
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 April 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1076997
This article is part of the Research Topic Heterogeneity in Breast Cancer: Clinical and Therapeutic Implications View all 20 articles
Background: Male breast cancer (MBC) is rare, which has restricted prospective research among MBC patients. With effective treatments, the prognosis of MBC patients has improved and developing a second primary malignancy (SPM) has become a life-threatening event for MBC survivors. However, few studies have focused on the prognosis of MBC patients and looked into the SPM issue in MBC survivors.
Method: We reviewed MBC patients diagnosed between 1990 and 2016 from the latest Surveillance, Epidemiology, and End Results (SEER) Plus database. Competing risk models and nomograms were conducted for predicting the risk of cancer-specific death and SPM occurrence. C-indexes, calibration curves, ROC curves, and decision curve analysis (DCA) curves were applied for validation.
Result: A total of 1,843 MBC patients with complete information were finally enrolled and 60 (3.26%) had developed an SPM. Prostate cancer (40%) was the most common SPM. The median OS of all the enrolled patients was 102.41 months, while the median latency from the initial MBC diagnosis to the subsequent diagnosis of SPM was 67.2 months. The patients who suffered from an SPM shared a longer OS than those patients with only one MBC (p = 0.027). The patients were randomly divided into the development cohort and the validation cohort (at a ratio of 7:3). The Fine and Gray competing risk model was used to identify the risk factors. Two nomograms were constructed and validated to predict the 5-year, 8-year, and 10-year survival probability of MBC patients, both of which had good performance in the C-index, ROC curves, calibration plots, and DCA curves, showing the ideal discrimination capability and predictive value clinically. Furthermore, we, for the first time, constructed a nomogram based on the competing risk model to predict the 5-year, 8-year, and 10-year probability of developing an SPM in MBC survivors, which also showed good discrimination, calibration, and clinical effectiveness.
Conclusion: We, for the first time, included treatment information and clinical parameters to construct a nomogram to predict not only the survival probability of MBC patients but also the probability of developing an SPM in MBC survivors, which were helpful in individual risk estimation, patient follow-up, and counseling in MBC patients.
Breast cancer is relatively uncommon in men. Approximately 2,000 men are diagnosed with breast cancer annually in the USA, accounting for 1% of all new breast cancer patients and 0.03% of all new malignant diseases in men (1). Male breast cancer (MBC) has a similar mortality rate to female breast cancer at 17% (2). Mortality rates in Europe remained fairly stable, but the USA indicated an increase in incidence (3, 4). This trend could result from an increase in longevity in the population, since age is the major determinant of risk for most solid tumors. The incidence of MBC had a similar increasing rate with that of female breast cancer, which is probably related to the popularity of mammography screening (5, 6). However, it was shown that the prognosis of MBC patients was worse than that of female breast cancer patients (7–9). Similar to female breast cancer, the incidence of MBC also has regional differences, which is higher in North America and Europe and lower in Asia (10). The majority of MBCs do not have specific risk factors, and some small-sample studies showed that a high level of estrogen and an imbalance of hormones may contribute to the development of MBC (11–13). Genetic factors may also have a possible connection to MBC, and BRCA2 mutations appear to be the strongest risk factor for breast cancer in men with a lifetime risk of 7%, which is approximately 80 times more than the general population (14).
The rarity of MBC has restricted prospective studies on it. Principles of treatments of MBC are derived largely from randomized trials carried out in women (15, 16). Ninety percent of MBCs are estrogen-receptor-positive; tamoxifen is the standard adjuvant therapy, and some individuals could also benefit from chemotherapy. Hormonal therapy is the main treatment for metastatic disease (17), while chemotherapy can also provide palliation (10). In addition, advances in early screening and treatments have caused a considerable proportion of MBC survivors. For some survivors, second primary malignancy (SPM) is one of the most potentially life-threatening outcomes (18). At present, no research has focused on the SPM in MBC survivors, and the prediction models of developing an SPM in MBC patients have not been provided. In this study, we developed two nomogram models to predict the survival probability of MBC patients using the competing risk method. Furthermore, we built an additional nomogram to predict the probability of an MBC survivor developing an SPM.
The data of the present research were obtained from the latest Surveillance, Epidemiology, and End Results (SEER) Plus database (SEER 9 Registries data, with additional treatment information, Nov 2021 sub). The SEER database is an authoritative source of information on cancer, covering approximately 34.6% of the population in the USA. The records of male patients diagnosed with breast carcinoma between 1990 and 2016 were extracted using the SEER*Stat software (version 8.4.1), ensuring long-term follow-up of at least 5 years to estimate the risk of developing a second primary cancer. The International Classification of Diseases for Oncology third edition (ICD-O-3) was used to identify breast malignancy by site code C50 (including C50.1 to C50.9). The three key variables “year of diagnosis”, “sequence number”, and “total number of in situ/malignant tumors for patient” of the SEER Plus database were used to determine the status of SPM. Cases that were diagnosed as synchronous cancers occurring as SPM within 2 months after initial diagnosis or those in which the breast malignancy was not the patients’ first primary malignancy were excluded. The inclusion criteria were as follows: (1) male breast malignancy was the only or the first primary malignancy; (2) histological diagnosis confirming the existence of breast malignancy; and (3) under treatment and the follow-up data were available. The exclusion criteria were as follows: (1) incomplete cases with missing information on important variables; (2) the SPM (if any) data were incomplete; (3) initially diagnosed with distant metastasis; and (4) synchronous cancers. The flowchart of case selection is shown in Supplementary Figure 1.
A total of 1,843 MBC patients were involved in this study. Variables such as age, race, marital status, year of diagnosis, sequence number, total number of in situ/malignant tumors for patient, histological type, tumor grade, TMN stage, surgery performance, radiotherapy performance, chemotherapy performance, months from diagnosis to treatment, the hormone receptor (HR) status, HER2 status, survival time, and cause of death were extracted. Age was regrouped into six groups (<45, 45–55, 55–65, 65–75, 75–85, and 85+). Race was regrouped into white, black, and other. Marital status included married, single, and divorced. Histological type was divided into infiltrating duct, adenocarcinoma, and other by the SEER Plus database. The HR status was classified as HR positive [estrogen receptor (ER) and/or progesterone receptor (PR) was positive] and HR negative (both ER and PR were negative). TMN stage was adjusted to the 6th AJCC staging edition by the SEER Plus database in the additional analysis. The site and the diagnosis time of the SPM were recorded. Overall survival (OS) refers to the time from the initial cancer diagnosis to cancer-specific death.
