
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 April 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1072943
This article is part of the Research Topic Biomarkers, Functional Mechanisms, and Therapeutic Potentials in Gastrointestinal Cancers View all 40 articles
 Jinglei Qu1,2,3,4†
Jinglei Qu1,2,3,4† Xin He1,2,3,4†
Xin He1,2,3,4† Ying Luo1,2,3,4
Ying Luo1,2,3,4 Ping Yu1,2,3,4
Ping Yu1,2,3,4 Ying Chen1,2,3,4
Ying Chen1,2,3,4 Jing Liu5
Jing Liu5 Xin Wang6
Xin Wang6 Chang Wang7
Chang Wang7 Tingting Liang7
Tingting Liang7 Yuxian Bai8
Yuxian Bai8 Yu Han8
Yu Han8 Li Man9
Li Man9 Chuanchun Leng10
Chuanchun Leng10 Caiyun Zhou11
Caiyun Zhou11 Lijie He12
Lijie He12 Xin Wang13
Xin Wang13 Yunpeng Liu1,2,3,4*
Yunpeng Liu1,2,3,4* Xiujuan Qu1,2,3,4*
Xiujuan Qu1,2,3,4*Objective: Apatinib and irinotecan are used as systematic therapies for advanced gastric adenocarcinoma (GAC) and gastroesophageal junction adenocarcinoma (GEJA), while the evidence for their combination as second-line therapy in these patients is limited. This study aimed to evaluate the efficacy and safety of second-line apatinib plus irinotecan for the treatment of GAC and GEJA.
Methods: In this prospective, multicenter phase II clinical study, 28 patients with advanced GAC or GEJA who received second-line apatinib plus irinotecan were recruited.
Results: In total, 1 (3.6%) patient achieved complete response, 7 (25.0%) patients achieved partial response, 13 (46.4%) patients had stable disease, and 4 (14.3%) patients showed progressive disease, while clinical response was not evaluable or not assessed in 3 (10.7%) patients. The objective response rate and disease control rate were 28.6% and 75.0%, respectively. Meanwhile, the median (95% confidence interval (CI)) progression-free survival (PFS) was 4.5 (3.9-5.1) months, and the median (95% CI) overall survival (OS) was 11.3 (7.4-15.1) months. By multivariate Cox regression analysis, male sex, liver metastasis, and peritoneal metastasis were independently associated with worse PFS or OS, while treatment duration ≥5 months was independently associated with better OS. In terms of the safety profile, 89.3% of patients experienced treatment-emergent adverse events of any grade, among which 82.1% of patients had grade 1-2 adverse events and 64.3% of patients had grade 3-4 adverse events.
Conclusion: Apatinib plus irinotecan as second-line therapy achieves a good treatment response and satisfactory survival with tolerable safety in patients with advanced GAC or GEJA.
Gastric adenocarcinoma (GAC) and gastroesophageal junction adenocarcinoma (GEJA) are prevalent and dangerous malignancies worldwide, with more than 1 million newly diagnosed cases and over 0.7 million deaths in 2020 (1–3). It has been reported that the incidence of GAC has declined, while that of GEJA has steadily increased in recent years (4–6). Moreover, there is a large proportion of GAC and GEJA patients with advanced disease at diagnosis who lose the opportunity for potentially curative surgical resection (7, 8). According to the guidelines recommended by the Chinese Society of Clinical Oncology, the first-line systematic therapy for advanced GAC and GEJA is fluorouracil-based chemotherapy plus immunotherapy (combined with trastuzumab if the tumor presents human epidermal growth factor receptor 2 (HER2)-positive), while the second-line therapy is limited and merely includes paclitaxel, docetaxel, and irinotecan monotherapy or their combination, as well as paclitaxel and ramucirumab combination (9, 10).
