- 1Department of Radiotherapy & Oncology, The Second Affiliated Hospital of Soochow University, Suzhou, China
- 2Institution of Radiotherapy & Oncology, Soochow University, Suzhou, China
- 3Laboratory for Combined Radiotherapy and Immunotherapy of Cancer, The Second Affiliated Hospital of Soochow University, Suzhou, China
Patients with chemo-refractory metastatic colorectal cancer (mCRC) have poor prognoses. The application of programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors encouragingly improved the survival of mCRC patients with microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR). Unfortunately, it was ineffective for mCRC with microsatellite-stable (MSS)/proficient mismatch repair (pMMR), which accounted for 95% of mCRC. Radiotherapy can promote local control by directly killing tumor cells and inducing positive immune activities, which might help synergistically with immunotherapy. We present the report of an advanced MSS/pMMR mCRC patient who had progressive disease (PD) after first-line chemotherapy, palliative surgery and second-line chemotherapy combined with targeted therapy. Then the patient received the therapy of PD-1 inhibitor combined with radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF). According to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST1.1), the patient showed a complete response (CR) after triple-combined therapy with progression-free survival (PFS) for more than 2 years so far. The patient had no other significant adverse reactions except for fatigue (Grade 1). The triple-combination therapy provided a promising strategy for metastatic chemo-refractory MSS/pMMR mCRC patients.
Introduction
By 2021, colorectal cancer (CRC) was the third most common cause of cancer mortality worldwide. Meanwhile, metastasis was found at the first diagnosis in 20% of CRC patients (1). Although the United States Food and Drug Administration (FDA) has approved PD-1 inhibitor pembrolizumab for the treatment of microsatellite instability-high (MSI-H) metastatic CRC (mCRC), about 95% of mCRC patients are MSS/pMMR and cannot benefit from PD-1 inhibitor monotherapy (2). The clinical trials of KEYNOTE-016 and KEYNOTE-028 showed no response in MSS mCRC patients treated with pembrolizumab (3, 4). The preclinical studies of MSS colorectal cancer mice models have shown synergy between radiotherapy and anti-PD-1 in modulating anti-tumor immune responses (5, 6). It provides a theoretical basis for the combination of radiotherapy and immunotherapy. Recently, several I/II clinical studies have shown that the combination of radiotherapy and immunotherapy could improve clinical outcomes in mCRC patients with MSS/pMMR with acceptable toxicity (7–9). Granulocyte-macrophage colony-stimulating factor (GM-CSF), known as an immunomodulatory cytokine, might improve the efficacy of immunotherapy in advanced biliary cancers (10). It is necessary to explore novel strategies for treating MSS/pMMR mCRC, and combining anti-PD-1 immunotherapy with radiotherapy and GM-CSF therapy might be a potential one.
We present the report of a refractory mCRC patient with MSS/pMMR who received PD-1 inhibitor combined with Radiotherapy and GM-CSF. The patient demonstrated a sustained tumor response and prolonged progression-free survival (PFS) for over 2 years so far.
Case presentations
A patient in his mid 40s was diagnosed with ascending colon adenocarcinoma through colonoscopy biopsy and pathological examination on 21 January 2020. Further Positron Emission Tomography/Computed Tomography (PET/CT) imaging showed metastasis of retroperitoneal and celiac lymph nodes (Figure 1A). According to the AJCC 8th TNM staging system, the patient was staged T3N2bM1a (c-Stage IVA). Immunohistochemical (IHC) staining was AE1/AE3(+), MSH2 (+), MSH6 (+), MLH1 (+), PMS2 (+), HER-2 (0), PD-L1 (+,CPS=10), CD8 (+,15%), CD68 (+,80%) (Supplementary Figure 1). Genetic testing of tumor tissue revealed that missense mutation A146T was found in exon 4 of the KRAS gene. No mutation was found in the BRAF/NRAS. The microsatellite state detection showed a microsatellite-stable (MSS) phenotype by Next Generation Sequencing (NGS).