The cumulative incidence of cancer-specific death and the occurrence of SPM were calculated based on the Fine and Gray competing risk model. The Kaplan–Meier method was constructed to estimate the difference in OS between MBC survivors with and without an SPM. The entire cohort was randomly divided into a development cohort (70%) and a validation cohort (30%) for the development and validation for the competing risk nomogram. Standardized mean differences (SMDs) were used to assess distributional differences in the baseline variables between the development and validation cohorts. As HER2 status is known to be tested after 2010, and HER2 status should be routinely diagnosed clearly in breast cancer patients nowadays, sensitivity analyses were carried out excluding those MBC patients whose HER2 was unknown or whose diagnosis was made prior to 2010.
The univariate and multivariate Cox regression analyses were firstly performed to identify variables that significantly affected the breast cancer-specific survival and occurrence of SPM. However, applying only univariate and multivariate Cox regression analyses was inadequate, because aside from the primary tumor, there were other factors that might threaten the patients’ lives, such as accidents and infectious or other serious diseases. As a result, death due to other causes acted as a competing risk event to death due to a specific cancer. Hence, the Cox proportional hazards model might overestimate the incidence rate of the outcome with the passage of time. Similarly, death due to primary breast cancer or other causes also acted as a competing event for the MBC patients to develop an SPM—only those cured from MBC could have the probability of developing an SPM during their long survival time. In this study, the additional Fine and Gray competing risk analysis was applied to compare the association among different causes of death with a competing risk framework: death due to breast cancer or death due to other causes. Then, as for the occurrence of SPM in MBC survivors, the Fine and Gray method was also applied: death due to primary breast cancer or other causes was the competing event in the development of an SPM.
In order to help clinicians predict the survival probability of MBC patients and their individual probability to develop an SPM, nomograms were established based on the multivariate competing risk models. Next, we identified low-risk and high-risk survivors by calculating the 50th quantiles of total points of the nomograms and compared the difference of their survival time. Validation of these nomograms was performed by calculating the concordance index (C-index) and plotting calibration curves by a bootstrapping method with 1,000 resamples. Furthermore, the receiver operating characteristic (ROC) curves were drawn to estimate the predictive value by calculating the area under the ROC curves (AUCs). Meanwhile, decision curve analyses (DCAs) were conducted to show the clinical effectiveness of the nomogram models.
All analyses were performed using R software (version 4.21, https://www.r-project.org/). Significance level was set as p < 0.05.
A total of 1,843 MBC patients, who were initially diagnosed between 1990 and 2016, were finally enrolled in the present study. Among these MBC patients, 60 (3.26%) developed at least one SPM. A total of 339 (18.39%) patients died from MBC, and 707 (38.4%) patients died from other causes. Among those survivors who suffered from an SPM, prostate cancer represented 24 (40%) of all SPMs, followed by lung and bronchus cancer at 6 (10.0%), melanoma of the skin at 5 (8.3%), secondary breast cancer at 4 (6.7%), liver cancer at 3 (5.0%), urinary bladder cancer at 3 (5.0%), kidney and renal pelvis cancer at 2 (3.3%), NHL at 2 (3.3%), pancreas cancer at 2 (3.3%), rectal cancer at 2 (3.3%), and stomach cancer at 2 (3.3%). The SPM details of these MBC survivors are shown in Figure 1. The median OS of all the enrolled patients was 102.41 months. The median latency from diagnosis of initial breast primary cancer to subsequent diagnosis of the SPM was 67.2 months. The detailed information of these MBC patients is summarized in Tables 1, 2.
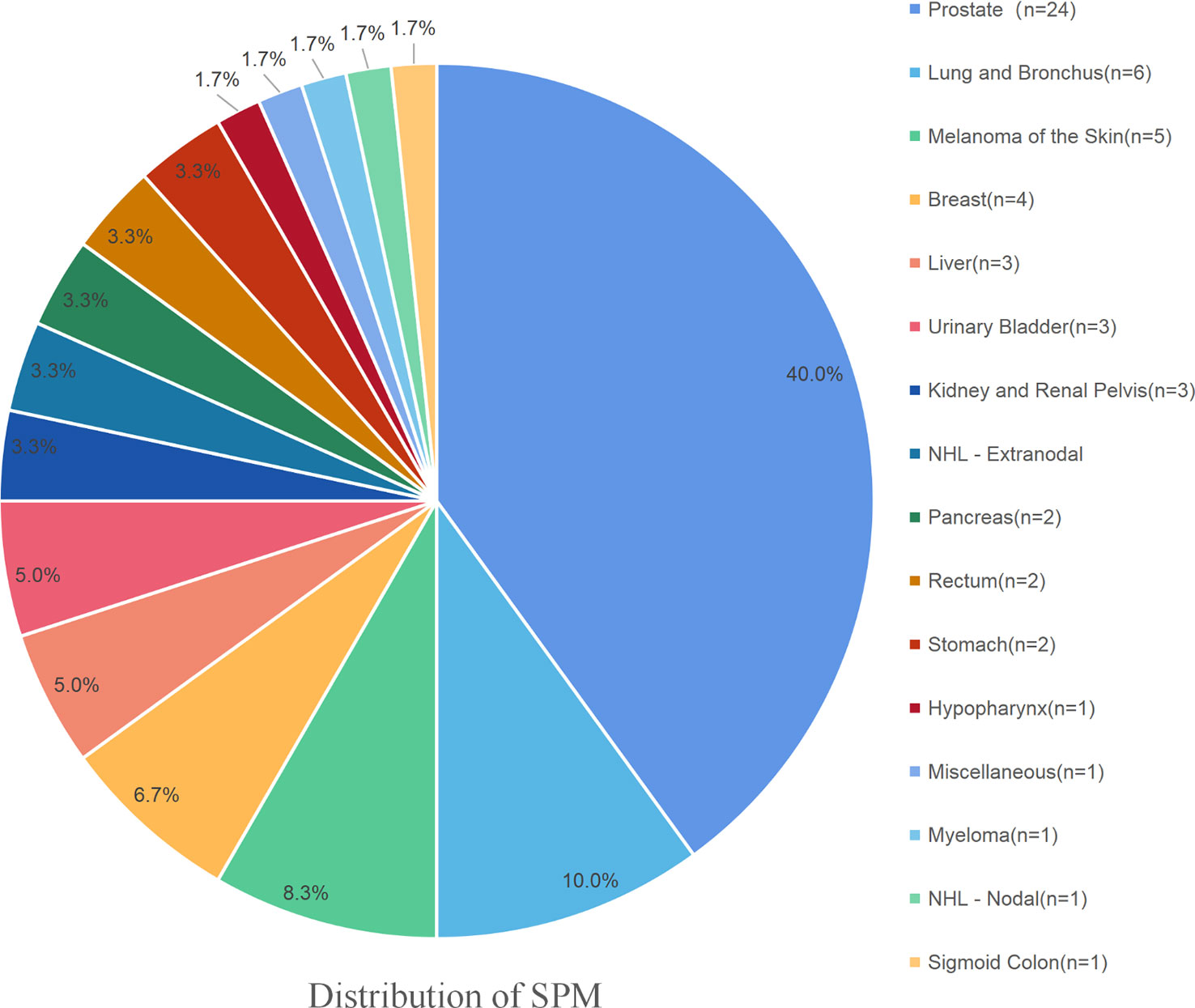
Figure 1 The detailed distribution of the SPMs among MBC survivors. Prostate cancer represented 24 (40%) of all SPMs, followed by lung and bronchus at 6 (10.0%), melanoma of the skin at 5 (8.3%), the secondary breast cancer at 4 (6.7%), liver at 3 (5.0%), urinary bladder at 3 (5.0%), kidney and renal pelvis at 2 (3.3%), NHL at 2 (3.3%), pancreas at 2 (3.3%), rectum at 2 (3.3%), and stomach at 2 (3.3%).
