
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 27 March 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1081258
This article is part of the Research TopicMethods in Genitourinary OncologyView all 6 articles
Objective: Our aim is to evaluate the safety and efficacy of iodine-125 seed strand for intraluminal brachytherapy on ureteral carcinoma.
Methods: From November 2014 to November 2021, 22 patients with ureteral cancer not suitable for surgical resection were enrolled. Iodine-125 seed strand was inserted under c-arm CT and fluoroscopic guidance. The technical success rate, complications, disease control rate, and survival time were evaluated. Hydronephrosis Girignon grade and ureteral cancer sizes before and after treatment were compared.
Results: A total of 46 seed strands were successfully inserted and replaced, with a technical success rate of 100% and median procedure time of 62 min. No procedure-related death, ureteral perforation, infection, or severe bleeding occurred. Minor complications were observed in eight (36.4%) patients, and migration of seed strand was the most common complication. Six months after seed strand brachytherapy, one complete response, three partial responses, and five stable diseases were evaluated, and the disease control rate was 64.3%. The Girignon grade of hydronephrosis was significantly improved 1 to 3 months after seed strand insertion. Disease control rates were 94.4, 62.5, and 64.3% at 1-, 3-, and 6-month follow-up. Twenty patients were successfully followed up, with a mean follow-up of 18.0 ± 14.5 months. The median overall survival and progress-free survival were 24.7 and 13.0 months, respectively.
Conclusion: Iodine-125 seed strand is safe and effective for intraluminal brachytherapy and can be used as an alternative to patients with ureteral carcinoma who are not suitable for surgical resection or systemic combined therapy.
Ureter transitional cell carcinoma is a relative rare disease, which account for about 1–3% of urinary transitional cell carcinoma cases (1). Surgical resection is the standard treatment for ureteral carcinoma. Radical ureterectomy with excision of the kidney and bladder cuff resection is generally recommended (2), considering that transitional cell carcinoma may develop metachronously or synchronously in a multifocal manner at different urinary tract sites. However, radical resection causes the loss of kidney to patient, which is not an easy choice for patients with renal insufficiency, isolated kidney, or bilateral ureteropathy. Some patients do not undergo radical nephroureterectomy due to advanced age, comorbidities, or intolerance or refusal of the procedure (3).
Traditional external radiotherapy is usually recommended for patients with advanced ureteral carcinoma and may be advantageous in several aspects. For example, adjuvant radiotherapy in the setting of locally advanced ureteral carcinoma improves locoregional control following definitive surgery (4). However, in ureteral lesions often close to intestines, spinal cord, and blood vessels, long-term high-dose radiotherapy may result in intestinal injury, spinal cord damage, or retroperitoneal fibrosis (5). As a novel kind of radiotherapy, intraluminal brachytherapy with radioactive iodine-125 seed strand has been used in some malignant tumors patients who cannot undergo or refuse surgical resection and shows encouraging efficacy (6), including advanced/recurrent esophageal cancer (7), malignant biliary obstruction (8), and hepatocellular carcinoma with portal vein tumor thrombus (9). Inspired by previous intraluminal brachytherapy, we wanted to explore whether iodine-125 seed strand brachytherapy is also suitable for the treatment of malignant ureteral obstruction. We herein presented 22 patients with ureteral carcinoma who were treated with intraluminal brachytherapy by iodine-125 seed strand insertion.
From November 2014 to November 2021, all patients with ureteral carcinoma treated by intraluminal brachytherapy were enrolled. Contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) were carried out at baseline (Figures 1A–C, 2A–C, 3A). The inclusion criteria: patients with age > 18 years, with an estimated life expectancy exceeds 3 months; patients with renal insufficiency, isolated kidney, or bilateral ureteropathy; patients not suitable for radical nephroureterectomy or combined therapy (GC chemotherapy + PD-1) due to advanced age, comorbidities; and patients with intolerance or refusal of surgical resection. Exclusion criteria: severe cardiovascular comorbidities including unstable angina, myocardial infarction, or uncontrolled hypertension; coagulopathy or bleeding diathesis; and severe infection. Ethical approval was waived by the Institutional Review Board of University due to its retrospective nature. Informed consent had been obtained from all patients before procedure.
