, however, was either unchanged or lowered (3, 4). PTMC refers to papillary thyroid carcinoma with a maximum diameter of ≤10 mm (5). The characteristics of low-risk PTMC are as follows: (I) the primary tumor is a single lesion (II) the maximum diameter of the primary tumor is less than 10mm. (III) Rather than being close to the thyroid capsule or trachea, the primary tumor is located in the core of the thyroid gland. (IV) there is no regional lymph node metastasis after evaluation (6).
Guidelines from professional societies recommend surgery as the ultimate treatment (7). Although thyroid lobectomy is effective, various complications (such as hypoparathyroidism and damage to the laryngeal recurrent nerve) can happen following surgery, which can significantly impact the quality of the patient’s life (8). Microwave ablation (MWA) has shown good efficacy in the treatment of PTMC, especially in low-risk PTMC (9). Guided by imaging technology, MWA uses a microwave magnetic field to coagulate, and dehydrate eradicate tumor tissues to achieve the purpose of treatment. In recent years, MWA has been gradually carried out in some patients with benign thyroid nodules, some low-risk PTMC, and neck metastatic lymph nodes. MWA is associated with less blood loss, shorter procedure time, and shorter hospital stay compared to open procedures. In order to evaluate the efficacy and safety of thermal ablation and surgery for the treatment of low-risk PTMCs, Kim et al. conducted a meta-analysis. Both thermal ablation and surgery are effective and risk-free options for the treatment of low-risk PTMCs, with thermal ablation having a lower complication rate. Only four studies, totaling 653 individuals who received thermal ablation or surgery, were considered (10). Thermal ablation for the treatment of PTMC was compared to routine surgery in a comprehensive review and meta-analysis for cost, efficacy, and safety. They found that, for the treatment of PTMC, thermal ablation offers a typically secure and affordable alternative to surgery (11). Microwave, radiofrequency, and laser ablation are all types of thermal ablation, and so on. Our study mainly focused on the effect of MWA in PTMC. There was no evidence of differences in disease progression and major complications between MWA and surgery (12). The role of MWA remains a controversial topic (13), and many related studies have been published recently So an updated meta-analysis is necessary.
The Cochrane Library, Embase, PubMed, and Google Scholar were all completely searched as of October 2022. The following search terms were employed: Surgery, microwave ablation (MWA), and papillary thyroid microcarcinoma are the first three (PTMC). The Boolean operators “AND” and “OR” were used in the technique to combine all of these terms. The language of the literature search was limited to English. Two of the authors designed the search technique, which also included a literature review and reference list screening, but it should be highlighted that not all pertinent research may have been included.
The studies incorporated into our meta-analysis met the following requirements: (I) Patients from PTMC who are at the stable stage are also included, as is a MWA test group., (II) included a surgery control group, (III) observed the primary outcome indicators of intraoperative outcomes (such as length of the procedure, blood loss, and incision size), postoperative outcomes (like the length of the hospital stay and the cost of the hospital stay), and follow-up results (such as the frequency of complications, recurrences, and lymph node metastasis), and (IV) used either a prospective or retrospective study design. Our meta-analysis did not include the following studies: (I) there wasn’t enough data, (II) the research compared other thermal ablation, such as RFA and laser ablation, (III) there weren’t enough therapies to assess, and (IV) The research was either an abstract, review, or duplicate publication.
The data were separately extracted by two researchers, and any disagreements were resolved through discussion with a third reviewer or by reaching a consensus. The planned data components from each investigation were extracted using a structured data abstraction form. The information that was gathered included information on the study’s authors, design, patient characteristics (such as age and gender), duration of the research year, and follow-up period. Since all of the included publications were randomized clinical trials, the risk-of-bias evaluation was finished using the Cochrane Risk of Bias 2.0.
In accordance with the characteristics of the included research, sensitivity analysis was used to investigate the origins of heterogeneity. After properly removing one of the included studies, the pooled analysis was then repeated. The new pooled effect size was contrasted with the initial pooled effect size to ascertain how the excluded studies affected the pooled effect size.
Stata (version 17.0) was used in our study’s meta-analysis to calculate the various impacts on patients with PTMC in the MWA and surgery groups. In essence, we used the odds ratio (OR) to account for the pooling effects of binary variables and the weighted mean difference (WMD) for continuous variables. The I2 statistic, which measures discrepancies in the research findings and indicates the percentage of the total variance in study projections that may be attributed to heterogeneity rather than sampling mistakes, was used to evaluate the disparity in the statistics between the studies.If there was no statistical heterogeneity, the meta-analysis was conducted using a fixed-effect model. (I2 ≤ 50) between the included researches. If there existed statistical heterogeneity, however, a random-effect model was employed. (I2 > 50). If there were more than 10 included studies, the Egger’s test and a funnel plot were used to investigate potential publication bias 10.
There were 373 distinct studies found in the literature search, and after duplicate files were removed, 167 papers were checked for eligibility. Further 139 were eliminated from consideration after the titles and abstracts were checked. To identify the kind of paper, the relevant outcome data, and the research design, the entire texts of 28 articles were examined. Ultimately, data was extracted from 7 studies (14–20) that were included in our meta-analysis. Figure 1 displayed the method for conducting a literature search, the rules for inclusion and exclusion, and the final studies that were included.
Table 1 provided a comprehensive summary of the meta-analysis papers. The 7 studies were all randomized prospective or retrospective studies. The meta-analysis comprised 1,567 patients in total (779 MWA patients and 788 surgery patients). The studies’ sample sizes varied from 87 to 644. The majority of the research publications on MWA for PTMC have been published after 2018, as it is a relatively new therapy.
The quality and bias risks of the listed studies were assessed using Cochrane Risk of Bias 2.0 (21). Six of the seven publications were retrospective studies, one was a prospective study, and all were randomized clinical trials. Three studies also had scant or no follow-up. Table 2 displayed the summary bias evaluations of all included studies.
Six of the seven studies (including 1,256 patients) focused on operation time. In comparison to the surgery group, the MWA group took considerably less operation time (WMD =-53.47, 95% CI: -67.62 to -39.32; P<0.05; random effects model; as shown in Figure 2).
