
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 31 March 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1081786
This article is part of the Research TopicMapping Symptom Networks among Co-occurrence of Psychological and Somatic SymptomsView all 6 articles
Background: Endocrine therapy-related symptoms are associated with early discontinuation and quality of life among breast cancer survivors. Although previous studies have examined these symptoms and clinical covariates, little is known about the interactions among different symptoms and correlates. This study aimed to explore the complex relationship of endocrine therapy-related symptoms and to identify the core symptoms among breast cancer patients.
Methods: This is a secondary data analysis conducted based on a multicenter cross-sectional study of 613 breast cancer patients in China. All participants completed the 19-item Chinese version of the Functional Assessment of Cancer Therapy-Endocrine Subscale (FACT-ES). Multivariate linear regression analysis was performed to identify significant factors. A contemporaneous network with 15 frequently occurring symptoms was constructed after controlling for age, payment, use of aromatase inhibitors, and history of surgery. Network comparison tests were used to assess differences in network structure across demographic and treatment characteristics.
Results: All 613 participants were female, with an average age of 49 years (SD = 9.4). The average duration of endocrine therapy was 3.6 years (SD = 2.3) and the average symptom score was 18.99 (SD = 11.43). Irritability (n = 512, 83.52%) and mood swings (n = 498, 81.24%) were the most prevalent symptoms. Lost interest in sex (mean = 1.95, SD = 1.39) and joint pain (mean = 1.57, SD = 1.18) were the most severe symptoms. The edges in the clusters of emotional symptoms (“irritability-mood swings”), vasomotor symptoms (“hot flashes-cold sweats-night sweats”), vaginal symptoms (“vaginal discharge-vaginal itching”), sexual symptoms (“pain or discomfort with intercourse-lost interest in sex-vaginal dryness”), and neurological symptoms (“headaches-dizziness”) were the thickest in the network. There were no significant differences in network structure (P = 0.088), and global strength (P = 0.330) across treatment types (selective estrogen receptor modulators vs. aromatase inhibitors). Based on an evaluation of the centrality indices, irritability and mood swings appeared to be structurally important nodes after adjusting for the clinical covariates and after performing subgroup comparisons.
Conclusion: Endocrine therapy-related symptoms are frequently reported issues among breast cancer patients. Our findings demonstrated that developing targeted interventions focused on emotional symptoms may relieve the overall symptom burden for breast cancer patients during endocrine therapy.
Due to advances in treatment and care, the five-year survival rate of breast cancer has reached 85% or above (1). Of note, endocrine therapy – including tamoxifen and aromatase inhibitors – is essential for reducing recurrence and mortality rates (2). Approximately 84% of patients with breast cancer express hormone receptors. Endocrine therapy (with a standard five-year duration after breast surgery, chemotherapy, or radiation therapy) has been proven to be helpful in treating endocrine-sensitive breast cancer (3, 4). However, reports have shown that breast cancer patients who receive endocrine therapy often experience a high level of symptom burden (5, 6). These symptoms inversely can influence drug compliance, anatomical status, and quality of life (7, 8).
Endocrine therapy-related symptoms (ESs) are defined as treatment-related side effects experienced by women receiving endocrine therapy that negatively affect health-related quality of life and adherence to therapy (9). Previous studies (3, 5, 9–11) have reported that ESs mainly include gynecologic symptoms (e.g., vaginal discharge, sexual dysfunction, and vaginal dryness), menopausal symptoms (e.g., hot flashes, night sweats, cold sweats, mood swings, irritability), and musculoskeletal symptoms (e.g., joint pain, bone pain). Among these symptoms, hot flashes were the most common side effects regardless of the treatment received (9). Other frequently reported side effects, such as vaginal discharge, vaginal dryness, dyspareunia, and arthralgia, vary in prevalence between different types of medicines (9).
According to previous studies (11, 12), patients undergoing endocrine therapy usually suffer from more than 10 cooccurring symptoms. A considerable amount of work has been performed to investigate cooccurring symptoms (5, 13, 14), symptom clusters (15–17) or some prevalent individual symptoms (18–21) for ESs. Understanding how symptoms interact with each other will be helpful for preventing the occurrence of related symptoms. Recent advances in network analysis provide a new way to gain insight into the complex nature of comorbid symptoms and clusters of symptoms and to identify core symptoms. The paradigm in symptom science called “symptom networks” has been used to explore the intricate connections between symptoms linked to chronic illnesses and psychopathology (22–25).
Network analysis could be used to construct a partial correlation model of the relationship between observed variables and then visualize the importance of each variable in the network and its complex association from the overall perspective in the form of a graph (26, 27). The partial correlation network model is based on the weighted correlation network, which accounts for the possibility that the correlation between two nodes is affected by another node and provides a way to further accurately explore the relationship between nodes (28). In recent years, the partial correlation network model has been used to study symptoms among cancer patients. For instance, de Rooij et al. (29) demonstrated that fatigue was the most common core symptom among cancer survivors, with moderate links to other symptoms such as emotional or cognitive symptoms, appetite loss, dyspnea, and pain.
Investigating core symptoms related to endocrine therapy could activate other symptoms in the network, which could be helpful for identifying targets for symptom intervention and delivering efficient symptom management (28, 30). Therefore, the main aim of the current study was to 1) explore the core symptoms of breast cancer patients with endocrine therapy and 2) assess differences in symptom networks among different demographic and clinical covariates. To our knowledge, this is the first study to explore the core symptoms among breast cancer patients undergoing endocrine therapy.