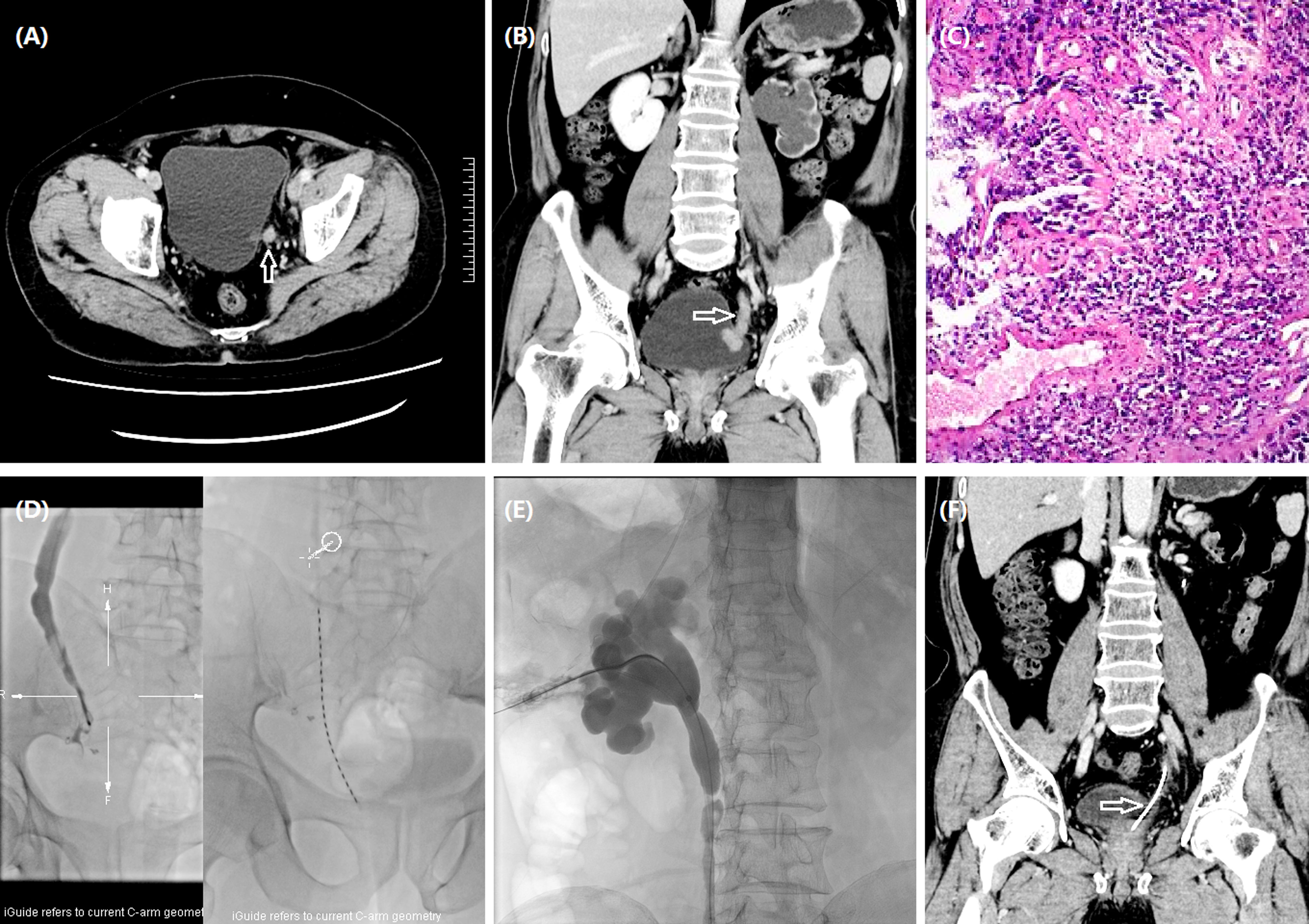
Figure 1 Iodine-125 seed strand placement for a 70-year man with ureteral carcinoma. (A) A left ureteral carcinoma (arrow) was shown by CT. (B) The tumor (arrow) was located in the left lower ureteric segment and bladder entrance with significantly dilated renal pelvis and thinning of renal cortex. (C) Pathology showed low-grade papillary urothelial carcinoma of the ureter without definite infiltration. (D) Urothrography revealed a 54.7-mm-long occlusion in the left lower ureter, and an iodine-125 seed strand with 23 seeds was inserted. (E) Nephrostomy was performed under fluoroscopic guidance. (F) Complete response (arrow) was detected by CT after 5.9 months.