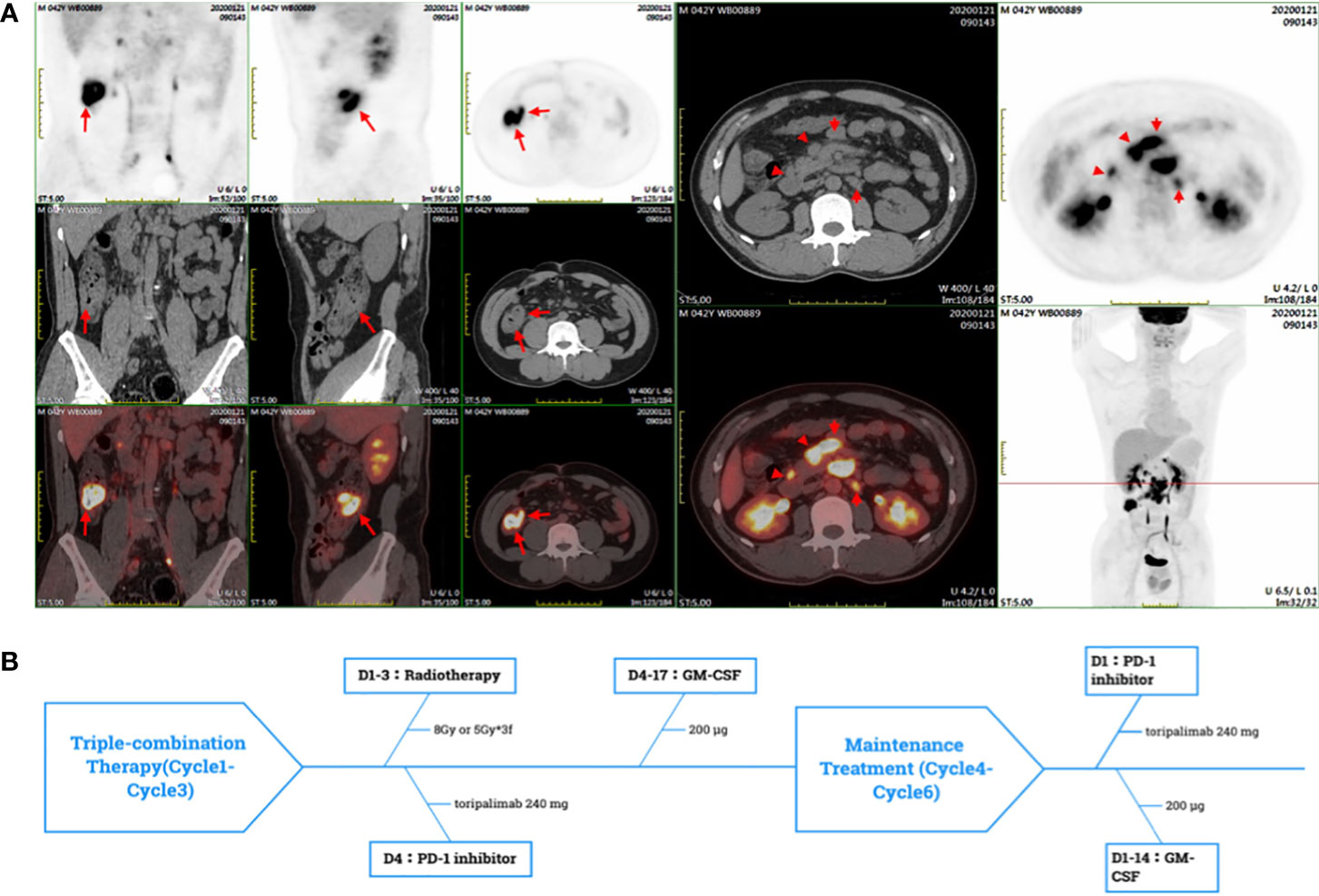
Figure 1 (A) The PET-CT showed the high SUVmean of ascending colon and enlarged lymph nodes of retroperitoneal and peritoneal. (B) In a PRaG cycle, radiotherapy was delivered for metastases, followed by GM-CSF subcutaneous (sc) injection once daily for two weeks, and toripalimab was intravenous(iv) once within one week after radiotherapy. PRaG Therapy was repeated every three weeks, and three cycles of triple-therapy were administered. Subsequently, the patient underwent three cycles of PD-1 inhibotor and GM-CSF maintenance treatment.