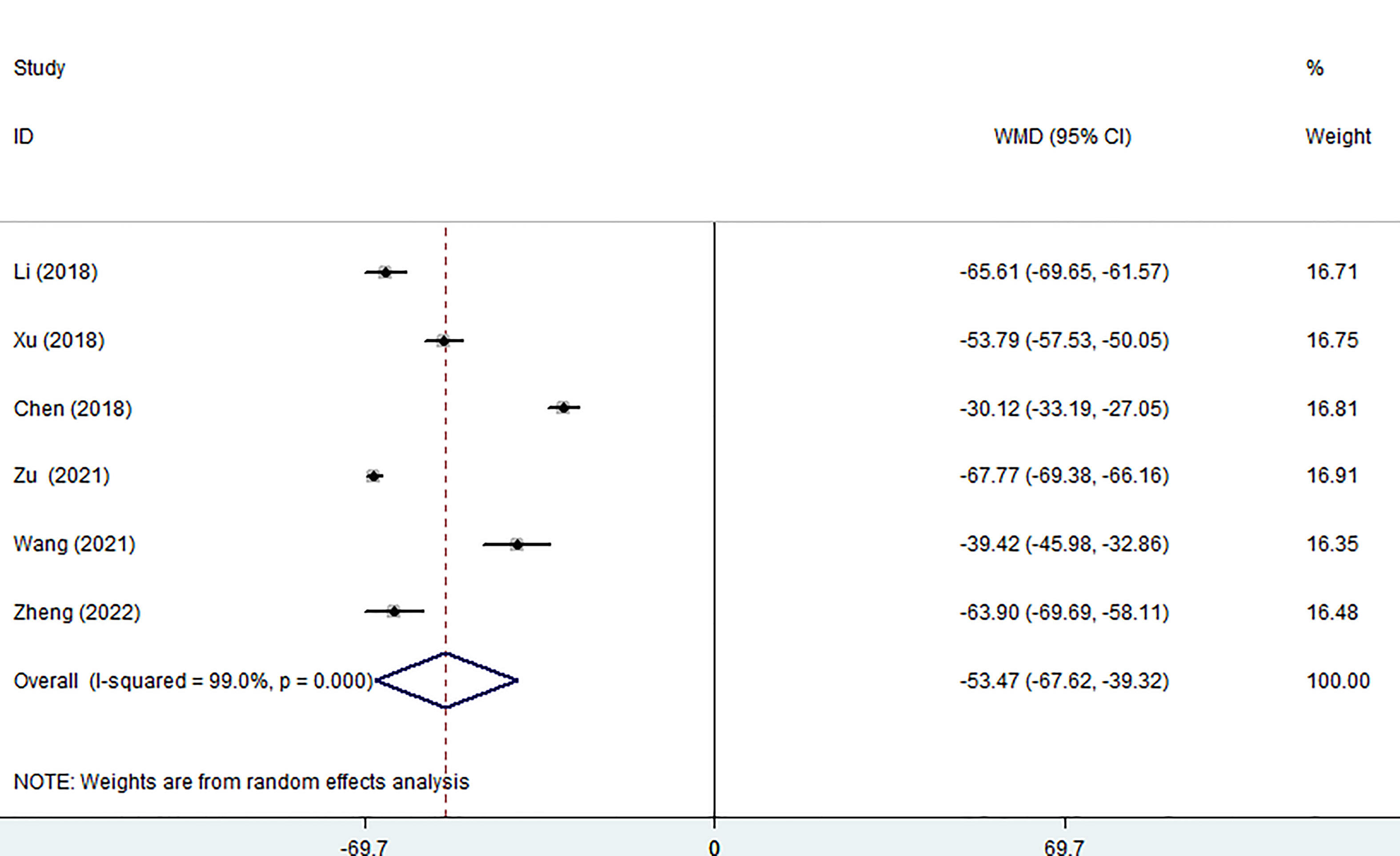
Figure 2 Forest plot of the operation time for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
Five of the seven studies examined blood loss. The pooled data showed that the MWA group lost significantly less blood than the surgical group using a random effects model. (WMD =-28.07, 95% CI: -33.77 to -22.38; P<0.05; as shown in Figure 3).
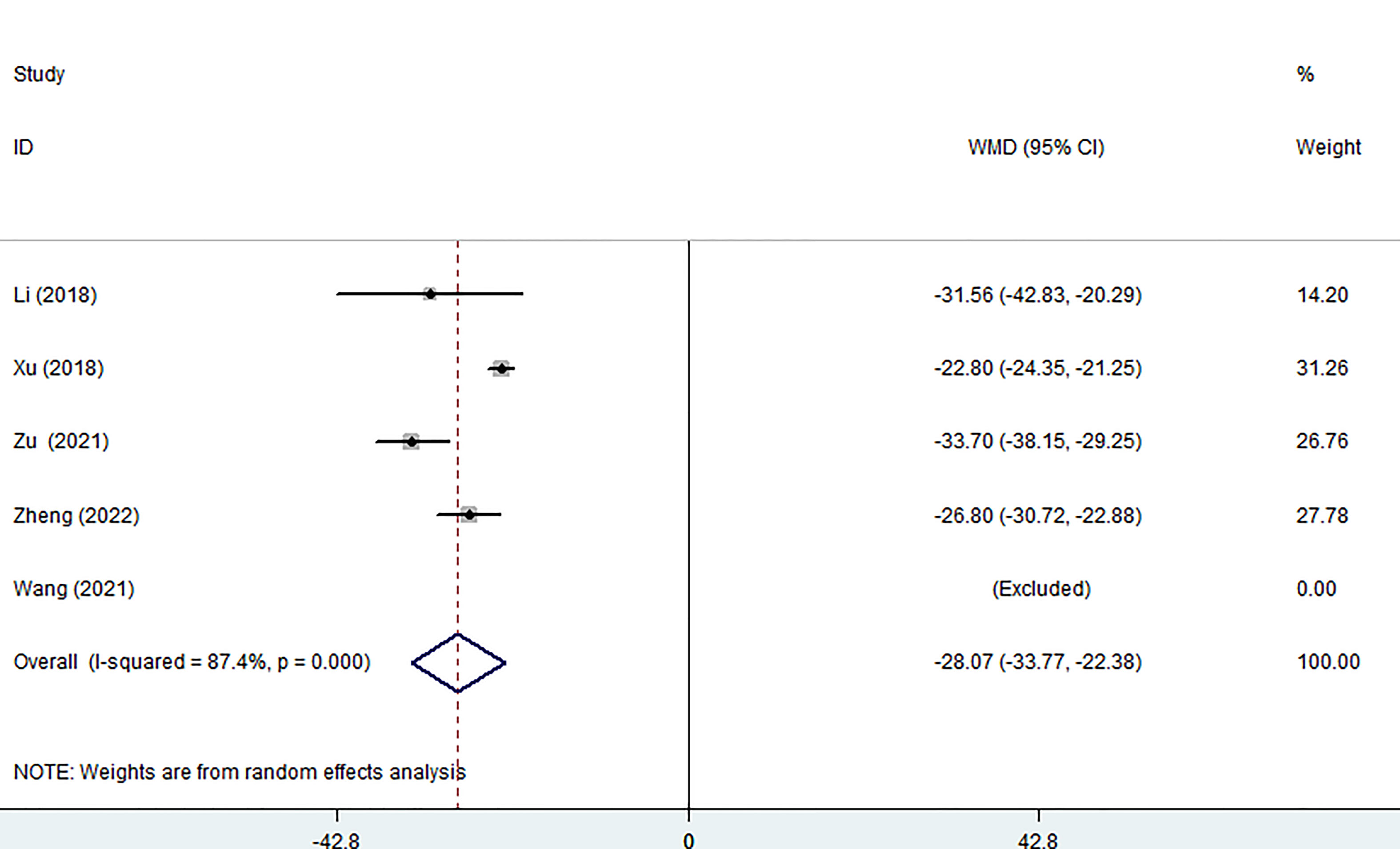
Figure 3 Forest plot of the blood loss for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
Three of the seven studies (including 882 patients) reported on the size of surgical incisions. The combined estimate revealed that the MWA group’s incision size was considerably smaller than that of the surgery group (WMD =-59.69, 95% CI: -67.79 to -51.59; P<0.05, random effects model; as shown in Figure 4).