Angiogenesis mediated by vascular endothelial growth factor (VEGF) has been identified as a key factor that facilitates the progression and metastasis of GAC and GEJA (11). Therefore, the application of antiangiogenic agents for the treatment of GAC and GEJA has received close attention. For instance, ramucirumab, a monoclonal antibody targeting VEGF receptor-2, has been approved by the Food and Drug Administration (FDA) for the treatment of advanced GAC and GEJA (12, 13). Meanwhile, apatinib, an oral tyrosine kinase inhibitor that also targets VEGF receptor-2, has been recommended as the third-line treatment for advanced GAC and GEJA in China since it dramatically prolongs progression-free survival in patients with advanced GAC and GEJA (9, 14). In the last two years, some clinical studies have shown that advanced GAC or GEJA patients who receive second-line apatinib plus chemotherapy present satisfactory response and survival, suggesting that apatinib plus chemotherapy may serve as a potential second-line therapy for advanced GAC and GEJA (14–21). In addition, a recent phase II study investigating ramucirumab plus irinotecan as second-line therapy in patients with advanced GAC also reveals the efficacy and safety of VEGF receptor-2 inhibitor plus irinotecan in those patients (22). However, more evidence is needed to facilitate the application of second-line apatinib plus chemotherapy for the treatment of advanced GAC or GEJA.
The current prospective, multicenter, phase II clinical study aimed to evaluate the treatment response, survival benefit, and adverse events of second-line apatinib plus irinotecan in advanced GAC or GEJA patients.
Between July 2017 and January 2022, 28 patients with advanced GAC or GEJA who received apatinib plus irinotecan as second-line treatment were recruited in this prospective, multicenter, single-arm, phase II clinical study. The inclusion criteria were as follows (1): 18-70 years old; (2) diagnosed with locally advanced or metastatic GAC/GEJA; (3) more than one measurable objective tumor lesion by spiral CT examination under Response Evaluation Criteria In Solid Tumors (RECIST) v1.1; (4) first-line chemotherapy failure before recruitment; (5) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; (6) acceptable hepatic and renal function; and (7) expected survival of more than 3 months. In consideration of safety, each subject should undergo UGT1A1*28 and UGT1A1*6 testing before enrollment. Patients with UGT1A1*28 (6/6) and *6 (G/G), UGT1A1*28 (6/6) and *6 (G/A), or UGT1A1*28 (6/7) and *6 (G/G) were eligible for enrollment. The exclusion criteria were (1) hypersensitivity to apatinib or irinotecan; (2) prior exposure to irinotecan or vascular endothelial growth factor receptor (VEGFR) inhibitors (such as apatinib, sorafenib, sunitinib); (3) uncontrolled hypertension (> 140/90 mmHg); (4) bleeding tendency; (5) received thrombolytics or anticoagulants; and (6) pregnant or lactating women. The study was approved by the First Hospital of China Medical University Institutional Review Board (No. 2016-197-10) with registration number NCT03116555 (https://clinicaltrials.gov). Each subject signed informed consent.
Patients received oral apatinib (Jiangsu Hengrui Medicine, Jiangsu, China) 250 mg once daily every 3 weeks (as a treatment cycle). Irinotecan (Jiangsu Hengrui Medicine, Jiangsu, China) was administered 180 mg/m2 intravenously on the first day every 3 weeks (once every three weeks as a treatment cycle). In each treatment cycle, apatinib intermittent dose discontinuous were allowed if severe adverse events occurred, and patients would continue to treatment after the symptoms of adverse events disappeared or were ameliorated. The treatment was continued until progressive disease, the occurrence of intolerable toxicities, or the patient refused treatment.
The patients received radiographic evaluations every 6 weeks until progressive disease or intolerant toxicities. The treatment response was calculated through RECIST (v1.1). Each subject was closely followed up until death or lost to follow-up. The last follow-up date was June 1, 2022. The primary endpoint was progression-free survival (PFS), referring to the interval from treatment beginning to progressive disease or death. The secondary endpoints included overall survival (OS), objective response rate (ORR), and disease control rate (DCR). OS was defined as the interval from treatment beginning to death. The National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0) was applied for grading adverse event severity.
The sample size was calculated via a hypothesis based on a previous study that the proportion of ORR was 26.9% with a confidence interval width of 0.369 (23). With a significance (α) level of 0.05, the minimum sample size was 25, adjusted to 28 for drop-out possibility. The statistical analyses were conducted using SPSS v27.0 (IBM Corp., USA), and the figures were created using GraphPad Prism v8.01 (GraphPad Software Inc., USA). PFS and OS are shown via Kaplan−Meier curves and were analyzed using the log-rank test. The survival analyses were performed via univariable and multivariable Cox regression analyses (backward stepwise methods; all factors were included). Comparison analyses were completed using the chi-square test or Fisher’s exact test. A P value <0.05 indicated statistical significance.