Considering that the patient was young and was willing to receive surgery, two cycles of conversion therapy of mFOLFOX4 were administered from January to February 2020. Bevacizumab was not added to the conversion therapy, which may cause surgical complications such as bleeding and gastrointestinal perforation. After the conversion chemotherapy, the patient underwent palliative surgery (R2 resection) in February 2020. All of the primary lesion and the part of the mesenteric lymph nodes were resected. The mesenteric and retroperitoneal lymph nodes were not resected. Then the patient received six cycles of chemotherapy of capecitabine plus irinotecan (mXELIRI) combined with Bevacizumab from March to July 2020. CT scan observed new lymph node metastases at mesenteric and retroperitoneal in August 2020, which indicated progressive disease (PD). It suggested the patient was insensitive to chemotherapy combined with vascular-targeted Therapy. In addition, neutropenia and gastrointestinal reaction (Grade 2) were observed during chemotherapy.
Considering the side effects of chemotherapy, the patient refused to continue the chemotherapy. Then the patient was enrolled in a prospective phase II clinical trial which was conducted to assess the clinical efficacy and safety of PD-1 (toripalimab) inhibitor combined with Radiotherapy and GM-CSF (Recombinant Human Interleukin-2(I) for Injection) in patients with advanced metastatic solid tumors on 10 August 2020 (ChiCTR1900026175, http://www.chictr.org.cn/index.aspx). We defined the triple-combined therapy of PD-1 inhibitor combined with Radiotherapy and GM-CSF as PRaG therapy. The patient underwent three