
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 26 January 2023
Sec. Cancer Epidemiology and Prevention
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1087700
This article is part of the Research TopicMolecular biomarkers in hepatobiliary and pancreatic cancers: Implications of non-coding RNAs and its therapeutic opportunitiesView all 6 articles
Objective: Cancer of the pancreas is a life-threatening condition and has a high distant metastasis (DM) rate of over 50% at diagnosis. Therefore, this study aimed to determine whether patterns of distant metastases correlated with prognosis in pancreatic ductal adenocarcinoma (PDAC) with metastatic spread, and build a novel nomogram capable of predicting the 6, 12, 18-month survival rate with high accuracy.
Methods: We analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database for cases of PDAC with DM. Kaplan-Meier analysis, log-rank tests and Cox-regression proportional hazards model were used to assess the impact of site and number of DM on the cancer-specific survival (CSS) and over survival (OS). A total of 2709 patients with DM were randomly assigned to the training group and validation group in a 7:3 ratio. A nomogram was constructed by the dependent risk factors which were determined by multivariate Cox-regression analysis. An assessment of the discrimination and ability of the prediction model was made by measuring AUC, C-index, calibration curve and decision curve analysis (DCA). In addition, we collected 98 patients with distant metastases at the time of initial diagnosis from Ningbo University Affiliated LiHuili Hospital to verify the efficacy of the prediction model.
Results: There was a highest incidence of liver metastases from pancreatic cancer (2387,74.36%), followed by lung (625,19.47%), bone (190,5.92%), and brain (8,0.25%). The prognosis of liver metastases differed from that of lung metastases, and the presence of multiple organ metastases was associated with poorer prognosis. According to univariate and multivariate Cox-regression analyses, seven factors (i.e., diagnosis age, tumor location, grade of tumor differentiation, T-stage, receipt of surgery, receipt of chemotherapy status, presence of multiple organ metastases) were included in our nomogram model. In internal and external validation, the ROC curves, C-index, calibration curves and DCA were calculated, which confirmed that this nomogram can precisely predict prognosis of PDAC with DM.
Conclusion: Metastatic PDAC patients with liver metastases tended to have a worse prognosis than those with lung metastases. The number of DM had significant effect on the overall survival rate of metastatic PDAC. This study had a high prediction accuracy, which was helpful clinicians to analyze the prognosis of PDAC with DM and implement individualized diagnosis and treatment.
The mortality rate of all cancer has decreased significantly with progress in medicine and health care, save for one. As per the latest report of American Cancer Statistics in 2022 published by the ACS (American Cancer Society) (1), pancreatic cancer remains elusive to treatment and is emerging as the third leading cause in terms of mortality for men and women combined, and it will become the second leading cause of cancer death, according to some estimates (2, 3). Due to the rich supply of blood and lymph vessels in the pancreas, the cells of pancreatic cancer can easily invade large blood vessels and nerves surrounding the organ (4). This is why the vast majority of patients (over 50%) diagnosed with pancreatic cancer have distant metastasis even when the tumor is small (5, 6). Based on 2008-2014 reports, the stage distribution for selected cancers reported being confined to the metastasis (47%), followed by regional stage (29%), primary site (13%) and unstaged (11%) (1). And the percentage of five years survival rate is lowest in people with cancer in the metastasis (3%), followed by regional (33%), and localized (64%), based on patients selected from 2011 to 2017 (1). Metastatic pancreatic cancer has the worst prognoses and only limited progress has been made in revealing the relationship between pancreatic cancer metastasis and prognosis.
Based on previous studies, the liver is the organ most commonly affected by metastatic disease of pancreas, followed by the lungs, abdominal lymph nodes, peritoneum/omentum, bone and adrenal glands (7). In 90% of patients with pancreatic cancer, distant metastases are discovered at autopsy (8). Thus, it shouldn’t come as any surprise that nearly all patients with pancreatic cancer die from metastatic cancer (9). In our knowledge, however, there are few studies providing reliable insights into the relationship between metastatic pattern and pancreatic cancer prognosis, and neither a predictive model nor a prognostic model was derived for pancreatic cancer with DM. Pancreatic ductal adenocarcinoma (PDAC) is the most common pathological type of pancreatic tumor, therefore, evaluating the prognosis of PDAC with DM and constructing accurate models to predict risks are urgent and imperative.
The nomogram, a prediction model that can intuitively quantify the likelihood of specific events of interest, which transforms traditional statistical predictive models into visualized probability estimates tailored to each patient, is currently widely used in the prediction of clinical efficacy of various diseases (10–12). Our study goal is to investigate the effect of different site of the metastasis, number of sites and other relevant factors on patient prognosis, identifying a representative cohort from the Surveillance Epidemiology, and End Results (SEER) database. Finally, a nomogram was established to facilitate potential clinical applications for PDAC patients with DM.
The data of metastatic PDAC patients currently studied were extracted from the SEER database between January 1, 2010, and December 31, 2015. SEER is supported by the Surveillance Research Program (SRP) in NCI’s Division of Cancer Control and Population Sciences (DCCPS), providing information on cancer statistics in an effort to reduce the cancer burden in the entire human population. Institutional Review Board approval was not required for any of the data because they were publicly available. An enormous, population-based cancer registry like SEER is able to extrapolate results to a broader population than single-center studies because more generalizable data of patients are available.
In addition, a total of 98 patients with initial diagnosis of metastatic PDAC were collected from Ningbo University Affiliated LiHuili Hospital, China, from October 1, 2017 to October 31, 2019.
The following criteria were used to determine inclusion: (1) The primary cancer sites were classified according to the 3rd edition of the International Classification of Diseases in Oncology (i.e., ICD-O-3: C25.0-Head of pancreas, C25.1-Body of pancreas, C25.2-Tail of pancreas, C25.3-Pancreatic duct, C25.7-Other specified parts of pancreas, C25.8-Overlapping lesion of pancreas, C25.9-Pancreas). (2) Retrieval was restricted to patients with pathology of ICD-O-3 histology/behavior codes of 8140/3 (Adenocarcinoma), 8500/3 (Infiltrating duct carcinoma). (3) All the patients were diagnosed as stage IV by 7th edition of the American Joint Committee on Cancer (AJCC, 7th edition). These criteria were used for exclusion: (1) All individuals without microscopically confirmed pancreatic malignancy were excluded. (2) Those with a first malignancy other than pancreatic cancer were excluded. (3) Patients with unknown distant metastatic sites and whose tumor grade, race, or survival time were unavailable were also excluded. Furthermore, the dataset was omitted from patients with unknown surgery and adjuvant chemotherapy status. (4) Autopsies and death certificates were excluded from the study.
These criteria established our present study, 2709 patients were diagnosed as metastatic PDAC, including 2260 diagnosed with single organ DM. The 2260 patients were analyzed to determine if the site of DM was associated with the prognosis of patients, and all study subjects were randomly selected to participate in the training set (70%) and the validation set (30%) to explore the impact of the number of distant metastatic sites and other relevant factors on the survival time of patients with metastatic PDAC.
The following parameters were collected after identifying cases for inclusion in this study: age at diagnosis (<50/50~64/>65), sex (Male/Female), ethnicity (White/Black/Other), primary site of the tumor (Pancreatic Head/Body-Tail/Other), tumor differentiation grade ((I-II/III-IV), pathological primary tumor T-Stage according to AJCC 7th ed (T1/T2/T3/T4), receipt of surgery (Yes/No), receipt of chemotherapy status (Yes/No or Unknown), the sites of distant metastases (Liver/Lung/Bone or Brain), number of distant metastatic sites (1/>1), survival status (Alive/Dead), cause of death (Pancreatic/Other), and survival time in month (i.e., a period of time from the diagnosis of a disease to death).
The cancer-specific survival (CSS) rate is calculated after excluding those who have died from causes other than cancer. PCSS (pancreas cancer-specific survival) is defined as death due to pancreatic cancer. In the study cohort of 2260 patients with one site of DM, the hazard ratio (HR) and associated 95% confidence intervals (CIs) for PCSS and OS were examined using Cox-proportional hazards regression model by univariable analysis. The comparison of OS and CSS among groups with different metastasis sites was conducted using Kaplan-Meier method with log-rank test. The 2709 PDAC patients were randomly divided into training and validation sets in a ratio of 7:3 by R software. All hypothesis tests were 2-sided with a significance level of 0.05.
In the entire study cohort, a multivariate analysis of the variables with p <0.05 in the univariate analysis was carried out (i.e., Cox-proportional hazards regression model). We also performed subgroup analysis and used forest plots to show HR values for DM patterns in specific subgroups. The comparison of OS and CSS between groups with or without multiple sites of DM was also conducted using Kaplan-Meier method with log-rank test. Besides, nomogram was created by running the R-package rms in R software by integrating variables with p <0.05 in multivariate analysis to form a personalized prediction model. The model was retested for internal validation using bootstrap, with 1000 bootstrap replicates, and the established nomogram was then calibrated using calibration curves and assessed by concordance indexes (C-index), area under receiver operating characteristic curves (AUC) and decision curve analysis (DCA). Observed results were compared to predicted probabilities using calibration curves. Generally, models with C-indices closer to 1 have improved predictive power. Similarly, nomograms with a calibration curve that is more closely aligned with the 45° diagonal have better predictive ability. Additionally, we applied 98 Chinese patients meeting the above criteria as external cohort to validate the effectiveness of this prediction model.