As is shown in Figure 2A, there was no significant difference in OS between the development and validation cohorts (p = 0.83). The OS of MBC patients who did not suffer from an SPM was 101.87 ± 68.17 months, while the OS of those who suffered from an SPM was 118.63 ± 75.76 months. Those who developed an SPM have a significantly longer OS (Figure 2B, p = 0.027).
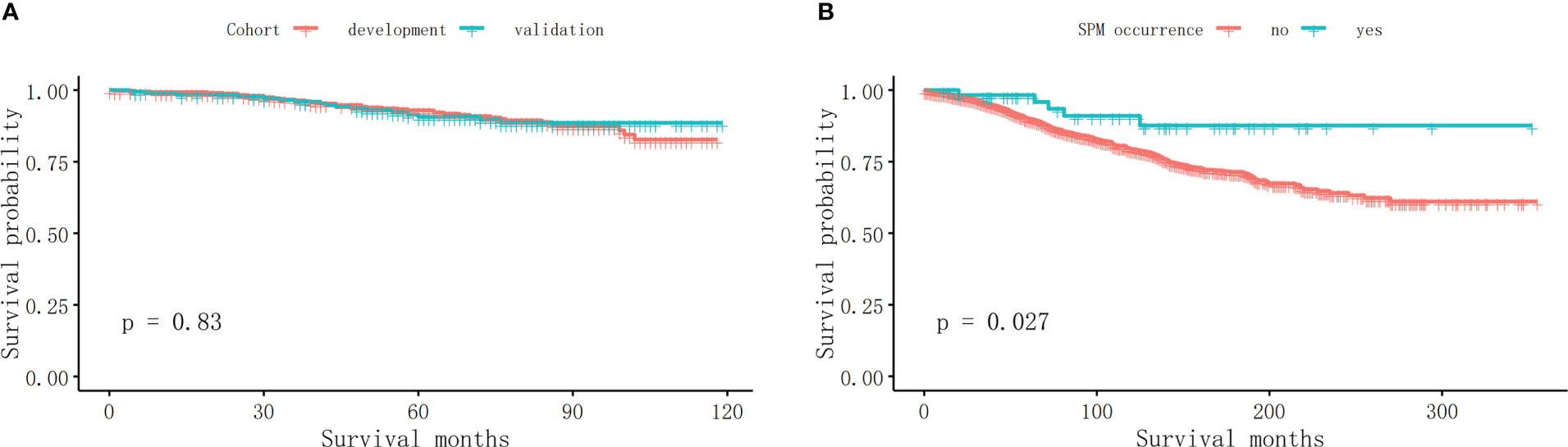
Figure 2 The survival analyses. (A) There was no significant difference in OS between the development and validation cohort (p = 0.83). (B) Those who developed an SPM have a significantly longer OS in MBC survivors (p = 0.027).
Univariate and multivariate Cox regression were applied in the development cohort to select the predictive variables for the prediction models of cancer-specific death. MBC patients whose HER2 was unknown or diagnosed prior to 2010 were excluded in the following sensitivity analyses as mentioned above. As is shown in Table 3, tumor grade, TMN stage, surgery, and chemotherapy were related to OS in the univariate analysis, while in the multivariate Cox regression, chemotherapy failed to show a significant relation with OS.
Univariate and multivariate Cox regression were also applied to select the predictive variables for the occurrence of SPM. As is shown in Table 4, marital status showed a significant relation with the occurrence of SPM in the univariate analysis. Moreover, in the multivariate Cox regression, age, race, tumor differentiated grade, histological type, TMN stage, chemotherapy, and the waiting time from diagnosis to begin treatment were significant.
The Fine and Gray method was used to estimate the risk predictors for cancer-specific death and the occurrence of SPM. The results of the characteristics are provided in Table 5. Age, race, marital status, histological type, TMN stage, therapy, the waiting time from diagnosis to begin treatment, HR status, and HER2 status were the significant risk factors for both cancer-specific death and the development of an SPM.
The first two nomograms were established based on the previously mentioned risk factors to predict the survival probability of MBC patients. Age, race, marital status, tumor differentiated grade, histology, TMN stage, surgery, radiotherapy, chemotherapy, duration to begin treatment, HR status, and HER2 status, which were selected by the Fine and Gray method, were enrolled in nomogram model 1 to predict the 5-year, 8-year, and 10-year survival probability of MBC patients (Figure 3A). Meanwhile, age, tumor differentiated grade, TMN stage, and surgery, which were selected by the multivariate Cox regression, were included in nomogram model 2 (Figure 3B) to predict the same survival probability above. The C-index of model 1 was 0.710 in the development cohort and 0.703 in the validation cohort, while model 2 had a C-index at 0.728 in the development cohort and 0.718 at the validation cohort. Both model 1 (AUC = 0.713) and model 2 (AUC = 0.757) achieved a better predictive value than the AJCC TMN staging system (AUC = 0.689) in the ROC analysis shown in Figure 4A. The integrated discrimination improvement (IDI) and net reclassification improvement (NRI) between model 1 and TMN stage were 0.610 (95% CI 0.490–0.258) and 0.333 (95% CI 0.182–0.508), respectively. Meanwhile, The IDI and NRI between model 2 and TMN stage were 0.059 (95% CI 0.036–0.193) and 0.290 (95% CI 0.154–0.513), respectively. The calibration curves show that both model 1 and model 2 had good agreement between predicted probability and the observed outcome (Figures 4B, C). The DCA also showed that model 1 (Figures 4D, E) and model 2 (Figures 4F, G) had a good discrimination in both the development and validation cohorts. We divided the patients into a low-risk group and a high-risk group at the 50th percentile of nomogram total points and compared the difference of the survival time among these subgroups. Figures 5A, B show that there were significant differences in survival time between different risk groups in both the development cohort (p = 0.0022) and the validation cohort (p = 0.002), based on nomogram model 1 (Figure 3A). Meanwhile, Figures 5C, D also show the survival time difference between different risk groups in the development cohort (p = 0.001) and the validation cohort (p = 2e-04), based on nomogram model 2 (Figure 3B), which indicated that both of these nomogram models had a good discrimination capability for the survival probability of the MBC patients. The details of these two nomograms are shown in Supplementary Tables 1, 2.