cycles of PRaG therapy in August 2020 and September 2020. In the PRaG cycle, radiotherapy (8Gy or 5Gy/d, d1-3) was delivered for lymph node metastases, followed by subcutaneous injection of GM-CSF (200μg once daily, d4-17) and intravenous injection of toripalimab (240mg, d4). PRaG regimen was repeated every three weeks (Figure 1B). After the 3 cycles of PRaG therapy, the patient achieved partial response (PR) according to RECIST1.1 by CT scan. CT showed a significant decrease of irradiated lymph node metastases in retroperitoneal and celiac (Figures 2A, B), and a reduction of nonirradiated lymph node metastases in celiac was also observed (Figure 2C). Moreover, tumor markers of carcinoembryonic antigen (CEA) and carbohydrate antigen 242 (CA242) decreased to normal range after two cycles of PRaG therapy (Figure 3A).
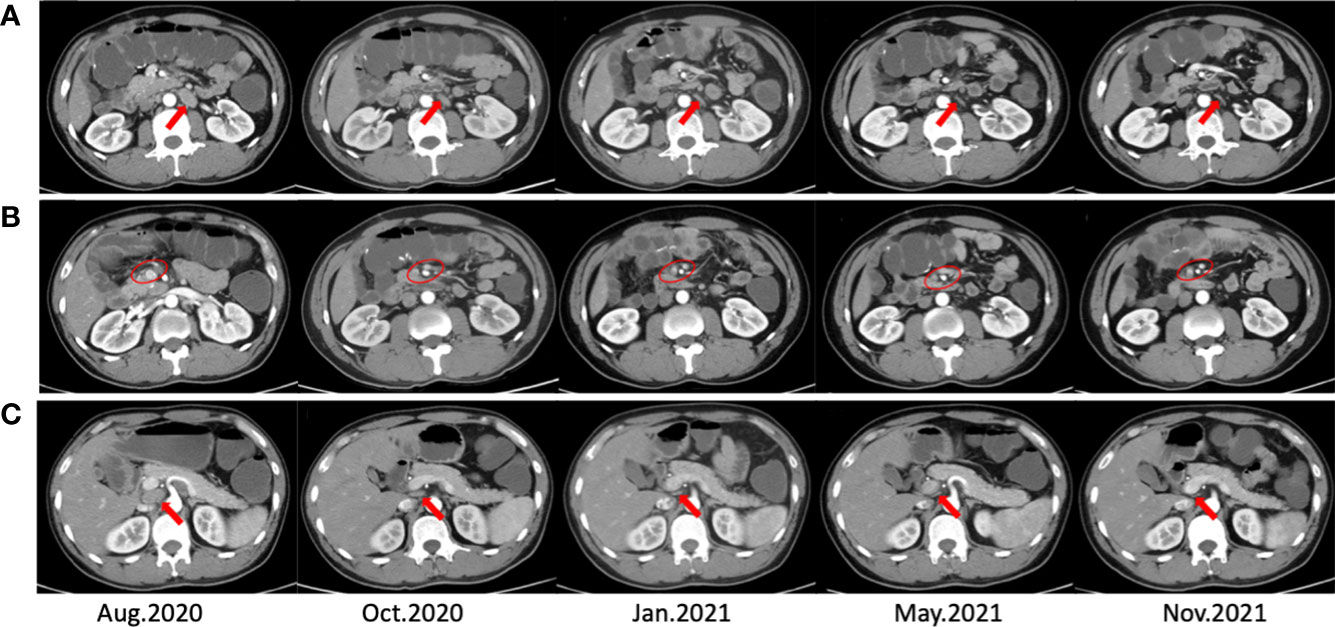
Figure 2 CT scans before, during, and after the PRaG therapy. The CT scans (A, B) showed shrunk and disappeared of irradiated lymph node metastases. The CT scans (C) showed shrunk and disappeared of nonirradiated lymph node metastases. The arrows point to individual lymph nodes, and the circles include fusion lymph nodes.
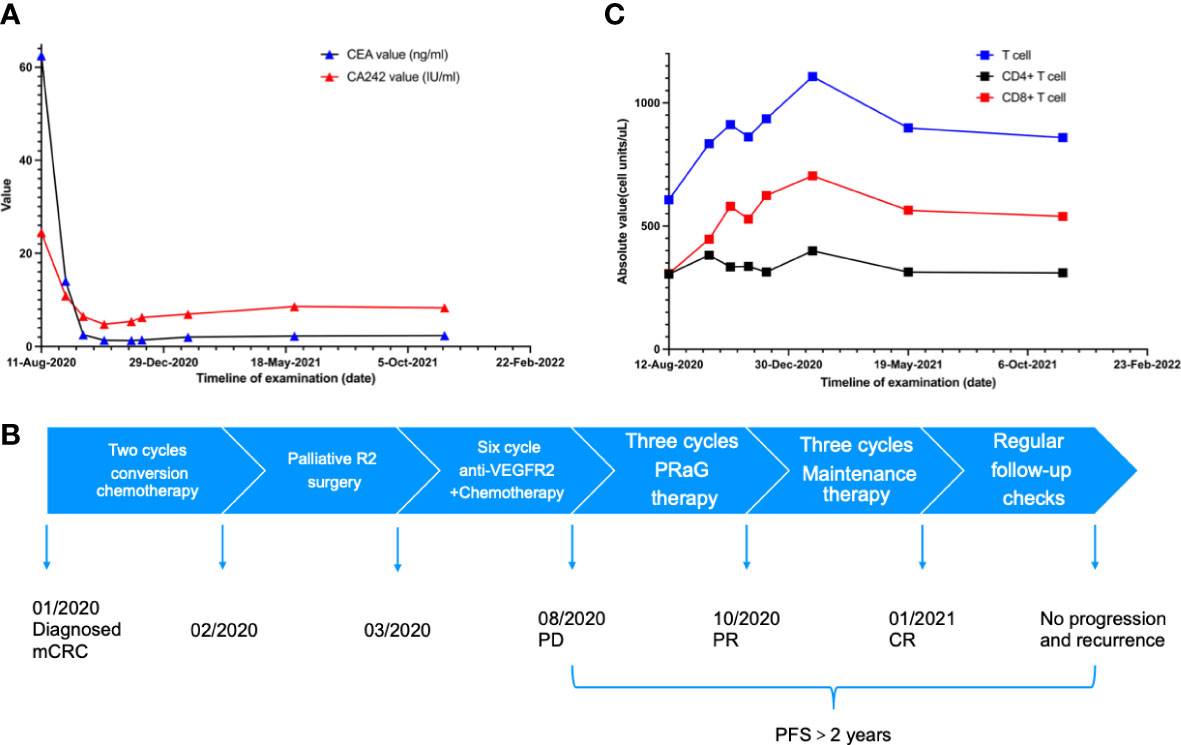
Figure 3 (A) The carcinoembryonic antigen (CEA) dropped to the normal range after two cycles of PRaG Therapy. In the whole course of treatment, the patients had no obvious adverse reactions. Due to the influence of COVID-19, the re-examination interval was longer than expected. (B) The scheme shows the complete treatment process of the patient. (C) The number and activation of lymphocytes are related to the efficacy of immunotherapy.