Between 2010 and 2015, this study included 2709 patients with metastatic PDAC based on the criteria described above. Most patients (2260,83.43%) had single-organ DM and were listed separately as a study cohort. In addition, all patients were divided into a training set and a validation set, regardless of whether they had single-organ metastases or multi-organ metastases. As shown in Table 1, the demographic and clinicopathological characteristics of study patients are presented. Training and validation sets were mostly composed of older people >65 years of age (59.0% in the training set and 59.5% in the validation set), primary pancreatic tumors occur most often in the head of pancreatic tissue (49.5% in the training set and 44.9% in the validation set), followed by pancreatic body and tail (38.0% in the training set and 41.2% in the validation set). As for tumor grading, grading of tumor differentiation was most common in grades III-IV (55.5% in the training set and 54.9% in the validation set). As for the treatment strategies for PDAC with DM at initial diagnosis, 90 patients (3.32%) were treated with the aggressive surgical treatment alone, 160 (5.90%) with surgery combined with adjuvant chemotherapy, 1460 (53.89%) with adjuvant chemotherapy alone. Approximately, 10.40% of patients with single metastatic site were treated surgically, significantly higher than those with multiple metastases (15,3.34%). The Chi-square test showed that the deviations were purely random. In addition, Table 2 demonstrates the demographic and clinicopathological characteristics of 98 Chinese patients.
The SEER database records 3210 distant metastasis sites in the 2709 patients. In consistent with previous reports, in most cases, PDAC metastasizes to the liver (2387,74.36%), followed by lung (625,19.47%), bone (190,5.92%), brain (8,0.25%). Most patients in this study were diagnosed with PDAC combined with single-organ metastases (2260,83.43%), followed by two sites (398,14.69%), and the proportion of three and four sites was 1.85% and 0.04%, respectively, as shown in Table 3. Liver metastases combined with lung metastases were the most common type of metastatic disease in patients with two distant sites (306,11.30%). Table 3 shows the distribution of distant metastatic sites in greater detail.
To determine if the metastatic site was a robust prognostic factor, we analyzed 2260 eligible patients with single-site DM. A total of 2040 patients died of PDAC, and the causes of death other than PDAC were competitive risk events. The univariate Cox regression analysis indicated that, using liver metastases as the reference, there was a longer survival rate among patients with lung metastases (for CSS: HR, 0.79; 95% CI, 0.69-0.90; p =0.0006)(for OS: HR, 0.81; 95% CI, 0.71-0.93; p =0.002). (Table 4). Meanwhile, Kaplan-Meier survival analyses were carried to compare CSS and OS among patients with different metastatic sites (Figures 1A–H). For CSS, the median survival time was 4, 6, and 3.5 months for patients with liver, lung, bone/brain metastases, respectively (liver vs lung metastases: p <0.001; liver vs bone/brain metastases: p =0.92; lung vs bone/brain metastases: p =0.14). For OS, the median survival time was 4, 6, and 5 months for patients with liver, lung, bone/brain metastases, respectively (liver vs lung metastases: p =0.002; liver vs bone/brain metastases: p =0.21; lung vs bone/brain metastases: p =0.91). The effect of the sites of DM on overall survival by subgroup is detailed in Figure 2A.
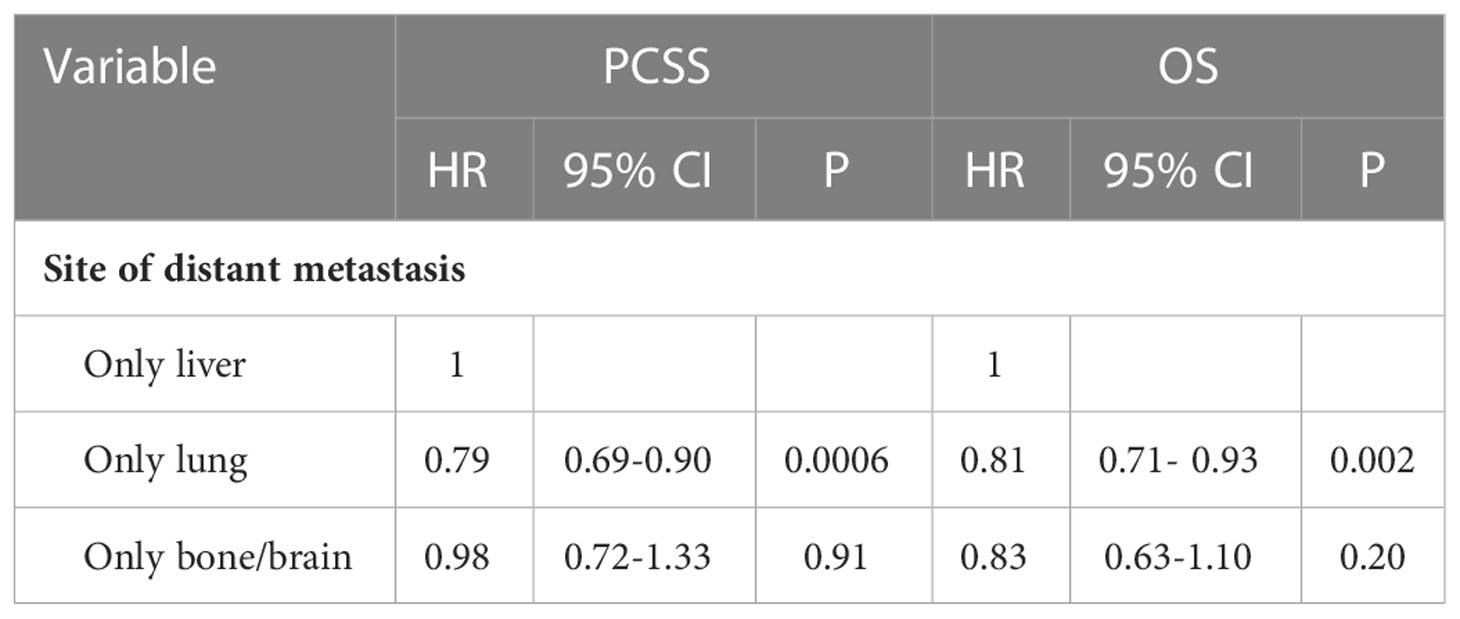
Table 4 Univariate Cox-regression analysis of the site of DM for cancer-specific survival and overall survival.
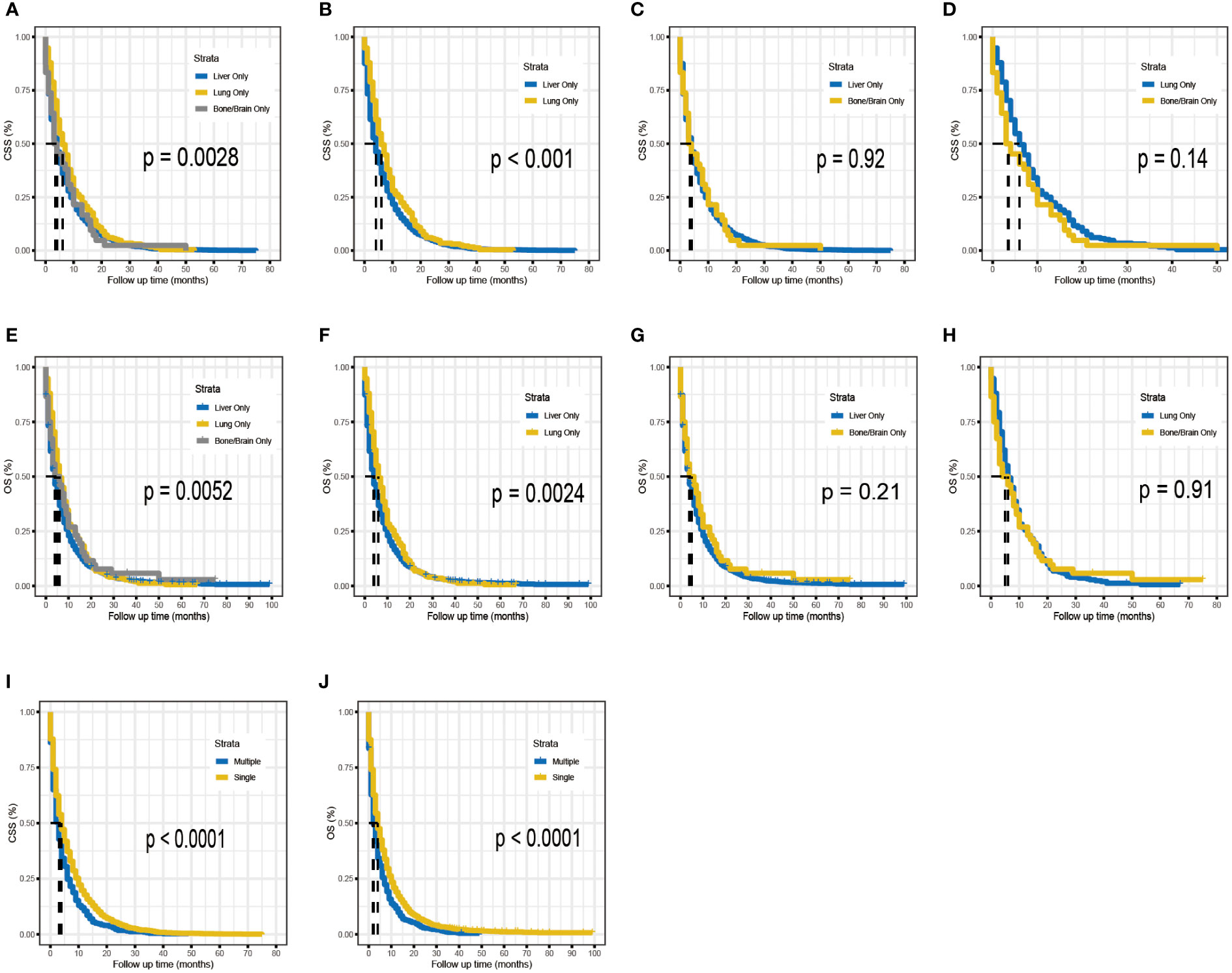
Figure 1 Kaplan–Meier analysis for CSS (A–D) and OS (E–H) of metastatic PDAC according to the metastatic site. Kaplan–Meier analysis for CSS (I) and OS (J) of metastatic PDAC according to the number of distant metastases.