This study involved secondary data analyses of data collected from the Symptom Profiles and Quality of Life among Breast Cancer Patients Undergoing Endocrine Therapy study (10). Patients were recruited from two tertiary hospitals and one cancer patient group between November 2019 and April 2020 in Shanghai, China. Participants had to meet the following criteria to be included: 1) at least 18 years old; 2) diagnosed with breast cancer and expressed estrogen receptors; 3) receiving endocrine therapy for more than six months (e.g., aromatase inhibitors, selective estrogen receptor modulators, combinations of these drugs); and 4) volunteered for this study. Patients who were diagnosed with other malignancies or those who were unable to complete the survey were excluded. The questionnaires were sent to eligible participants via an online follow-up platform. Two researchers checked the quality of the questionnaires together. A total of 685 questionnaires were distributed, and 613 were valid, thus yielding an effective recovery rate of 89.49%.
We used the Functional Assessment of Cancer Therapy-Endocrine Subscale (ES) to assess the occurrence and intensity of symptoms in the past 7 days among breast cancer patients undergoing endocrine therapy; this tool included 19 items (i.e., I have hot flashes; I have cold sweats; I have night sweats; I have vaginal discharge; I have vaginal itching/irritation; I have vaginal bleeding or spotting; I have vaginal dryness; I have pain or discomfort with intercourse; I have lost interest in sex; I have gained weight; I feel dizzy; I have been vomiting; I have diarrhea; I get headaches; I feel bloated; I have breast sensitivity/tenderness; I have mood swings; I am irritable; and I have pain in my joints) (31). A 4-point Likert scale (i.e., 0=“not at all” or “no symptom”, 1=“a little bit”, 2=“some-what”, 3=“quite a bit”, 4=“very much”) was used to evaluate symptom occurrence and severity. The total score ranged from 0 to 76, and higher scores indicated more severe symptoms. Additionally, we used a self-report questionnaire to collect the sociodemographic and clinical characteristics of participants (i.e., age, body mass index, education level, religion, marital status, living status, employment status, family monthly income, payment, cancer stage, time since endocrine therapy, type of endocrine therapy, history of breast cancer treatment, menopausal status) (10).
Network analyses were conducted using R version 4.2.1 with the “qgraph” package. We used regularized partial correlation network analyses to estimate the networks of symptoms for the total sample (including and excluding clinical covariates) and for subgroups separately (29). To generate a sparse network, we applied least absolute shrinkage and selection operator (LASSO) regression with the extended Bayesian information criteria (32). The hyperparameter γ was set at 0.1 to minimize spurious connections (33). To improve the accuracy and stability of the network, we excluded the symptoms with low prevalence rates (i.e., vomiting, diarrhea, bloating, vaginal bleeding or spotting) in the current study; these symptoms may not be induced by endocrine therapy according to previous studies and clinical experience (11, 12). Moreover, we used a multilinear regression analysis to test the statistical significance of clinical covariates for overall symptom severity. Those factors that were significant (P < 0.001) in the regression analysis were selected a priori as clinical covariates in the network analysis. However, clinical covariates were included in the total sample network model but not for subgroup networks because of variations between groups (29). Undirected association networks were generated using the Fruchterman-Reingold algorithm and spring layout (34). To make all networks more comprehensible, a maximum edge value of 0.45 and a minimum value of 0.03 were used for each network. In the network, the red nodes represented the top 2 highest node strengths, orange nodes represented node strengths > 0, green nodes represented node strengths < 0, and gray nodes represented clinical covariates. A green edge indicates a positive relationship, and a red edge indicates a negative relationship; thicker edges indicate stronger relationships (29).
The approach to symptom network analysis was most concerned with which symptom activation was more likely to activate other symptoms in the network. Three common centrality measures were strength, closeness, and betweenness (35). The strength centrality was the total direct connection of the symptom with other symptoms, that is, the ability of the symptom to influence other symptoms. Closeness centrality was used to reflect the inverse of the distance between the symptom and other symptoms, that is, the core position of the symptom in the network. The betweenness centrality was used to reflect the number of shortest paths through the symptom, that is, the symptom’s bridging function in the network. Symptoms with the highest centrality coefficients were identified as the core symptoms. Because the order of strength centrality was estimated more reliably in a previous study (24), we focused our interpretation of the most relevant symptoms on node strength centrality (rs) in the remainder of the report.
We estimated the accuracy and stability of the networks using the novel R package bootnet. An evaluation of the accuracy and stability of centrality measurements was conducted by bootstrapping (nBoots = 1000). First, edge weights with 95% confidence intervals (CIs) were bootstrapped to measure the edge’s accuracy. Second, a subsetting bootstrap was used to determine the centrality stability of the coefficient (CS-coefficient). In general, it is recommended that a CS coefficient should be no less than 0.25 and ideally higher than 0.50 (32).
To formally test for between-group network differences, we performed the network comparison test (NCT) using the R package NetworkComparisonTest (36). The NCT is a two-tailed permutation test that examines differences between two networks concerning network structure, edge strength, and global strength. A p value < 0.05 indicates a significant difference. The NCT can lose power when sample sizes are not equal (37). Therefore, to ensure balanced sample sizes between subgroups (n > 200), which were needed to enable comparisons between networks, we decided to include the covariate of type of endocrine therapy (selective estrogen receptor modulators vs. aromatase inhibitors) based on the current data distributions.
There were 613 participants involved in the analysis. All participants were female and aged 30 to 79 years, with an average age of 49 years (SD = 9.4). The mean BMI was 22.9 kg/m2 (SD = 2.9). The average duration of endocrine therapy was 3.6 years, and the range was 0.5 to 10 years. The majority of participants (50.6%) had a college or higher education level, had no religious belief (76.3%), were married (95.4%) and were living with families (94.8%). Over half of the participants were retired or unemployed (68.2%), had a family monthly income (Chinese Yuan) of less than 10,000 (56.5%), and reported basic medical insurance as the main source of medical expenses (88.6%). Most participants were premenopausal (76.5%). Stage II breast cancer (46.8%) was the most prevalent stage, followed by stage I (24.5%) and stage III or above (22.0%). Regarding the therapies received, most participants had been treated with surgery (95.6%), chemotherapy (83.8%), or radiation therapy (52.0%) and were taking selective estrogen receptor modulators (41.9%) or aromatase inhibitors (45.7%). More details about the sociodemographic and clinical characteristics of the participants are described in Table 1.