Three different metastatic sites were chosen for irradiation in three cycles. In the first two cycles, radiotherapy (8Gy/d, d1-3) was delivered for celiac metastatic lymph node and retroperitoneal metastatic lymph node. Considering the tolerance dose constraints of normal tissues, the radiation dose was reduced to 5Gy/d (d1-3) for celiac lymph node in the third cycle.
After the PRaG therapy, the CT scan showed that lymph node metastases in retroperitoneal and celiac almost disappeared compared with before (Figure 2). The clinical response of PRaG therapy was complete remission (CR) based on RECIST1.1. Due to the significant decrease of the lesions, no lesions can be irradiated in the follow-up treatment. Subsequent maintenance therapy was implemented with toripalimab and GM-CSF for three cycles from October 2020 to January 2021. The patient exhibited no tumor progression or recurrence in the next two re-examinations by CT scan. The patient had no other significant adverse reactions except for fatigue (Grade 1). The PFS has more than 2 years so far (Figure 3B). Due to the influence of COVID-19, the re-examination interval was longer than expected. The recent follow-up in August 2022 showed that the patient maintained a good physical condition and exhibited sustained CR.
Discussion
Backline treatment of MSS mCRC is a hard nut to crack. Regorafenib or trifluridine and tipiracil (TAS-102) were recommended, but the survival benefit was still limited. A phase II trial of TAS-102 combined with nivolumab showed no tumor response in MSS/pMMR mCRC (11). The mPFS was 2.8 months (95% CI,1.8 to 5.1). 72% of the patients experienced grade grade ≥3 adverse events (AEs). The REGONIVO study from Japan showed that the combination of regorafenib and nivolumab achieved encouraging results in treating MSS mCRC (12). The mPFS was 7.9months (95%CI,2.9 to not reached [NR]), but outcomes of this trial were not reproduced in subsequent clinical studies. These clinical trials showed that the existing combined therapies were limited in overcoming the immune resistance of MSS/pMMR mCRC. A recent study revealed that MSS mCRC patients might benefit from the combination of radiotherapy, anti-PD-1 and anti-CTLA-4 immunotherapy (8). It is necessary to develop suitable combination strategies to improve the efficacy of immunotherapy for the MSS/pMMR mCRC.
Radiotherapy is an essential local tumor control treatment method. In recent years, studies have found that adding radiotherapy can enhance the anti-tumor effect of immunotherapy. Radiation can transform tumor cells into in-situ vaccines that can promote tumor cells to release tumor-associated antigens (TAAs) and induce immunogenic cell death (ICD) (13). The PEMBRO-RT study has reported the synergistic effect between immunotherapy and radiotherapy in advanced metastatic NSCLC. The ORR of combined therapy was 36% vs. 18% of the control group (pembrolizumab alone). The combined therapy’s mPFS and mOS were better than the control group (mPFS: 6.6 vs. 1.9 months, mOS: 15.9 vs. 7.6 months). The adverse reactions between the combined and control groups have no significant difference (14). The same results were obtained in PEMBRO-RT and MDACC clinical trials pooled analysis. The combined therapy prolonged the mPFS and mOS than the pembrolizumab alone in patients with metastatic NSCLC (PFS: 9.0 vs. 4.4 months, median OS: 19.2 vs. 8.7 months) (15).