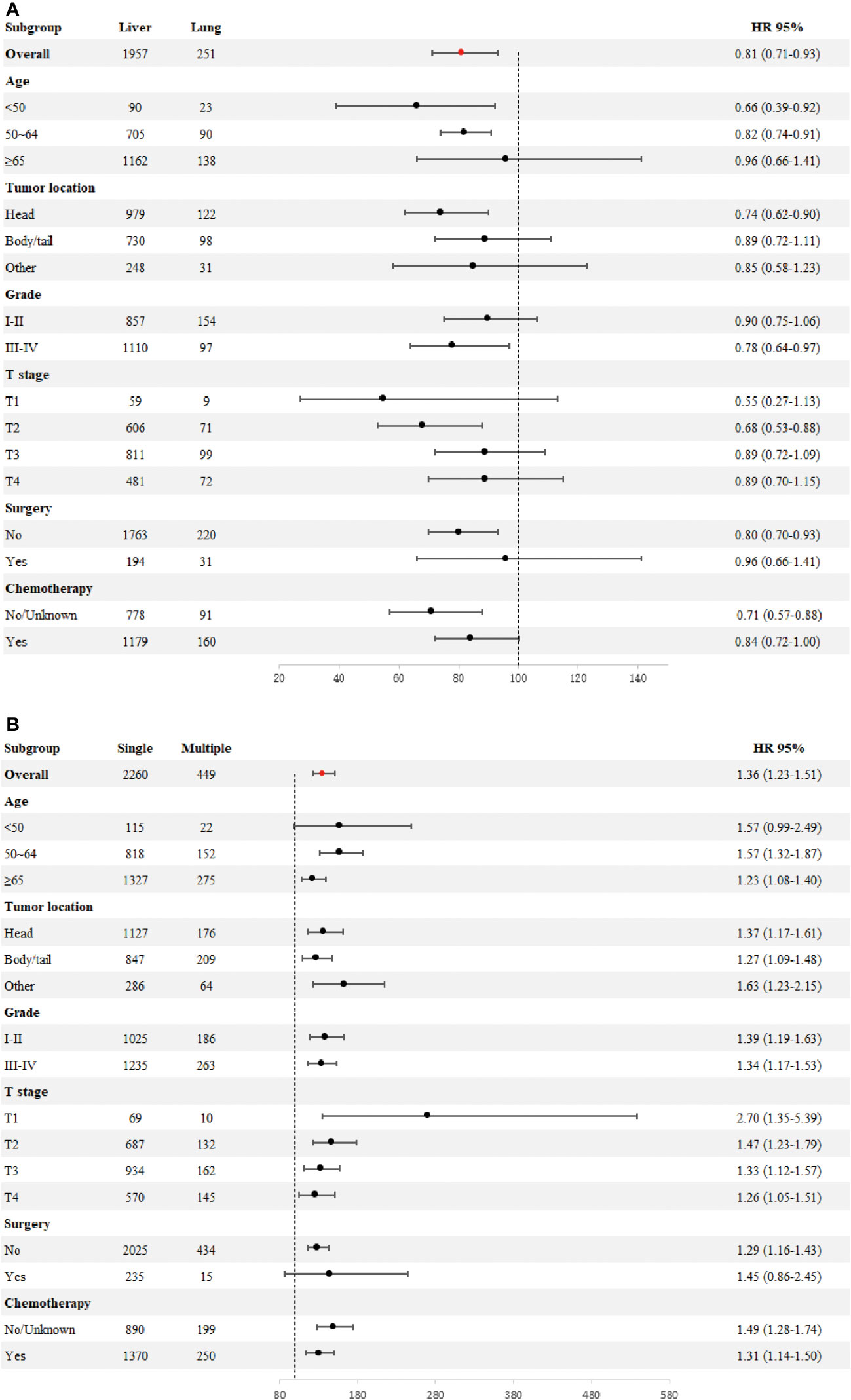
Figure 2 Forest plot for subgroup analysis of the sites of distant metastases (A). Forest plot for subgroup analysis of the number of distant metastases (B).
Across the entire cohort, single-site and multiple-site DM patients had a median survival time of 4 (95% CI, 4-4) and 3 (95% CI, 2-3) months, respectively (p <0.0001; Figures 1I, J). Besides, data regarding age at diagnosis, sex, ethnicity, primary site of tumor, tumor differentiation grade, size of tumor, receipt of surgery, receipt of chemotherapy, and number of sites of metastases were included in the multivariate Cox-regression analysis, which revealed that higher age (p <0.001), black race (p =0.049), tumors located in the body/tail (p =0.016), higher tumor grade and size (p <0.001), absence of surgery (p <0.001), absence of chemotherapy (p <0.001) and multiple sites of DM were significant prognostic factors for metastatic PDAC. Using one site of DM as the reference, there was a shorter overall survival rate among patients with multiple sites of DM (HR, 1.36; 95% CI, 1.23-1.51; p <0.001). Considering the limitations of univariate analysis, multivariable Cox-analyses were carried out to identify independent prognostic factors. Our findings showed longer survival benefit after aggressive surgical treatment and adjuvant chemotherapy. However, race did not appear to be an independent prognostic factor in multivariate Cox-regression, contrary to the result of univariate Cox-regression (Table 5). Subgroup comparisons for overall survival of age, tumor location, tumor grade, T-Stage, surgery and chemotherapy are shown in Figure 2B. Poorer prognosis was found in the multiple sites of DM group, in every subgroup analysis of overall survival, compared with the one site of DM group. Multiple metastases did not have a statistically significant prognosis for patients with PDAC in the <50 years age group (115 patients in the one site of DM group, median overall survival 8 months, 95% CI 7–10; 22 patients in the multiple sites of DM group, median overall survival 3.5 months, 95%CI 2–11; HR, 1.57; 95% CI, 0.99–2.49), Multiple metastases had a greater prognostic impact on PDAC patients in the 50~64 years age group (818 patients in the one site of DM group, median overall survival 5 months, 95% CI 4–6; 152 patients in the multiple sites of DM group, median overall survival 3 months, 95%CI 2–4; HR,1.57; 95% CI, 1.32-1.87) than in the ≥ 65 years age group (1327 patients in the one site of DM group, median overall survival 3 months, 95% CI 3-4; 275 patients in the multiple sites of DM group, median overall survival 2 months, 95%CI 2–3; HR, 1.23; 95% CI, 1.08-1.40). The effect of the number of DM on overall survival by subgroup is detailed in Figure 2B.

Table 5 Univariate and multivariate Cox-analyses of prognostic factors for overall survival in metastatic PDAC patients.