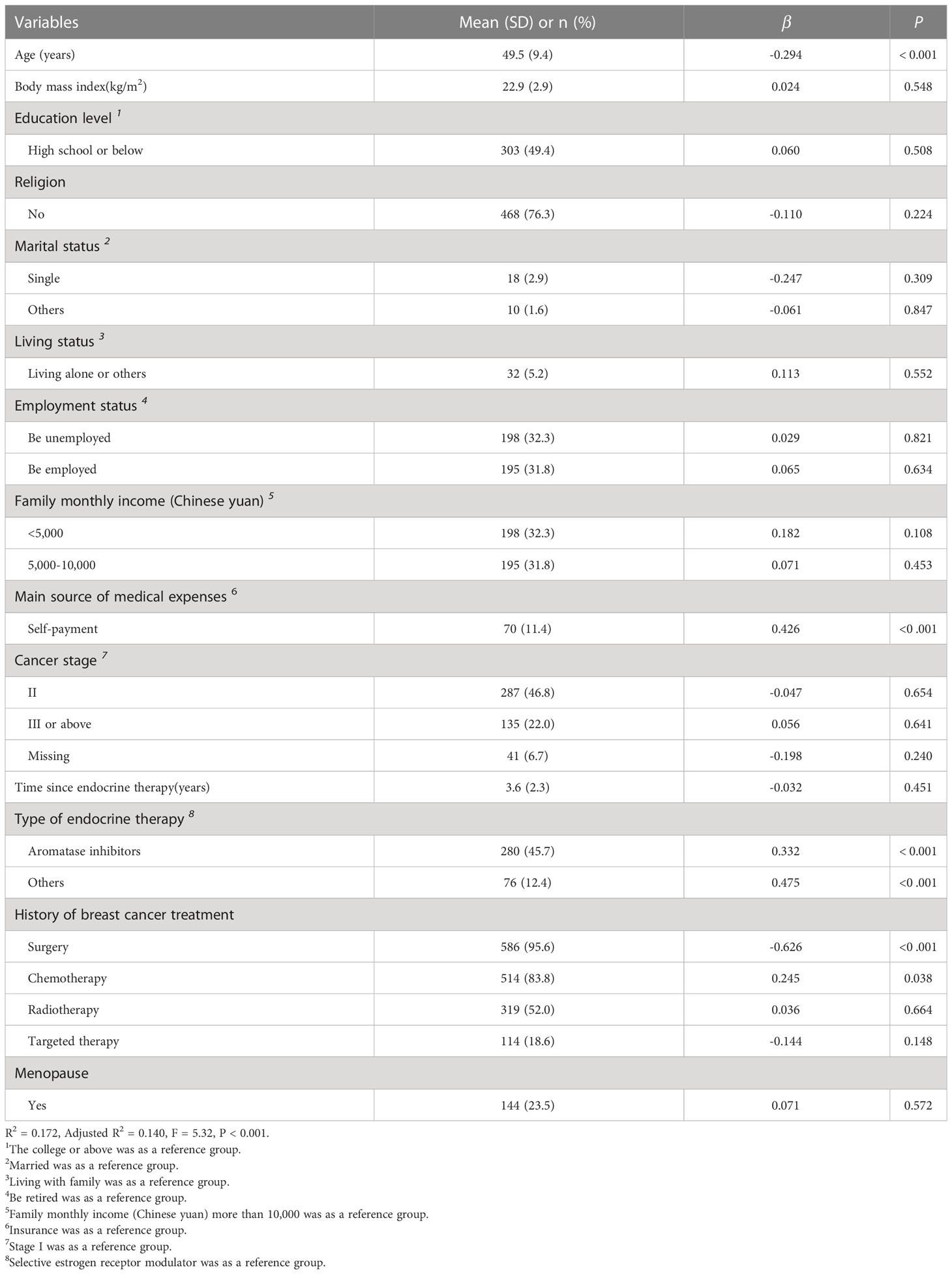
Table 1 Characteristics of the participants and linear regression analysis of overall symptom severity (n = 613).
Irritability (n = 512, 83.52%) and mood swings (n = 498, 81.24%) were the most prevalent symptoms. With regard to symptom severity, lost interest in sex (mean = 1.95, SD = 1.39) and joint pain (mean = 1.57, SD = 1.18) were the most severe symptoms. The incidence and severity of all symptoms among the subjects are shown in Table 2. In addition, the average score of the ES was 18.99 (SD = 11.43). Age (β = - 0.294, P < 0.001), self-payment (β = 0.426, P < 0.001), receiving aromatase inhibitors (β = 0.332, P < 0.001), and history of surgery (β = - 0.626, P < 0.001) significantly influenced the overall symptom severity. The results of the linea regression analysis are presented in Table 1.
The partial correlation network models (see Figure 1) showed that in the total sample (n = 613), mood swings had a strong connection with irritability (r = 0.70); lost interest in sex had a strong connection with vaginal dryness (r = 0.58) and a moderate connection with pain or discomfort with intercourse (r = 0.35); dizziness had a moderate connection with headaches (r = 0.43); and vaginal discharge had a moderate connection with vaginal itching/irritation (r = 0.45). In addition, there were moderate connections among hot flashes, night sweats and cold sweats (r = 0.28, 0.28, 0.15). After the addition of clinical covariates to the network, the weight of each connection in the network decreased, but the connections between symptoms were almost identical. It is worth noting that connections between aromatase inhibitors and age (r = 0.25), between aromatase inhibitors and joint pain (r = 0.13), and between aromatase inhibitors and vaginal discharge (r = - 0.17) appeared. However, payment and history of surgery had weak connections to all the symptoms. More details about the weight of each connection in the network with and without clinical covariates are presented in Supplementary Tables 1, 2. Moreover, the results of our centrality analyses (see Supplementary Table 3) indicated that on the basis of strength, mood swings (rs = 1.44 vs. rs = 1.33) and irritability (rs = 1.40 vs. rs = 1.16) were the most central symptoms in both networks without and with clinical covariates.

