
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 17 February 2023
Sec. Cancer Metabolism
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1086846
This article is part of the Research Topic Linking Cellular Metabolism to Hematological Malignancies, volume II View all 11 articles
Background: Fms-like tyrosine kinase 3 (FLT3) gene mutations occur in approximately 30% of all patients with acute myeloid leukemia (AML). Internal tandem duplication (ITD) in the juxtamembrane domain and point mutations within the tyrosine kinase domain (TKD) are two distinct types of FLT3 mutations. FLT3-ITD has been determined as an independent poor prognostic factor, but the prognostic impact of potentially metabolically related FLT3-TKD remains controversial. Hence, we performed a meta-analysis to investigate the prognostic significance of FLT3-TKD in patients with AML.
Methods: A systematic retrieval of studies on FLT3-TKD in patients with AML was performed in PubMed, Embase, and Chinese National Knowledge Infrastructure databases on 30 September 2020. Hazard ratio (HR) and its 95% confidence intervals (95% CIs) were used to determine the effect size. Meta-regression model and subgroup analysis were used for heterogeneity analysis. Begg’s and Egger’s tests were performed to detect potential publication bias. The sensitivity analysis was performed to evaluate the stability of findings in meta-analysis.
Results: Twenty prospective cohort studies (n = 10,970) on the prognostic effect of FLT3-TKD in AML were included: 9,744 subjects with FLT3-WT and 1,226 subjects with FLT3-TKD. We found that FLT3-TKD revealed no significant effect on disease-free survival (DFS) (HR = 1.12, 95% CI: 0.90–1.41) and overall survival (OS) (HR = 0.98, 95% CI: 0.76–1.27) in general. However, meta-regressions demonstrated that patient source contributed to the high heterogeneity observed in the prognosis of FLT3-TKD in AML. To be specific, FLT3-TKD represented a beneficial prognosis of DFS (HR = 0.56, 95% CI: 0.37–0.85) and OS (HR = 0.63, 95% CI: 0.42–0.95) for Asians, whereas it represented an adverse prognosis of DFS for Caucasians with AML (HR = 1.34, 95% CI: 1.07–1.67).
Conclusion: FLT3-TKD revealed no significant effects on DFS and OS of patients with AML, which is consistent with the controversial status nowadays. Patient source (Asians or Caucasians) can be partially explained the different effects of FLT3-TKD in the prognosis of patients with AML.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults with the features that poorly differentiated cells from hematopoietic system infiltrate in bone marrow, blood, and other tissues (1). Nowadays, it tends to be assumed that valuable and accurate prognostic assessments benefit patients with AML by providing optimized treatments for their survivals. Hence, more and more recurrent genetic mutations, such as FLT3-ITD, Nucleophosmin (NPM1), and CCAAT enhancer-binding protein alpha (CEBPA), have been used to guide disease management and refine individual prognosis.
Fms-like tyrosine kinase 3 (FLT3) is a potential prognostic genetic marker, which encodes a class 3 receptor tyrosine kinase to plays a crucial role in hematopoiesis. FLT3 gene mutations occur in approximately 30% of all patients with AML (2). There are two distinct forms of FLT3 mutations: internal tandem duplication (ITD) in the juxtamembrane domain and point mutations within the activation loop of the tyrosine kinase domain (TKD), affecting D835 in most cases (3). These gain-of-function mutations lead to ligand-independent activation of FLT3, which contributes to uncontrolled proliferation of AML blasts (2, 4, 5). Numerous studies have found that FLT3-ITD is an independent factor for adverse prognosis (6). However, the prognostic value of FLT3-TKD remains controversial due to the relatively low incidence and limitations of single center studies (2). The relationship between FLT3-TKD and cytoplasmic Src family tyrosine kinases has been confirmed recently (7) while we have known about the associations between Src family members and multiple nutrient metabolism, including glucose (8), lipid (9), and glutamine (10). From our perspectives, FLT3-TKD is regarded as a potentially metabolically related mutation in tumorigenesis and progression of AML. For this reason, we performed a meta-analysis within the published studies before 30 September 2020 to investigate the prognostic significance of FLT3-TKD in patients with AML.
Two independent investigators implemented a systematic search in PubMed, Embase, and Chinese National Knowledge Infrastructure (CNKI) databases systematically, with the last search updated on 30 September 2020. The following terms “(acute myeloid leukemia) or (acute myeloblastic leukemia) or (acute myelocytic leukemia) or (acute myelogenous leukemia) or (acute nonlymphoblastic leukemia) or AML”, “FLT3 or CD135 or (fms-like tyrosine kinase 3) or (fetal liver kinase-2) or (fetal liver kinase-3) or (human stem cell tyrosine kinase-1)”, “(tyrosine kinase domain mutation) or (TKD mutation) or D835 or I836”, and “prognosis or prognoses or (prognostic factors) or (prognostic implication) or (prognostic element)” were retrieved in PubMed entries in the National Institutes of Health and European Embase databases without any limitation applied. The key words “急性髓系白血病or急性髓性白血病or急性非淋巴细胞性白血病”, “FLT3”, and “TKD突变” were retrieved in CNKI. The reference lists in retrieved studies and relevant reviews were also manually searched for more eligible studies.
All literature studies involved in AML and FLT3-TKD were electronically retrieved for the next filter. Afterward, prospective cohort studies were identified according to their titles and abstracts. The full texts of the literature studies that fulfilled the inclusion criteria were perused to validate their eligibility. Inclusion criteria were as follows: (a) the evaluation of association between prognosis of AML and FLT3-TKD; (b) untreated patients with AML were included in study; (c) complete original materials with specific explanation of sample size; (d) they provided data of all enrolled subjects on either or both of overall survival (OS) and disease-free survival (DFS) after a period of follow-up in the study; (e) with survival information based on the FLT3 status: FLT3-TKD and wild type; and (f) prospective cohort study focusing on human being. Exclusion criteria were as follows: (a) not conforming to inclusion criteria; (b) abstract, review article, letter, comment, and editorial; (c) duplicate publication of previous publications; (d) family-based studies of pedigrees; (e) without detailed FLT3 status data (FLT3-TKD and wild type); (f) with incomplete specific explanation or without specific explanation of sample size; and (g) studies were excluded if they focused exclusively on acute promyelocytic leukemia (APL) (M3). For multiple publications from a same population, the largest study was included only to exclude duplicate studies or overlapping data. According to the inclusion and exclusion criteria, the two independent investigators accomplished study selection independently by screening title, abstract, and full text. Any dissent was solved by discussion. If agreement could not be reached, then a third researcher was consulted. Four studies were discussed and excluded after discussion (11–14).