Based on seven independent predictors, which are statistically significant in the multivariate Cox-regression analysis, a novel nomogram was developed for predicting OS in metastatic PDAC (Figure 3). In the next step, we established ROC curves for both training set and validation set, and the AUCs of the nomogram in the training set for the 6, 12 and 18 months reached 0.771, 0.743 and 0.741 (Figure 4A), and 0.808, 0.794 and 0.816, respectively, in the validation set (Figure 4B), indicating a high degree of prediction accuracy. Meanwhile, we calculated the total score of each patient according to nomogram and got the median number of 128.1 in the training set. Then we divided them into high-risk (total score > 128.1) and low-risk (total score ≤ 128.1) groups, and explored the survival difference between them by Kaplan Meier survival analyses (Figures 4C, D). More importantly, the C-indices for both training and validation sets were 0.713 and 0.735 respectively and survival predictions and actual observations were well matched by the calibration curves of this predictive model (Figures 5A–C, 6A–C). Furthermore, DCA curves confirmed the clinical value of nomograms (Figures 5D, 6D).
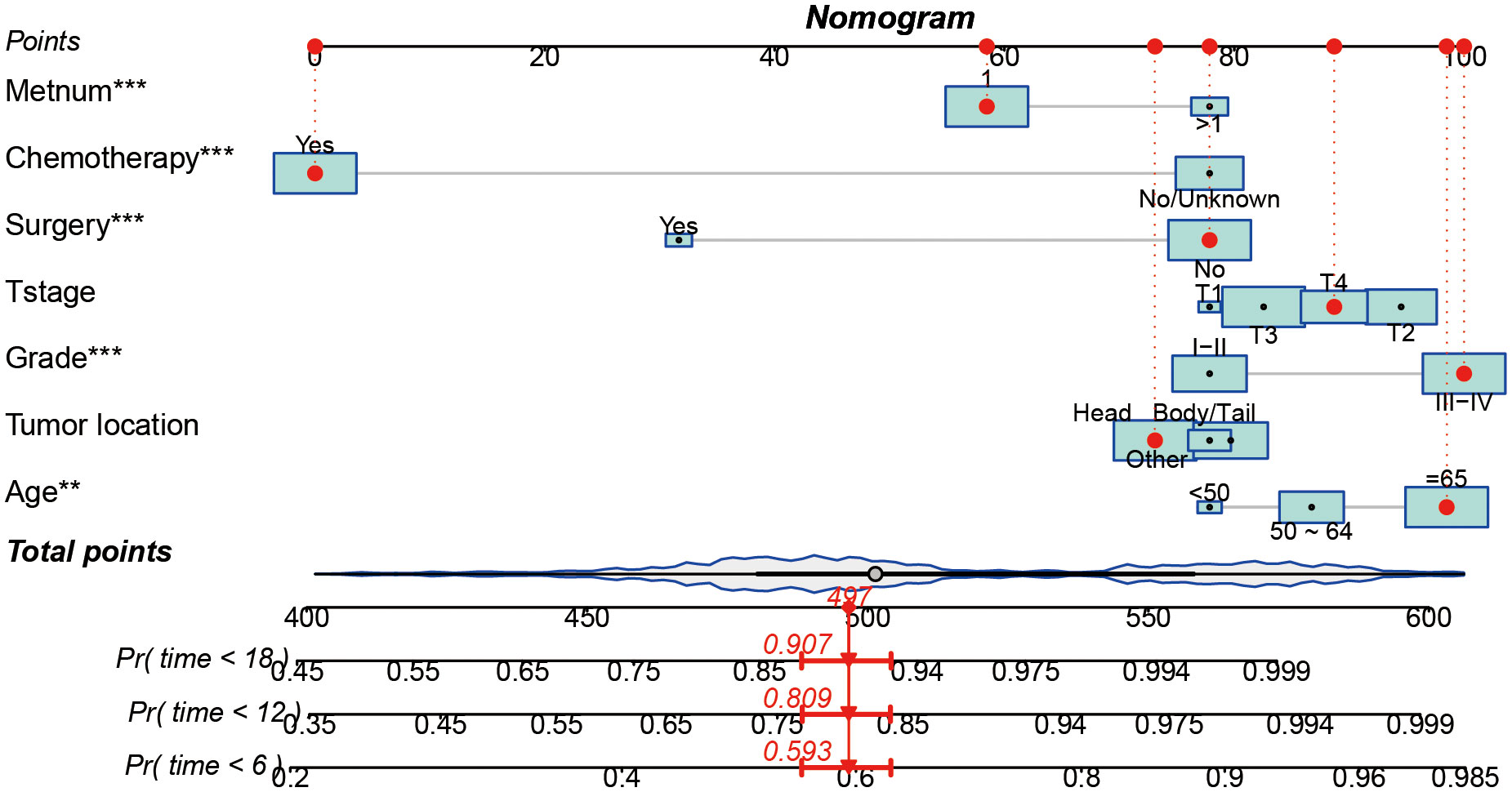
Figure 3 A prognostic nomogram for predicting the OS of metastatic PDAC patients for the 6, 12, and 18 months.
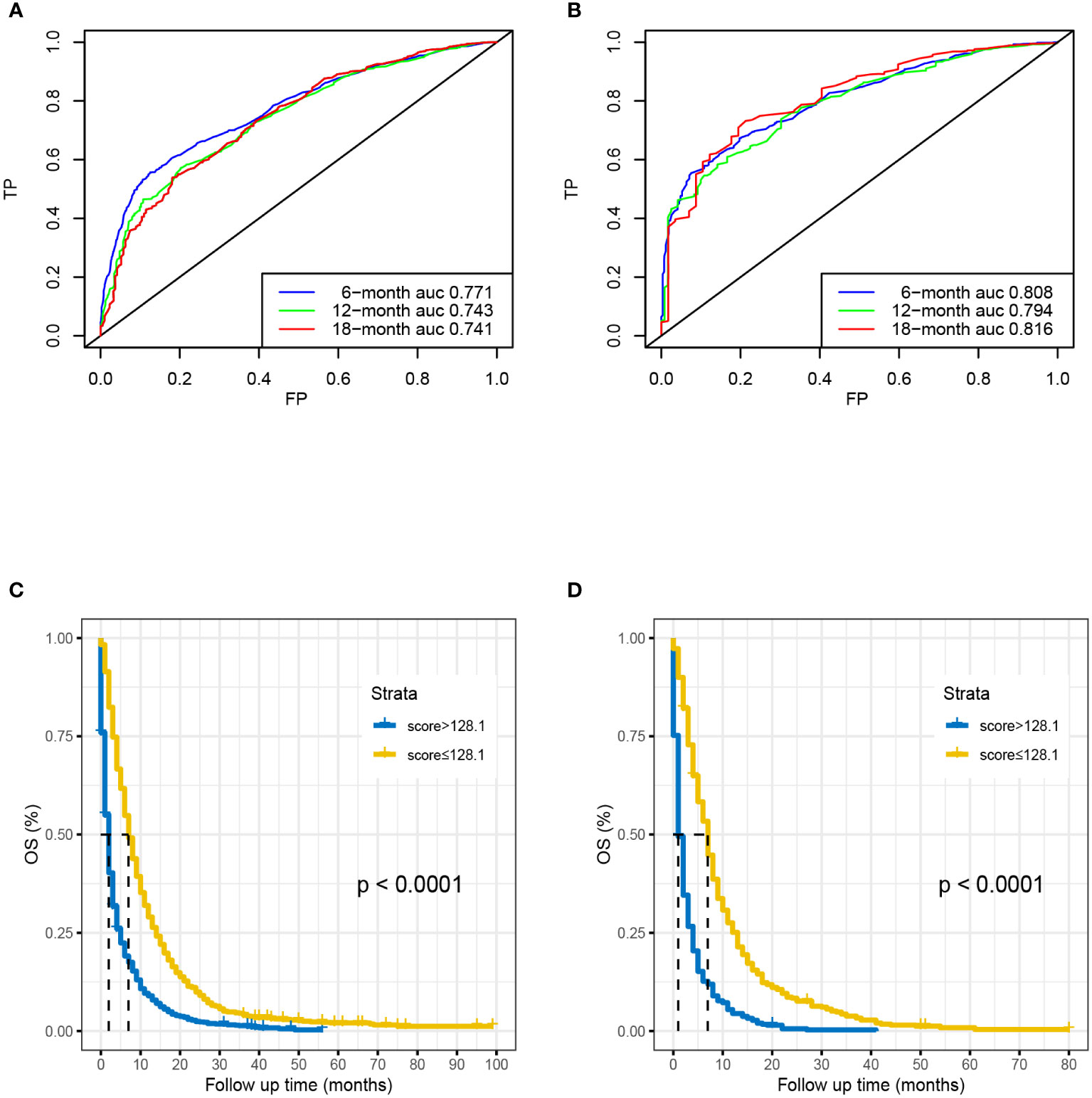
Figure 4 Time-dependent ROC curve analysis of the nomogram for the 6, 12, and 18 months in the training set (A) and the validation set (B). The Kaplan–Meier survival curves of high-risk group and low-risk group in the training set (C) and in the validation set (D).