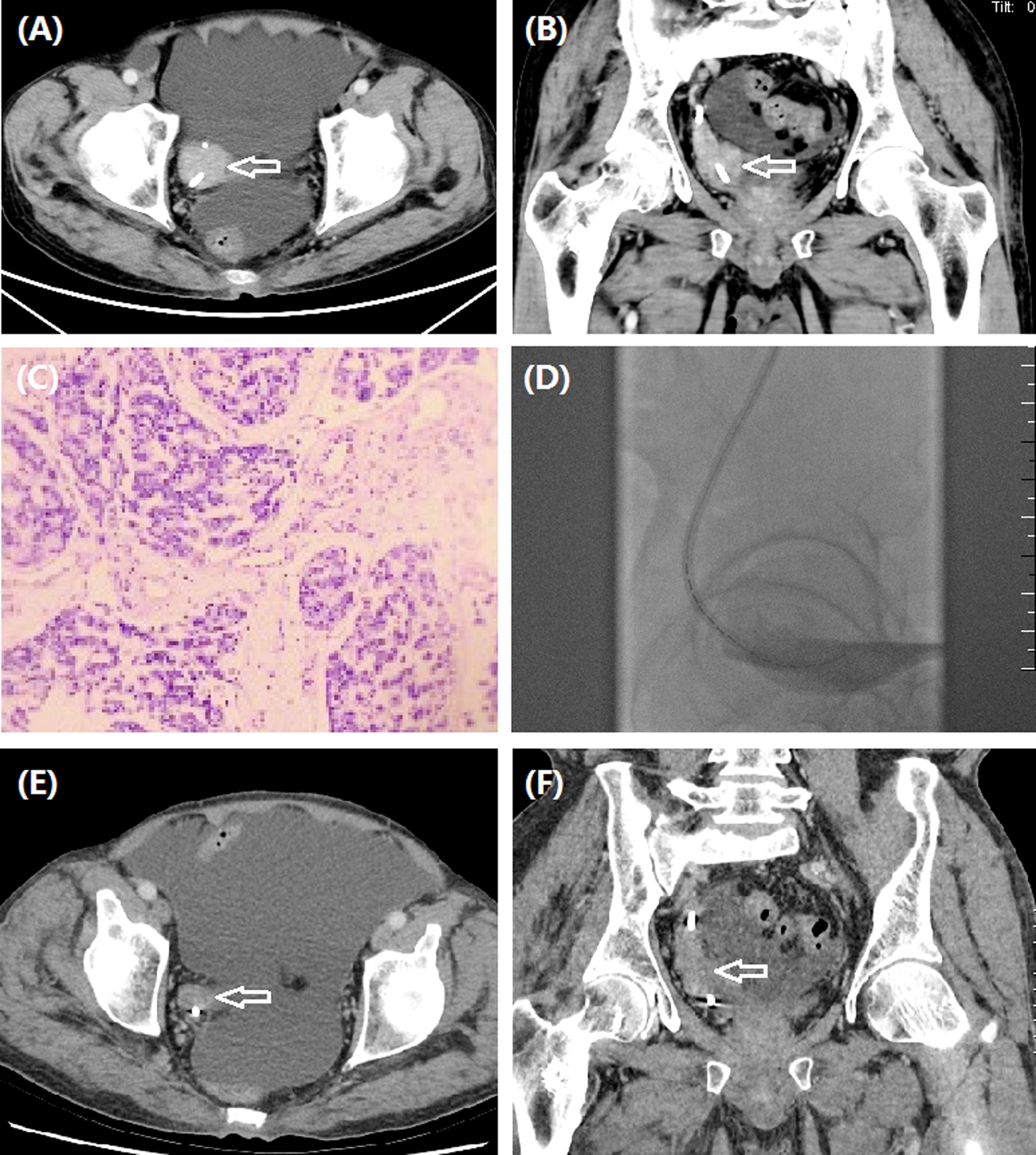
Figure 2 A 61-year man treated by iodine-125 seed strand for right ureteral carcinoma. (A, B) CT revealed ureteral carcinoma (arrow) of right lower ureter. (C) Pathology was confirmed to be ureteric papillary urothelial carcinoma with tumor invasion in the local lamina propria. (D) Iodine-125 seed strand with 15 seeds was inserted in the occlusive segment. (E–F) Stable tumor (arrow) was detected by CT after 6.0 months.
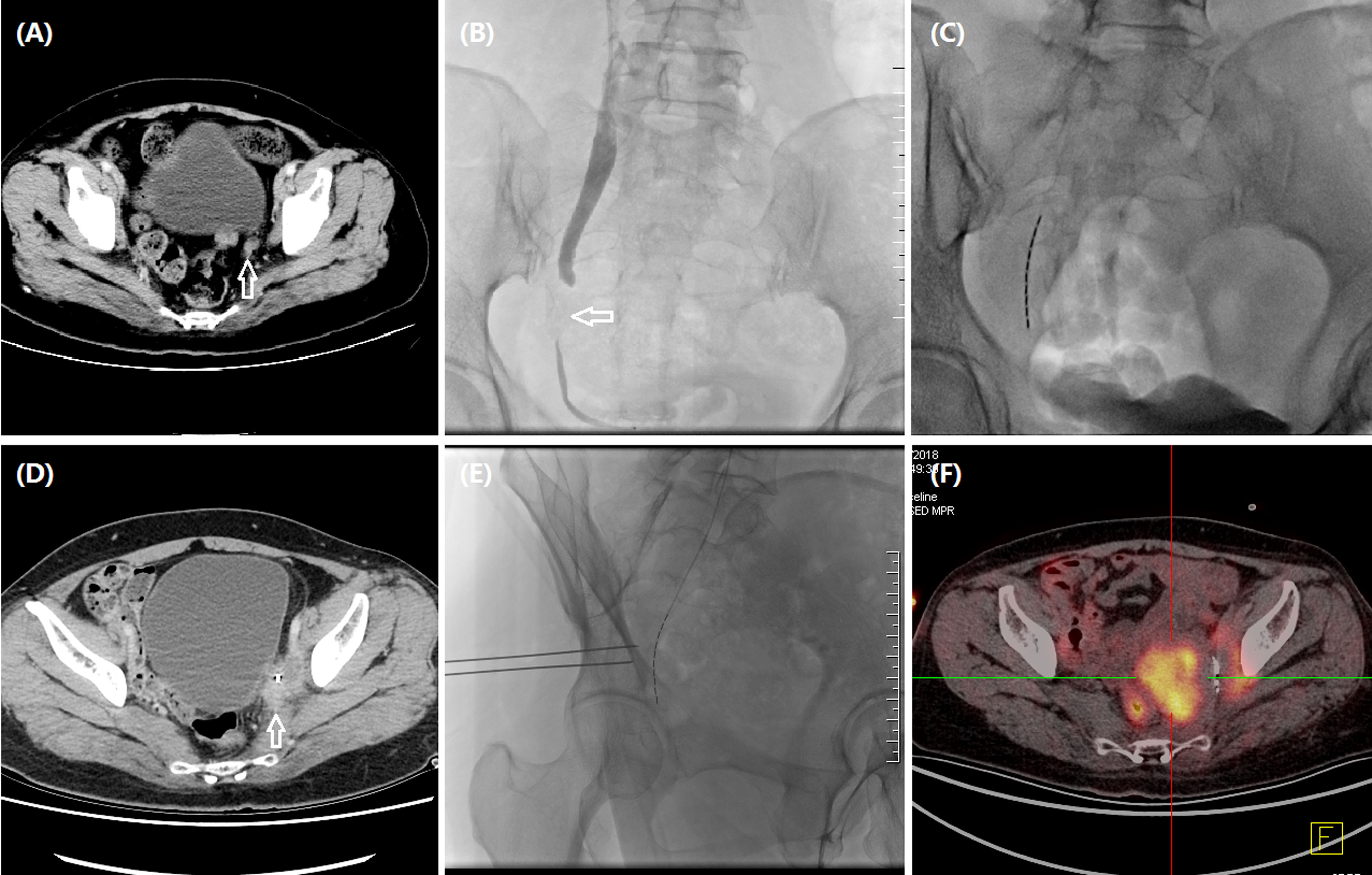
Figure 3 A 48-year woman treated by iodine-125 seed strand for metastatic carcinoma of ureter. (A) CT revealed metastatic carcinoma of left ureter due to recurrent cervical carcinoma (arrow). (B) Urothrography revealed a segmental occlusion (arrow) in the lower segment of left ureteral segment, approximately 27.2 mm in length. (C) Iodine-125 seed strand with 10 seeds was inserted in the occlusive segment. (D) CT revealed an enlarged tumor (arrow) 2.2 months later. (E) Progressive tumors were treated with percutaneous particle implantation, and a total of 10 particles were implanted. (F) Tumor progression was detected by PET-CT after 12.0 months’ follow-up.
The patient was prone on the examination table, and percutaneous renal pelvis puncture was performed under the virtual navigation of c-arm CT iGuide and fluoroscopic guidance. The upper segment of the renal pelvis and the ureter were visible by urography, and the involved ureter was shown as local stenosis or occlusion. The lesion in the ureter was shown as filling defect of the contrast agent. The