The data of the eligible studies were extracted in duplicate by two independent researchers. The data extracted comprised first author’s name, publication year, diagnostic criteria for AML, the resource of the subjects, genotyping methods, the number of subjects, the FLT3 status (FLT3-TKD or wild type) of subjects, the number of OS and/or DFS from all subjects (if any), hazard ratio (HR) with 95% confidence interval (CI) of OS and/or DFS, and the baseline data of all subjects in all included prospective cohort studies [age, sex, patient source, other mutations (if any), the usage of chemotherapeutics (if any), and so on] (Table S1). HR and its 95% CI were extracted directly or calculated the observed minus expected (O-E) according to the ratio of event or were extracted by using Engauge Digitizer according to the Cox curve (15). Various patient source descents were classified as Caucasian and Asian. Two investigators would check the extracted data and reach to consensus on all collected data. If a dispute existed, then original data of the included studies would be rechecked and be discussed again to reach consensus. If the dispute still existed, then the third investigators would be appointed as the decider to adjudicate the disagreements.
The Newcastle-Ottawa (NOS) scale was used to score the strength of all included studies by the three independent investigators (16). The scale has nine items classified into three major categories: selection (four items), comparability (two items), and outcome (three items) (Supplement 1. Method). In this scoring system, selection, comparability, and outcome categories could be awarded a maximum of four, two, and three points, respectively. High quality was considered as six or more points that each cohort study scored. Any discrepancies were resolved among authors. The results of quality assessment are displayed in Table S2.
Potential publication bias was checked by Begg’s funnel plots (17) and Egger’s test (18). An asymmetric plot with p-value less than 0.05 was considered a significant publication bias. Moreover, sensitivity analysis was performed on the pooled HRs to evaluate the effect of each study, in which the results of the meta-analysis were recalculated after removal of each study in a turn.
Among entire conduction of the meta-analysis, we strictly abided by the PRISMA checklists as a guideline (19). All statistical analyses were performed with Stata 16.0 software (StataCorp, College Station, TX, USA). A two-tailed p < 0.05 was considered significant except for specified conditions, where a certain p-value was declared. HR and corresponding 95% CI were applied to assess the prognostic impact of FLT3-TKD in patients with AML. Furthermore, HR > 1 was considered as poorer prognosis in patients with FLT3-TKD than patients with FLT3-WT, whereas HR < 1 was considered as beneficial prognosis in patients with FLT3-TKD than patients with FLT3-WT. The heterogeneity of the studies was assessed by I2 statistic (I2 = 0%–25%, no heterogeneity; I2 = 25%–50%, moderate heterogeneity; I2 = 50%–75%, large heterogeneity; and I2 = 75%–100%, extreme heterogeneity) (20). When the heterogeneity was statistically significant (I2 > 50%), the random-effects model was used for assessing information; otherwise, the fixed-effects was conducted (21). Heterogeneity was analyzed by meta-regression model including age and patient source, and subgroup analysis was stratified by age and patient source. In addition, Begg’s and Egger’s tests were performed to detect potential publication bias. Furthermore, sensitivity analysis was conducted to determine the stability of findings in meta-analysis.
The study selection process for the meta-analysis about the prognosis of FLT3-TKD in AML is shown in Figure 1. Following the initial retrieval of 917 publications through database search (253 from PubMed, 638 from Embase, and 26 from CNKI), 696 relevant publications were selected after the removal of duplicates. Moreover, after a careful review of the title and abstract, 152 publications were rejected because of their irrelevance to this meta-analysis. The remaining 544 publications were full-text–reviewed; of these, 524 were excluded. The reasons for excluding are shown in Figure 1. Finally, 20 prospective cohort study studies (22–41) consisting of 10,970 participants (FLT3-WT = 9,744; FLT3-TKD = 1,226) were included in our meta-analysis. The general characteristics of the 20 studies are shown in Table 1, and additional information is shown in Table S1.
Before analysis, we determined two outcomes: one of them is relapse of AML or death from AML relapse as the outcome of DFS and the other one is death from all causes as the outcome of OS to represent the prognosis of AML in this meta-analysis. In all the patients with AML from included studies, we analyzed two outcomes between the group with FLT3-WT and the group with only FLT3-TKD in FLT3 gene in random-effects model. The pooled HR of DFS is 1.12 (95% CI: 0.90–1.41; I2 = 70.7%, p = 0.000) (Figure 2A). In same model, the pooled HR of OS is 0.98 (95% CI: 0.76–1.27; I2 = 79.9%, p = 0.000) (Figure 2B).
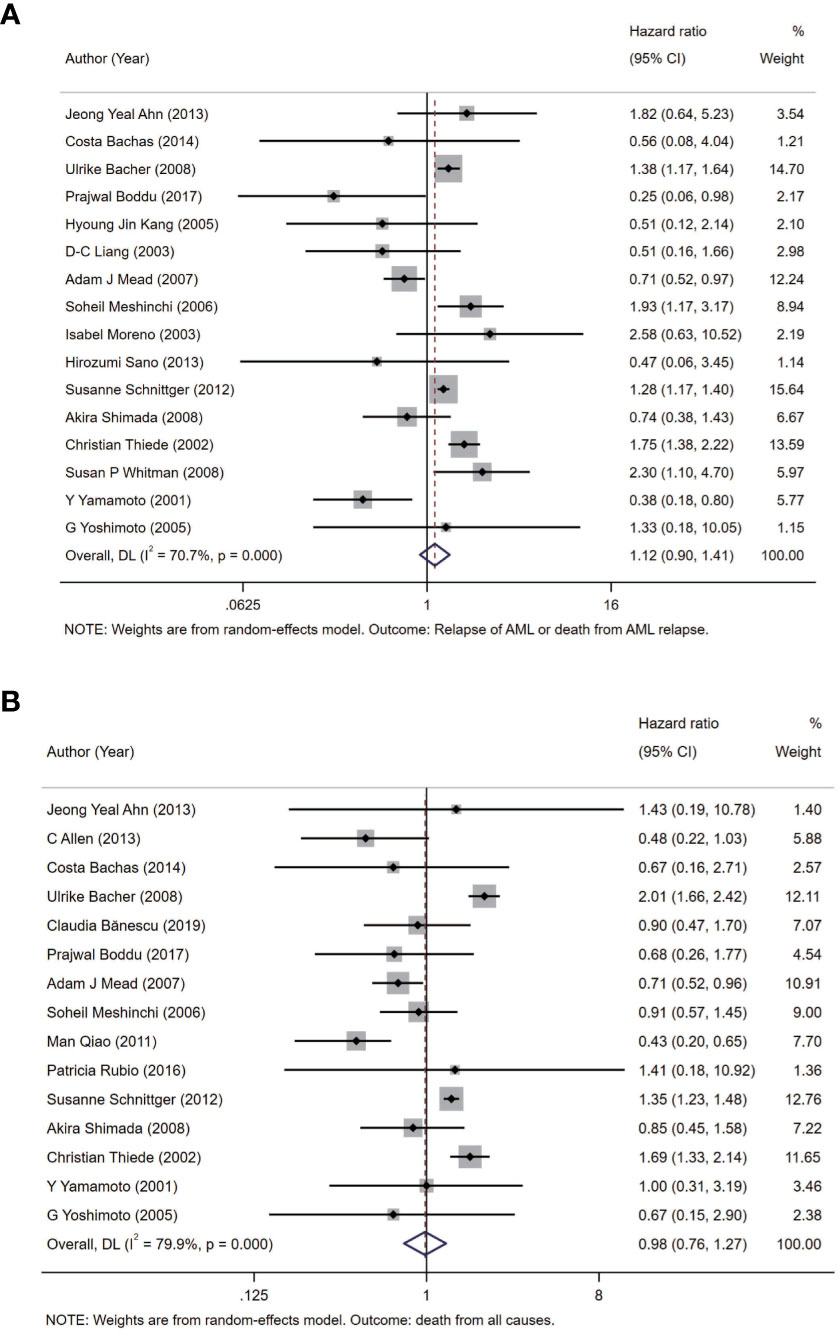
Figure 2 Forest plots of HRs and 95% CI for DFS (A) and OS (B) in patients with AML. The size of blocks or diamonds represents the weight in this meta-analysis, and the length of straight line segment represents the width of 95% CI. HR, hazard ratio; CI, confidence intervals; DFS, disease-free survival; OS, overall survival; AML, acute myeloid leukemia.