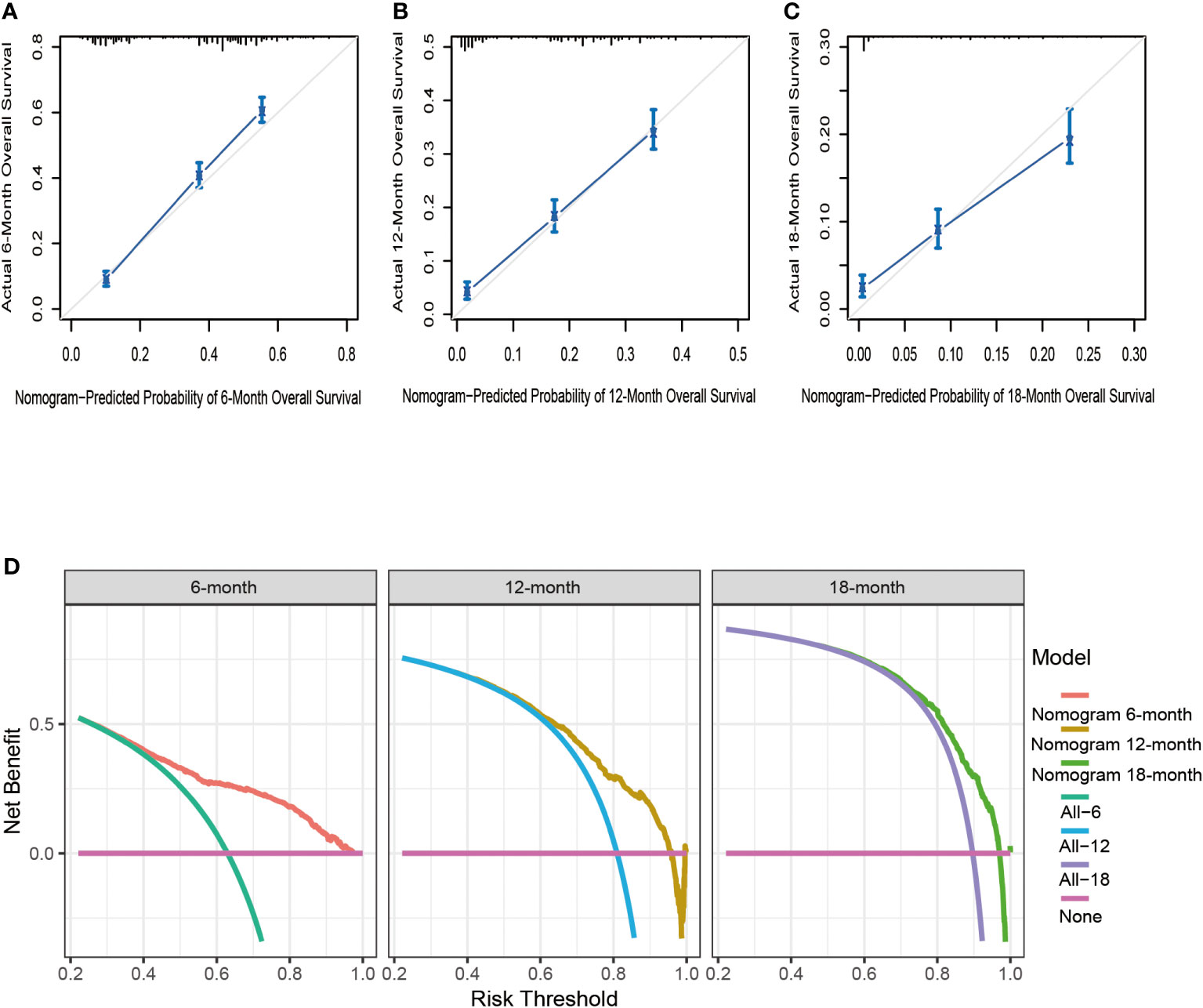
Figure 5 The calibration curves of the nomogram for the 6 (A), 12 (B), and 18 months (C) in the training set. The decision curve analysis of the nomogram for the 6, 12, and 18 months in the training set (D).
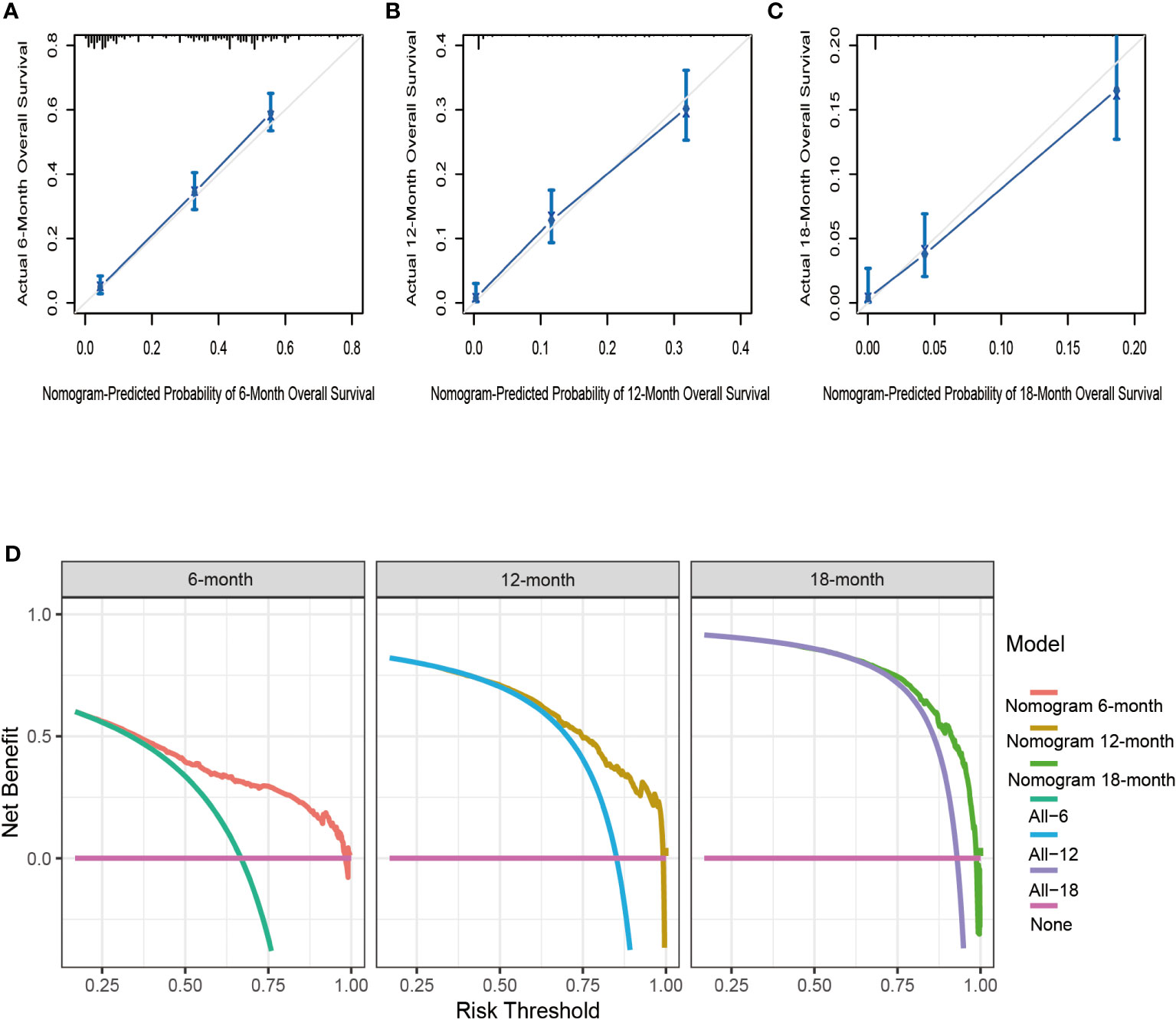
Figure 6 The calibration curves of the nomogram for the 6 (A), 12 (B), and 18 months (C) in the validation set. The decision curve analysis of the nomogram for the 6, 12, and 18 months in the validation set (D).
Kaplan-Meier survival analyses were carried to compare OS among 98 Chinese patients with different metastatic sites. The median survival time was 4.5, 8.5, and 10 months for patients with liver, peritoneum/omentum, other (i.e., lung, bone, spleen) metastases, respectively (p =0.80; Figure 7A). Single-site and multiple-site DM patients had a median survival time of 5 (95% CI, 4-10) and 1 (95% CI, 1-4) months, respectively (p <0.001; Figure 7B). Due to the limitation of sample size, we only plotted the ROC curve, calibration curve and DCA for 6 months and 12 months (Figures 7C–F), and the C-index of external cohort was 0.752, indicating its high clinical predictive accuracy and clinical applicability. To further determine whether the nomogram is generalizable and reliable, we also divided this cohort into high-risk (total score > 128.1) and low-risk (total score ≤ 128.1) groups and explored the survival difference between them by Kaplan Meier survival analyses (Figure 7G). The results showed that high-risk (n = 38) and low-risk (n = 60) patients had a median survival time of 1 (95% CI, 1-2) and 6 (95% CI, 5-11) months, respectively (p <0.05). It follows that the established nomogram can be widely suggested.