The patients had a mean age of 58.3 ± 7.5 years, consisting of 6 (21.4%) females and 22 (78.6%) males. There were 22 (78.6%) patients with tumors in the gastric and 6 (21.4%) patients with tumors in the gastroesophageal junction. Meanwhile, 7 (25.0%), 5 (17.9%), 5 (17.9%), 8 (28.6%), and 7 (25.0%) patients had tumor metastases on the liver, lung, peritoneum, lymph node, and others. In addition, 16 (57.1%) patients had <2 metastatic sites, while 12 (42.9%) patients had ≥2 metastatic sites. Regarding treatment history, 18 (64.3%) patients had a history of surgery; 20 (71.4%) patients received treatment <5 months, and 8 (28.6%) patients received treatment ≥5 months. Other baseline characteristics are specifically shown in Table 1. The specific previous first-line treatment regimens are shown in Supplementary Table 1.
The median treatment duration of apatinib plus irinotecan was 3.8 months, with a range of 0.6-17.2 months. After treatment, clinical response was not evaluable or not assessed in 3 (10.7%) patients. There was 1 (3.6%) patient who achieved complete response (CR) and 7 (25.0%) patients who achieved partial response (PR). Meanwhile, 13 (46.4%) patients had stable disease (SD), and 4 (14.3%) patients showed progressive disease (PD). Therefore, the ORR was 28.6%, and the DCR was 75.0% (Table 2).
Survival-related information was recorded, and PFS and OS were calculated to evaluate the long-term efficacy of second-line apatinib plus irinotecan. The data showed that the median (95% confidence interval (CI)) PFS was 4.5 (3.9-5.1) months (Figure 1A). Meanwhile, the median (95% CI) OS was 11.3 (7.4-15.1) months (Figure 1B). Moreover, apatinib intermittent dose suspension did not affect ORR, DCR, PFS, or OS (all P>0.05) (Supplementary Table 2).
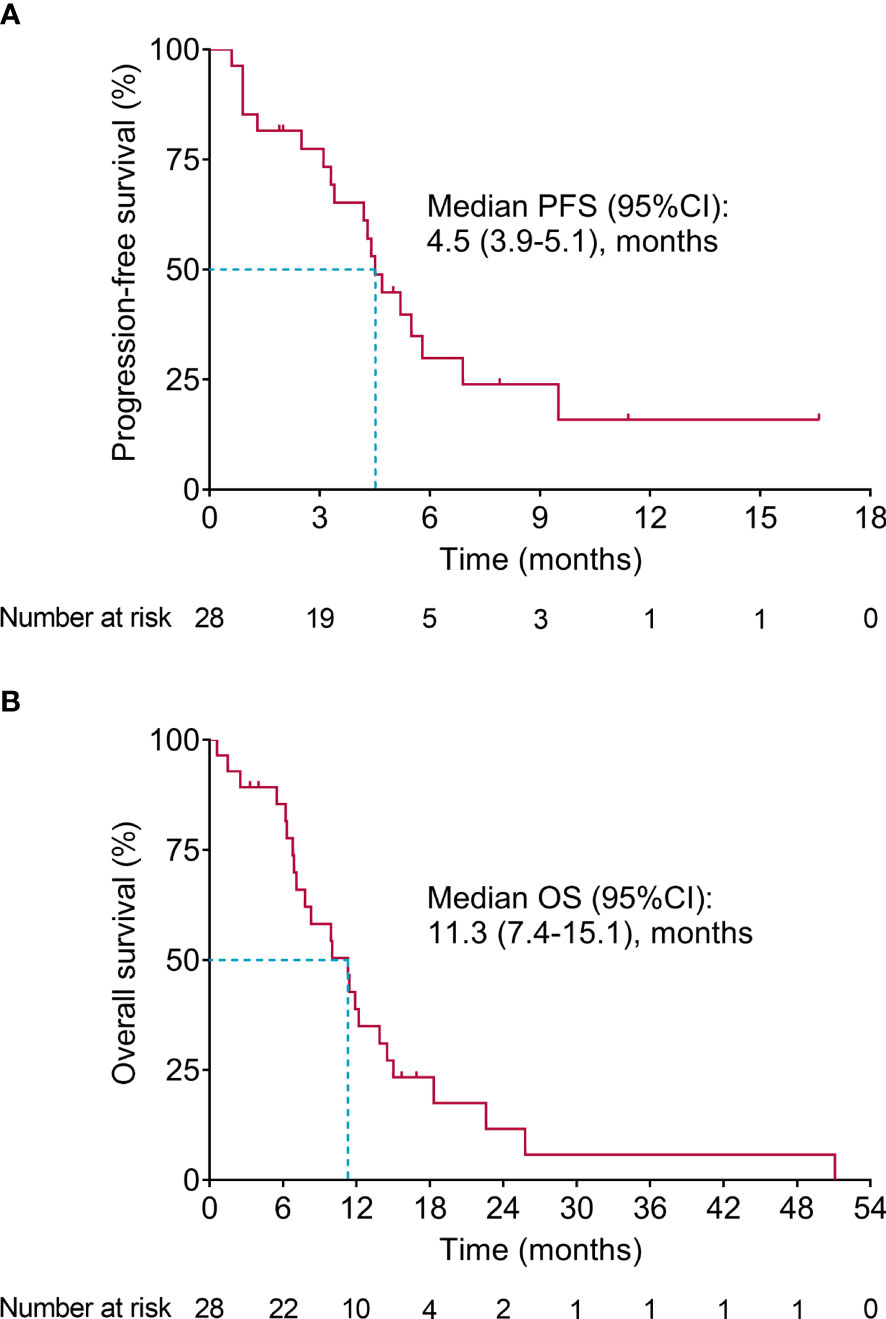
Figure 1 Survival. PFS (A) and OS (B) in advanced GAC or GEJA patients receiving second-line apatinib plus irinotecan.