ureteral obstruction length was measured to assess the number of seed placements. For severe upper urinary tract obstruction patients, a 0.035 inch guidewire and a 5F catheter were used to carefully pass through the ureteral stenosis or occlusion segment into the bladder. A stiff guidewire was then exchanged, and 8F short sheath was inserted. Another stiff guidewire was introduced via the short sheath. After removal of short sheath, an 8.5F drainage tube was inserted along a stiff guidewire into renal pelvis for urine drainage.
The seed strand was introduced into the lesion segment along another stiff guidewire and fixed to avoid migration. The iodine-125 seeds (seed activity: 0.7 mCi, Saide Biological Technology Co., Ltd., Tianjin, China) are 0.8 mm in diameter and 4.8 mm in length and have a half-life of 59.6 days and an irradiation distance of 17 mm. A seed implantation gun was used to push the seeds into a 3F catheter, arranging the iodine-125 seeds in a row within the catheter to form a seed strand. The length of both strand ends was 2 cm longer than the lesion segment. Both ends of the seed strand are sealed with fire to avoid seed migration. The following formula was used to assess the number (N) of seeds implanted: N = (lesion segment length + 2 cm + 2 cm)/0.45. In this study, the mean D90 (the dose covering 90% of the gross tumor volume) and the organ at risk (OAR) doses were 50.7 and 3.8 Gy, respectively. The seed strand and the drainage tube were removed or exchanged 2–3 months after the insertion (Figures 1D, E, 2D, 3B, C, E).