Figure 1 Symtom networks of the total sample (n=613) without and with clinical covarities. (A) Total without covariates (n=613). (B) Total with covariates (n=613).
The network was estimated accurately according to the edge weight bootstrap: there was a significant overlap between 95% CIs of the edge weights (see Figure 2). In terms of the subset bootstrap, the CS coefficient of node strength was 0.52 and 0.67 for the networks without and with clinical covariates, respectively (see Figure 3). Our results revealed that the order of strength centrality was more stable than the order of closeness and betweenness (24).
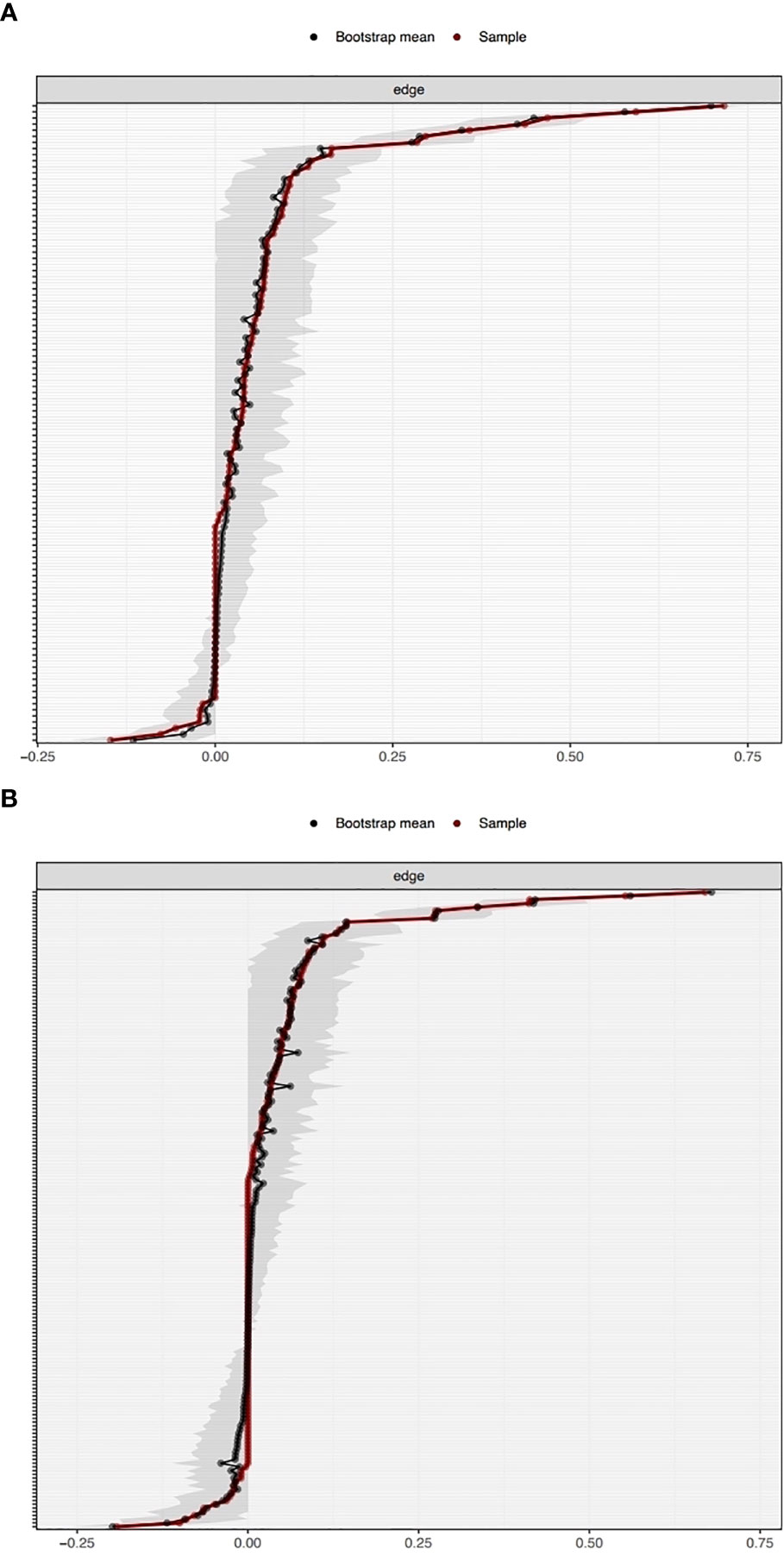
Figure 2 Bootstrapped confidence intervals of the edge weights in the networks without clinical covariates (A) and with clinical covariates (B).