Univariate Cox regression analysis showed that gender (male vs. female) (P=0.020, hazard ratio (HR)=11.306) and liver metastasis (yes vs. no) (P=0.001, HR=7.375) were associated with worse PFS, while current treatment duration (≥5 months vs. <5 months) (P=0.007, HR=0.185) was associated with better PFS. Multivariate Cox regression analysis showed that gender (male vs. female) (P=0.004, HR=43.299), liver metastasis (yes vs. no) (P=0.005, HR=14.965), and peritoneal metastasis (yes vs. no) (P=0.002, HR=23.177) were independently associated with worse PFS, whereas current treatment duration (≥5 months vs. <5 months) (P=0.029, HR=0.043) was independently associated with better PFS (Table 3).
In terms of OS, liver metastasis (yes vs. no) (P=0.001, HR=8.377) and peritoneal metastasis (yes vs. no) (P=0.021, HR=3.948) were associated with shorter OS. Further multivariate Cox regression analysis confirmed that liver metastasis (yes vs. no) (P<0.001, HR=30.140) and peritoneal metastasis (yes vs. no) (P=0.002, HR=14.600) were independently associated with unfavorable OS (Table 4).
A total of 89.3% of patients experienced treatment-emergent adverse events of any grade, among which 82.1% of patients had grade 1-2 adverse events and 64.3% of patients had grade 3-4 adverse events.
Regarding hematological adverse events, the incidences of leukopenia, neutropenia, anemia, and thrombocytopenia of any grade were 60.7%, 60.7%, 50.0%, and 35.7%, respectively. Meanwhile, the incidences of grade 3-4 leukopenia, neutropenia, anemia, and thrombocytopenia were 28.6%, 46.4%, 14.3%, and 14.3%, respectively.
In terms of non-hematological adverse events, the most common ones of any grade were nausea (46.4%), elevated g-glutamyltransferase (GGT) (42.9%), diarrhea (42.9%), and fatigue (39.3%). In addition, grade 3-4 non-hematological adverse events were elevated GGT (14.3%), diarrhea (10.8%), elevated bilirubin (7.1%), elevated alanine aminotransferase (ALT) (3.5%), elevated aspartate aminotransferase (AST) (3.5%), hypertension (3.5%), and hand-foot syndrome (3.5%) (Table 5).