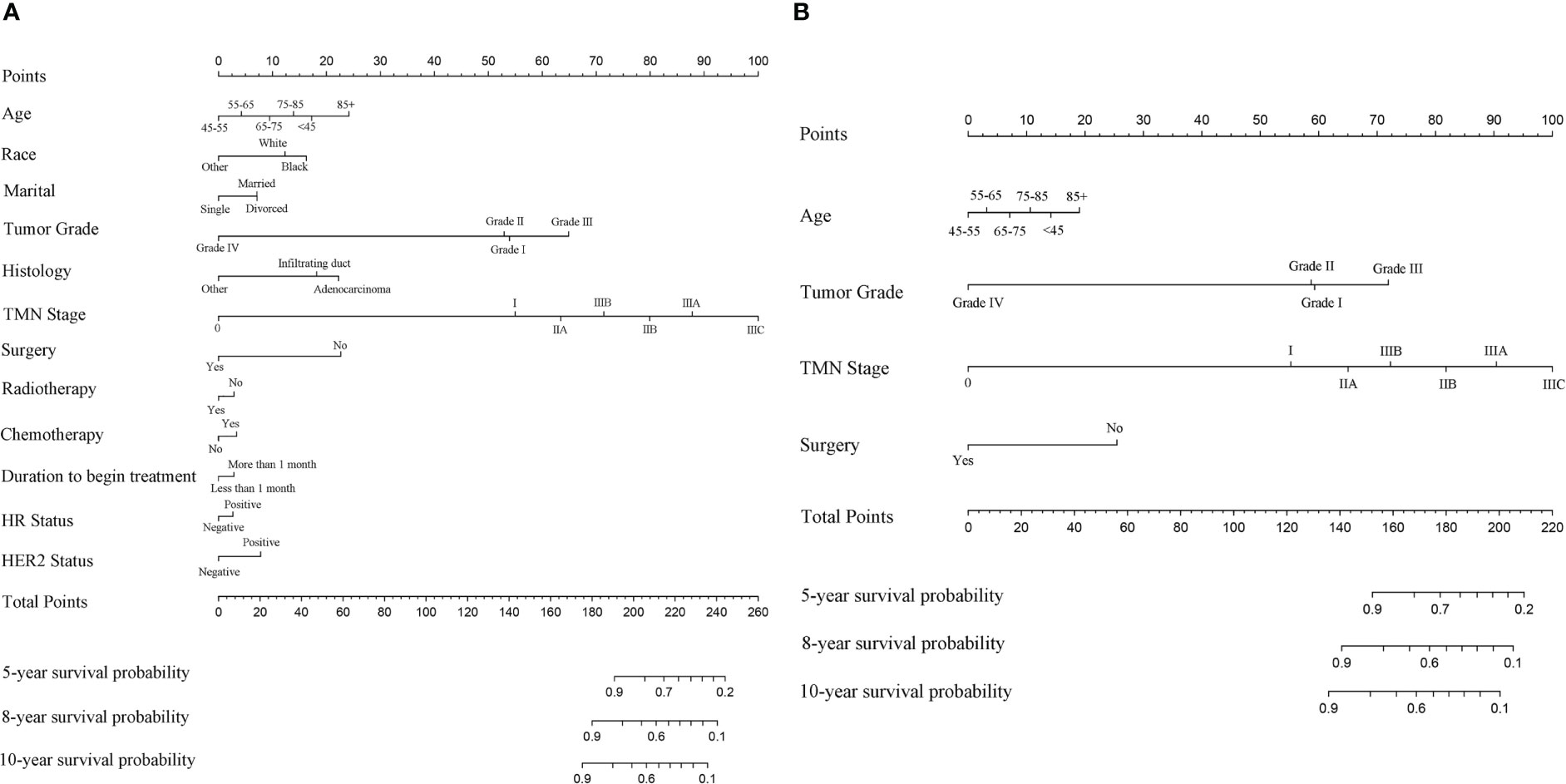
Figure 3 (A) The nomogram model 1 to predict the 5-year, 8-year, and 10-year survival probability of MBC patients based on the Fine and Gray method. (B) The nomogram model 2 to predict the same survival probability of MBC patients based on the multivariate Cox regression.
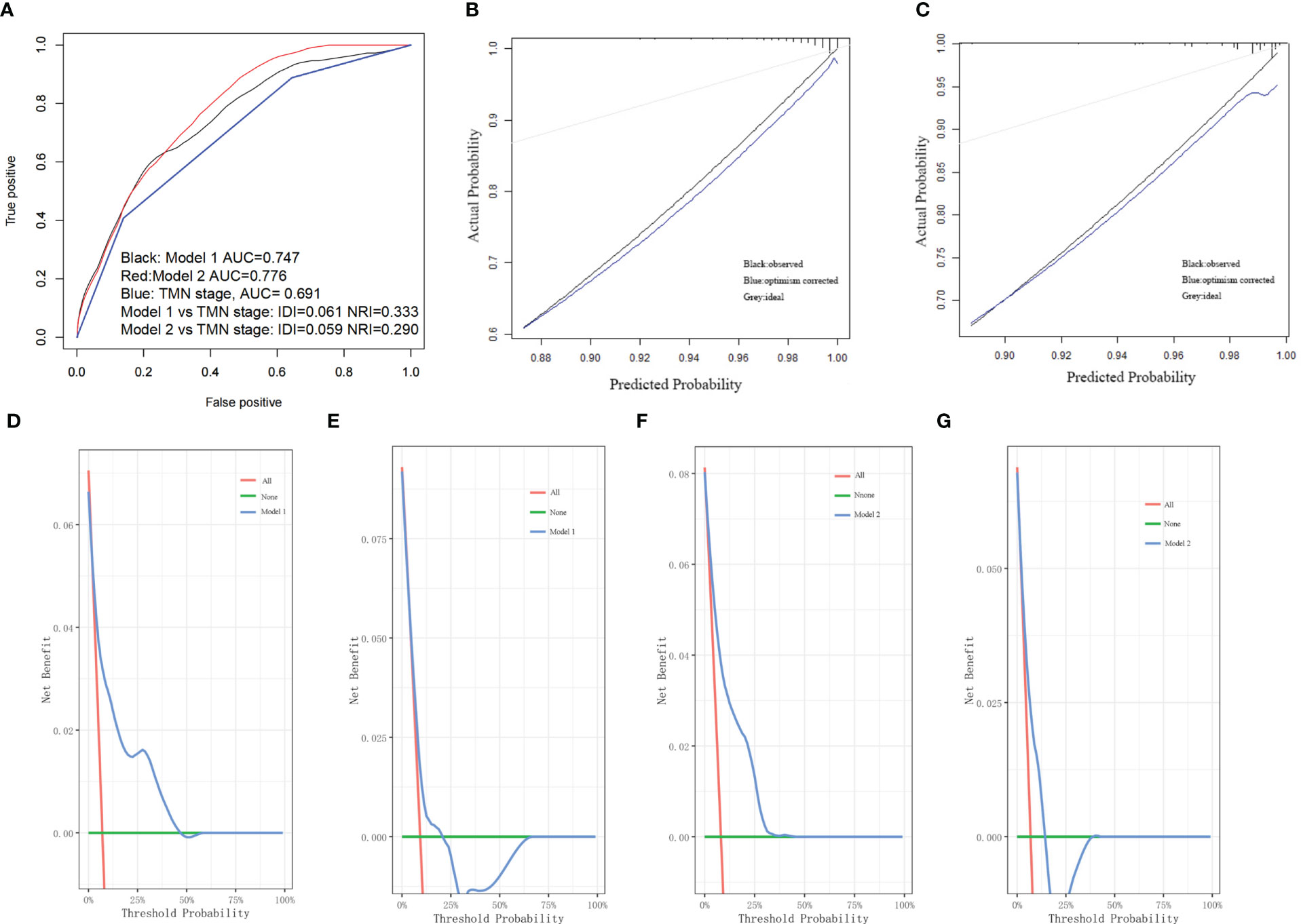
Figure 4 (A) Both model 1 and model 2 showed better predictive value than TMN stage in the ROC analyses. (B) The calibration curve of model 1. (C) The calibration curve of model 2. (D) The DCA of model 1 in the development cohort. (E) The DCA of model 1 in the validation cohort. (F) The DCA of model 2 in the development cohort. (G) The DCA of model 2 in the validation cohort.
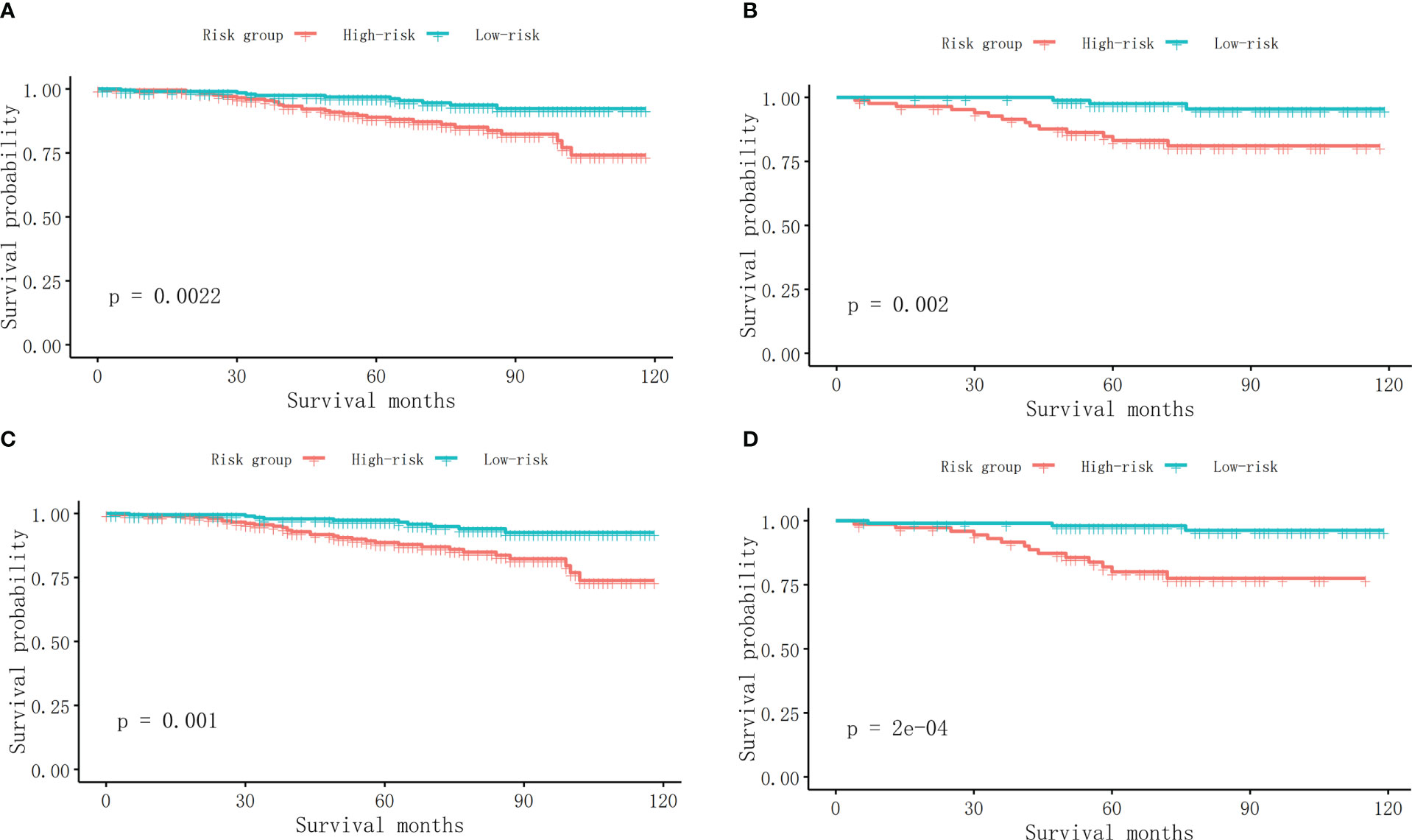
Figure 5 (A) The survival curves between different risk groups in the development cohort in model 1. (B) The survival curves between different risk groups in the validation cohort in model 1. (C) The survival curves between different risk groups in the development cohort in model 2. (D) The survival curves between different risk groups in the validation cohort in model 2.
An additional nomogram model 3 was established to predict the probability of MBC survivors developing an SPM within 10 years after the initial diagnosis. All of the risk factors selected by the Fine and Gray method were included in model 3 (Figure 6A). The C-index of model 3 was 0.909 in the development cohort and 0.494 in the validation cohort. The AUC of the ROC curve in model 3 is 0.934 (Figure 6B). The calibration curve is shown in Figure 6C. The DCA curve is shown in Figure 6D in the development cohort and in Figure 6E in the validation cohort. The details of these risk factors are shown in Supplementary Table 3.