Age and patient source were included in meta-regression model for heterogeneity analysis. The coefficient of age is −0.005 (p = 0.497) and 0.008 (p = 0.299) in patients of AML for DFS and OS, respectively, and the coefficient of patient source is −1.004 (p = 0.015) and −0.358 (p = 0.324) in patients of AML for DFS and OS, respectively (Table 2, Figure 3).
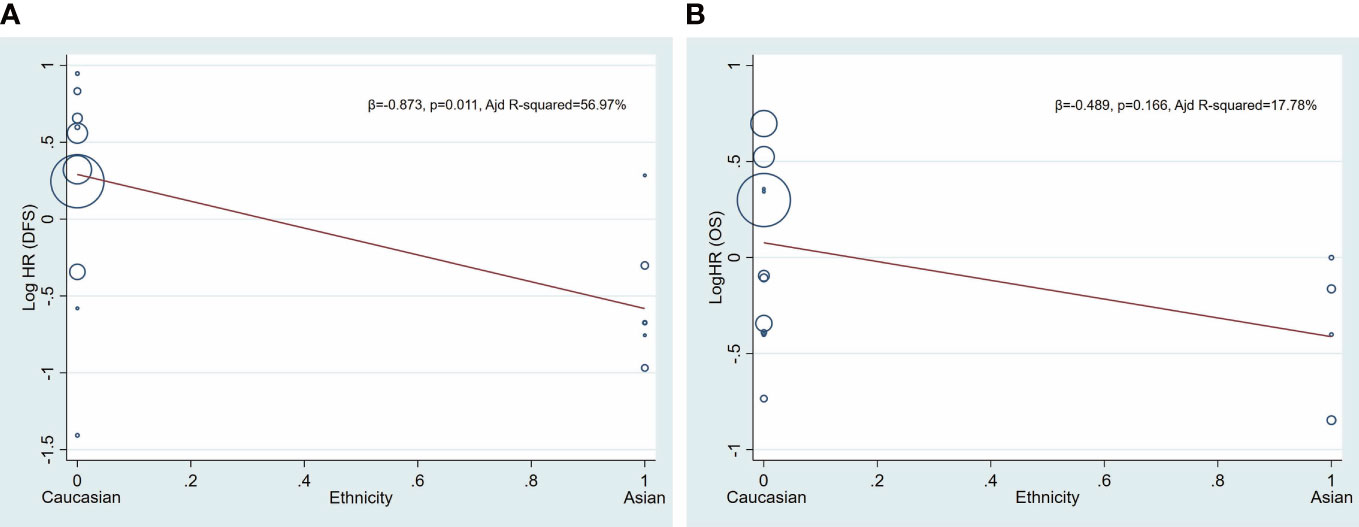
Figure 3 Univariate meta−regression analysis. Log HR of DFS and OS according patient source (ethnicity). A circle represents a study, and the diameter of circle represents sample capacity. Various ethnicity (patient source) descents were classified as Caucasian and Asian. HR, hazard ratio; DFS, disease-free survival; OS, overall survival.
To further analyze heterogeneity, considering with the background knowledge of FLT3-TKD, we conducted meta-analysis in subgroups according to the patient source (ethnicity) of all participants included (Table S1). The pooled HR of DFS is 1.34 (95% CI: 1.07–1.67; I2 = = 72.9%, p = 0.000) in the Caucasian subgroup, and the pooled HR of DFS is 0.56 (95% CI: 0.37–0.85; I2 = 0.0%, p = 0.777) in the Asian subgroup (Figure 4A). The pooled HR of OS is 1.11 (95% CI: 0.85–1.44; I2 = 80.5%, p = 0.000) in the Caucasian subgroup, and the pooled HR of OS is 0.63 (95% CI: 0.42–0.95; I2 = 5.1%, p = 0.368) in the Asian subgroup (Figure 4B).