Irinotecan is one of the standard second-line chemotherapies for advanced GAC or GEJA, which could be metabolized into SN38, a DNA topoisomerase I inhibitor, thus suppressing the duplication of DNA and synthesis of RNA and exerting antitumor efficacy (24). Because of its spectral antitumor efficacy and wide application in digestive cancers, the current study chose irinotecan in combination with apatinib, a VEGFR-2 inhibitor that specifically represses angiogenesis to hinder tumor progression (25). In addition, a previous study suggested that irinotecan-based chemotherapy plus ramucirumab (another VEGFR-2 inhibitor) achieves a better treatment response than paclitaxel plus ramucirumab (26), which implied the potential superiority of irinotecan plus a VEGFR-2 inhibitor versus other chemotherapies plus a VEGFR-2 inhibitor. According to previous studies, the common dose of apatinib is 500 mg daily (15, 18, 19). Meanwhile, considering that advanced GAC or GEJA patients might be impossible to tolerate 150-180 mg/m2 irinotecan on the first day every 2 weeks, the dosage in our phase I clinical trial (data not published) was set as 500 mg apatinib daily and 180 mg/m2 irinotecan on the first day every 3 weeks (as a treatment cycle) to promote tolerability. However, all 3 patients experienced grade 3 adverse events, which mainly included diarrhea, hypertension, and granulocytopenia. The adverse events resulted in drug cessation in 2 of the 3 patients, and the dose of apatinib was reduced to 250 mg daily in the other patient. Therefore, in the current phase II study, the dose of apatinib was set as 250 mg daily and that of irinotecan was set as 180 mg/m2 on the first day every 3 weeks to increase the tolerability.
The application of second-line apatinib plus chemotherapy for the treatment of GAC and GEJA has become a hotspot in recent years. For instance, it has been reported that in patients with advanced GAC or GEJA refractory to first-line chemotherapy, the ORR is 21.6%, and the DCR is 83.8% (18). Meanwhile, a randomized, controlled trial revealed that second-line apatinib plus docetaxel achieved an ORR of 18.4% and DCR of 60.5% in advanced GCA or GEJA patients who failed first-line chemotherapy (17). Moreover, in advanced GAC patients who receive second-line apatinib plus chemotherapy (including docetaxel, paclitaxel, oxaliplatin, capecitabine, and tegafur), a total of 18.5% of patients achieve an objective response, and 92.6% of patients achieve disease control (15). In addition, a recent retrospective, real-world study investigated second-line or above apatinib plus irinotecan in patients with advanced gastric cancer (27). The current study was a prospective, multicenter, phase II clinical study focusing on second-line apatinib plus irinotecan in patients with advanced GAC or GEJA, which could further verify the therapeutic potential of second-line apatinib plus irinotecan for the treatment of these patients. The data revealed that the ORR and DCR were 28.6% and 75.0%, respectively, in advanced GAC or GEJA patients who received second-line apatinib plus irinotecan, which was within the range of previous study-reported ORR and DCR (15–21). In addition, the ORR and DCR were numerically higher than those in advanced GAC patients receiving second-line chemotherapy (19). A possible explanation could be that apatinib inhibited the progression of GAC and GEJA by suppressing angiogenesis, while chemotherapy directly killed tumor cells (24, 28). Therefore, the combination of these two antitumor agents with different mechanisms of performance might exert good effects on treating GAC and GEJA. Thus, the treatment response was acceptable in patients receiving second-line apatinib plus irinotecan. In the current study, 3 patients were excluded from treatment response evaluation. The reasons were as follows (1): The first patient was nonlocal. He/she failed to be followed up due to COVID-19, and the treatment response was not evaluated in this patient. (2) The second patient had poor drug consistency after 2 cycles of treatment. In addition, treatment response was not evaluated in this patient. (3) The third patient died after 1 cycle of treatment, which was considered to be associated with disease progression but not the drugs. Treatment response was not evaluated in this patient.
The survival of patients with advanced GAC or GEJA is quite unfavorable; it has been reported that the 5-year survival rate in these patients ranges from 5% to 30% (6, 29, 30), which is partly caused by the limited treatment choice after failure of first-line therapy. Previous studies have shown that second-line apatinib plus chemotherapy achieves certain survival in GAC or GEJA patients. For instance, in advanced GAC or GEJA patients receiving second-line apatinib plus chemotherapy (including docetaxel, paclitaxel, oxaliplatin, capecitabine, and tegafur), the median PFS and OS are 3.06 and 6.51 months, respectively (15). Meanwhile, in advanced GAC patients who receive second-line apatinib plus S-1, the median PFS and OS are 143.1 days (approximately 4.77 months) and 211.6 days (approximately 7.05 months), respectively (20). In the current phase II clinical study, it was observed that the median PFS and OS were 4.5 and 11.7 months, respectively, in advanced GAC or GEJA patients receiving second-line apatinib plus irinotecan. The PFS in the current study was within the range in previously reported studies, while the OS was numerically longer than that previously reported (15–21), which could be explained by the fact that some of the patients were locally advanced in our study and might have longer survival. Another possible explanation might be that compared with other studies, patients in the current study tended to have milder disease conditions and better performance status, such as younger age, lower ECOG PS score, and fewer metastatic sites, which contributed to a longer OS. Additionally, data from multivariate Cox regression analysis revealed that male sex, liver metastasis, and peritoneal metastasis were independent risk factors for worse PFS or OS, which were all well-recognized risk factors for mortality in advanced GAC or GEAJ patients in previous studies (31, 32). However, patients with these factors should not be excluded from the upcoming trial since these factors were prevalent in patients with advanced GAC or GEJA. Conversely, clinicians should pay close attention to patients with these factors to achieve better management in those patients.