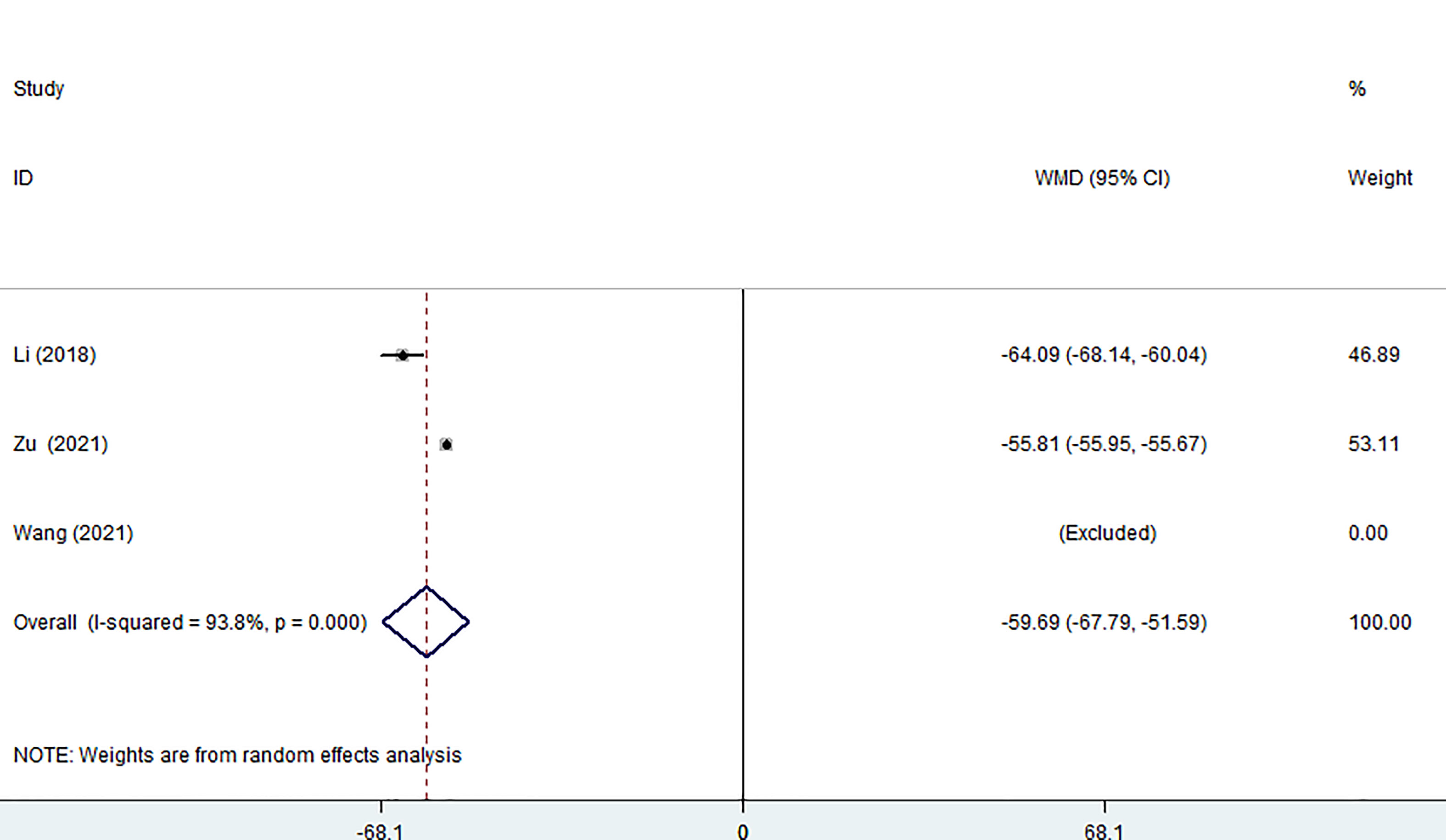
Figure 4 Forest plot of the size of surgical incision for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
Five of the seven studies (including 1,058 patients) evaluated hospital stays. According to the combined findings, MWA significantly shortened patients’ stays in the hospital (WMD =-4.59, 95% CI: -6.40 to -2.77; P<0.05, random-effects model; as shown in Figure 5).
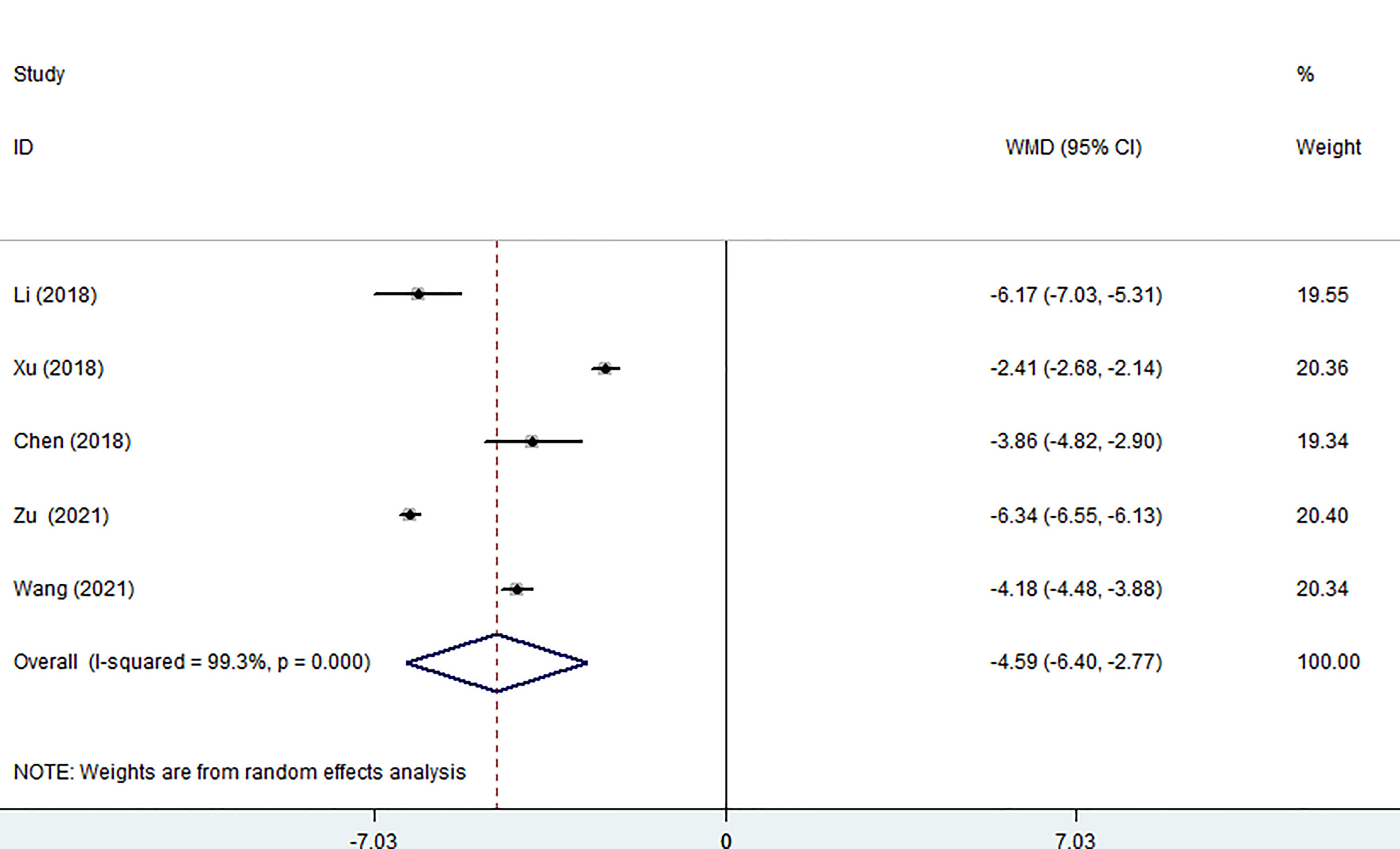
Figure 5 Forest plot of the hospital stay for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
Four of the seven studies (including 1,023 patients) evaluated hospitalization costs. Hospitalization costs for the MWA group were considerably lower than for the surgery group, according to the pooled data (WMD= -70.06, 95% CI: -90.93 to -49.19; P<0.05, random-effects model; as shown in Figure 6).