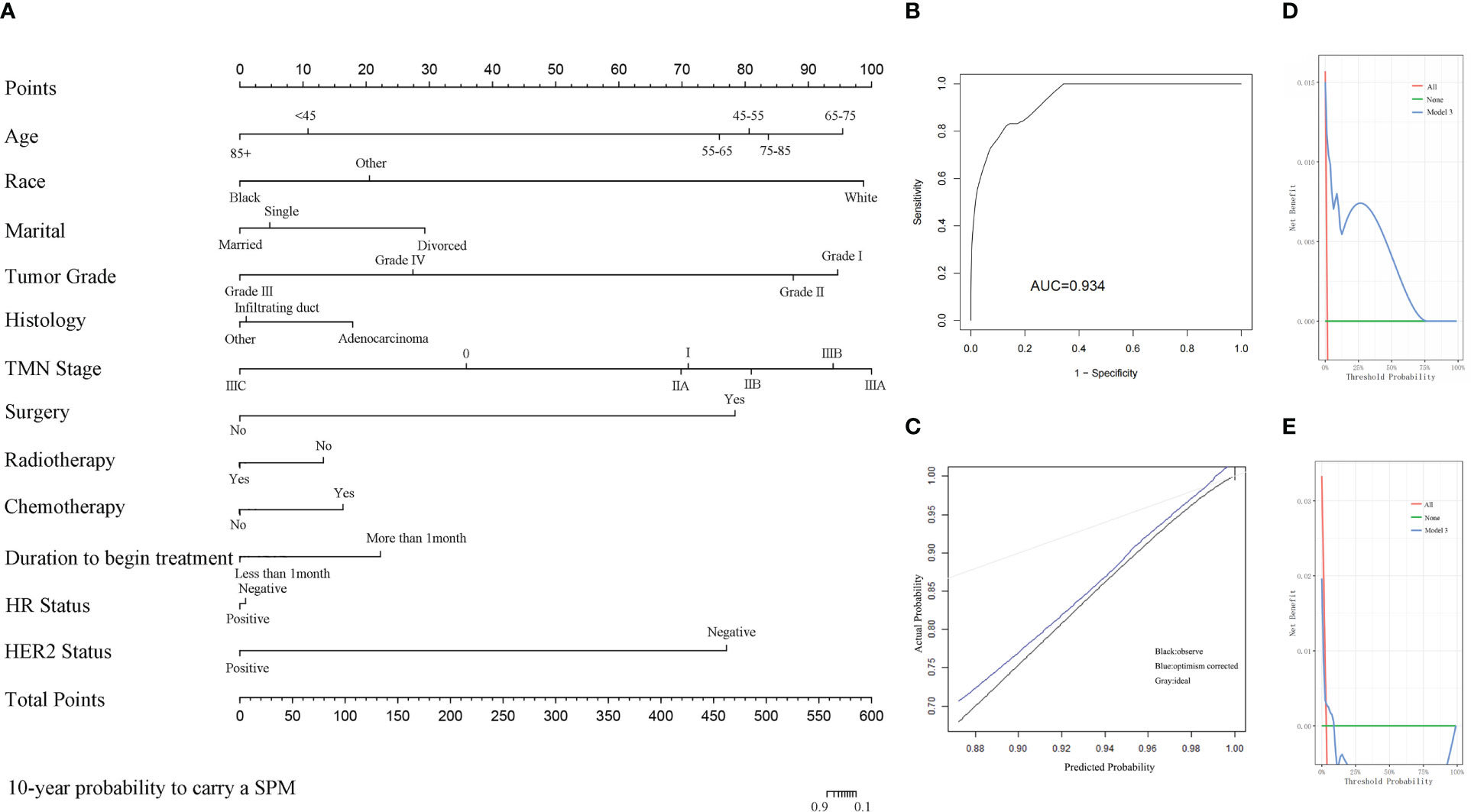
Figure 6 (A) The nomogram of model 3 for predicting the 10-year probability of MBC survivors who suffer from an SPM. (B) The ROC curve of model 3. (C) The calibration curve of model 3. (D) The DCA of model 3 in the development cohort. (E) The DCA of model 3 in the validation cohort.
MBC is a rare disease whose causes remain incompletely characterized and understood. Because of the limitation of large-scale randomized prospective research, MBC treatment largely follows the guidelines of female breast cancer (19). By applying sufficient therapies, such as surgery, chemotherapy, radiotherapy, endocrine therapy, targeted therapy, and immunotherapy, the prognosis of MBC survivors has improved in the past 25 years (20). With the longevity of the MBC survivors, SPM has become a life-threatening event. In the present study, we enrolled 1,843 MBC patients who were randomly divided into a development and a validation group at a ratio of 7:3. No difference was found between these two groups (Table 1). At present, a few studies focused on the prognosis of MBC patients. Wang et al. developed a nomogram to predict distant metastasis in MBC patients, based on univariate and multivariate logistic regression analyses, but did not focus on the probability of survival and the development of an SPM (21). Chen et al. constructed a nomogram to predict the prognosis of MBC patients based on univariate and multivariate Cox regression (22). Similar research was published by Zhang et al. (23). However, as we mentioned above, applying only Cox regression analysis was inadequate and would overestimate the risk of cancer-specific death, because aside from the primary tumor, there were other factors that might threaten their life (24), and death due to other causes actually acted as a competing event to death caused by MBC. In this study, two nomograms were constructed to predict the survival probability of MBC patients based on the Fine and Gray competing risk analysis and multivariate Cox regression, respectively, to correct this bias. Sun et al. performed a competing risk analysis in MBC patients but failed to include treatment information (25). As is shown in the present study, treatments influenced cancer-specific death and the occurrence of SPM. Different clinical circumstances with different treatment strategies might lead to different outcomes.
A few studies estimated the effect of initial treatment on the development of SPM in female breast cancer patients (26, 27), but no research has focused on the development of SPM in MBC survivors. To our knowledge, this is the first available nomogram for developing an SPM in MBC survivors in the presence of competing events.
In this study, 60 survivors developed an SPM. Prostate cancer was the most common SPM. Interestingly, previous research showed that prostate cancer was also the most common SPM in colon cancer survivors treated with colectomy (28). However, prostate cancer had a bigger portion in SPM patients than in the whole population (29). The efficiency of endocrine therapy, along with the high proportion of HR-positive status in MBC patients (17), warrants further study to clarify whether the endocrine status is related to the occurrence of the SPM. It is also worth noting that patients who suffered from an SPM shared a longer OS than those patients with only one MBC (Figure 2, p = 0.027), which indicated that the cumulative incidence of developing an SPM increased with the prolonged survival time.