The technical success rate, complications, disease control rate, and survival time were evaluated. Hydronephrosis Girignon grade and ureteral cancer sizes before and after treatment were compared. Technical success rate was defined as the successful placement of nephrostomy and seed strand. Two to 3 months later, abdominal CT scan was performed to evaluate clinical efficacy. Tumor evaluation was performed by using RECIST to evaluate the efficacy (Figures 1F, 2E, F, 3D, F).
From November 2014 to November 2021, 22 patients with ureteral cancer not suitable for surgical resection were enrolled in this study, including 12 men and 10 women (mean age 68.1 ± 11.1 years, range 46–84 years). Disease characteristics and baseline demographics are shown in Table 1. Local metastasis was present in 10 patients (45.5%), and distant metastasis was found in three patients (13.6%). Twelve patients were confirmed by ureteroscopy or percutaneous forceps biopsy as ureter transitional cell carcinoma. Nine patients (40.9%) complained of gross hematuria, and median duration of symptom was 6.0 months. Five patients (22.7%) received synchronous radiochemotherapy. Five patients with metastatic carcinoma of ureter from cervical cancer (22.7%) received prior radiochemotherapy, including concurrent radiochemotherapy (TP chemotherapy + intracavitary brachytherapy 15Gy/3F) in four patients and DP chemotherapy (Docetaxel + nedaplatin) in the remained one patient.
As shown in Table 2, a total of 46 seed strand sessions were successfully inserted and replaced in 22 patients, with a mean of 2.1 ± 1.5 sessions. Ten patients (45.5%) completed two to five sessions of seed strand replacement or removal, with median indwelling interval of 3.0 months. The median procedure time was 62 min, and each strand was loaded with 20 iodine-125 seeds. Eighteen patients (81.8%) received other interventional treatments, including transcatheter arterial chemoembolization (n = 10) and percutaneous seed implantation (n = 3), and both (n = 5) for progressive and migrated tumors.
Minor complications were observed in eight (36.4%) patients; no severe adverse events occurred such as procedure-related death, ureteral perforation, infection, or severe bleeding. Migration of seed strand was the most common complication, which was observed in five patients (22.7%). Seed strand adjustment, replacement, or removal was performed for migrated strand. Mild abdominal pain of renal puncture site was observed in three patients (13.6%), and fever was observed in two patients (9.1%). One patient showed obstruction of nephrostomy tube, and the tube was successfully replaced.
Six months after seed strand brachytherapy, one complete response, three partial responses and five stable diseases were evaluated, and the disease control rate was 64.3%. The Girignon grade of hydronephrosis was significantly improved 1–3 months after seed strand insertion. One patient achieved a complete response 6 months after seed strand placement according to the RECIST criteria. Partial response was observed in four, five, and three patients at 1-, 3-, and 6-month follow-up. The objective response rates were 22.2, 31.3, and 28.6%, respectively, at 1, 3, and 6 months. The disease control rates were 94.4, 62.5, and 64.3%, respectively at 1, 3, and 6 months (Table 3).
Two patients were lost of follow-up, with a mean follow-up of 18.0 ± 14.5 months and follow-up rate of 90.9%. Seven patients (31.8%) died of tumor progression. The median overall survival and progress-free survival were 24.7 and 13.0 months, respectively (Figure 4). The 6-, 12-, and 36-month overall survival rates were 95.0, 88.2, and 30.5%, respectively. The 6-, 12-, and 36-month progression-free survival rates were 60.5, 44.8, and 22.4%, respectively.