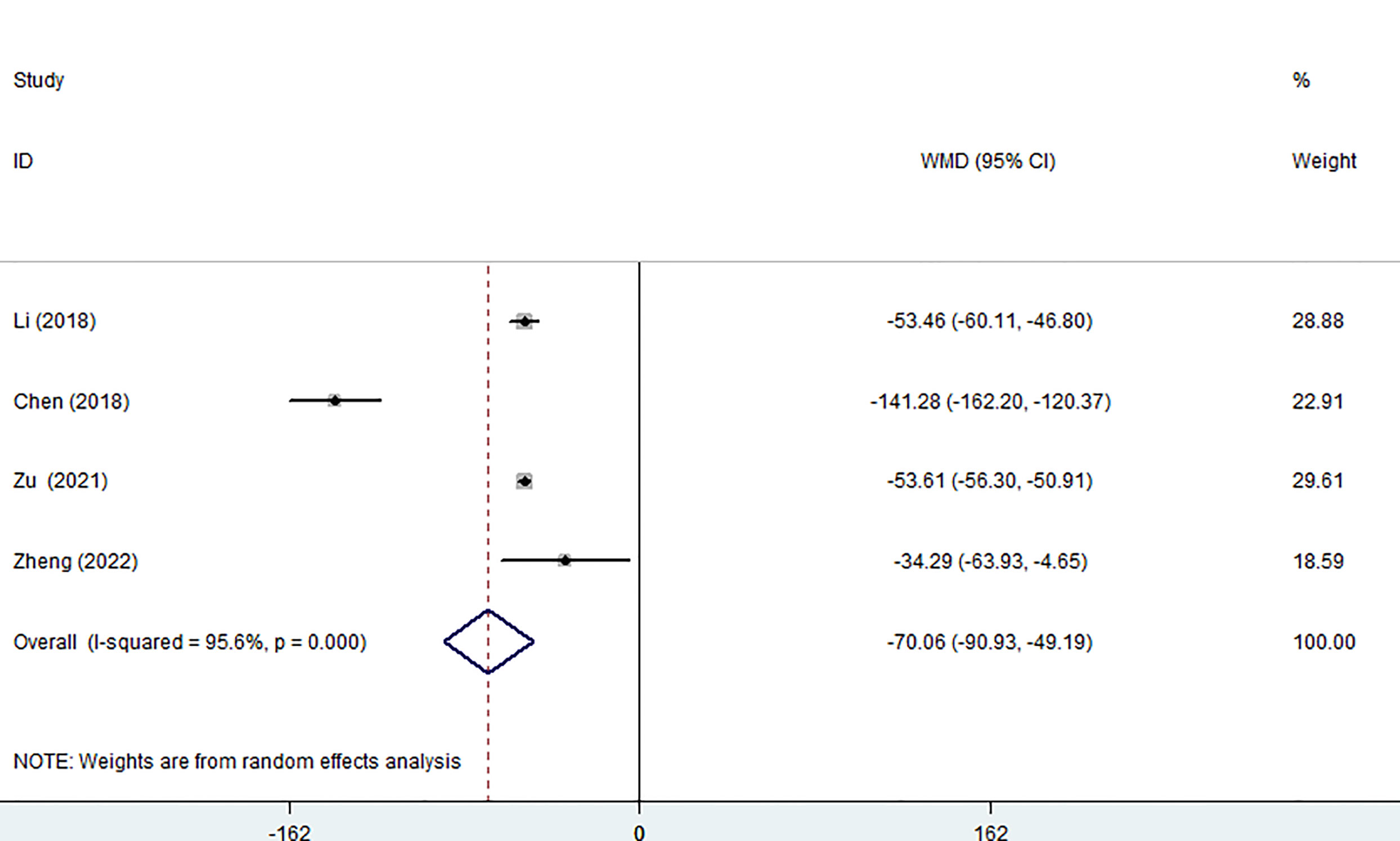
Figure 6 Forest plot of the hospitalization costs for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
Six of the seven studies (1,421 patients) focused on complications. The complication rate in the MWA group was significantly lower than that in the surgical group. (OR = 0.28; 95% CI: 0.18 to 0.42; P<0.05, fixed effects model; as shown in Figure 7). There was no discernible difference between the MWA group and the surgery group in the four studies that assessed the recurrence rate (a total of 1,193 patients) (OR= 0.86, 95% CI: 0.38 to 1.95; P>0.05, fixed-effects model; as shown in Figure 8). Similar to this, a meta-analysis of the variations in lymph