According to previous reports, the hematological adverse events of apatinib plus chemotherapy mainly refer to leukopenia, neutropenia, anemia, and thrombocytopenia, whose incidences range from approximately 15% to 60% (14–21, 33). Regarding the non-hematological adverse events of apatinib plus chemotherapy, the common ones are fatigue, nausea and vomiting, elevated transaminase, diarrhea, etc., whose incidence ranges from approximately 30% to 75% (14–21, 33). The current phase II clinical study showed that the category of hematological and non-hematological adverse events was in line with previous studies; meanwhile, the incidences of these adverse events were also within the range of previous reports (14–21, 33). Meanwhile, the incidence of febrile neutropenia was 3 (10.7%). Growth factors were allowed for the treatment, but not for the prevention, of neutropenia. In addition, there were 12 patients who continued the original treatment regimen until the end of the study, 5 patients who stopped treatment due to treatment-emergent adverse events, and 11 patients with dose reduction due to treatment-emergent adverse events. These findings suggested that second-line apatinib plus irinotecan was tolerated in advanced GAC or GEJA patients.
This study might serve as vital evidence for the use of second-line apatinib plus irinotecan in patients with advanced GAC or GEJA, while some limitations of this study should be clarified. First, the sample size of this study was not large enough; further studies should verify our findings in a larger cohort. The possible reasons for slow accrual for the trial are listed as follows: a. most patients with GAC or GEJA were resectable, and the number of patients with advanced GAC or GEJA was small; b. with the continuously widening application of PD-1 inhibitors in China, an increasing number of patients were willing to receive PD-1 inhibitors. Therefore, the current study only enrolled 28 patients. Second, this study was a prospective, single-arm study; thus, further randomized controlled trials or meta-analyses might be needed to validate the efficacy and safety of second-line apatinib plus irinotecan. Third, the patients in this study received different first-line treatments, which could be a potential confounding factor. Fourth, the underlying mechanisms of apatinib plus irinotecan in inhibiting GAC or GEJA should be further explored by in vitro and in vivo experiments.
Collectively, apatinib plus irinotecan as second-line therapy achieves a good treatment response and satisfactory survival with tolerable safety in patients with advanced GAC or GEJA, which could serve as a potential treatment option for these patients. However, these findings should be further verified in studies with larger sample sizes to provide more reliable evidence to support second-line apatinib plus irinotecan in patients with advanced GAC or GEJA.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the First Hospital of China Medical University Institutional Review Board (No. 2016-197-10) with the registration number NCT03116555 (https://clinicaltrials.gov). The patients/participants provided their written informed consent to participate in this study.
XQ and YLi contributed to the study conception and design. Material preparation was performed by JQ, XH and YLu. Data collection was performed by PY, YC and JL. Data analysis was performed by XW (7th author), CW and TL. YB, YH and LM were responsible for the interpretation of the data. The first draft of the manuscript was written by CL and CZ. LH and XW (16 author) contributed to manuscript revisions of the intellectual content. All authors contributed to the article and approved the submitted version.