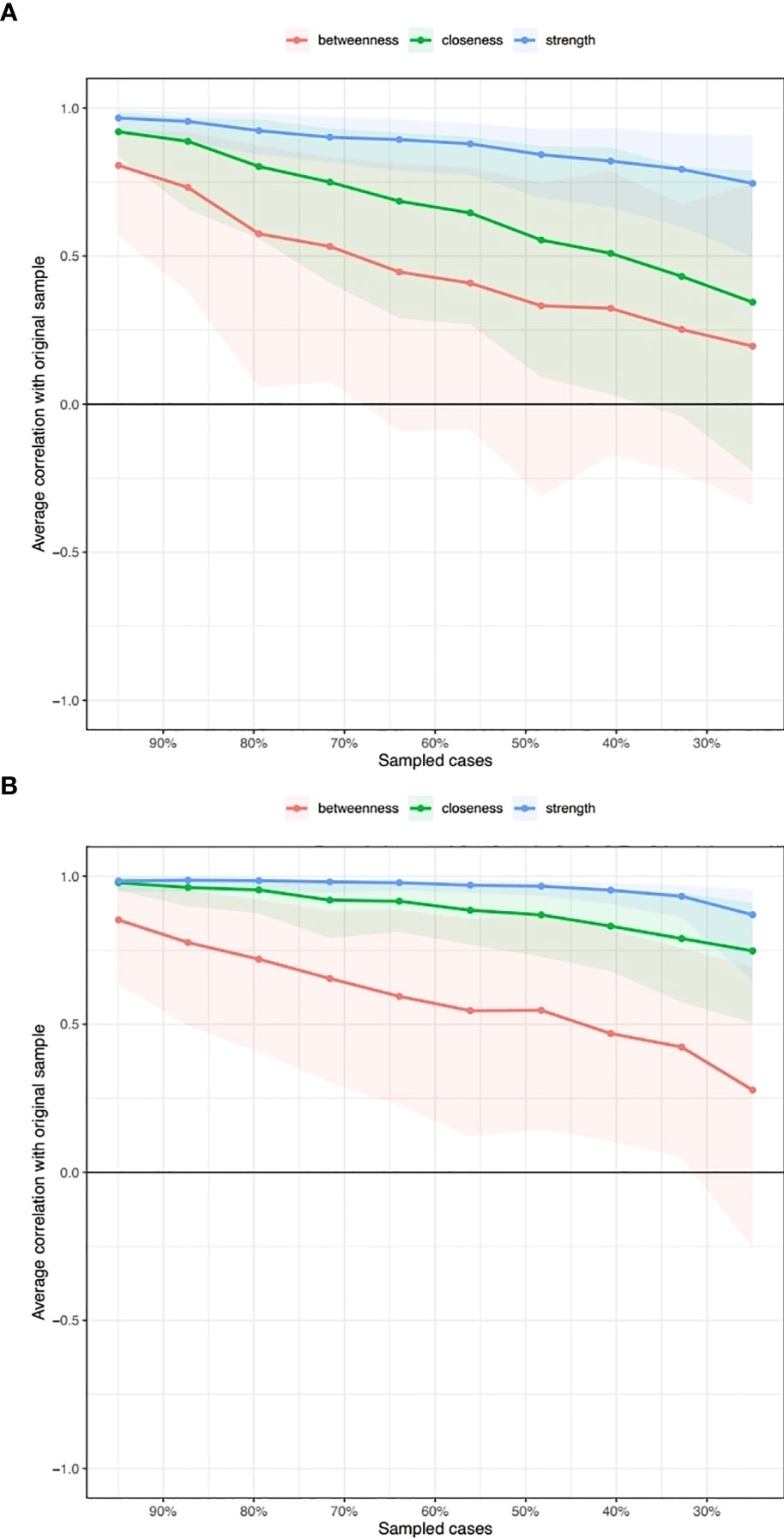
Figure 3 Subsetting bootstrap for the network without clinical covariates (A) and with clinical covariates (B).
There were no significant differences between symptom networks with selective estrogen receptor modulators versus aromatase inhibitors based on the network invariance test (P = 0.088) and global strength invariance test (P = 0.330). However, regarding the edge invariance test, the network model (see Figure 4) of patients who had received aromatase inhibitors (n = 280) compared with those who had received selective estrogen receptor modulators (n = 257) showed strong additional connections between vaginal discharge and cold sweats (P = 0.001), between vaginal discharge and lost interest in sex (P = 0.001), between vaginal dryness and lost interest in sex (P < 0.001). In addition, mood swings (rs = 1.20 vs. rs = 1.45) and irritability (rs = 1.50 vs. rs = 1.52) were still the most central symptoms in both the selective estrogen receptor modulator subgroup and the aromatase inhibitor subgroup.
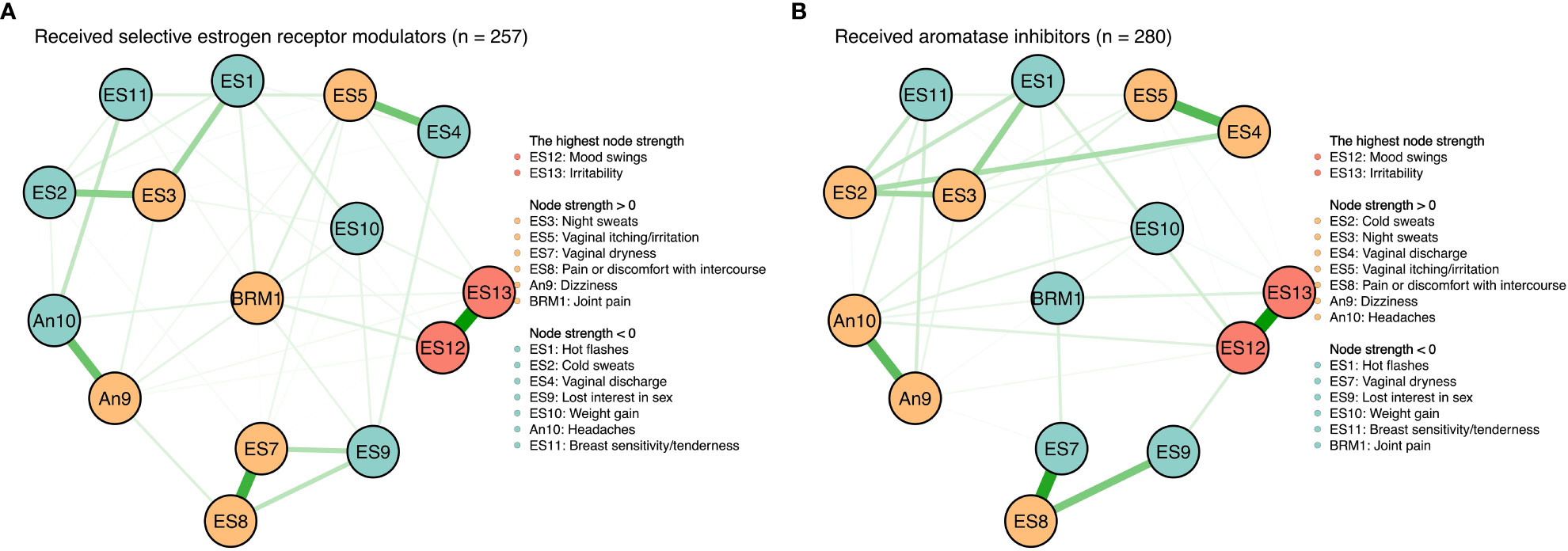
Figure 4 Symtom networks by different treatment regimens. (A) Received selective estrogen receptor modulators (n=257). (B) Received aromatase inhibitors (n=280).