node metastasis between the two groups was conducted, but no appreciable difference was found. (OR =0.71, 95% CI: 0.26 to 1.95; P>0.05; as shown in Figure 9).
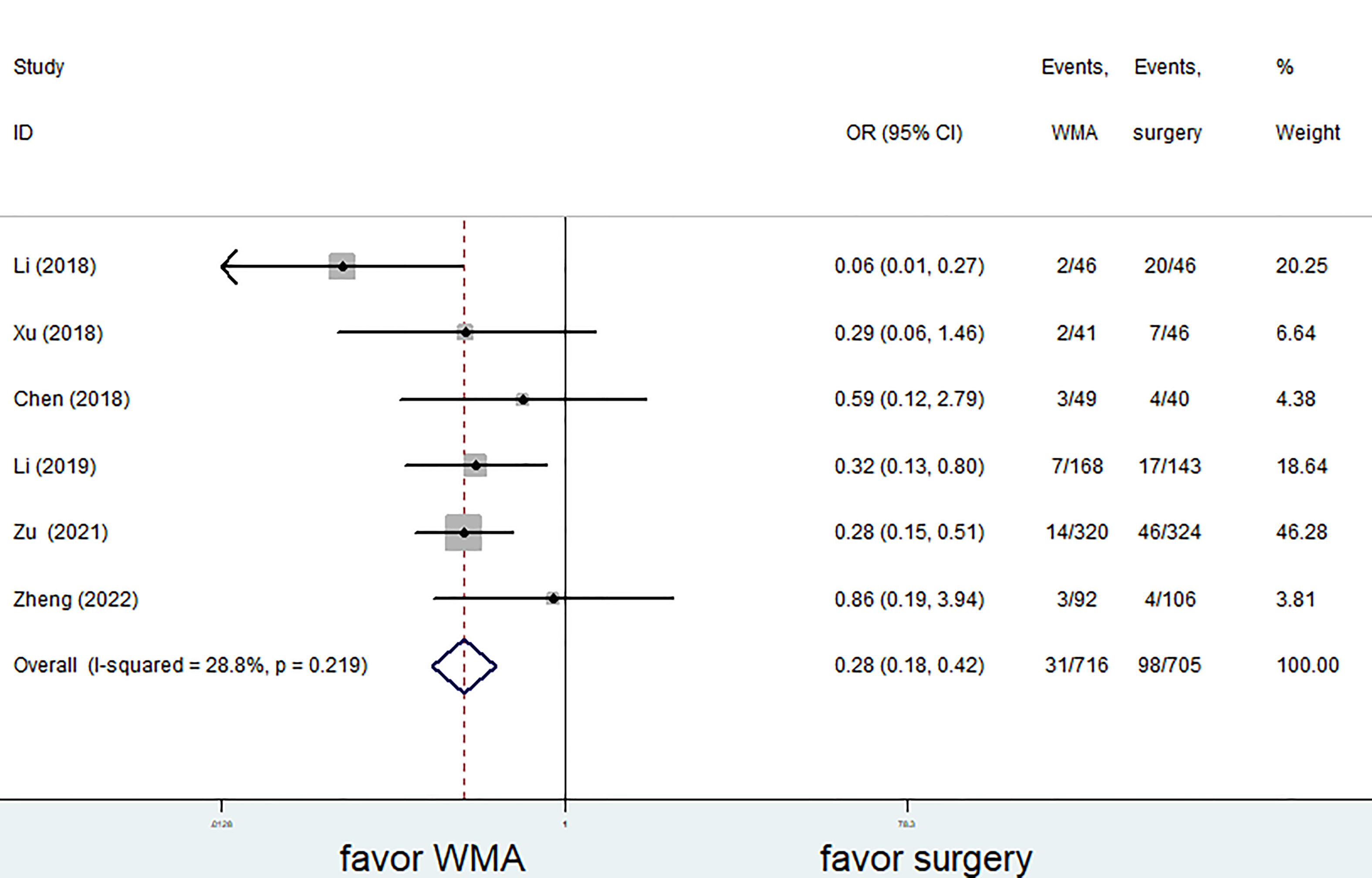
Figure 7 Forest plot of complications for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
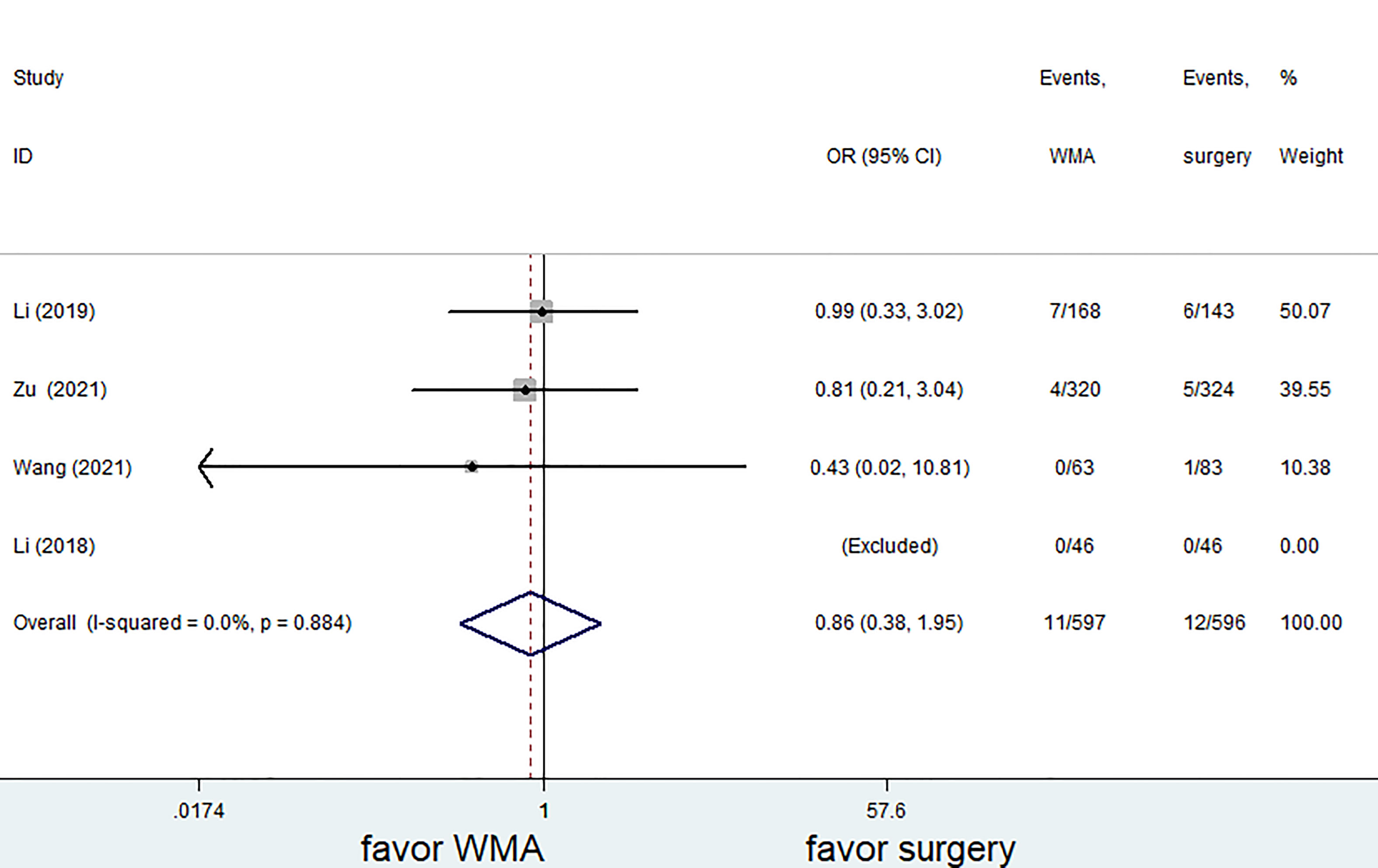
Figure 8 Forest plot of recurrence rates for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.

Figure 9 Forest plot of lymph node metastasis for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
The results of the sensitivity analysis are shown in Figures 10–13. The results showed that after eliminating Chen’s and Zu’s study, the recombined effect size changed greatly, so we considered Chen’s and Zu’s study studies as the possible causes of heterogeneity.