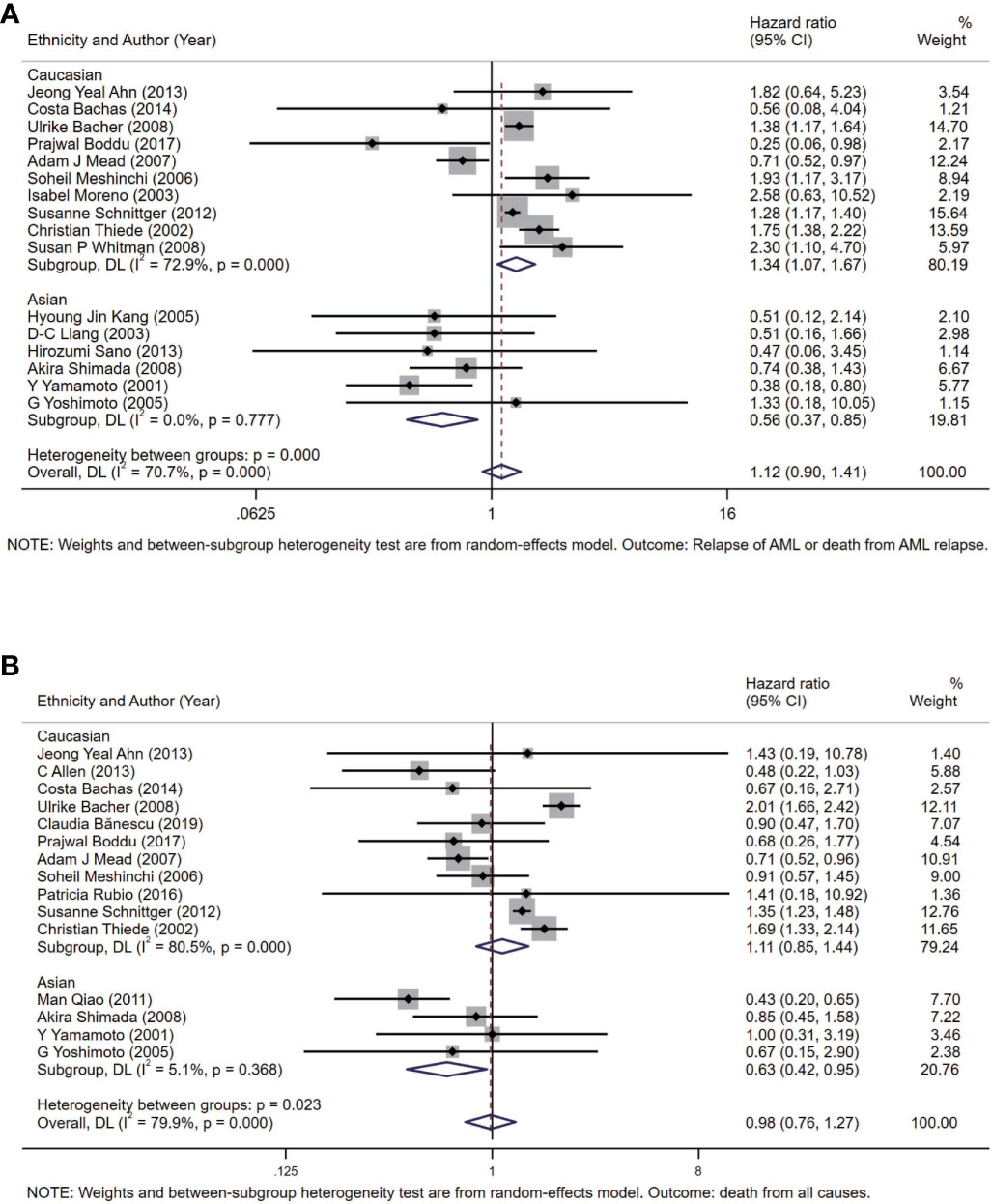
Figure 4 Forest plots of HRs and 95% CI for DFS (A) and OS (B) in patients with AML in the Asian subgroup and the Caucasian subgroup. The size of blocks or diamonds represents the weight in this meta-analysis, and the length of straight line segment represents the width of 95% CI. HR, hazard ratio; CI, confidence intervals; DFS, disease-free survival; OS, overall survival; AML, acute myeloid leukemia.
Begg’s and Egger’s tests have been used to detect any publication bias that indicated that there was no significant bias between studies of FLT3-TKD in prediction of DFS (p = 0.499 of Begg’s and p = 0.233 of Egger’s) and also of OS (p = 0.488 of Begg’s and p = 0.061 of Egger’s). Symmetrical Begg’s funnel plot was obtained (Figure 5). We also conducted sensitivity analysis to examine the stability of the meta-analysis to determine the influence of each study on pooled HRs in patients with AML by deleting a single study in every model. It showed no individual study affected the pooled HR of DFS (Figure 6A) and OS (Figure 6B) in all patients with AML.
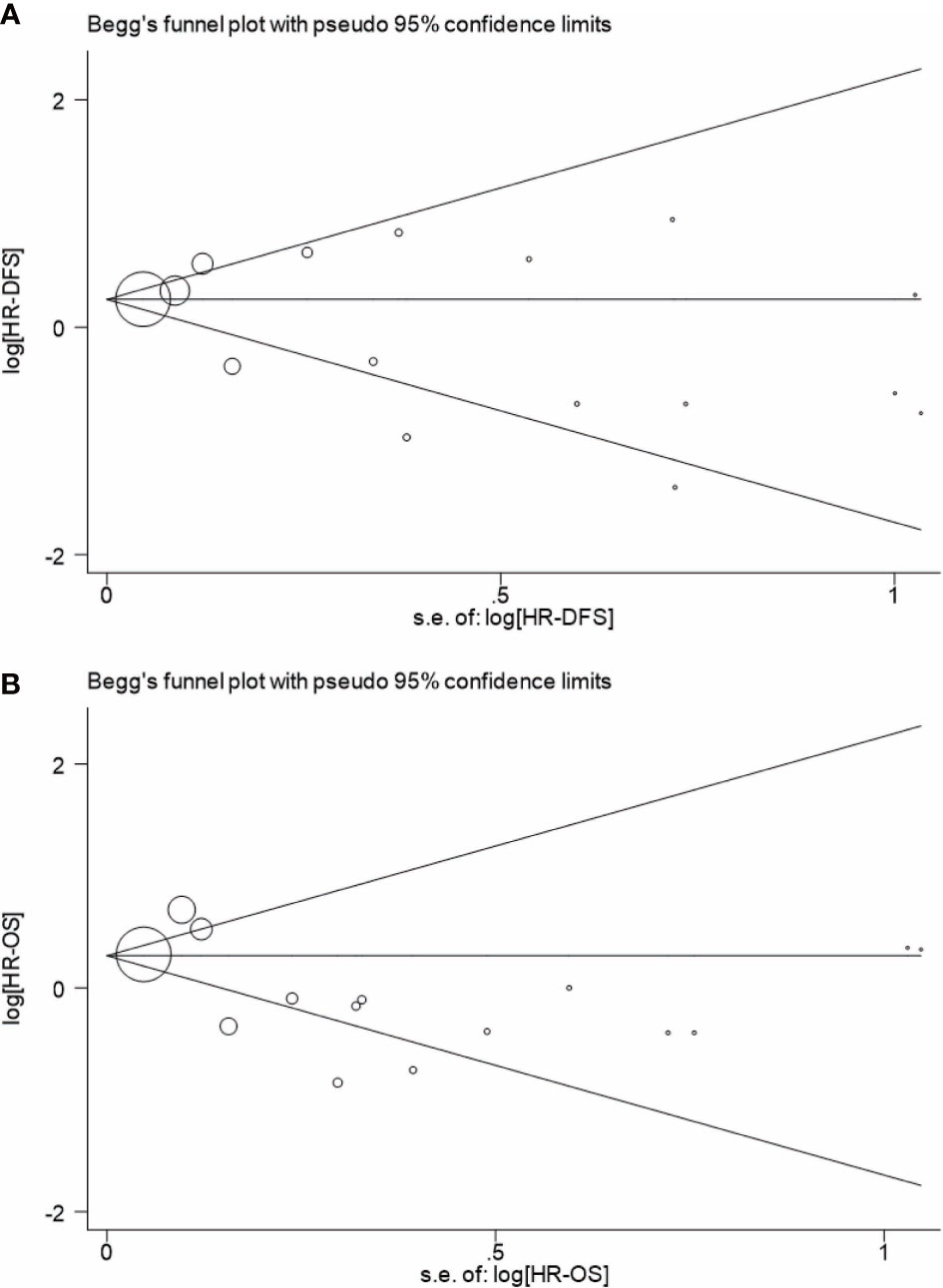
Figure 5 Begg’s funnel plot for analyzing publication bias of DFS (A) and OS (B) in patients with AML. A circle represents a study, and the diameter of circle represents sample capacity. DFS, disease-free survival; OS, overall survival; AML, acute myeloid leukemia.