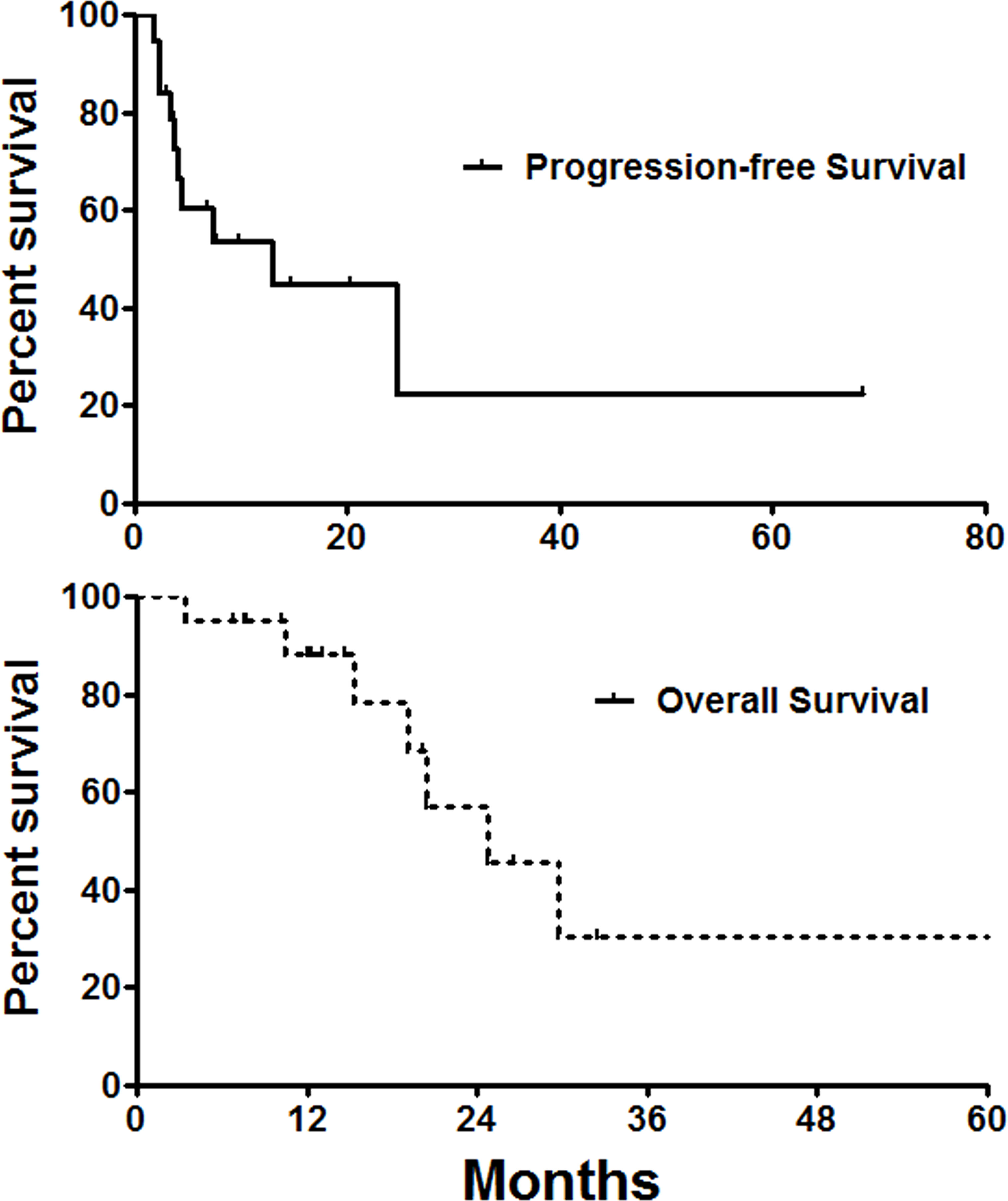
Figure 4 Survival follow-up. The median overall survival and progress-free survival were 24.7 and 13.0 months, respectively.
Since the renal pelvis, ureter, and bladder all originate from the mesoderm, with similar biological and morphology behavior, radical resection of bladder and ureteral carcinoma requires simultaneous resection of the bladder and ureter to reduce tumor metastasis and recurrence. A recurrence rate of 15–50% is observed in ureteral carcinoma after surgical resection of unilateral upper urinary carcinoma, and even reported a high rate of up to 70% (2, 10). Radical nephroureterectomy is traumatic and costly, patients have to lose their kidneys, but the recurrence rate is still high. This is not an easy choice for patients with renal insufficiency, isolated kidney, or bilateral ureteropathy. Some patients do not undergo radical nephroureterectomy due to advanced age, comorbidities, or intolerance or refusal of the procedure (3). In addition, locoregional urothelial malignancies of urinary tract was treated by adjuvant radiotherapy or combined with chemotherapy (5, 11, 12), and confocal laser endomicroscopy has been used for upper tract urothelial carcinoma (13, 14), although radiotherapy is rarely used for ureter tumors.
Currently, more minimally invasive alternative methods are urgently needed in clinical practice for such patients with ureteral carcinoma who are not suitable for surgical resection. Minimally invasive treatment using interstitial puncture and the implantation of iodine-125 seeds has become a common clinical technique for the treatment of various solid malignancies (15), such as oral and maxillofacial malignant tumors (16). However, it remains unclear whether malignant tumors of cavity organs such as ureteral carcinoma can be treated by radioactive seeds brachytherapy, although cavity organs such as esophageal cancer have been treated with low-dose-rate brachytherapy with radioactive stent (17) and nutrient tube (7). It has also been reported that iodine-125 seeds loading inside the catheter to form a seed strand structure to treat malignant bile duct obstruction (18, 19) and portal vein tumor thrombus (20) showed satisfactory clinical results.
The ureter is a thin, long, and tubular shape organ, and ureteral carcinoma can easily cause ureteral obstruction and hydrops, even when the tumor is very small. Inspired by previous intraluminal brachytherapy, we aimed to explore whether iodine-125 seed strand brachytherapy is also suitable for the treatment of malignant ureteral obstruction (21). The iodine-125 seeds have an irradiation distance within 1.7 cm; intraluminal low-dose brachytherapy may theoretically play a safer and better role for ureteral carcinoma (21). We herein presented 22 patients with ureteral carcinoma who were treated with intraluminal brachytherapy by iodine-125 seed strand insertion.