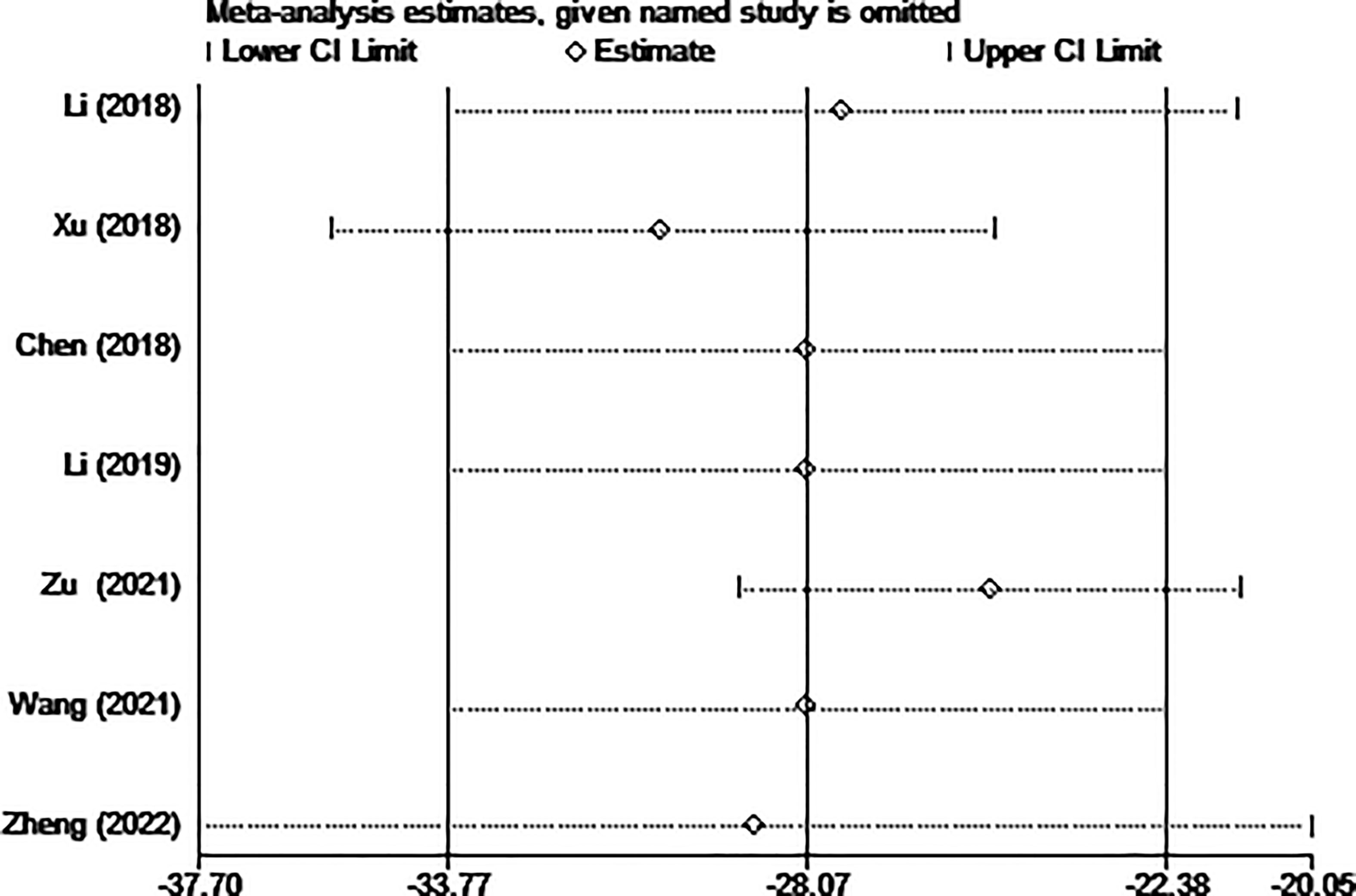
Figure 10 Sensitivity analysis of blood loss for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
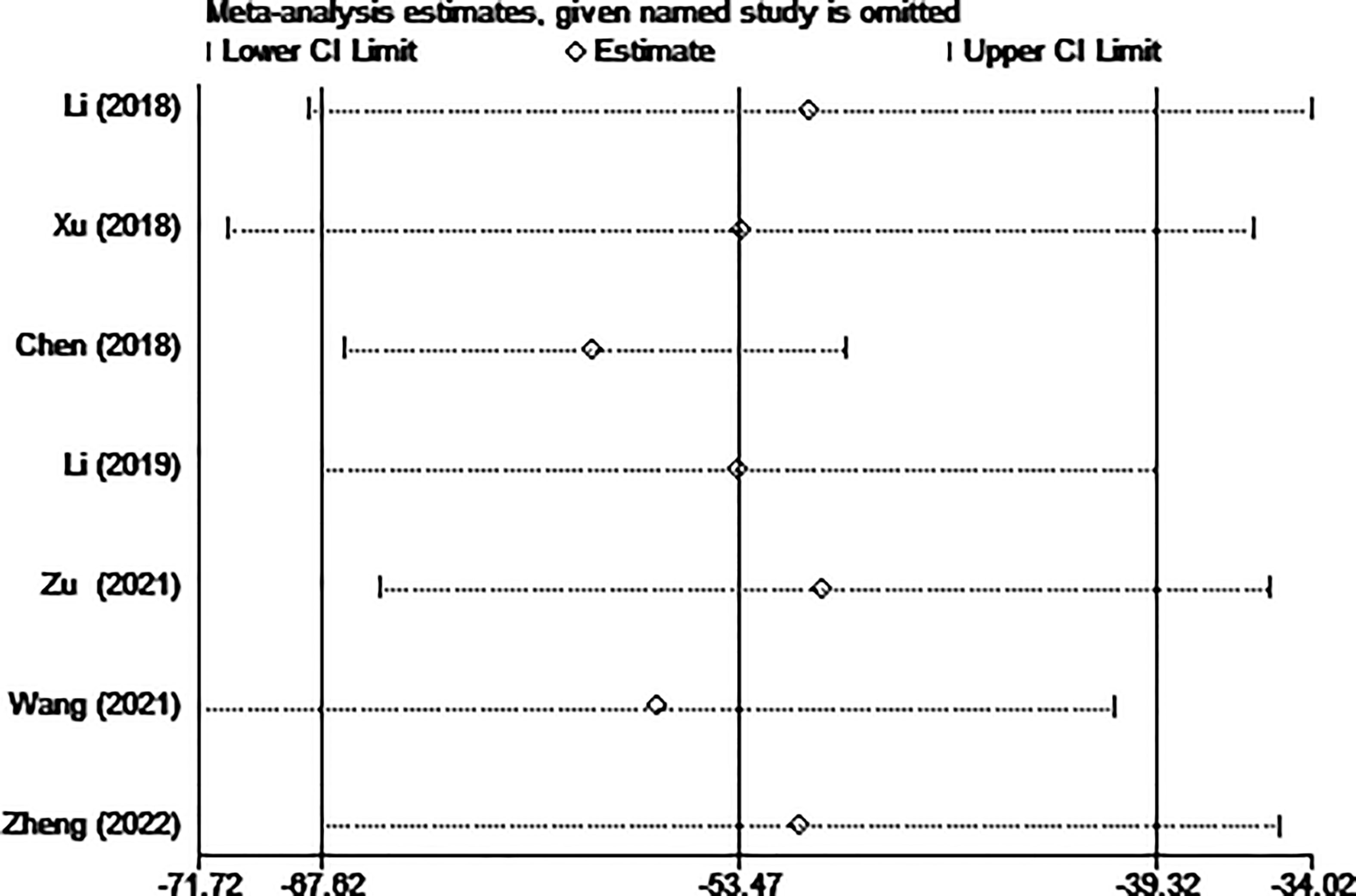
Figure 11 Sensitivity analysis of operation time for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.

Figure 12 Sensitivity analysis of hospitalization costs for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
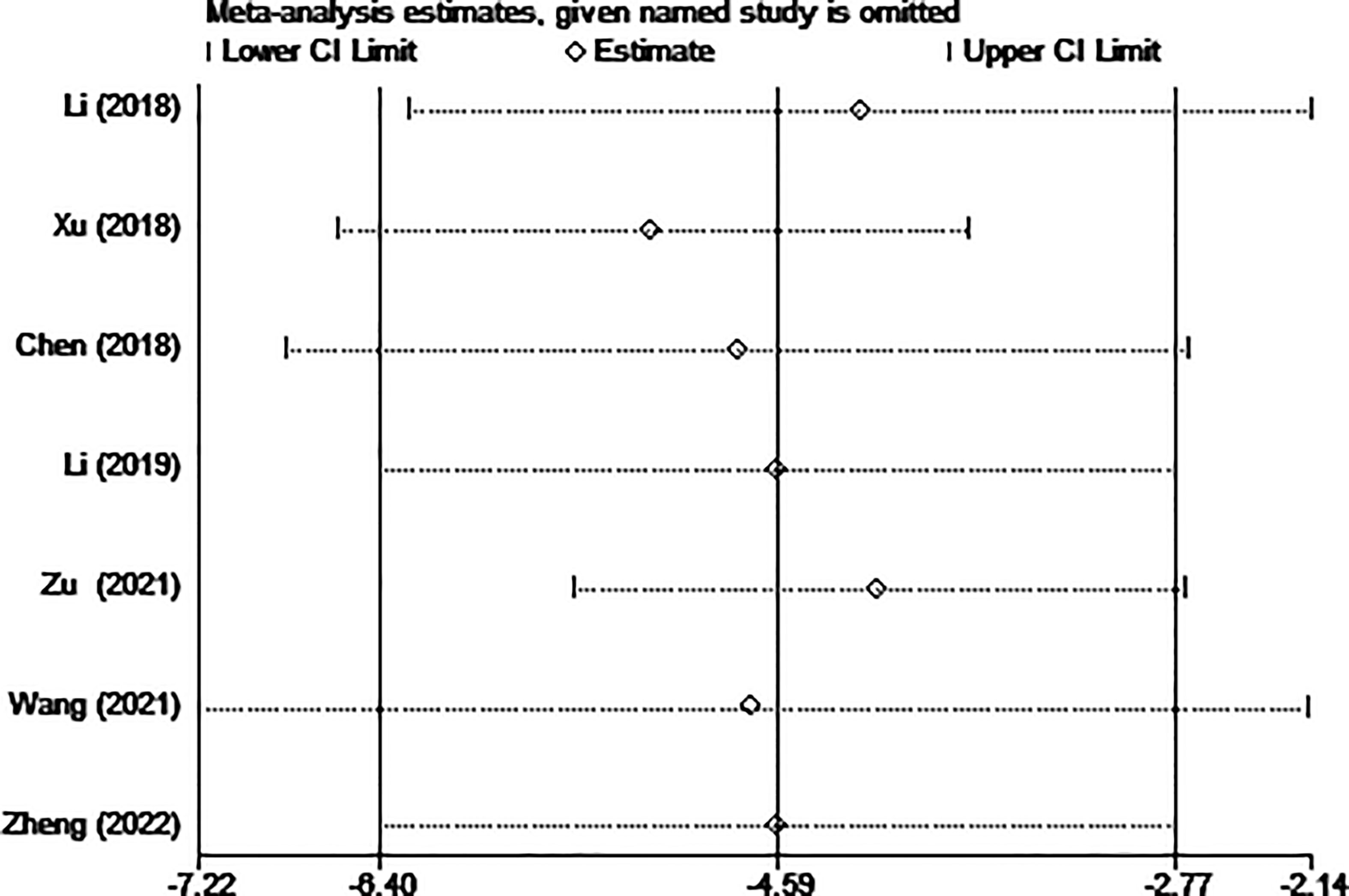
Figure 13 Sensitivity analysis of hospital stay for comparing MWA with surgery in patient with PTMC. MWA, microwave ablation, PTMC, papillary thyroid microcarcinoma.