To the best of our knowledge, this is the first report to analyze the ESs network among breast cancer patients. The main finding of this study is that irritability and mood swings were the most prevalent and central symptoms. Developing interventions targeted at emotional symptoms may be essential for reducing the overall symptom burden among breast cancer patients undergoing endocrine therapy.
In the overall symptom networks, we identified five clusters: emotional symptoms (i.e., irritability and mood swings), sexual symptoms (i.e., lost interest in sex, vaginal dryness, and pain or discomfort with intercourse), vaginal symptoms (i.e., vaginal discharge and vaginal itching/irritation), vasomotor symptoms (i.e., hot flashes, night sweats, and cold sweats), and neurological symptoms (i.e., dizziness and headaches). There were a few inconsistencies with previous results. For example, Li et al. (15) identified seven clusters of musculoskeletal, vasomotor, urinary, sexual, psychological, neurocognitive, and weight symptoms across the 18-month follow-up period of aromatase inhibitor therapy using exploratory factor analyses. Wen et al. (17) identified three groups of breast cancer patients receiving aromatase inhibitors by principal component analysis, namely, the disease symptom cluster, treatment-related psychological symptom cluster, and gastrointestinal symptom cluster. The inconsistencies may be caused by the instruments and data analysis method. Li et al. (15) evaluated 47 symptoms by the Breast Cancer Prevention Trial Symptom Checklist, Patient’s Assessment of Own Functioning Inventory, Beck Depression Inventory-II, and Profile of Mood, States Tension/Anxiety and Fatigue/Inertia subscales, and Wen et al. (17) assessed 13 symptoms by the MD Anderson Symptom Inventory; however, we used the Functional Assessment of Cancer Therapy-Endocrine Subscale (ES). The content of symptoms that were included to conduct clusters was different. Moreover, previous studies identified symptom clusters usually assuming a common underlying factor, while network analysis provided a more dynamic approach with the assumption that symptoms cluster because they mutually interact (29).
The most central symptoms of breast cancer patients during endocrine therapy were emotional symptoms regardless of treatment regimens (selective estrogen receptor modulators versus aromatase inhibitors). Previous studies also revealed that breast cancer patients undergoing endocrine therapy experienced a range of emotional distress (11, 38) The widely used endocrine treatment reduces the postmenopausal estrogen levels by nearly complete inhibition of the enzyme aromatase or anti-estrogenic effects on breast cancer cells that contain estrogen receptors by blocking this receptor (3). Possibly beneficial effects of estrogens on brain function may be, among others, the result of estrogenic activity through estrogen receptors in brain regions that are important for cognitive functioning, effects on neurotransmitters, protection against ischemic damage, and increased survival of brain cells (21, 39). According to network analysis theory (40), core symptoms have the greatest impact on the other symptoms over the entire network. Regarding those patients undergoing endocrine therapy, emotional symptoms might have strong interactions with sexual symptoms, vaginal symptoms, vasomotor symptoms, and neurological symptoms. The findings were also confirmed in the network analysis of gastric cancer patients, which showed that treating psychological distress and enhancing emotional well-being can be high-impact intervention targets throughout the cancer trajectory (41). Therefore, further interventions targeting emotional symptoms, such as psychosocial support, may be the optimal strategy to reduce the overall symptom burden.
Although previous studies provided evidence that receiving aromatase inhibitors could induce musculoskeletal symptoms frequently and vaginal discharge was more frequent in selective estrogen receptor modulator therapy (2, 9, 13, 42), we did not detect any significant differences in the network structure and global strength between treatment subgroups. One reason was network approaches in which constructs were modeled in terms of interactions between their constituent factors rather than the incidence or severity (36), and the other reason might be that we could not control all the other clinical covariates in the subgroup network analyses (29). However, it is worth noting that the edge difference test suggested that compared with the selective estrogen receptor modulator group, the network of patients treated with aromatase inhibitors had increased connections among vaginal and sexual symptoms, which implied that selective estrogen receptor modulators and aromatase inhibitors potentially have distinctive effects on vaginal and sexual domains. To overcome vaginal and sexual issues, education consulting, using vaginal lubricant, and combining pelvic floor muscle relaxation exercises may be potential approaches (9, 43).
First, according to Epskamp et al. (32), in a 20-node and 15-node network, 210 parameters and 120 parameters needed to be estimated, respectively. To reliably estimate these parameters, the number of observations needed typically exceeds the number available in characteristic psychological data. Therefore, our sample size was appropriate. Nevertheless, network analysis is a data-driven approach, and generally, the larger the sample size is, the more stable the network. Therefore, it is necessary to verify the results using different algorithms in other independent data with larger samples. Second, we excluded four symptoms based on low prevalence and clinical experience, and we did not include clinical covariates in our subgroup networks because they were not consistent across treatment regimens (36), which could introduce information bias and variable selection bias. Third, we applied an online survey to collect data using convenience sampling, which attracted many younger and higher education level participants, thus potentially introducing participant selection bias. Last but not least, it was a cross-sectional study that limited causality determination among symptoms and the generalizability of the findings. Thus, it is necessary to conduct longitudinal research to develop dynamic networks in the future.