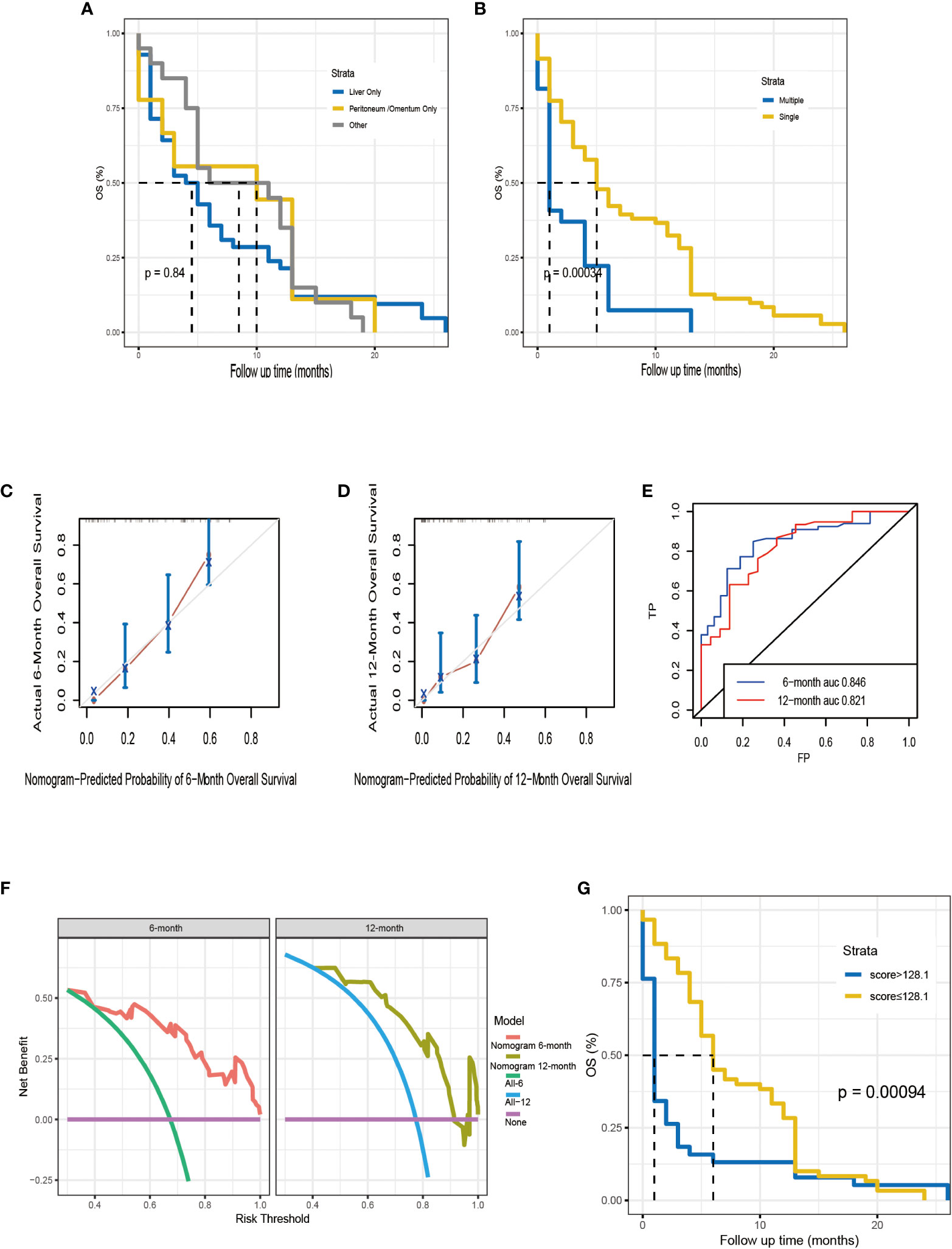
Figure 7 Kaplan–Meier analysis for OS of Chinese patients according to the metastatic site (A). Kaplan–Meier analysis for OS of Chinese patients according to the number of distant metastases (B). The calibration curves of the nomogram for the 6, 12 months in the external validation set (C, D). Time-dependent ROC curve analysis of the nomogram for the 6, 12 months in the external validation set (E). The DCA of the nomogram for the 6, 12 months in the external validation set (F). The Kaplan–Meier survival curves of high-risk group and low-risk group in the Chinese cohort (G).
Invasion and growth of tumors are key characteristics of pancreatic cancer, and more than 80% of patients with pancreatic cancer have already missed the chance to undergo surgical resection at the time of diagnosis (13, 14). The survival rate of patients sharply declines once the disease has metastasized (1). Research has increasingly focused on prognostic factors to predict pancreatic cancer survival and prognosis in recent years. However, their cohort studies were limited to individuals with early-stage or resectable carcinoma (15–19). Their summarized findings indicate that clinicopathologic factors such as resection margins status, perineural and blood vessel invasion, positive lymph nodes and perioperative blood transfusions are statistically significant prognostic variables. Nevertheless, above variables are unlikely to be available in patients with advanced pancreatic cancer who have lost access to surgery. Moreover, numerous studies focused on the molecular level rather than clinicopathologic features. H Friess et al. determined that Bax, a promoter of apoptosis, prolonged survival times in patients with pancreatic cancer by involving in the regulation of apoptosis (20). S. Dhara et al. conclude that PDAC could be predicted by ATAC-array (Assay for Transposase-Accessible Chromatin) (21). Jun Yu et al. found that high expression of miR-200c and E-cadherin was associated with a better prognosis of patients with pancreatic cancer (22). Fangfang Jin et al. also found that tRNA-derived small RNAs (tsRNAs) may serve as valuable biomarkers for predicting survival time of patients after surgery (23). However, it should be noted that these studies had low statistical power due to the sample size and study designs were single-center that should be used for further verification. More importantly, prognostic indicators at the molecular level are less convenient and practical than the easily available clinicopathological indicators. Thus, our data highlights the inclusion of clinicopathological factors in the prognostic assessment and our established prognostic model has more advantages in applying to clinical practice.
A MPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial) study revealed that the presence or absence of liver metastases were independent predictors of survival (24), and Josep Tabernero et al. reached the same conclusion. Subsequently, Renata D’Alpino Peixoto et al. found that in comparison to only local progression, development of peritoneal or distant metastases was associated with shorter OS (25). However, few studies have analyzed the connection between the patterns of DM and prognosis of metastatic PDAC. 2260 patients diagnosed with single DM were finally included for analysis the effect of different metastatic sites on the prognosis of metastatic PDAC in the present study. It has been demonstrated that the site of metastasis is a strong factor in the prognosis of certain metastatic diseases, such as ovarian cancer, prostate cancer, lung cancer, and testicular cancer (26–30). Our study suggested that metastatic PDAC with lung metastases had better survival outcomes (both CSS and OS) compared to metastatic PDAC with liver metastases. In addition, the number of DM sites also affected the survival outcome (both CSS and OS) of metastatic PDAC. The lung, as an organ with a rich blood supply, may be able to prolong the survival time of metastatic PDAC patients by buffering the invasion of metastatic cancer, even though patients with significant metastatic disease have a higher mortality rate due to organ failure or cachexia (31). It has been shown that metastatic PDAC patients with liver metastases have high levels of expression of epidermal growth factor receptor, E-cadherin and laminin, resulting in a tendency for multiple metastases and a poorer prognosis, which may be one of the reasons for the poorer prognosis of liver metastases compared with lung metastases (32).
Previous studies have shown that prognosis of pancreatic cancer may vary depending on several factors, including age, occupation, history of disease, location of the tumor, surgery method, perioperative complications, and TNM stage (33). In present study, we found that patients with the most advanced disease characteristic generally had the poorer prognosis (i.e., those diagnosed at a higher age, tumors located in the body/tail, higher tumor differentiation grade, larger tumor volume, presence of multiple metastatic sites), which is consistent with previous researches (34, 35). In the 2019 WHO Classification of Tumors of the Digestive System, the incidence of PDAC is about 85%, pancreatic neuroendocrine carcinoma (pNEC) account for about 5%, and other types of tumors account for the remaining 10% (36). The 5-year survival rate for PDAC is less favorable, at only 8% (36). Although the biological behavior and treatment of pNEC are very different from PDAC and often associated with a better prognosis, it has a significant risk of distant metastasis, even higher than PDAC. It has been mentioned in the literature that pNEC is often accompanied by metastasis, presenting with synchronous metastases in up to 65% of patients (37), aggressive treatment (surgery, radio-frequency ablation, etc.) will prolong the survival of patients with pNEC even in the presence of liver metastases (38, 39). Regarding the impact of PDAC location on patient prognosis, a previous study has found that cancers of the body and tail of the pancreas have a poorer prognosis. Previous studies evaluating a large cohort showed that less than 10% of patients had successful removal of pancreatic cancer from the body or tai (40, 41). Takeo Fujita et al. pointed out that these tumors in the body or tail become symptomatic much later than those in other areas such as the head of the pancreas, so that the tumors often cannot be removed due to extra-pancreatic involvement or distant metastases (42). In contrast, tumors located in the head of the pancreas induce obstructive jaundice at an early stage, which usually leads to seeking medical attention earlier, making them more curable and thus leading to a more favorable prognosis (43). As with previous findings, our research showed that tumors located in the head of the pancreas tended to have a better prognosis than the body of the pancreas, even when patients with PDAC had DM at the time of initial diagnosis.