GM-CSF is a cytokine used to promote the growth of leukopenia or neutropenia and is widely used to promote the production of granulocytes or APCs. Preclinical studies supported that GM-CSF combined with immune checkpoint inhibitors (ICI) can improve the activity of innate immune cells, and indirectly recruit T cells by promoting the antigen cross-presentation (16, 17). Ipilimumab combined with GM-CSF can prolong the OS of advanced melanoma more than ipilimumab alone (mOS: 17.5 vs. 12.7 months) (18). In addition, a prospective clinical study has shown that local radiotherapy combined with GM-CSF can improve the prognosis of patients with advanced metastatic solid tumors (19).
The doses and frequency of radiation have not been standardized when radiotherapy is combined with PD-1/PD-L1 inhibitors. Preclinical studies have shown that hypo-fractionated radiotherapy (5Gy × 3f) boosted more proliferation and activation of antigen-presenting cells compared with conventionally fractionated radiotherapy (2Gy × 5f) (20). The conventional fraction also caused more lymphocyte death than hypo-fraction regimens, which affects the response to immunotherapy (21). When the fraction dose of radiation exceeds 5Gy, radiotherapy can indirectly promote the ICD of the tumor (22). However, a higher fraction dose did not represent a better response for treatment. Studies have shown that the increase of Tregs will offset the local control effect when therapy with a single high dose (15Gy × 1f), while the medium fraction regimen (7.5-10Gy × 2-3f) can maintain the low level of Treg and activate the immune response effectively (23). In the PEMBRO-RT study, the fraction regimen (8Gy × 3f) combined with PD-1 inhibitors had excellent clinical efficacy in advanced metastatic NSCLC (14). No additional adverse reactions of immunotherapy were added at this fraction dose. The fraction regimen (3×8Gy or 3×5Gy) we used in PRaG Therapy could be a reasonable choice.
Considering the heterogeneity of the tumor, irradiation of a single site may not induce sufficient exposure to TAAs. Chang et al. suggested multisite radiotherapy of metastases to enhance the synergistic effect (24). However, multisite irradiation may increase the volume of irradiation and lead to a higher incidence of adverse reactions. The number and activation of lymphocytes are related to the efficacy of immunotherapy (25). The decrease in lymphocyte number caused by lymph node irradiation directly reduces the efficacy of immunotherapy (26). Considering side effects and lymphocyte depletion caused by irradiation, it is difficult to irradiate all sites in one cycle for patients with large masses and multiple metastases. Therefore, we chose one lesion to irradiate each cycle. The treatment consists of multiple cycles. Compared to conventional radiotherapy, the range of irradiated lesions was smaller and the total radiation dose was lower (Supplementary Figures 2–4). We suggested multiple cycles of radiotherapy, with each cycle targeting a small volume that might protect the lymphocytes and produce sustained immune activation (27). Moreover, irradiation of the Tumor-draining lymph nodes (TDLNs) can benefit patients with lymph node metastases. In this case, there was no significant decrease in lymphocytes, which may be one reason for the excellent efficacy (Figure 3C).
After three irradiation cycles achieved an excellent local control effect with a significant decrease in the irradiated lymph node metastases. At the same time, the regression of the nonirradiated lesion was also observed. Regression of the nonirradiated tumor was called the abscopal effect, which was not frequently in patients with radiotherapy alone (28, 29). However, we cannot be sure that the regression of the nonirradiated lesion was caused by abscopal effect in this case. The regression of nonirradiated lesions might be due to the sensitization of radiotherapy or GM-CSF to immunotherapy. Moreover, MSS CRCs were divided into multiple subtypes by consensus molecular subtypes (CMS) consortium. Most of MSS CRCs are immune desert and has no immune cell infiltration, a small portion of them do have CD8+ cell infiltration but suppressed by TME (30). According to the results of IHC and NGS, the immune phenotype of this patient was low CD8+ cell infiltrated. We think that only a subgroup of MSS mCRCs should be considered for this combinational therapy of anti-PD1, radiotherapy and GM-CSF. The specific mechanisms remain to be further studied.