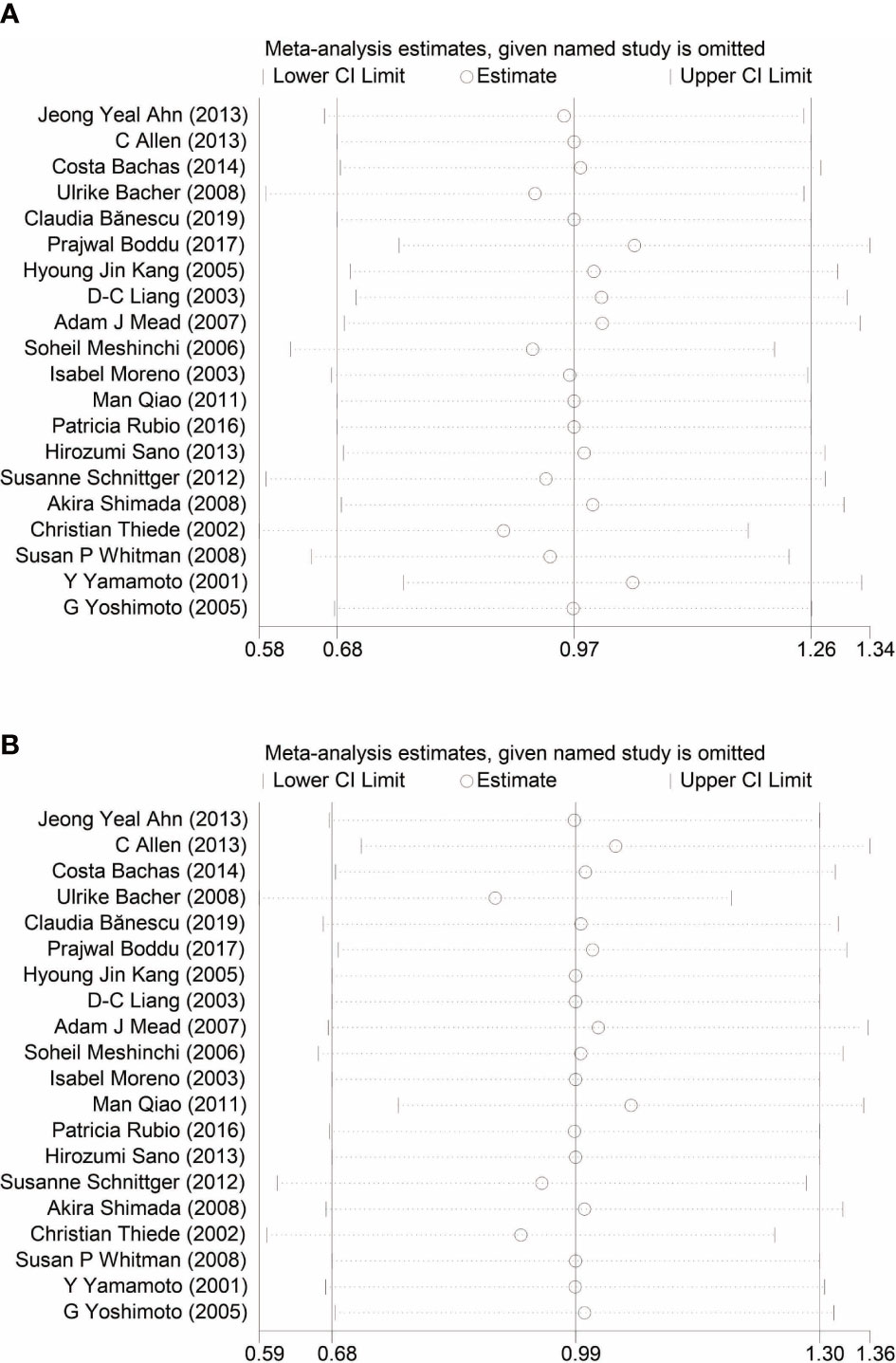
Figure 6 Sensitivity analysis of DFS (A) and OS (B) in patients with AML. DFS, disease-free survival; OS, overall survival; AML, acute myeloid leukemia.
In this meta-analysis, 20 prospective cohort studies (n = 10,970) on FLT3-TKD in AML were included: 9,744 subjects with FLT3-WT and 1,226 subjects with FLT3-TKD (22–41). The incidence of FLT3-TKD in this meta-analysis was 11.2%, which was nearly consistent with previous studies (approximately 7%–10% of all AML cases) (2). Our results indicated that, within 20 cohort studies (n = 10,970) included, the pooled HR of DFS was 1.12 (95% CI: 0.90–1.41; I2 = 70.7%, p = 0.000) and OS was 0.98 (95% CI: 0.76–1.27; I2 = 79.9%, p = 0.000), which revealed no significant effect of FLT3-TKD on DFS and OS of patients with AML by random effect models. However, meta-regressions demonstrated that patient source associated with the prognosis effect of FLT3-TKD in patients with AML. To be specific, FLT3-TKD represented a beneficial prognosis of DFS (HR = 0.56, 95% CI: 0.37–0.85) and OS (HR = 0.63, 95% CI: 0.42–0.95) for Asians with AML, whereas FLT3-TKD represented an adverse prognosis of DFS for Caucasians with AML (HR = 1.34, 95% CI: 1.07–1.67). However, the results of DFS from Caucasians ought to be interpreted with caution due to the heterogeneity (I2 = 72.9%, p = 0.000).
In general, FLT3-TKD reveals no significant effects on DFS and OS of patients with AML, which is consistent with the controversial statue nowadays. The controversies of prognostic impacts on FLT3-TKD in patients with AML were supported by two-sided laboratory evidence of FLT3-TKD. On one hand, many studies indicated that FLT3-TKD associated a beneficial prognosis of AML. In some cases, patients with FLT3-TKD companying with other mutations, such as NPM1 mutation, showed a favorable prognosis (27) with the reasons that the localization and signaling of FLT3-TKD was changed by NPM1c in AML (42). On the other hand, other research studies manifested that FLT3-TKD was considered as a harmful mutation in prognosis of AML. Since FLT3-ITD was first recognized as a frequently mutated gene in AML in 1996 (43), growing studies indicated that a lot of FLT3-ITD–positive patients with AML relapse with the appearance of FLT3-TKD after initial response to FLT3 inhibitor treatments. Moreover, several FLT3 inhibitors including Sorafenib and Quizartinib potently had effects on inhibiting FLT3-ITD but were not effective toward FLT3-TKD (2, 44, 45). The phenomenon was plausibly interpreted as the coexistence of two kinds of FLT3 mutations and the presence of FLT3-TKD in a very low level at initial stage of disease, which subsequently became prevalent after FLT3-ITD–positive leukemic cells, are eliminated (46).