In this study, 46 seed strands were successfully inserted and replaced, with a technical success rate of 100%. The Girignon grade of hydronephrosis was significantly improved 1–3 months after seed strand insertion. Disease control rates were 94.4, 62.5, and 64.3% at 1-, 3-, and 6-month follow-up. The median overall survival and progression-free survival were 24.7 and 13.0 months, respectively. Our results suggest that intraluminal brachytherapy by iodine-125 seed strand is feasible and effective alternative to patients who are not suitable for surgical resection.
Regarding safety, it is also reported that iodine-125 brachytherapy may cause radiation damage to vascular (22), nerve (23), and tracheal structures (24), but the results all showed mild target and surrounding organ damage. In our study, no procedure-related death or severe complications occurred. Only eight (36.4%) patients had minor complications, and migration of seed strand was the most common complication.
There were several limitations in this study: (1) Only 22 patients were studied in this retrospective study; further studies with larger sample size are required. (2) Less than half of patients received more than two sessions of seed strand, which may contribute to confounding factor affecting efficacy evaluation. (3) The radiation distance of the seed is short, and it may not be enough for metastatic cancer or large lesions; other local or systematic treatments are required. (4) The combined therapy is recommended for advanced ureteral carcinoma. In this study, patients are not suitable for radical nephroureterectomy or combined therapy (GC chemotherapy + PD-1) due to advanced age, comorbidities. Theoretically, the combination of local iodine-125 strand therapy with the systemic combined therapy is likely to achieve better efficacy for advance ureteral carcinoma.
As a localized and palliative therapy, iodine-125 seed strand is safe and effective for intraluminal brachytherapy and can be used as an alternative to patients with ureteral carcinoma who are not suitable for surgical resection or systemic combined therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was waived by the Institutional Review Board of University due to its retrospective nature. The patients/participants provided their written informed consent to participate in this study.
XH and JR conceptualized the study. YB, DJ, and JZ developed the methodology. DJ and JZ validated the study. YB, DJ, and JZ performed the formal analysis. YB and DJ wrote and prepared the original draft. KG and XT wrote, reviewed, and edited the article. XH and JR conducted the visualization. XH and JR supervised the study. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ito Y, Kikuchi E, Tanaka N, Miyajima A, Mikami S, Jinzaki M, et al. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J Urol (2011) 185:1621–6. doi: 10.1016/j.juro.2010.12.035
2. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol (2015) 68:868–79. doi: 10.1016/j.eururo.2015.06.044
4. Zalay O, Yan M, Sigurdson S, Malone S. FE Vera-Badillo,A mahmud, adjuvant radiotherapy for upper tract urothelial carcinoma: Systematic review and meta-analysis. Curr Oncol (2022) 30:19–36. doi: 10.3390/curroncol30010002
5. Gustin P, Yossi S, Lafont M, Peyraga G, Tremolieres P, Rousseau D, et al. [Use of chemotherapy and radiotherapy in the treatment of urothelial carcinoma of the upper urinary tract]. Cancer Radiother (2015) 19:120–6. doi: 10.1016/j.canrad.2014.11.012
6. Li Q, Liang Y, Zhao Y, Gai B. Interpretation of adverse reactions and complications in Chinese expert consensus of iodine-125 brachytherapy for pancreatic cancer. J Cancer Res Ther (2019) 15:751–4. doi: 10.4103/jcrt.JCRT_884_18
7. Bi Y, Zhu X, Yu Z, Jiao D, Yi M, Han X, et al. Radioactive feeding tube in the palliation of esophageal malignant obstruction. Radiol Med (2020) 125:544–50. doi: 10.1007/s11547-020-01151-9
8. Jiao D, Wu G. J Ren,X han, study of self-expandable metallic stent placement intraluminal (125)I seed strands brachytherapy of malignant biliary obstruction. Surg Endosc (2017) 31:4996–5005. doi: 10.1007/s00464-017-5481-5
9. Zhang ZH, Zhang W, Gu JY, Liu QX, Ma JQ, Liu LX, et al. Treatment of hepatocellular carcinoma with tumor thrombus with the use of iodine-125 seed strand implantation and transarterial chemoembolization: A propensity-score analysis. J Vasc Interv Radiol (2018) 29:1085–93. doi: 10.1016/j.jvir.2018.02.013