Conclusions
The MSS mCRC patient achieved terrific results through PRaG triple-combination therapy with well-tolerated. The efficacy and safety of PRaG therapy for MSS mCRC patients need to be confirmed in future prospective studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JY composed the manuscript as the first authorship. LZ designed and conducted the study as corresponding authors. PX and YK helped with data collection and interpretation. MX collected and sorted out part of the image data. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82171828), the Key R&D plan of Jiangsu Province (Social Development, BE2021652), Suzhou Radiotherapy Clinical Medical Center (Szlcyxzx202103), Open project of the State Key Laboratory of Radiology and Radiation Protection of Soochow University (GZK1202014), Open Project of Provincial Key Laboratory of Soochow University (KJS1961), the Subject construction support project of the Second Affiliated Hospital of Soochow University (XKTJ-RC202001, XKTJ- HRC20210011), the Suzhou Science and Technology Development Plan (SYS2020143), Chinese Society of Clinical Oncology Research Foundation of Beijing (Y-XD202002/ZB- 0015), Wu Jieping Medical Foundation (320.6750.2021-01-12), Open project of Provincial Key Laboratory of Soochow University (KJS1961), and the special project of “Technological Innovation” project of CNNC Medical Industry Co. Ltd (ZHYLTD2021001). CNNC Medical Industry Co. Ltd was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The study was also supported by Suzhou Science and Education Health Project (KJXW2021018) Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22_1508). Csco fund (Y-pierrefabre202102-0113).
Acknowledgments
The authors would like to thank the participant for her willingness to participate in this study. The authors acknowledge the patient and his family for consenting to publish this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1078915/full#supplementary-material
Abbreviations
mCRC, metastatic colorectal cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; MSI-H, microsatellite instability-high; dMMR, mismatch repair-deficient; MSS, microsatellite-stable; pMMR, proficient mismatch repair; GM-CSF, granulocyte-macrophage colony-stimulating factor; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial remission; PD, progressive disease; PFS, progression-free survival; PET/CT, Positron Emission Tomography/Computed Tomography; IHC, Immunohistochemical; NGS, Next Generation Sequencing; CEA, Carcinoembryonic antigen; CA242, Carbohydrate antigen 242; AEs, adverse events; NSCLC, non-small cell lung cancer; ORR, Objective response rate; APCs, antigen presenting cells; TDLNs, Tumor-draining lymph nodes; FDA, Food and Drug Administration; CMS, consensus molecular subtypes.
References
1. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA (2021) 325(7):669–85. doi: 10.1001/jama.2021.0106
2. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-Deficient/Microsatellite instability-high metastatic colorectal cancer. J Clin Oncol (2018) 36(8):773–9. doi: 10.1200/JCO.2017.76.9901
3. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
4. O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PloS One (2017) 12(12):e0189848. doi: 10.1371/journal.pone.0189848
5. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest (2014) 124(2):687–95. doi: 10.1172/JCI67313
6. Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res (2015) 21(16):3727–39. doi: 10.1158/1078-0432.CCR-14-2824
7. Floudas CS, Brar G, Mabry-Hrones D, Duffy AG, Wood B, Levy E, et al. A pilot study of the PD-1 targeting agent AMP-224 used with low-dose cyclophosphamide and stereotactic body radiation therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer (2019) 18(4):e349–60. doi: 10.1016/j.clcc.2019.06.004
8. Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer (2021) 2(11):1124–35. doi: 10.1038/s43018-021-00269-7