This study was supported by the Shenyang Clinical Medical Research Center for Comprehensive Treatment of Cancer and the Provincial Science and Technology Innovation Platform Project of Liaoning Province.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1072943/full#supplementary-material
1. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol (2020) 38(23):2677–94. doi: 10.1200/JCO.20.00866
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, et al. Global incidence and mortality of gastric cancer, 1980-2018. JAMA Netw Open (2021) 4(7):e2118457. doi: 10.1001/jamanetworkopen.2021.18457
4. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol (2020) 18(3):534–42. doi: 10.1016/j.cgh.2019.07.045
5. Lin D, Khan U, Goetze TO, Reizine N, Goodman KA, Shah MA, et al. Gastroesophageal junction adenocarcinoma: Is there an optimal management? Am Soc Clin Oncol Educ Book (2019) 39:e88–95. doi: 10.1200/EDBK_236827
6. Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience (2018) 12:883. doi: 10.3332/ecancer.2018.883
7. Huang RJ, Hwang JH. Improving the early diagnosis of gastric cancer. Gastrointest Endosc Clin N Am (2021) 31(3):503–17. doi: 10.1016/j.giec.2021.03.005
8. Martin-Richard M, Carmona-Bayonas A, Custodio AB, Gallego J, Jimenez-Fonseca P, Reina JJ, et al. SEOM clinical guideline for the diagnosis and treatment of gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJA) (2019). Clin Transl Oncol (2020) 22(2):236–44. doi: 10.1007/s12094-019-02259-9
9. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) (2021) 41(8):747–95. doi: 10.1002/cac2.12193
10. Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol (2023) 41(7):1470–91. doi: 10.1200/JCO.22.02331
11. Forma A, Tyczynska M, Kedzierawski P, Gietka K, Sitarz M. Gastric carcinogenesis: a comprehensive review of the angiogenic pathways. Clin J Gastroenterol (2021) 14(1):14–25. doi: 10.1007/s12328-020-01295-1
12. Cao F, Hu C, Xu ZY, Zhang YQ, Huang L, Chen JH, et al. Current treatments and outlook in adenocarcinoma of the esophagogastric junction: a narrative review. Ann Transl Med (2022) 10(6):377. doi: 10.21037/atm-22-1064
13. Hess LM, Grabner M, Wang L, Liepa AM, Li XI, Cui ZL, et al. Reliability of conclusions from early analyses of real-world data for newly approved drugs in advanced gastric cancer in the united states. Pragmat Obs Res (2020) 11:27–43. doi: 10.2147/POR.S241427
14. Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol (2016) 34(13):1448–54. doi: 10.1200/JCO.2015.63.5995
15. Zhang Y, Xu J, Wang Q, Ling G, Mao Y, Cai M, et al. Efficacy and safety of second-line therapy with apatinib combined with chemotherapy as second-line therapy in advanced gastric cancer: a single-arm, open-label, prospective, multicenter study. Ann Transl Med (2022) 10(11):641. doi: 10.21037/atm-22-2752
16. Jing C, Wang J, Zhu M, Bai Z, Zhao B, Zhang J, et al. Camrelizumab combined with apatinib and s-1 as second-line treatment for patients with advanced gastric or gastroesophageal junction adenocarcinoma: a phase 2, single-arm, prospective study. Cancer Immunol Immunother (2022) 71(11):2597–608. doi: 10.1007/s00262-022-03174-9
17. Yan Y, Li H, Wu S, Wang G, Luo H, Niu J, et al. Efficacy and safety of intermittent versus continuous dose apatinib plus docetaxel as second-line therapy in patients with advanced gastric cancer or gastroesophageal junction adenocarcinoma: a randomized controlled study. Ann Transl Med (2022) 10(4):205. doi: 10.21037/atm-22-546
18. Jing C, Bai Z, Zhang J, Jiang H, Yang X, Yan S, et al. Apatinib plus s-1 for previously treated, advanced gastric or gastro-oesophageal junction adenocarcinoma: a phase 2, single-arm, prospective study. J Gastrointest Oncol (2021) 12(5):2035–44. doi: 10.21037/jgo-21-186
19. Chen H, Xue L, Liu L, Li P. Efficacy of apatinib combined with tegafur gimeracil and oteracil potassium in the second-line treatment of advanced gastric cancer. J BUON (2021) 26(3):917–23.