10. Campi R, Cotte J, Sessa F, Seisen T, Tellini R, Amparore D, et al. Robotic radical nephroureterectomy and segmental ureterectomy for upper tract urothelial carcinoma: a multi-institutional experience. World J Urol (2019) 37:2303–11. doi: 10.1007/s00345-019-02790-y
11. Hahn AW, Giri S, Pathak R, Bhatt VR, Martin MG. Effect of adjuvant radiotherapy on survival in patients with locoregional urothelial malignancies of the upper urinary tract. Anticancer Res (2016) 36:4051–5. doi: 36/8/4051
12. Maehata Y, Kuriyama K, Aoki S, Araya M, Marino K, Onishi H. Stereotactic body radiotherapy for localized ureter transitional cell carcinoma: Three case reports. Case Rep Urol (2015) 2015:519897. doi: 10.1155/2015/519897
13. Bui D, Mach KE, Zlatev DV, Rouse RV, Leppert JT, Liao JC. A pilot study of In vivo confocal laser endomicroscopy of upper tract urothelial carcinoma. J Endourol (2015) 29:1418–23. doi: 10.1089/end.2015.0523
14. Villa L, Cloutier J, Cote JF, Salonia A, Montorsi F, Traxer O. Confocal laser endomicroscopy in the management of endoscopically treated upper urinary tract transitional cell carcinoma: Preliminary data. J Endourol (2016) 30:237–42. doi: 10.1089/end.2015.0644
15. Zhang H, Dev D, Yu H, Di X, Liang Y, Zhang L, et al. Feasibility of three-dimensional-printed template-guided (125)I seed brachytherapy and dosimetric evaluation in patients with malignant tumor. J Cancer Res Ther (2019) 15:793–800. doi: 10.4103/jcrt.JCRT_347_18
16. Wang Y, Kang P, He W, Li R. MR-guided 125I seed implantation treatment for maxillofacial malignant tumor. J Appl Clin Med Phys (2021) 22:92–9. doi: 10.1002/acm2.13112
17. Bi Y, Li J, Yi M, Yu Z, Han X, Ren J. Self-expanding segmental radioactive metal stents for palliation of malignant esophageal strictures. Acta Radiol (2020) 61:921-6. doi: 10.1177/0284185119886315
18. Hu X, Pang Q, Liu H, Qian Z, Jin H, Zhou L, et al. Inflammation-based prognostic scores in patients with extrahepatic bile duct lesions treated by percutaneous transhepatic biliary stenting combined with (125)I seeds intracavitary irradiation. Clin Transl Oncol (2019) 21:665–73. doi: 10.1007/s12094-018-1969-2
19. Jiao D, Zhou X, Li Z, Bi Y, Zhang Q, Li J, et al. A newly designed biliary brachytherapy drainage catheter for patients with malignant biliary obstruction: A pilot study. J Cancer Res Ther (2020) 16:286–91. doi: 10.4103/jcrt.JCRT_804_19
20. Wu YF, Wang T, Yue ZD, Zhao HW, Wang L, Fan ZH, et al. Stents combined with iodine-125 implantation to treat main portal vein tumor thrombus. World J Gastrointest Oncol (2018) 10:496–504. doi: 10.4251/wjgo.v10.i12.496
21. Jiao D, Yao Y, Xu K, Lei Q, Li Z, Han X, et al. Investigation of a novel brachytherapy ureteral stent: trial studies on normal beagle dogs. J Cancer Res Clin Oncol (2021) 147:1115–23. doi: 10.1007/s00432-021-03513-w
22. Zhang W, Luo J, Liu Q, Ma J, Qu X, Yang M, et al. Brachytherapy with iodine-125 seeds strand for treatment of main portal vein tumor thrombi: an experimental study in a rabbit model. Am J Cancer Res (2016) 6:587–99.
23. Jiao L, Zhang T, Wang H, Zhang W, Fan S, Huo X, et al. Implanting iodine-125 seeds into rat dorsal root ganglion for neuropathic pain: neuronal microdamage without impacting hind limb motion. Neural Regener Res (2014) 9:1204–9. doi: 10.4103/1673-5374.135326
Keywords: iodine-125 seed strand, intraluminal brachytherapy, ureteral carcinoma, nephrostomy, renal pelvis
Citation: Bi Y, Jiao D, Zhang J, Ren J, Han X, Guo K and Tu X (2023) Safety and efficacy of iodine-125 seed strand for intraluminal brachytherapy on ureteral carcinoma. Front. Oncol. 13:1081258. doi: 10.3389/fonc.2023.1081258
Received: 01 November 2022; Accepted: 14 March 2023;
Published: 27 March 2023.
Edited by:
Amin Nassar, Yale New Haven Health System, United StatesReviewed by:
Jure Murgic, Sisters of Charity Hospital, CroatiaCopyright © 2023 Bi, Jiao, Zhang, Ren, Han, Guo and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianzhuang Ren, cnJqanp6anJrQDEyNi5jb20=; Xinwei Han, ZHJlYW13ZWF2ZXIwOEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors share co-correspondence authorship
§ORCID: Jianzhuang Ren, orcid.org/0000-0003-0432-2640
Xinwei Han, orcid.org/0000-0002-8411-6427
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.