Surgery is known as the cornerstone of traditional treatment for patients with PTMC (22). However, it may cause a series of complications, namely hypoparathyroidism, hypothyroidism, paralysis of the recurrent laryngeal nerve, or ugly scars (23). It may have a negative impact on the patient’s quality of life, and mental health. MWA has been applied in liver, lung, kidney, and bone marrow lesions with the advancement of technology (10, 24). MWA is gaining more and more recognition in thyroid treatment (25).
In this study, we found that MWA could reduce operation time, hospital stay, hospitalization costs, incision size, blood loss, and complications without increasing the risk of recurrence and lymph node metastasis. Moreover, the latest expert consensus on thyroid thermal ablation recommends that PTMC be ablated to avoid complications after surgical treatment, such as choking on drinking water, low tone, hoarse voice, difficulty in breathing (26).
Recurrent laryngeal nerve damage following thyroid surgery has been found to occur at a rate of 0.3%-15.4% (27). The common causes of recurrent laryngeal nerve injury include tumor adhesion, nerve invasion, surgical operation, and so on. Damage to the recurrent laryngeal nerve may result in hoarseness and breathing difficulties (28). After surgery, the patient might have a deep voice and water choking if the superior laryngeal nerve has been injured. In addition, hypothyroidism and hypocalcemia following surgery are quite frequent. The incidence of hypothyroidism is reported to be as high as 75%, while the incidence of permanent hypocalcemia ranges from 0% to 3.1% (29, 30).
The duration of the procedure is also a factor in the safety equation because, in some aspects, a lengthy procedure increases the risk of bleeding, tissue damage, and anesthesia complications. Our research revealed that the operation time of the MWA group was significantly shorter than that of the surgery group (p<0.05). The primary factor is that a portion of MWA can be carried out as an outpatient surgery, and the rapid turnaround time means that general anesthesia is not required. In addition, postoperative hospital stay and perioperative costs were also compared, and the differences were statistically significant. We found that the MWA group had shorter hospital stays and lower costs after treatment, suggesting that PTMC patients recovered more quickly after surgery. In considering the medical resources and the cost of patient care, especially the high incidence of PTMC, MWA may be a better choice for PTMC patients. MWA has the above advantages for the following reasons: firstly, MWA is treated with fine needles, which means it is more discrete and straightforward to approach the aim. In addition, the energy deployment of MWA is predictable and precise. The relatively small necrotic area after MWA means faster recovery, shorter hospital stays, and ultimately lower costs (8, 31). Both results (I2 = 99.3%, I2 = 95.6%) demonstrated high heterogeneity, which might be brought on by an imbalance in how different regions are developing economically, the difference in medical technology level, and the experience of different surgeons.
Since MWA is treated with ablation needles, the incision is naturally smaller than surgery. Besides, our study also found that the intraoperative blood loss of MWA was less than that of surgery, which indicated that MWA was less traumatic to the human body and more conducive to prevent the occurrence of postoperative neck hematoma, so as to avoid the risk of asphyxia caused by hematoma compression of the trachea. Additionally, because to the fibrosis brought on by scar tissue and the distortion of postoperative normal tissue, repeated surgery for tumor recurrence after thyroid surgery is usually difficult (17, 32). Therefore, postoperative tumor recurrence is also an important follow-up index. Some scientists advocated that compared with surgery, MWA may lead to incomplete treatment and therefore a higher rate of recurrence, enduring or even remote metastases during extended follow-up (33). However, our findings revealed that there was no statistically significant distinction between individuals who underwent MWA and surgery in terms of tumor recurrence or lymph node metastasis. Consequently, a randomized controlled trial (RCT) with a large sample size and lengthy follow-up period is urgently needed to confirm whether there are differences between MWA and surgery in recurrence and lymph node metastasis after PTMC. Although there are many advantages about MWA, there are also disadvantages. The disadvantages of MWA are mainly as follows: Firstly, it is impossible to make pathological judgment, and even if there is “puncture”, the diagnosis is not comprehensive and reliable. The understanding of benign, malignant and metastasis of thyroid tumors is insufficient. Besides, it cannot provide three-dimensional observation and judgment and all-round understanding and control of the tumor and its surrounding tissues during operation. Finally, it is only applicable to low-risk PTMC, not to other types of thyroid cancer.
Our study has the following limitations, first of all, all the included studies focused on China. The results of our meta-analysis may not be comparable to that in other areas where medical technology and levels may differ because of our study’s lack of extensibility. The results would be more consistent if high-quality research were carried out in the United States, Europe, or any other nations and included in this meta-analysis. Second, the number of the included studies were relatively limited, with most studies being retrospective cohort studies. If RCTs were included, the meta-overall analysis’s result would be more trustworthy. Third, there is considerable range in operation duration, postoperative hospitalization, and perioperative expenditures, which may be a result of regional economic conditions, variations in medical training, and patient age differences. Subgroup analysis couldn’t be done since there weren’t enough studies included. The source of heterogeneity could be found if more comparable studies were conducted and included in the meta-analysis. Fourth, MWA for PTMC is a relatively new technique with a limited mean follow-up time. Studies with extended follow-up are required to determine whether the findings are enduring or repeatable. Preferably more than five years of follow-up.
In conclusion, our meta-analysis showed that MWA had a considerable reduction in the rate of complications, operative time, postoperative hospital stay, and hospitalization costs compared with surgery. It also showed a recurrence rate and risk of lymph node metastasis similar to surgery. MWA may therefore be an efficient, secure, and cost-effective treatment for low-risk PTMC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JF designed the study. JF and YJ pooled the data. JF and YF analyzed the data. JF wrote and reviewed the article. All authors contributed to the article and approved the submitted version.