Our results indicated that FLT3-TKD represented a beneficial prognosis for Asians with AML, whereas it represented an adverse prognosis of DFS for Caucasians with AML, but with heterogeneity. First, in the Caucasian subgroup, the pooled HR of DFS was 1.34 (95% CI: 1.07–1.67; I2 = 72.9%, p = 0.000), and the pooled HR of OS was 1.11 (95% CI: 0.85–1.44; I2 = 80.5%, p = 0.000) (Figure 4). There were 635 Asians from four different countries and 10,335 Caucasians from eight different countries in this meta-analysis, which revealed that Caucasians outnumbered Asians. Hence, we speculated that confounding factors from large sample capacity of the Caucasian subgroup accounted for the heterogeneity in Caucasians (I2 = 72.9%, p = 0.000). In this situation, if related confounding factors were well controlled, then the conclusion might be more convinced. However, it was indicated that Caucasians reveals a distinct genetic alteration profiles of AML than Eastern Asian population (47), but what we focused on is the ratio of FLT3-TKD in AML, which would not be influence by population base too much at this meta-analysis. Second, according to different therapeutic guidelines of AML, the chemotherapy regimens for patients with AML vary internationally. On one hand, included studies from Japan accounted for a large population in the Asian subgroup in this meta-analysis. The conventional “3 + 7” induction is regarded as basic regimens for complete remission (CR) with anthracyclines for 3 days and standard dose of cytarabine for 7 days. We noticed that the dose of cytarabine and anthracyclines in Japan was less than the dose in Caucasian countries according to practical guideline for AML (48, 49). Cytarabine of 100 mg/m2 was recommended for 7 days in Japan, whereas cytarabine of 100–200 mg/m2 was recommended for 7 days in Caucasian countries; and daunorubicin of 50 mg/m2 was recommended for 3 days in Japan, whereas daunorubicin of 60–90 mg/m2 for 3 days was recommended in Caucasian countries (48, 49). Hence, we speculated prognosis of AML in different countries or regions might attribute to the dose of cytarabine and daunorubicin, which consist of the conventional “3 + 7” induction. On another hand, anthracyclines have been used extensively with standard dose of cytarabine to induce remission of patients with AML worldwide (48, 50). However, clinicians in China tend to attach great importance to homoharringtonine (HHT) and apply HHT-based induction regimens to induce the CR of patients with AML (not APL), which was considered as another discrepancy between Asian countries and Caucasian countries in the treatments of AML (48, 50). Concretely, clinical hematologists and oncologists in China are apt to replace anthracyclines with HHT in CR induction of AML or added HHT upon prime chemotherapy regimens for higher CR rate of AML. HHT is a kind of alkaloid deriving from genus Cephalotaxus and exerts selective antileukemia effects on patients with AML, especially on these carrying FLT3-ITD and elderly patients with AML (51–53). What is noteworthy is that the FLT3-TKD status associates with chemotherapy regimens, especially in relapse (2), whereas some salvage therapies for relapsed AML include HHT as the basic members of chemotherapy regimens, such as HAA regimen (54). Hence, we speculated that prognosis of AML in different ethnicities may also due to the clinical usage of HHT partially. Third, race diversity is the result of different genetic backgrounds, not only the gene encoding FLT3. Patients with AML usually were with genomic anomaly or sporadic gene mutations (42). Currently, the contribution of different genetic backgrounds to the occurrence and progression of AML remains unclear. In conclusion, FLT3-TKD exerts impacts on contrasting prognosis of AML in different ethnicities due to multiple reasons, which deserve further explorations.
FLT3-TKD is considered as a potentially metabolically related mutation in AML. FLT3-TKD associates with cytoplasmic Src family tyrosine kinases by increasing the phosphorylation of activating tyrosines, such as FGR and HCK (7). In addition, Src family members are involve in multiple nutrients metabolisms, including glucose (8), lipid (9), and glutamine (10), indicating that FLT3-TKD is a potentially metabolically related mutation. As for glucose metabolism, Src is able to regulate cyclin B1, N-cadherin, and E-cadherin under high glucose as a response to hyperglycemia in colorectal cancer (8). For glutamine metabolism, epidermal growth factor receptor (EGFR)-promoted tumor progression is considered as being Src signaling pathway related by influencing glutamine metabolism in multiple malignancies (10). For lipid metabolism, Src is able to being recruited by CDCP1 into lipid rafts, which affect HGF receptor Met via the activation of STAT3 (9). Overall, FLT3-TKD is considered as being metabolically related based on the functions of Src family tyrosine kinases, which can be used to explain FLT3-TKD status in AML.
Our meta-analysis has several strengths. First, we selected 20 eligible prospective cohort studies, which was considered as an ideal epidemiological method to predict prognosis of AML. In addition, selection bias was well controlled by two independent investigator and an unlimited literature search. Furthermore, most included studies were of high quality with regard to quality assessment of the NOS scale (16). Moreover, no evident publication bias was identified by either Begg’s funnel plot or Egger’s test. Finally, we conducted sensitivity analysis by deleting a single study in every model, and we did not find obvious abnormal studies contributing to the pooled HR.
Begg’s funnel plot was used to detect publication bias in this meta-analysis (Figure 5): As is shown in Figure 5, publication bias is not evident when DFS was regarded as an evaluated end point (Figure 5A); however, it indicated a publication bias when OS was regarded as an evaluated end point (Figure 5B). Indeed, OS reflects the multiple influences to individuals, not just AML. The OS of AML is able to attribute to multiple factors, which make the manuscript easy to publish. Moreover, OS can be concluded according to the status of the patients with AML (live/dead), whereas DFS is usually diagnosed on the basis of medical examination, such as bone marrow biopsy. The difference contributed to the difficulty of obtaining data from AML, which also may lead to the publication bias. All in all, DFS of AML is considered as a better evaluated index in the prognosis AML, and DFS of AML in this meta-analysis did not indicate a publication bias.
There are several limitations of this study that should be acknowledged. First, there was a heterogeneity in Caucasians (I2 = 72.9%, p = 0.000), which may attribute to the fact that Caucasians outnumbered Asians: 635 Asians from four different countries and 10,335 Caucasians from eight different countries in this meta-analysis. In our opinion, the heterogeneity from Caucasian group was from some unknown confounding factors in the united prospective cohorts of Caucasians. In this situation, if related confounding factors were well controlled, then the conclusion might be more convinced. In addition, we failed to get the specific information about chemotherapy from 10,970 participants including chemotherapy regimens, chemotherapy dose, and chemotherapy time, because different types of treatment may exert distinct impacts on the prognosis of the patients with AML. However, FLT3-TKD does not result from general chemotherapies, so the FLT3-TKD in AML in this study is regarded as being from individuals’ genetic backgrounds. Furthermore, because of the limitation from the extracted data, we were unable to perform more stratification analysis according to other confounding factors. Although confounding factors work on the ending events, it is considered as a common problem in clinical research studies because we cannot predict everything before the start.