Surgical resection of metastatic liver cancer has proven to be a treatment method that is effective for colorectal cancer (44) and gastric cancer (45), but no clear consensus has been reached on the treatment strategy of pancreatic cancer with simultaneous liver metastasis (46–48). However, surgeons continue to strive for longer survival time for patients with DM through aggressive surgical resection. A study of 69 participants with concurrent liver metastases showed that participants who underwent simultaneous resection had a longer survival time significantly compared to those who did not undergo surgical resection (14 months vs 8 months, p < 0.01) (49). An analysis of the SEER database suggests that CDC (cancer–directed surgery) is capable of prolonging survival of oligometastatic PDAC patients (50). In addition, locally-ablative treatments like microwave ablation (MWA) and radiofrequency ablation (RFA) are considered complementary to radical surgery (51). As for pancreatic cancer with lung metastases, a retrospective analysis of patients with pulmonary metachronous metastasis after PDAC collected from a database of two high-volume pancreatic cancer centers demonstrates that patients might benefit from surgical approaches of metachronous pulmonary metastasis (52). On the basis of the present data, it is also warranted that aggressive surgical treatment is associated with a better prognosis, but predictions of surgical outcomes for advanced pancreatic cancer with DM remain a challenge and many patients do not benefit from surgery.
In this study, chemotherapy remained one of the primary treatments for most patients with advanced pancreatic cancer and resulted in longer overall survival significantly. A MPACT, reported by von Hoff et al. (24) in 2013, is a phase III clinical trial of combined gemcitabine plus nab-paclitaxel therapy versus gemcitabine alone for first-line treatment in pancreatic cancer patients with DM. The findings indicated that the overall survival of patients receiving the combined regimen was significantly prolonged. In recent years, FOLFIRINOX regimen has also been recommended for the treatment of advanced pancreatic cancer with metastases (53).
Patients and their families rely on clinicians to predict the clinical course of their illness, and accurate predictions can prevent barriers to patient-doctor communication. To the present author’s knowledge, few studies have selected metastatic PDAC patients who lack effective treatment as study subjects, and there is still no prognostic prediction model developed for metastatic PDAC. Our study successfully developed a novel prognostic nomogram to predict the prognosis of metastatic PDAC, and this model was internally and externally validated to have an extremely high AUC, which demonstrated that this predictive model may provide a new source of clinical decision-making and personalized assessment. It is worth noting that there appears to be no logical order to T stage. It is easy to explain this: T stage is not a continuous variable and the recorded T stage was inaccurate because it is difficult to determine size measurements, and the distinction between T3 and T4 is now (AJCC, 7th edition) resectability, not size or invasion per se. Furthermore, note that we have not considered N stage for inclusion in our analysis. Because there are many factors affecting N stage, and the number of lymph nodes dissected is one of the main factors. The result of Valsangkar et al. (54) showed that predicting prognosis with N stage was ineffective when the number of lymph nodes dissected was less than 5 or 9. Strobel et al. (55) also found that the detection of more lymph nodes was more helpful to determine the N stage and predict the prognosis more accurately. Obviously, N stage was not applicable to the majority of patients, who missed the opportunity for radical surgical resection at the time of diagnosis.
We acknowledge that limitations exist with this study. First of all, the retrospective design of this study led many confounding variables and may render bias inevitable. Second, the limited number of pancreatic cancer patients with single brain/bone metastasis (N=52) may contributed to possible errors. Third, data from the SEER database do not provide information on disease progression because the information collected was about the disease at the time of initial diagnosis. Fourth, due to the limitations of the SEER database, only four site-specific distant metastases were included in our study. However, in addition to these sites, distant lymph nodes and peritoneum/omentum can also be metastasized in patients with metastatic pancreatic cancer (7), but the database we searched did not contain any detailed information about these distant metastatic sites. Although we further analyzed the prognostic differences between metastatic PDAC with hepatic and abdominal metastases, this study is a single-center retrospective study with its own limitations, and the relevant results still need to be further confirmed in a multicenter, large-sample randomized controlled trial. Additionally, detailed systemic treatment information, such as surgical modalities, palliative care, treatment regimens for adjuvant chemotherapy and their types, is not available.
In summary, our study demonstrated that metastatic PDAC with lung metastases had better survival outcomes (both CSS and OS) compared to metastatic PDAC with liver metastases, and muti-organ metastases significantly shortened patient survival time. Notably, based on multivariate Cox-regression analysis, we developed a new nomogram, which can be used as a visual graphical tool for metastatic PDAC to quantitatively assess the risk and prognosis of metastatic PDAC and to guide clinical decision-making.
This study analyzed publicly available datasets. This data can be found here: https://seer.cancer.gov/data/.
The studies involving human participants were reviewed and approved by The Affiliated Lihuili Hospital, Ningbo University, Ningbo, Zhejiang, China. The ethics committee waived the requirement of written informed consent for participation.
Study conception: LZ and DY, Data collection: LZ and RJ, Statistical analysis: LZ and XY, Article writing and revision: LZ and DY. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Ningbo Natural Science Foundation (2019A610208).
We are very grateful to the SEER program for approving the registration and the SEER database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1087700/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol (2018) 24(43):4846–61. doi: 10.3748/wjg.v24.i43.4846
3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res (2014) 74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155
4. Wang L, Xiong L, Wu Z, Miao X, Liu Z, Li D, et al. Expression of Ugp2 and Cfl1 expression levels in benign and malignant pancreatic lesions and their clinicopathological significance. World J Surg Oncol (2018) 16(1):11. doi: 10.1186/s12957-018-1316-7
5. Strobel O, Neoptolemos J, Jager D, Buchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol (2019) 16(1):11–26. doi: 10.1038/s41571-018-0112-1
6. Antoniou E, Margonis GA, Sasaki K, Andreatos N, Polychronidis G, Pawlik TM, et al. Is resection of pancreatic adenocarcinoma with synchronous hepatic metastasis justified? A review of current literature. ANZ J Surg (2016) 86(12):973–7. doi: 10.1111/ans.13738
7. Embuscado EE, Laheru D, Ricci F, Yun KJ, de Boom Witzel S, Seigel A, et al. Immortalizing the complexity of cancer metastasis: Genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther (2005) 4(5):548–54. doi: 10.4161/cbt.4.5.1663
8. Giovannetti E, van der Borden CL, Frampton AE, Ali A, Firuzi O, Peters GJ. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol (2017) 44:43–59. doi: 10.1016/j.semcancer.2017.04.006
9. Rocha Lima CM, Green MR, Rotche R, Miller WH Jr., Jeffrey GM, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol (2004) 22(18):3776–83. doi: 10.1200/JCO.2004.12.082
10. Yang XG, Feng JT, Wang F, He X, Zhang H, Yang L, et al. Development and validation of a prognostic nomogram for the overall survival of patients living with spinal metastases. J Neurooncol (2019) 145(1):167–76. doi: 10.1007/s11060-019-03284-y
11. Zhang HR, Wang F, Yang XG, Xu MY, Qiao RQ, Li JK, et al. Establishment and validation of a nomogram model for aseptic loosening after tumor prosthetic replacement around the knee: A retrospective analysis. J Orthop Surg Res (2019) 14(1):352. doi: 10.1186/s13018-019-1423-3
12. Zhang HR, Zhang JY, Yang XG, Qiao RQ, Li JK, Hu YC. Predictive value of the nomogram model in patients with megaprosthetic failure around the knee: A retrospective analysis. J Arthroplasty (2020) 35(10):2944–51. doi: 10.1016/j.arth.2020.05.016