20. Qiu ZY, Qin R, Tian GY, Zhang Z, Chen M, He H, et al. Apatinib combined with s-1 as second-line therapy in advanced gastric cancer. Med (Baltimore) (2021) 100(17):e25630. doi: 10.1097/MD.0000000000025630
21. Zhang F, Yin Y, Ni T, Zhang M, Zhou Z, Sun X, et al. Treatment effect of apatinib combined chemotherapy as second-line or above therapy in patients with advanced gastric cancer or adenocarcinoma of the gastroesophageal junction. Pharmazie (2020) 75(8):389–94. doi: 10.1691/ph.2020.0403
22. Kawamoto Y, Yuki S, Sawada K, Nakamura M, Muto O, Sogabe S, et al. Phase II study of ramucirumab plus irinotecan combination therapy as second-line treatment in patients with advanced gastric cancer: HGCSG1603. Oncologist (2022) 27(8):e642–e9. doi: 10.1093/oncolo/oyac086
23. Nie C, Lv H, Chen B, Xu W, Wang J, Wang S, et al. High DCR and better survival in patients with advanced or metastatic gastric cancer receiving anti-angiogenic TKI plus chemotherapy: A real-world study. Technol Cancer Res Treat (2023) 22:15330338221150561. doi: 10.1177/15330338221150561
24. Kciuk M, Marciniak B, Kontek R. Irinotecan-still an important player in cancer chemotherapy: A comprehensive overview. Int J Mol Sci (2020) 21(14):4919. doi: 10.3390/ijms21144919
25. Scott LJ. Apatinib: A review in advanced gastric cancer and other advanced cancers. Drugs (2018) 78(7):747–58. doi: 10.1007/s40265-018-0903-9
26. Lorenzen S, Thuss-Patience P, Pauligk C, Gokkurt E, Ettrich T, Lordick F, et al. FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab as second-line therapy for patients with advanced or metastatic gastroesophageal adenocarcinoma with or without prior docetaxel - results from the phase II RAMIRIS study of the German gastric cancer study group at AIO. Eur J Cancer (2022) 165:48–57. doi: 10.1016/j.ejca.2022.01.015
27. Caiyun N. 102P - a real-world study comparing apatinib combined with irinotecan versus irinotecan as second-line or above therapy in patients with advanced or metastatic gastric cancer. ESMO Asia 2022 (2022).
28. Fathi Maroufi N, Rashidi MR, Vahedian V, Akbarzadeh M, Fattahi A, Nouri M. Therapeutic potentials of apatinib in cancer treatment: Possible mechanisms and clinical relevance. Life Sci (2020) 241:117106. doi: 10.1016/j.lfs.2019.117106
29. Diaz Del Arco C, Ortega Medina L, Estrada Munoz L, Garcia Gomez de Las Heras S, Fernandez Acenero MJ. Is there still a place for conventional histopathology in the age of molecular medicine? Lauren classification, inflammatory infiltration and other current topics in gastric cancer diagnosis and prognosis. Histol Histopathol (2021) 36(6):587–613. doi: 10.14670/HH-18-309
30. Kahraman S, Yalcin S. Recent advances in systemic treatments for HER-2 positive advanced gastric cancer. Onco Targets Ther (2021) 14:4149–62. doi: 10.2147/OTT.S315252
31. Wei J, Wu ND, Liu BR. Regional but fatal: Intraperitoneal metastasis in gastric cancer. World J Gastroenterol (2016) 22(33):7478–85. doi: 10.3748/wjg.v22.i33.7478
32. Wu Z, Cheng H, Shan F, Ying X, Miao R, Dong J, et al. In-hospital mortality risk model of gastric cancer surgery: Analysis of a nationwide institutional-level database with 94,277 Chinese patients. Front Oncol (2019) 9:846. doi: 10.3389/fonc.2019.00846
Keywords: second-line apatinib plus irinotecan, gastric adenocarcinoma, gastroesophageal junction adenocarcinoma, treatment efficacy, safety
Citation: Qu J, He X, Luo Y, Yu P, Chen Y, Liu J, Wang X, Wang C, Liang T, Bai Y, Han Y, Man L, Leng C, Zhou C, He L, Wang X, Liu Y and Qu X (2023) Evaluation of second-line apatinib plus irinotecan as a treatment for advanced gastric adenocarcinoma or gastroesophageal conjunction adenocarcinoma: a prospective, multicenter phase II trial. Front. Oncol. 13:1072943. doi: 10.3389/fonc.2023.1072943
Received: 18 October 2022; Accepted: 27 March 2023;
Published: 24 April 2023.
Edited by:
Kui Zhang, The University of Chicago, United StatesReviewed by:
Chunyan Hua, Wenzhou Medical University, ChinaCopyright © 2023 Qu, He, Luo, Yu, Chen, Liu, Wang, Wang, Liang, Bai, Han, Man, Leng, Zhou, He, Wang, Liu and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujuan Qu, eGpxdUBjbXUuZWR1LmNu; Yunpeng Liu, eXBsaXVAY211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.