In conclusion, we found that emotional symptoms (i.e., mood swings and irritability) were frequently reported by breast cancer patients during endocrine therapy and were consistently central in the symptom networks across not adjusting and adjusting clinical covariates and treatment subgroups. It was suggested that emotional symptoms could be an important target for reducing the overall symptom burden in breast cancer patients during endocrine therapy. Although causal conclusions cannot be drawn, if the interrelatedness of these symptoms is assumed, then developing interventions targeting emotional symptoms may reduce multiple other symptoms through a negative feedback loop of other interrelated symptoms. The findings of this study need to be verified by larger samples and using different algorithms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Feng Jing, MjExMTExNzAwMDFAbS5mdWRhbi5lZHUuY24=.
The studies involving human participants were reviewed and approved by Institutional Review Boards of Fudan University School of Nursing (No.IRB#2017-05-01). The patients/participants provided their written informed consent to participate in this study.
The manuscript was written by FJ. ZZ provided statistical method guidance. JQ and LT contributed to the data collection. LX provided critical feedback. YH and WX directed the writing and revision of the paper. All authors contributed to the article and approved the submitted version.
The National Natural Science Foundation of China (Grant Number: 72004034; 82272922), and the China Medical Board Open Competition Program (Grant Number: #20-371) provided funding for this research.
The researchers would like to express their gratitude to the participants for their involvement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1081786/full#supplementary-material
Supplementary Table 1 | Weight of each connection in GLASSO network without clinical covariates.
Supplementary Table 2 | Weight of each connection in GLASSO network with clinical covariates.
Supplementary Table 3 | Node strength of the network without and with clinical covariates (n = 613).
1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (2018) 391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3
2. Early Breast Cancer Trialists' Collaborative G. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol (2022) 23(3):382–92. doi: 10.1016/S1470-2045(21)00758-0
3. Krauss K, Stickeler E. Endocrine therapy in early breast cancer. Breast Care (Basel). (2020) 15(4):337–46. doi: 10.1159/000509362
4. American Cancer society. In: Breast cancer facts & figures 2019-2020, vol. 2. Atlanta: American Cancer Society, Inc. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf.
5. Berkowitz MJ, Thompson CK, Zibecchi LT, Lee MK, Streja E, Berkowitz JS, et al. How patients experience endocrine therapy for breast cancer: An online survey of side effects, adherence, and medical team support. J Cancer Surviv. (2021) 15(1):29–39. doi: 10.1007/s11764-020-00908-5
6. Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: Observational data over 12 months from the mind-body study. J Clin Oncol (2016) 34(8):816–+. doi: 10.1200/JCO.2015.64.3866
7. Martino G, Catalano A, Agostino RM, Bellone F, Morabito N, Lasco CG, et al. Quality of life and psychological functioning in postmenopausal women undergoing aromatase inhibitor treatment for early breast cancer. PloS One (2020) 15(3):e0230681. doi: 10.1371/journal.pone.0230681
8. Kadakia KC, Snyder CF, Kidwell KM, Seewald NJ, Flockhart DA, Skaar TC, et al. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist (2016) 21(5):539–46. doi: 10.1634/theoncologist.2015-0349
9. Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat (2008) 107(2):167–80. doi: 10.1007/s10549-007-9548-1
10. Jing F, Zhu Z, Qiu J, Tang L, Xu L, Xing W. Symptom profiles and related factors among breast cancer patients undergoing endocrine therapy: A latent profile analysis. Cancer Nursing. (2022). doi: 10.1097/NCC.0000000000001125
11. Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist (2015) 20(6):598–604. doi: 10.1634/theoncologist.2015-0007
12. Zhu Y, Cohen SM, Rosenzweig MQ, Bender CM. Symptom map of endocrine therapy for breast cancer: A scoping review. Cancer Nurs. (2019) 42(5):E19–e30. doi: 10.1097/NCC.0000000000000632
13. Morales L, Neven P, Timmerman D, Christiaens MR, Vergote I, Van Limbergen E, et al. Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anticancer Drugs (2004) 15(8):753–60. doi: 10.1097/00001813-200409000-00003
14. Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA, et al. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer-Am Cancer Soc (2014) 120(16):2403–11. doi: 10.1002/cncr.28756
15. Li HJ, Sereika SM, Marsland AL, Conley YP, Bender CM. Symptom clusters in women with breast cancer during the first 18 months of adjuvant therapy. J Pain Symptom Manage (2020) 59(2):233–41. doi: 10.1016/j.jpainsymman.2019.10.002
16. Borreani C, Alfieri S, Infante G, Miceli R, Mariani P, Bosisio M, et al. Aromatase inhibitors in postmenopausal women with hormone receptor-positive breast cancer: Profiles of psychological symptoms and quality of life in different patient clusters. Oncology (2021) 99(2):84–95. doi: 10.1159/000509651
17. Wen C, Wang Y, Sun H, Yu X. Symptom clusters and the effects on quality of life among breast cancer patients receiving aromatase in hibitors [in Chinese]. Chin J Nurs. (2021) 56(08):1201–7.