Univariate and multivariate Cox regression analyses were insufficient, and in this study, we applied additional Fine and Gray competing risk analysis to show the differences among the risk factors associated with OS and the occurrence of SPM. We have constructed two nomogram models to predict the OS of the MBC patients: model 1 based on the risk factors selected by the Fine and Gray method, and model 2 based on the multivariate analysis. Both of these nomogram models achieved good C-index. Model 2 had an even better predictive value than model 1 and the TMN stage in the combined ROC analysis (Figure 4A). The calibration plots, the DCA curves, and the survival curves of different risk groups altogether showed that both of these models had an ideal discrimination capability and predictive value. Model 1 included more clinical details while model 2 was more simplified. According to our study, higher age at diagnosis, higher TMN stage, absence of surgery and radiotherapy, more than 1 month waiting time to begin treatment, and being HR and HER2 positive contributed to a poorer prognosis in MBC patients.
An additional nomogram model 3 was constructed based on the Fine and Gray method to predict the probability of the occurrence of an SPM. Li et al. focused on the SPM on female breast cancer patients and constructed a nomogram to predict the SPM probability of female breast cancer patients (30). A similar study was published by Bao et al. on female breast cancer patients (31). Mellemkjær et al. investigated whether pregnancy near the time of the initial female breast cancer diagnosis would increase the risk of an SPM and obtained a negative result (32). Chen et al. found that germline pathogenic variants in BRCA1, BRCA2, and ERCC2 increased the risk for female breast cancer patients of developing an SPM (33). Nevertheless, no similar research had been published in MBC patients and few studies had focused on the SPM issue in MBC patients. Satram-Hoang et al. found that there is a general tendency towards higher risks of SPM among younger men compared to older men but did not provide a predictive model (34). Hung et al. found that the risk of SPM was significantly higher for both male and female breast cancer patients compared with the general population (35). In this study, we constructed an available nomogram to predict the SPM probability of MBC patients. There were 36 SPM patients in the development cohort and 24 in the validation (Table 1). Nomogram model 3 achieved good performance in the C-index and DCA curve in the development cohort and attained an ordinary score in the validation cohort, which was attributed to the rarity of MBC and the small number of the enrolled SPM patients. However, the present study is still the first research to look into the SPM of MBC patients, and achieved an AUC at 0.934 (Figure 6B), which indicated a good predictive value of the predictive model.
A nomogram had been widely used for the prediction of certain clinical outcomes because it is convenient and reliable. In this study, we, for the first time, constructed competing risk nomograms including both the treatment information and the clinicopathological parameters to predict the prognosis of MBC patients and, for the first time, developed a competing risk nomogram to predict the probability of developing an SPM in MBC patients, which was thought to be helpful for both clinicians and the patients to estimate the risk and manage their strategies about treatment and follow-up.
There are some limitations in our study. First, this study was a population-based retrospective study using the SEER Plus database, which had missed some important variables of some of the patients, leading to more than 1,000 MBC patients being excluded because of the incomplete information. Second, some important risk factors for SPM that were rapidly developing or widely used in clinical practice nowadays, such as diet and lifestyle, family history of cancer, oncogene test, radiotherapy or chemotherapy protocols, and the performance of endocrine therapy, targeted therapy, or immunotherapy, were not included in the SEER Plus database. Additionally, MBC is a rare disease, and the SEER Plus database did not involve a larger population worldwide, which had restricted the scale of the present study and might lead to bias. An additional larger study is needed to determine the mechanism of SPM in MBC patients.
Our study for the first time included the treatment information and clinical parameters needed to construct an external validation competing risk nomogram to predict the survival probability of MBC patients, according to which higher age at diagnosis, higher TMN stage, absence of surgery and radiotherapy, more than 1 month waiting time to begin treatment, and being HR and HER2 positive contributed to a poorer prognosis in MBC patients. This study also, for the first time, constructed a nomogram to predict the probability of developing an SPM in MBC survivors, which was helpful in individual risk estimation, patient follow-up, and counseling in MBC patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Guangzhou Red Cross Hospital of Jinan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HH, ZL, ZH, and LH performed the study, analyzed the data, prepared figures and/or tables, and authored or reviewed drafts of the paper. WL, GL, and YM conceived and designed the study, performed the study, authored or reviewed drafts of the paper, and approved the final draft. All authors contributed to the article and approved the submitted version.
This study was funded by the Guangzhou Health Science and Technology project (grant numbers 20221A010014 and 20211A011020), the Guangzhou Science and Technology Bureau Program (grant number 202201010806), the research grants of Excellent Science and Technology Talents Project of Guangzhou Red Cross Hospital (WL), and the Research-oriented Hospital Program of Guangzhou (RHPG05). General Health Research Project in Huadu District, Guangzhou (grant number 21-HDWS-066) and Guangzhou Science and Technology Plan Project (grant number 2023A04J0632).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1076997/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin (2016) 66(1):7–30. doi: 10.3322/caac.21332
2. Expert Panel on Breast Imaging, Niell BL, Lourenco AP, Moy L, Baron P, Didwania AD, et al. ACR appropriateness criteria(®) evaluation of the symptomatic Male breast. J Am Coll Radiol (2018) 15(11s):S313–s320. doi: 10.1016/j.jacr.2018.09.017
3. Chichura A, Attai DJ, Kuchta K, Nicholson K, Kopkash K, Pesce C, et al. Male Breast cancer patient and surgeon experience: The Male WhySurg study. Ann Surg Oncol (2022) 29(10):6115–31. doi: 10.1245/s10434-022-12135-6
4. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer (2004) 101(1):51–7. doi: 10.1002/cncr.20312
5. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male Breast cancer: a population-based comparison with female breast cancer. J Clin Oncol (2010) 28(2):232–9. doi: 10.1200/JCO.2009.23.8162
6. Kreiter E, Richardson A, Potter J, Yasui Y. Breast cancer: trends in international incidence in men and women. Br J Cancer (2014) 110(7):1891–7. doi: 10.1038/bjc.2014.66
7. Fentiman IS. Prognostic difficulties of men with breast cancer. Breast J (2021) 27(12):877–82. doi: 10.1111/tbj.14297
8. Fox S, Speirs V, Shaaban AM. Male Breast cancer: an update. Virchows Arch (2022) 480(1):85–93. doi: 10.1007/s00428-021-03190-7
9. Iorfida M, Bagnardi V, Rotmensz N, Munzone E, Bonanni B, Viale G, et al. Outcome of male breast cancer: a matched single-institution series. Clin Breast Cancer (2014) 14(5):371–7. doi: 10.1016/j.clbc.2014.02.008
10. Fentiman IS, Fourquet A, Hortobagyi GN. Male Breast cancer. Lancet (2006) 367(9510):595–604. doi: 10.1016/S0140-6736(06)68226-3
11. Clarke CN, Cortina CS, Fayanju OM, Dossett LA, Johnston FM, Wong SL. Breast cancer risk and screening in transgender persons: A call for inclusive care. Ann Surg Oncol (2022) 29(4):2176–80. doi: 10.1245/s10434-021-10217-5
12. Ewertz M, Holmberg L, Tretli S, Pedersen BV, Kristensen A. Risk factors for male breast cancer–a case-control study from Scandinavia. Acta Oncol (2001) 40(4):467–71. doi: 10.1080/028418601750288181
13. Anderson WF, Devesa SS. Breast carcinoma in men. Cancer (2005) 103(2):432–3. doi: 10.1002/cncr.20797
14. Johansen Taber KA, Morisy LR, Osbahr AJ 3rd, Dickinson BD. Male Breast cancer: risk factors, diagnosis, and management (Review). Oncol Rep (2010) 24(5):1115–20. doi: 10.3892/or_00000962
15. Yadav S, Sangaralingham L, Payne SR, Giridhar KV, Hieken TJ, Boughey JC, et al. Surveillance mammography after treatment for male breast cancer. Breast Cancer Res Treat (2022) 194(3):693–8. doi: 10.1007/s10549-022-06645-w
16. Healy NA, Parag Y, Wallis MG, Tanner J, Kilburn-Toppin F. Outcomes of male patients attending the symptomatic breast unit: adherence to local and national imaging guidelines and effectiveness of clinical examination and imaging in detecting male breast cancer. Clin Radiol (2022) 77(1):e64–74. doi: 10.1016/j.crad.2021.09.018
17. Zattarin E, Ligorio F, Nichetti F, Bianchi G, Capri G, de Braud F. Prolonged benefit from palbociclib plus letrozole in heavily pretreated advanced male breast cancer: case report. Tumori (2021) 107(6):Np15–np19. doi: 10.1177/0300891620976981
18. Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol (2012) 30(30):3734–45. doi: 10.1200/JCO.2012.41.8681
19. Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer (2013) 132(8):1918–26. doi: 10.1002/ijc.27841
20. O'Malley CD, Prehn AW, Shema SJ, Glaser SL. Racial/ethnic differences in survival rates in a population-based series of men with breast carcinoma. Cancer (2002) 94(11):2836–43. doi: 10.1002/cncr.10521
21. Wang D, Yang L, Yang Y, Chen M, Yang H. Nomogram for predicting distant metastasis of male breast cancer: A SEER population-based study. Med (Baltimore) (2022) 101(39):e30978. doi: 10.1097/MD.0000000000030978
22. Chen S, Liu Y, Yang J, Liu Q, You H, Dong Y, et al. Development and validation of a nomogram for predicting survival in Male patients with breast cancer. Front Oncol (2019) 9:361. doi: 10.3389/fonc.2019.00361
23. Zhang LP, Lin H, Wang AJ. Development and validation of a nomogram to predict survival for advanced male breast cancer. Andrologia (2022) 54(8):e14479. doi: 10.1111/and.14479
24. Li Z, Wu X, Huang H, Xu F, Liang G, Lin C, et al. MTHFR C677T polymorphism and cerebrovascular lesions in elderly patients with CSVD: A correlation analysis. Front Genet (2022) 13:987519. doi: 10.3389/fgene.2022.987519
25. Sun W, Cheng M, Zhou H, Huang W, Qiu Z. Nomogram predicting cause-specific mortality in nonmetastatic Male breast cancer: A competing risk analysis. J Cancer (2019) 10(3):583–93. doi: 10.7150/jca.28991
26. Molina-Montes E, Requena M, Sánchez-Cantalejo E, Fernández MF, Arroyo-Morales M, Espín J, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol (2015) 136(1):158–71. doi: 10.1016/j.ygyno.2014.10.029
27. Rubino C, de Vathaire F, Diallo I, Shamsaldin A, Lê MG. Increased risk of second cancers following breast cancer: role of the initial treatment. Breast Cancer Res Treat (2000) 61(3):183–95. doi: 10.1023/A:1006489918700
28. Zhang B, Guo K, Zheng X, Sun L, Shen M, Ruan S. Risk of second primary malignancies in colon cancer patients treated with colectomy. Front Oncol (2020) 10:1154. doi: 10.3389/fonc.2020.01154
29. Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin (2021) 71(6):466–87. doi: 10.3322/caac.21695
30. Li D, Weng S, Zhong C, Tang X, Zhu N, Cheng Y, et al. Risk of second primary cancers among long-term survivors of breast cancer. Front Oncol (2019) 9:1426. doi: 10.3389/fonc.2019.01426
31. Bao S, Jiang M, Wang X, Hua Y, Zeng T, Yang Y, et al. Nonmetastatic breast cancer patients subsequently developing second primary malignancy: A population-based study. Cancer Med (2021) 10(23):8662–72. doi: 10.1002/cam4.4351
32. Mellemkjær L, Eibye S, Albieri V, Kjær SK, Boice JD Jr. Pregnancy-associated cancer and the risk of second primary cancer. Cancer Causes Control (2022) 33(1):63–71. doi: 10.1007/s10552-021-01500-7
33. Chen F, Park SL, Wilkens LR, Wan P, Hart SN, Hu C, et al. Genetic risk of second primary cancer in breast cancer survivors: The multiethnic cohort study. Cancer Res (2022) 82(18):3201–8. doi: 10.1158/0008-5472.CAN-21-4461
34. Satram-Hoang S, Ziogas A, Anton-Culver H. Risk of second primary cancer in men with breast cancer. Breast Cancer Res (2007) 9(1):R10. doi: 10.1186/bcr1643
Keywords: male breast cancer, second primary malignancy, prognosis, survival probability, nomogram
Citation: Huang H, Li Z, Huang Z, Huang L, Liu W, Liu G and Mo Y (2023) Development and validation of nomograms to predict the survival probability and occurrence of a second primary malignancy of male breast cancer patients: a population-based analysis. Front. Oncol. 13:1076997. doi: 10.3389/fonc.2023.1076997
Received: 22 October 2022; Accepted: 30 March 2023;
Published: 20 April 2023.
Edited by:
Anna Diana, Ospedale del Mare, ItalyReviewed by:
Giuseppe D’Ermo, Sapienza University of Rome, ItalyCopyright © 2023 Huang, Li, Huang, Huang, Liu, Liu and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Liu, bHdlaTdAbWFpbDIuc3lzdS5lZHUuY24=; Guolong Liu, ZXlnbGxpdUBzY3V0LmVkdS5jbg==; Yuzhen Mo, Z3pteXoyMDE2QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.