In conclusion, our results showed that FLT3-TKD revealed no significant effect on DFS and OS of patients with AML. However, meta-regressions demonstrated that patient source associated with the prognosis effect of FLT3-TKD in patients with AML. To be specific, FLT3-TKD represented a beneficial prognosis of DFS and OS for Asians with AML, whereas FLT3-TKD represented an adverse prognosis of DFS for Caucasians with AML. However, the results of DFS from Caucasians ought to be interpreted with caution due to the heterogeneity. This meta-analysis provided new information about the distinct prognosis of patients with AML between Asians and Caucasians. From our perspectives, the Caucasians with FLT3-TKD at the initial diagnostic stage of AML could be recommended the Asians dose of cytarabine and daunorubicin (cytarabine of 100 mg/m2 + daunorubicin of 50 mg/m2) in conventional “3 + 7” induction, so that they could receive a better prognosis of AML for survivals. An adequately designed prospective study including a large population with AML with clear FLT3 gene statue is needed to confirm our results.
The funder of the study supported the study design, data analysis, and publication of the manuscript.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
SL was involved in project conceiving, data collection, data curation, literature assessment, investigation, formal analysis, software performance, visualization, and original manuscript writing. NL was involved in data collection, data curation, and literature assessment. YC was involved in project conceiving, study supervision, and manuscript editing. ZZ was involved in study supervision and funding acquisition. YG was involved in project conceiving, literature assessment, study supervision, investigation, funding acquisition, and manuscript editing. All of authors read and approved the final manuscript.
We thank National Natural Science Foundation of China (No. 82202579 and No. 81900673) and the Shenzhen Science and Technology Innovation Committee of Guangdong Province of China (Grant No. JCYJ20180307150634856, JCYJ20210324123200003) to support the study design, data analysis and publication of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1086846/full#supplementary-material
1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med (2015) 373(12):1136–52. doi: 10.1056/NEJMra1406184
2. Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia. (2019) 33(2):299–312. doi: 10.1038/s41375-018-0357-9
3. Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. (2005) 19(8):1345–9. doi: 10.1038/sj.leu.2403838
4. Kiyoi H. FLT3 Inhibitors: Recent advances and problems for clinical application. Nagoya J Med Sci (2015) 77(1-2):7–17. doi: 10.6004/jnccn.2017.0116
5. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. (2017) 129(4):424–47. doi: 10.1182/blood-2016-08-733196
6. O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2017) 15(7):926–57. doi: 10.3324/%25x
7. Kramer MH, Zhang Q, Sprung R, Day RB, Erdmann-Gilmore P, Li Y, et al. Proteomic and phosphoproteomic landscapes of acute myeloid leukemia. Blood. (2022) 140(13):1533–48. doi: 10.1182/blood.2022016033
8. Chen YC, Ou MC, Fang CW, Lee TH, Tzeng SL. High glucose concentrations negatively regulate the IGF1R/Src/ERK axis through the MicroRNA-9 in colorectal cancer. Cells. (2019) 8(4). doi: 10.3390/cells8040326
9. Kajiwara K, Yamano S, Aoki K, Okuzaki D, Matsumoto K, Okada M. CDCP1 promotes compensatory renal growth by integrating src and met signaling. Life Sci alliance. (2021) 4(4). doi: 10.26508/lsa.202000832
10. Li B, Antonyak MA, Druso JE, Cheng L, Nikitin AY, Cerione RA. EGF potentiated oncogenesis requires a tissue transglutaminase-dependent signaling pathway leading to src activation. Proc Natl Acad Sci United States America. (2010) 107(4):1408–13. doi: 10.1073/pnas.0907907107
11. Colovic N, Tosic N, Aveic S, Djuric M, Milic N, Bumbasirevic V, et al. Importance of early detection and follow-up of FLT3 mutations in patients with acute myeloid leukemia. Ann Hematol (2007) 86(10):741–7. doi: 10.1007/s00277-007-0325-3
12. Group S-USLC. [FLT3 gene mutation and its prognostic implication in patients with acute leukemia.]. Zhonghua Xue Ye Xue Za Zhi (2010) 31(1):1–5. doi: 10.3760/cma.j.issn.0253-2727.2010.01.001
13. Chillón MC, Fernández C, García-Sanz R, Balanzategui A, Ramos F, Fernández-Calvo J, et al. FLT3-activating mutations are associated with poor prognostic features in AML at diagnosis but they are not an independent prognostic factor. Hematol J (2004) 5(3):239–46. doi: 10.1038/sj.thj.6200382
14. Shih LY, Huang CF, Wang PN, Wu JH, Lin TL, Dunn P, et al. Acquisition of FLT3 or n-ras mutations is frequently associated with progression of myelodysplastic syndrome to acute myeloid leukemia. Leukemia. (2004) 18(3):466–75. doi: 10.1038/sj.leu.2403274
15. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
16. Wells GA SB, O'Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2009). Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordhtm.