13. Song Z, Feng C, Lu Y, Lin Y, Dong C. Phgdh is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer. Gene (2018) 642:43–50. doi: 10.1016/j.gene.2017.11.014
14. Song Z, Feng C, Lu Y, Lin Y, Dong C. Corrigendum to "Phgdh is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer" [Gene 642 (2018) 43-50]. Gene (2020) 735:144401. doi: 10.1016/j.gene.2020.144401
15. Andren-Sandberg A. Prognostic factors in pancreatic cancer. N Am J Med Sci (2012) 4(1):9–12. doi: 10.4103/1947-2714.92893
16. Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg (2004) 240(2):293–8. doi: 10.1097/01.sla.0000133125.85489.07
17. Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg (1993) 165(1):68–72. doi: 10.1016/s0002-9610(05)80406-4
18. Venkat R, Puhan MA, Schulick RD, Cameron JL, Eckhauser FE, Choti MA, et al. Predicting the risk of perioperative mortality in patients undergoing pancreaticoduodenectomy: A novel scoring system. Arch Surg (2011) 146(11):1277–84. doi: 10.1001/archsurg.2011.294
19. Vollmer CM Jr., Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, et al. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg (2012) 16(1):89–102. doi: 10.1007/s11605-011-1753-x
20. Friess H, Lu Z, Graber HU, Zimmermann A, Adler G, Korc M, et al. Bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut (1998) 43(3):414–21. doi: 10.1136/gut.43.3.414
21. Dhara S, Chhangawala S, Chintalapudi H, Askan G, Aveson V, Massa AL, et al. Pancreatic cancer prognosis is predicted by an atac-array technology for assessing chromatin accessibility. Nat Commun (2021) 12(1):3044. doi: 10.1038/s41467-021-23237-2
22. Yu J, Ohuchida K, Mizumoto K, Sato N, Kayashima T, Fujita H, et al. Microrna, hsa-Mir-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer (2010) 9:169. doi: 10.1186/1476-4598-9-169
23. Jin F, Yang L, Wang W, Yuan N, Zhan S, Yang P, et al. A novel class of tsrna signatures as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol Cancer (2021) 20(1):95. doi: 10.1186/s12943-021-01389-5
24. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med (2013) 369(18):1691–703. doi: 10.1056/NEJMoa1304369
25. Peixoto RD, Speers C, McGahan CE, Renouf DJ, Schaeffer DF, Kennecke HF. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med (2015) 4(8):1171–7. doi: 10.1002/cam4.459
26. Deng K, Yang C, Tan Q, Song W, Lu M, Zhao W, et al. Sites of distant metastases and overall survival in ovarian cancer: A study of 1481 patients. Gynecol Oncol (2018) 150(3):460–5. doi: 10.1016/j.ygyno.2018.06.022
27. Koo KC, Park SU, Kim KH, Rha KH, Hong SJ, Yang SC, et al. Prognostic impacts of metastatic site and pain on progression to castrate resistance and mortality in patients with metastatic prostate cancer. Yonsei Med J (2015) 56(5):1206–12. doi: 10.3349/ymj.2015.56.5.1206
28. Li J, Zhu H, Sun L, Xu W, Wang X. Prognostic value of site-specific metastases in lung cancer: A population based study. J Cancer (2019) 10(14):3079–86. doi: 10.7150/jca.30463
29. Xu P, Wang J, Abudurexiti M, Jin S, Wu J, Shen Y, et al. Prognosis of patients with testicular carcinoma is dependent on metastatic site. Front Oncol (2019) 9:1495. doi: 10.3389/fonc.2019.01495
30. Zhan H, Zhao X, Lu Z, Yao Y, Zhang X. Correlation and survival analysis of distant metastasis site and prognosis in patients with hepatocellular carcinoma. Front Oncol (2021) 11:652768. doi: 10.3389/fonc.2021.652768
31. Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. Dpc4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol (2009) 27(11):1806–13. doi: 10.1200/JCO.2008.17.7188
32. Shibata K, Matsumoto T, Yada K, Sasaki A, Ohta M, Kitano S. Factors predicting recurrence after resection of pancreatic ductal carcinoma. Pancreas (2005) 31(1):69–73. doi: 10.1097/01.mpa.0000166998.04266.88
33. Tabernero J, Chiorean EG, Infante JR, Hingorani SR, Ganju V, Weekes C, et al. Prognostic factors of survival in a randomized phase iii trial (Mpact) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist (2015) 20(2):143–50. doi: 10.1634/theoncologist.2014-0394
34. Botsis T, Anagnostou VK, Hartvigsen G, Hripcsak G, Weng C. Modeling prognostic factors in resectable pancreatic adenocarcinomas. Cancer Inform (2010) 7:281–91. doi: 10.4137/cin.s3835
35. Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, et al. Outcome of distal pancreatectomy for pancreatic adenocarcinoma. Dig Surg (2008) 25(1):32–8. doi: 10.1159/000117821
36. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 who classification of tumours of the digestive system. Histopathology (2020) 76(2):182–8. doi: 10.1111/his.13975
37. Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology (2008) 135(5):1469–92. doi: 10.1053/j.gastro.2008.05.047
38. Deutsch GB, Lee JH, Bilchik AJ. Long-term survival with long-acting somatostatin analogues plus aggressive cytoreductive surgery in patients with metastatic neuroendocrine carcinoma. J Am Coll Surg (2015) 221(1):26–36. doi: 10.1016/j.jamcollsurg.2015.03.055
39. Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, et al. Neuroendocrine hepatic metastases: Does aggressive management improve survival? Ann Surg (2005) 241(5):776–83. doi: 10.1097/01.sla.0000161981.58631.ab
40. Matsuno S, Sato T. Surgical treatment for carcinoma of the pancreas. Exp 272 Patients. Am J Surg (1986) 152(5):499–504. doi: 10.1016/0002-9610(86)90214-x
41. Nordback IH, Hruban RH, Boitnott JK, Pitt HA, Cameron JL. Carcinoma of the body and tail of the pancreas. Am J Surg (1992) 164(1):26–31. doi: 10.1016/s0002-9610(05)80641-5
42. Fujita T, Nakagohri T, Gotohda N, Takahashi S, Konishi M, Kojima M, et al. Evaluation of the prognostic factors and significance of lymph node status in invasive ductal carcinoma of the body or tail of the pancreas. Pancreas (2010) 39(1):e48–54. doi: 10.1097/MPA.0b013e3181bd5cfa
43. Watanabe I, Sasaki S, Konishi M, Nakagohri T, Inoue K, Oda T, et al. Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas (2004) 28(2):160–5. doi: 10.1097/00006676-200403000-00007
44. McNally SJ, Parks RW. Surgery for colorectal liver metastases. Dig Surg (2013) 30(4-6):337–47. doi: 10.1159/000351442
45. Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, et al. Long-term outcomes after surgical resection for gastric cancer liver metastasis: An analysis of 64 macroscopically complete resections. Langenbecks Arch Surg (2012) 397(6):951–7. doi: 10.1007/s00423-012-0959-z
46. Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Group EGW. Pancreatic adenocarcinoma: Esmo-esdo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2012) 23 Suppl 7:vii33–40. doi: 10.1093/annonc/mds224
47. Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, et al. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol (2016) 34(23):2784–96. doi: 10.1200/JCO.2016.67.1412
48. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(4):439–57. doi: 10.6004/jnccn.2021.0017
49. Tachezy M, Gebauer F, Janot M, Uhl W, Zerbi A, Montorsi M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery (2016) 160(1):136–44. doi: 10.1016/j.surg.2016.02.019
50. Pausch TM, Liu X, Cui J, Wei J, Miao Y, Heger U, et al. Survival benefit of resection surgery for pancreatic ductal adenocarcinoma with liver metastases: A propensity score-matched seer database analysis. Cancers (Basel) (2021) 14(1). doi: 10.3390/cancers14010057
51. Timmer FEF, Geboers B, Nieuwenhuizen S, Schouten EAC, Dijkstra M, de Vries JJJ, et al. Locoregional treatment of metastatic pancreatic cancer utilizing resection, ablation and embolization: A systematic review. Cancers (Basel) (2021) 13(7). doi: 10.3390/cancers13071608
52. Ilmer M, Schiergens TS, Renz BW, Schneider C, Sargut M, Waligora R, et al. Oligometastatic pulmonary metastasis in pancreatic cancer patients: Safety and outcome of resection. Surg Oncol (2019) 31:16–21. doi: 10.1016/j.suronc.2019.08.010
53. Vaccaro V, Sperduti I, Milella M. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med (2011) 365(8):768–9. doi: 10.1056/NEJMc1107627
54. Valsangkar NP, Bush DM, Michaelson JS, Ferrone CR, Wargo JA, Lillemoe KD, et al. N0/N1, pnl, or lnr? the effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointestinal Surg (2012) 17(2):257–66. doi: 10.1007/s11605-012-1974-7
Keywords: pancreatic cancer, distant metastasis, predictor, nomogram, prognosis factors
Citation: Zhang L, Jin R, Yang X and Ying D (2023) A population-based study of synchronous distant metastases and prognosis in patients with PDAC at initial diagnosis. Front. Oncol. 13:1087700. doi: 10.3389/fonc.2023.1087700
Received: 02 December 2022; Accepted: 11 January 2023;
Published: 26 January 2023.
Edited by:
Caiming Xu, Dalian Medical University, ChinaCopyright © 2023 Zhang, Jin, Yang and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongjian Ying, eWluZ2RvbmdqaWFuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.