This study was supported by Xiamen Haicang hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gharib DH. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab (2008) 22(6):901–11. doi: 10.1016/j.beem.2008.09.019
2. Koshkina A, Fazelzad R, Sugitani I. Association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg (2021) 1:147. doi: 10.1001/jamaoto.2020.0368
3. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol (2016) 4(11):933–42. doi: 10.1016/S2213-8587(16)30180-2
4. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid (2015) 25(10):1127–36. doi: 10.1089/thy.2015.0116
5. Zhang H, Zheng X, Liu J, Gao M, Qian B. Active surveillance as a management strategy for papillary thyroid microcarcinoma. Cancer Biol Med (2020) 17(3):543. doi: 10.20892/j.issn.2095-3941.2019.0470
6. Wang TS, Goffredo P, Sosa JA, Roman SA. Papillary thyroid microcarcinoma: an over-treated malignancy? World J Surg (2014) 38(9):2297–303. doi: 10.1007/s00268-014-2602-3
7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
8. Zhang M, Tufano RP, Russell JO, Zhang Y, Zhang Y, Qiao Z, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years' follow-up. Thyroid (2020) 30(3):408–17. doi: 10.1089/thy.2019.0147
9. Teng DK, Li WH, Du JR, Wang H, Yang DY, Wu XL. Effects of microwave ablation on papillary thyroid microcarcinoma: a five-year follow-up report. Thyroid (2020) 30(12):1752–8. doi: 10.1089/thy.2020.0049
10. Kim HJ, Cho SJ, Baek JH. Comparison of thermal ablation and surgery for low-risk papillary thyroid microcarcinoma: a systematic review and meta-analysis. Korean J Radiol (2021) 22(10):1730. doi: 10.3348/kjr.2020.1308
11. Shen K, Xue S, Xie Y, Wang H, Li J, Sun Y, et al. Comparison of thermal ablation and routine surgery for the treatment of papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia (2020) 37(1):913–24. doi: 10.1080/02656736.2020.1777331
12. VanSonnenberg E, Simeone JF. Microwave ablation versus surgery for papillary thyroid carcinoma: More therapeutic options. More Controversies (2022) 304:714–5. doi: 10.1148/radiol.220896
13. Wei Y, Niu WQ, Zhao ZL, Wu J, Peng LL, Li Y, et al. Microwave ablation versus surgical resection for solitary T1N0M0 papillary thyroid carcinoma. Radiology (2022) 304(3):704–13. doi: 10.1148/radiol.212313
14. Li JM, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia (2018) 34(5):653–9. doi: 10.1080/02656736.2018.1453092
15. Xu B, Zhou NM, Cao WT, Gu SY. Comparative study on operative trauma between microwave ablation and surgical treatment for papillary thyroid microcarcinoma. World J Clin cases (2018) 6(15):936–43. doi: 10.12998/wjcc.v6.i15.936
16. Chen H, Chao Z, Huang P. Microwave ablation and surgical resection of papillary thyroid microcarcinoma: comparative analysis of clinical efficacy, safety and economy. Chin J Med Ultrasound (Electronic Edition) (2018) 15(04):275–80. doi: 1672-6448.2018.04.008
17. Li J, Liu Y, Liu J, Yang P, Hu X, Qian L. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia (2019) 36(1):639–45. doi: 10.1080/02656736.2019.1626492
18. Zu Y, Liu Y, Zhao J, Yang P, Li J, Qian L, et al. A cohort study of microwave ablation and surgery for low-risk papillary thyroid microcarcinoma. Int J Hyperthermia (2021) 38(1):1548–57. doi: 10.1080/02656736.2021.1996643
19. Wang X, Niu X, Mu S, Zhang M, Jiang W, Zhai L, et al. Analysis and evaluation of the efficacy of ultrasound-guided microwave ablation for papillary thyroid microcarcinoma. Int J Hyperthermia (2021) 38(1):1476–85. doi: 10.1080/02656736.2021.1988152
20. Zheng L, Dou JP, Liu FY, Yu J, Cheng ZG, Yu XL, et al. Microwave ablation vs. surgery for papillary thyroid carcinoma with minimal sonographic extrathyroid extension: a multicentre prospective study. Eur Radiol (2022) 33(1):233–43. doi: 10.1007/s00330-022-08962-6
21. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:14898. doi: 10.1136/bmj.l4898
22. Brito JP, Hay ID. Management of papillary thyroid microcarcinoma. Endocrinol Metab Clinics (2019) 48(1):199–213. doi: 10.1016/j.ecl.2018.10.006
23. Rafferty MA, Goldstein DP, Rotstein L, Asa SL, Panzarella T, Gullane P, et al. Completion thyroidectomy versus total thyroidectomy: is there a difference in complication rates? an analysis of 350 patients. J Am Coll Surgeons (2007) 205(4):602–7. doi: 10.1016/j.jamcollsurg.2007.05.030
24. Linos D, Economopoulos KP, Kiriakopoulos A, Linos E, Petralias A. Scar perceptions after thyroid and parathyroid surgery: comparison of minimal and conventional approaches. Surgery (2013) 153(3):400–7. doi: 10.1016/j.surg.2012.08.008
25. Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Problems Diagn Radiol (2009) 38(3):135–43. doi: 10.1067/j.cpradiol.2007.10.001
26. Xu D, Ge M, Yang A, Cheng R, Sun H, Wang H, et al. Expert consensus workshop report: guidelines for thermal ablation of thyroid tumors (2019 edition). J Cancer Res Ther (2020) 16(5):960. doi: 10.4103/jcrt.JCRT_558_19
27. Hayward NJ, Grodski S, Yeung M, Johnson WR, Serpell J. Recurrent laryngeal nerve injury in thyroid surgery: a review. ANZ J Surg (2013) 83(1-2):15–21. doi: 10.1111/j.1445-2197.2012.06247.x
28. Simó R, Nixon IJ, Rovira A, Vander Poorten V, Sanabria A, Zafereo M, et al. Immediate intraoperative repair of the recurrent laryngeal nerve in thyroid surgery. Laryngoscope (2021) 131(6):1429–35. doi: 10.1002/lary.29204
29. de Jong M, Nounou H, García VR, Christakis I, Brain C, Abdel-Aziz TE, et al. Children are at a high risk of hypocalcaemia and hypoparathyroidism after total thyroidectomy. J Pediatr Surg (2020) 55(7):1260–4. doi: 10.1016/j.jpedsurg.2019.06.027
30. Van Slycke S, Van Den Heede K, Brusselaers N, Vermeersch H. Feasibility of autofluorescence for parathyroid glands during thyroid surgery and the risk of hypocalcemia: first results in Belgium and review of the literature. Surg Innovation (2021) 28(4):409–18. doi: 10.1177/1553350620980263
31. Zhou W, Ni X, Xu S, Zhang L, Chen Y, Zhan W. Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int J Hyperthermia (2019) 36(1):896–903. doi: 10.1080/02656736.2019.1649475
32. Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated?: an analysis of 18,445 cases. Ann Surg (2011) 254(4):653–60. doi: 10.1097/SLA.0b013e318230036d
Keywords: microwave ablation, papillary, thyroid, microcarcinoma, PTC
Citation: Feng J, Jiang Y and Feng Y (2023) Latest evidence of microwave ablation for papillary thyroid microcarcinoma compared with surgery: A systematic review and meta-analysis. Front. Oncol. 13:1088265. doi: 10.3389/fonc.2023.1088265
Received: 03 November 2022; Accepted: 17 January 2023;
Published: 07 February 2023.
Edited by:
Xiang Xue, University of New Mexico, United StatesReviewed by:
Xicheng Song, Qingdao University, ChinaCopyright © 2023 Feng, Jiang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Feng, ZmVuZ2ppZTIxMzIxM0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.