17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50(4):1088–101. doi: 10.2307/2533446
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
22. Ahn JY, Seo K, Weinberg OK, Arber DA. The prognostic value of CXCR4 in acute myeloid leukemia. Appl Immunohistochem Mol Morphol. (2013) 21(1):79–84. doi: 10.1097/PAI.0b013e3182606f4d
23. Allen C, Hills RK, Lamb K, Evans C, Tinsley S, Sellar R, et al. The importance of relative mutant level for evaluating impact on outcome of KIT, FLT3 and CBL mutations in core-binding factor acute myeloid leukemia. Leukemia. (2013) 27(9):1891–901. doi: 10.1038/leu.2013.186
24. Bachas C, Schuurhuis GJ, Reinhardt D, Creutzig U, Kwidama ZJ, Zwaan CM, et al. Clinical relevance of molecular aberrations in paediatric acute myeloid leukaemia at first relapse. Br J Haematol (2014) 166(6):902–10. doi: 10.1111/bjh.12989
25. Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: The combination matters an analysis of 3082 patients. Blood. (2008) 111(5):2527–37. doi: 10.1182/blood-2007-05-091215
26. Bănescu C, Tripon F, Trifa AP, Crauciuc AG, Moldovan VG, Bogliş A, et al. Cytokine rs361525, rs1800750, rs1800629, rs1800896, rs1800872, rs1800795, rs1800470, and rs2430561 SNPs in relation with prognostic factors in acute myeloid leukemia. Cancer Med (2019) 8(12):5492–506. doi: 10.1002/cam4.2424
27. Boddu P, Kantarjian H, Borthakur G, Kadia T, Daver N, Pierce S, et al. Co-Occurrence of FLT3-TKD and NPM1 mutations defines a highly favorable prognostic AML group. Blood Adv (2017) 1(19):1546–50. doi: 10.1182/bloodadvances.2017009019
28. Kang HJ, Hong SH, Kim IH, Park BK, Han KS, Cho HI, et al. Prognostic significance of FLT3 mutations in pediatric non-promyelocytic acute myeloid leukemia. Leuk Res (2005) 29(6):617–23. doi: 10.1016/j.leukres.2004.11.006
29. Liang DC, Shih LY, Hung IJ, Yang CP, Chen SH, Jaing TH, et al. FLT3-TKD mutation in childhood acute myeloid leukemia. Leukemia. (2003) 17(5):883–6. doi: 10.1038/sj.leu.2402928
30. Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. (2007) 110(4):1262–70. doi: 10.1182/blood-2006-04-015826
31. Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. (2006) 108(12):3654–61. doi: 10.1182/blood-2006-03-009233
32. Moreno I, Martin G, Bolufer P, Barragán E, Rueda E, Román J, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. (2003) 88(1):19–24. doi: 10.3324/%25x
33. Qiao M, Li WY, Sun AN, Chen SN, Liang JY, Ding ZX, et al. [Analysis of tyrosine kinases gene mutations in core binding factor related acute myeloid leukemia and its clinical significance]. Zhonghua Xue Ye Xue Za Zhi. (2011) 32(10):679–83. doi: 10.3760/cma.j.issn.0253-2727.2011.10.008
34. Rubio P, Campos B, Digiorge JA, Gallego MS, Medina A, Rossi JG, et al. NPM1, FLT3 and CEBPA mutations in pediatric patients with AML from Argentina: incidence and prognostic value. Int J Hematol (2016) 104(5):582–90. doi: 10.1007/s12185-016-2064-5
35. Sano H, Shimada A, Tabuchi K, Taki T, Murata C, Park MJ, et al. WT1 mutation in pediatric patients with acute myeloid leukemia: a report from the Japanese childhood AML cooperative study group. Int J Hematol (2013) 98(4):437–45. doi: 10.1007/s12185-013-1409-6
36. Schnittger S, Bacher U, Haferlach C, Alpermann T, Kern W, Haferlach T. Diversity of the juxtamembrane and TKD1 mutations (exons 13-15) in the FLT3 gene with regards to mutant load, sequence, length, localization, and correlation with biological data. Genes Chromosomes Cancer. (2012) 51(10):910–24. doi: 10.1002/gcc.21975
37. Shimada A, Taki T, Tabuchi K, Taketani T, Hanada R, Tawa A, et al. Tandem duplications of MLL and FLT3 are correlated with poor prognoses in pediatric acute myeloid leukemia: A study of the Japanese childhood AML cooperative study group. Pediatr Blood Cancer. (2008) 50(2):264–9. doi: 10.1002/pbc.21318
38. Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. (2002) 99(12):4326–35. doi: 10.1182/blood.V99.12.4326
39. Whitman SP, Ruppert AS, Radmacher MD, Mrózek K, Paschka P, Langer C, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. (2008) 111(3):1552–9. doi: 10.1182/blood-2007-08-107946
40. Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. (2001) 97(8):2434–9. doi: 10.1182/blood.V97.8.2434
41. Yoshimoto G, Nagafuji K, Miyamoto T, Kinukawa N, Takase K, Eto T, et al. FLT3 mutations in normal karyotype acute myeloid leukemia in first complete remission treated with autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. (2005) 36(11):977–83. doi: 10.1038/sj.bmt.1705169
42. Rudorf A, Müller TA, Klingeberg C, Kreutmair S, Poggio T, Gorantla SP, et al. NPM1c alters FLT3-D835Y localization and signaling in acute myeloid leukemia. Blood. (2019) 134(4):383–8. doi: 10.1182/blood.2018883140
43. Stone RM, Manley PW, Larson RA, Capdeville R. Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv (2018) 2(4):444–53. doi: 10.1182/bloodadvances.2017011080
44. Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol (2017) 4(5):e225–e36. doi: 10.1016/S2352-3026(17)30027-3
45. Smith LL. 58th American society of hematology annual meeting. Lancet Haematol (2017) 4(1):e13–e4. doi: 10.1016/S2352-3026(16)30197-1
46. Guo Y, Sun H, Zhang D, Zhao Y, Shi M, Yang M, et al. Development of a highly sensitive method for detection of FLT3D835Y. biomark Res (2020) 8:30. doi: 10.1186/s40364-020-00210-7
47. Wei H, Wang Y, Zhou C, Lin D, Liu B, Liu K, et al. Distinct genetic alteration profiles of acute myeloid leukemia between Caucasian and Eastern Asian population. J Hematol Oncol (2018) 11(1):18. doi: 10.1186/s13045-018-0566-8
48. Network® NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Acute myeloid leukemia NCCN evidence blocks™ (2021). Available at: https://wwwnccnorg/patients/.
49. Kiyoi H, Yamaguchi H, Maeda Y, Yamauchi T. JSH practical guidelines for hematological malignancies, 2018: I. Leukemia-1. Acute myeloid leukemia (AML). Int J Hematol (2020) 111(5):595–613. doi: 10.1007/s12185-020-02856-3
50. [Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2017)]. Zhonghua Xue Ye Xue Za Zhi. (2017) 38(3):177–82. doi: 10.3760/cma.j.issn.0253-2727.2017.03.001
51. Lam SS, Ho ES, He BL, Wong WW, Cher CY, Ng NK, et al. Homoharringtonine (omacetaxine mepesuccinate) as an adjunct for FLT3-ITD acute myeloid leukemia. Sci Transl Med (2016) 8(359):359ra129. doi: 10.1126/scitranslmed.aaf3735
52. Jin J, Wang JX, Chen FF, Wu DP, Hu J, Zhou JF, et al. Homoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: A multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol (2013) 14(7):599–608. doi: 10.1016/S1470-2045(13)70152-9
53. Wang J, Lü S, Yang J, Song X, Chen L, Huang C, et al. A homoharringtonine-based induction regimen for the treatment of elderly patients with acute myeloid leukemia: a single center experience from China. J Hematol Oncol (2009) 2:32. doi: 10.1186/1756-8722-2-32
Keywords: AML, FLT3-TKD, meta-analysis, metabolism, prognosis
Citation: Li S, Li N, Chen Y, Zheng Z and Guo Y (2023) FLT3-TKD in the prognosis of patients with acute myeloid leukemia: A meta-analysis. Front. Oncol. 13:1086846. doi: 10.3389/fonc.2023.1086846
Received: 01 November 2022; Accepted: 02 February 2023;
Published: 17 February 2023.
Edited by:
Jian Yu, Beihang University, ChinaReviewed by:
Jia Wei, Huazhong University of Science and Technology, ChinaCopyright © 2023 Li, Li, Chen, Zheng and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Guo, Z3VveTc1QG1haWwuc3lzdS5lZHUuY24=; Zhihua Zheng, emh6aGlodWFAbWFpbC5zeXN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.