18. Schover LR, Baum GP, Fuson LA, Brewster A, Melhem-Bertrandt A. Sexual problems during the first 2 years of adjuvant treatment with aromatase inhibitors. J Sex Med (2014) 11(12):3102–11. doi: 10.1111/jsm.12684
19. Olufade T, Gallicchio L, MacDonald R, Helzlsouer KJ. Musculoskeletal pain and health-related quality of life among breast cancer patients treated with aromatase inhibitors. Support Care Cancer. (2015) 23(2):447–55. doi: 10.1007/s00520-014-2364-3
20. Frechette D, Paquet L, Verma S, Clemons M, Wheatley-Price P, Gertler SZ, et al. The impact of endocrine therapy on sexual dysfunction in postmenopausal women with early stage breast cancer: Encouraging results from a prospective study. Breast Cancer Res Treat (2013) 141(1):111–7. doi: 10.1007/s10549-013-2659-y
21. Ates O, Soylu C, Babacan T, Sarici F, Kertmen N, Allen D, et al. Assessment of psychosocial factors and distress in women having adjuvant endocrine therapy for breast cancer: the relationship among emotional distress and patient and treatment-related factors. Springerplus (2016) 486(2016). doi: 10.1186/s40064-016-2136-2
22. Rha SY, Lee J. Stable symptom clusters and evolving symptom networks in relation to chemotherapy cycles. J Pain Symptom Manage (2021) 61(3):544–54. doi: 10.1016/j.jpainsymman.2020.08.008
23. Zhu Z, Hu Y, Xing WJ, Guo MD, Zhao R, Han SY, et al. Identifying symptom clusters among people living with HIV on antiretroviral therapy in China: A network analysis. J Pain Symptom Manage (2019) 57(3):617–26. doi: 10.1016/j.jpainsymman.2018.11.011
24. Armour C, Fried EI, Deserno MK, Tsai J, Pietrzak RH. A network analysis of DSM-5 posttraumatic stress disorder symptoms and correlates in U.S. military veterans. J Anxiety Disord (2017) 45:49–59. doi: 10.1016/j.janxdis.2016.11.008
25. Solmi M, Collantoni E, Meneguzzo P, Degortes D, Tenconi E, Favaro A. Network analysis of specific psychopathology and psychiatric symptoms in patients with eating disorders. Int J Eat Disorder. (2018) 51(7):680–92. doi: 10.1002/eat.22884
26. Hevey D. Network analysis: A brief overview and tutorial. Health Psychol Behav Med (2018) 6(1):301–28. doi: 10.1080/21642850.2018.1521283
27. Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol Methods (2018) 23(4):617–34. doi: 10.1037/met0000167
28. Borsboom D, Cramer AO. Network analysis: An integrative approach to the structure of psychopathology. Annu Rev Clin Psychol (2013) 9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608
29. de Rooij BH, Oerlemans S, van Deun K, Mols F, de Ligt KM, Husson O, et al. Symptom clusters in 1330 survivors of 7 cancer types from the PROFILES registry: A network analysis. Cancer-Am Cancer Soc (2021) 127(24):4665–74. doi: 10.1002/cncr.33852
30. Bryant RA, Creamer M, O'Donnell M, Forbes D, McFarlane AC, Silove D, et al. Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: A network analysis. JAMA Psychiatry (2017) 74(2):135–42. doi: 10.1001/jamapsychiatry.2016.3470
31. Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-b. Breast Cancer Res Treat (1999) 55(2):189–99. doi: 10.1023/A:1006263818115
32. Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: A tutorial paper. Behav Res Methods (2018) 50(1):195–212. doi: 10.3758/s13428-017-0862-1
33. Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics (2008) 9(3):432–41. doi: 10.1093/biostatistics/kxm045
34. Zhu Z, Sun YL, Kuang Y, Yuan XY, Gu HY, Zhu J, et al. Contemporaneous symptom networks of multidimensional symptom experiences in cancer survivors: A network analysis. Cancer Med-Us. (2023) 12(1):663–73. doi: 10.1002/cam4.4904
35. Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: Generalizing degree and shortest paths. Soc Networks. (2010) 32(3):245–51. doi: 10.1016/j.socnet.2010.03.006
36. van Borkulo CD, van Bork R, Boschloo L, Kossakowski JJ, Tio P, Schoevers RA, et al. Comparing network structures on three aspects: A permutation test. Psychol Methods (2022). doi: 10.1037/met0000476
37. van Borkulo CD. A tutorial on r package NetworkComparisonTest (NCT). Groningen: University of Groningen (2019).
38. van Londen GJ, Beckjord EB, Dew MA, Cooper KL, Davidson NE, Bovbjerg DH, et al. Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support Care Cancer. (2014) 22(4):937–45. doi: 10.1007/s00520-013-2041-y
39. Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: Cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol (2009) 48(1):76–85. doi: 10.1080/02841860802314738
40. Weems CF. Commentary on the special issue on network analysis: Assessment, intervention, theory, and the nature of reality: Actualizing the potential of network perspectives on posttraumatic stress disorder. J Trauma Stress. (2020) 33(1):116–25. doi: 10.1002/jts.22482
41. Shim EJ, Ha H, Suh YS, Kong SH, Lee HJ, Yang HK, et al. Network analyses of associations between cancer-related physical and psychological symptoms and quality of life in gastric cancer patients. Psycho-Oncology (2021) 30(6):946–53. doi: 10.1002/pon.5681
42. Takei H, Ohsumi S, Shimozuma K, Takehara M, Suemasu K, Ohashi Y, et al. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National surgical adjuvant study of breast cancer 04 (N-SAS BC 04). Breast Cancer Res Tr. (2012) 133(1):227–36. doi: 10.1007/s10549-011-1943-y
Keywords: breast cancer, endocrine therapy, symptom network, network analysis, nursing
Citation: Jing F, Zhu Z, Qiu J, Tang L, Xu L, Xing W and Hu Y (2023) Contemporaneous symptom networks and correlates during endocrine therapy among breast cancer patients: A network analysis. Front. Oncol. 13:1081786. doi: 10.3389/fonc.2023.1081786
Received: 08 November 2022; Accepted: 21 March 2023;
Published: 31 March 2023.
Edited by:
Shibiao Wan, University of Nebraska Medical Center, United StatesReviewed by:
Daowei Yang, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2023 Jing, Zhu, Qiu, Tang, Xu, Xing and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijie Xing, eGluZ3dlaWppZUBmdWRhbi5lZHUuY24=; Yan Hu, aHV5YW5AZnVkYW4uZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.