9. Segal NH, Cercek A, Ku G, Wu AJ, Rimner A, Khalil DN, et al. Phase II single-arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair-proficient metastatic colorectal cancer. Clin Cancer Res (2021) 27(8):2200–8. doi: 10.1158/1078-0432.CCR-20-2474
10. Kelley RK, Mitchell E, Behr S, Hwang J, Keenan B, Cheung A, et al. Phase II trial of pembrolizumab (PEM) plus granulocyte macrophage colony stimulating factor (GM-CSF) in advanced biliary cancers (ABC). J Clin Oncol (2018) 36(4_suppl):386–6. doi: 10.1200/JCO.2018.36.4_suppl.386
11. Patel MR, Falchook GS, Hamada K, Makris L, Bendell JC. A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med (2021) 10(4):1183–90. doi: 10.1002/cam4.3630
12. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol (2020) 38(18):2053–61. doi: 10.1200/JCO.19.03296
13. Turgeon GA, Weickhardt A, Azad AA, Solomon B, Siva S. Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med J Aust (2019) 210(1):47–53. doi: 10.5694/mja2.12046
14. Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol (2019) 5(9):1276–82. doi: 10.1001/jamaoncol.2019.1478
15. Theelen W, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts J, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med (2021) 9(5):467–75. doi: 10.1016/S2213-2600(20)30391-X
16. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood (2012) 119(15):3383–93. doi: 10.1182/blood-2011-11-370130
17. Gurbatri CR, Lia I, Vincent R, Coker C, Castro S, Treuting PM, et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med (2020) 12(530):eaax0876. doi: 10.1126/scitranslmed.aax0876
18. Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA (2014) 312(17):1744–53. doi: 10.1001/jama.2014.13943
19. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol (2015) 16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6
20. Kulzer L, Rubner Y, Deloch L, Allgauer A, Frey B, Fietkau R, et al. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol (2014) 11(4):328–36. doi: 10.3109/1547691X.2014.880533
21. Pike LRG, Bang A, Mahal BA, Taylor A, Krishnan M, Spektor A, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys (2019) 103(1):142–51. doi: 10.1016/j.ijrobp.2018.09.010
22. Vanpouille-Box C, ilones KA, Wennerberg E, Formenti SC, Demaria S. In situ Vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine (2015) 33(51):7415–22. doi: 10.1016/j.vaccine.2015.05.105
23. Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys (2012) 83(4):1306–10. doi: 10.1016/j.ijrobp.2011.09.049
24. Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol (2019) 16(2):123–35. doi: 10.1038/s41571-018-0119-7
25. Menetrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer (2019) 7(1):85. doi: 10.1186/s40425-019-0549-5
26. Patel RR, Verma V, Barsoumian HB, Ning MS, Chun SG, Tang C. Use of multi-site radiation therapy for systemic disease control. Int J Radiat Oncol Biol Phys (2021) 109(2):352–64. doi: 10.1016/j.ijrobp.2020.08.025
27. Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol (2020) 13(1):105. doi: 10.1186/s13045-020-00940-z
28. Demaria S, Formenti SC. The abscopal effect 67 years later: from a side story to center stage. Br J Radiol (2020) 93(1109):20200042. doi: 10.1259/bjr.20200042
29. Pevzner AM, Tsyganov MM, Ibragimova MK, Litvyakov NV. Abscopal effect in the radio and immunotherapy. Radiat Oncol J (2021) 39(4):247–53. doi: 10.3857/roj.2021.00115
Keywords: colorectal cancer, immunotherapy, radiotherapy, GM-CSF, MSS/pMMR, case report
Citation: Yang J, Xing P, Kong Y, Xu M and Zhang L (2023) PD-1 inhibitor combined with radiotherapy and GM-CSF in MSS/pMMR metastatic colon cancer: a case report. Front. Oncol. 13:1078915. doi: 10.3389/fonc.2023.1078915
Received: 24 October 2022; Accepted: 12 April 2023;
Published: 28 April 2023.
Edited by:
Jinyan Wang, China Medical University, ChinaReviewed by:
Sun Tian, Carbon Logic Biotech Ltd., ChinaYong Yang, China Pharmaceutical University, China
Copyright © 2023 Yang, Xing, Kong, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyuan Zhang, emhhbmdsaXl1YW5Ac3VkYS5lZHUuY24=
 Jiabao Yang
Jiabao Yang Pengfei Xing
Pengfei Xing Yuehong Kong
Yuehong Kong Meiling Xu
Meiling Xu Liyuan Zhang
Liyuan Zhang