
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 June 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1072922
 Ji Hoon Kim1,2
Ji Hoon Kim1,2 Jin Hyoung Kim1*
Jin Hyoung Kim1* Hyun-Ki Yoon1
Hyun-Ki Yoon1 Gi-Young Ko1
Gi-Young Ko1 Ji Hoon Shin1
Ji Hoon Shin1 Dong Il Gwon1
Dong Il Gwon1 Heung-Kyu Ko1
Heung-Kyu Ko1 Hee Ho Chu1
Hee Ho Chu1 Seong Ho Kim1
Seong Ho Kim1 Gun Ha Kim1
Gun Ha Kim1 Yonghun Kim1
Yonghun Kim1 Shakir Aljerdah3
Shakir Aljerdah3Objectives: To evaluate the safety and efficacy of TACE and factors predicting survival in patients with advanced hepatocellular carcinoma (HCC) without macrovascular invasion (MVI) or extrahepatic spread (EHS).
Methods: This single-center retrospective study included 236 treatment-naïve patients who underwent TACE as first-line treatment for advanced HCC without MVI or EHS between January 2007 and December 2021.
Results: Following TACE, the median overall survival (OS) was 24 months. Multivariate Cox regression analyses revealed that tumor number ≥4 (risk point: 3), maximal tumor size >10 cm (risk point: 2), Child–Pugh class B (risk point: 2), alpha-fetoprotein (AFP) concentration ≥400 ng/mL (risk point: 2), and presence of HCC rupture (risk point: 2) were risk factors significantly associated with OS. The expected median OS among patients with <2, 2–4, and 5–9 risk points were 72, 29, and 12 months respectively. The major complication rates were significantly lower in patients with maximal tumor size ≤10 cm than in those with maximal tumor size >10 cm (4% [5/138] vs 21% [21/98], p = 0.001).
Conclusion: TACE may be safe and effective in selected patients with advanced HCC without MVI or EHS, with a median OS of 24 months. Patients with limited tumor burden, compensated liver function, absence of HCC rupture, and favorable biologic markers may benefit the most from TACE. TACE is not recommended for patients with huge HCCs (>10 cm) because of its high rate of major complications (21%).
The Barcelona Clinic Liver Cancer (BCLC) staging system is the most frequently used staging system for hepatocellular carcinoma (HCC), determining patient prognosis and suggesting treatment algorithms based on tumor-, patient-, and liver function-related factors (1). Advanced HCC (BCLC stage C) includes patients who have macrovascular invasion (MVI) and/or extrahepatic spread (EHS) and/or tumor-related symptoms, defined as an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1 or 2 (2). The BCLC staging system is applicable to patients with highly heterogeneous clinical and oncological features. However, despite this heterogeneity, only systemic therapy is currently recommended as first-line treatment for patients with advanced HCC (1, 3–5).
Several recent studies, however, have suggested that patients with symptomatic HCC without MVI or EHS should not be regarded as having advanced-stage disease. Furthermore, patients with advanced HCC without MVI or EHS in a real-world setting have frequently been treated with locoregional therapy, such as transarterial chemoembolization (TACE) (6–10). The efficacy of TACE, however, has been insufficiently defined, and factors associated with prognosis in patients treated with TACE remain unclear. The clinical need for a more effective treatment strategy of patients with advanced HCC have made it necessary to determine the role of TACE in this patient group. The present study therefore evaluated tumor responses and factors predicting survival after TACE in patients with advanced HCC without MVI or EHS.
The records of our institution were retrospectively searched to identify treatment-naïve patients who underwent TACE as first-line treatment for advanced HCC between January 2007 and December 2021. Advanced HCC was defined according to the BCLC staging system. Patients with HCC, with or without cancer-related symptoms (ECOG PS of 1 or 2), were included (1). Patients with MVI and/or EHS, those with prior or current malignancy other than HCC, and those lost to follow-up during the study period were excluded. The demographic and clinical characteristics of all patients, including age, sex, liver disease etiology, and biochemical parameters, were obtained at the time of HCC diagnosis. The study protocol was approved by the Institutional Review Board or our center, which waived the requirement for written informed consent.
TACE was performed through right femoral artery puncture and cannulation under local anesthesia. Superior mesenteric and common hepatic arteriographies were achieved with a 5-F catheter (Rősch hepatic catheter; Cook) to assess the direction of portal flow, the anatomy of the hepatic artery, tumor size and location, and feeding arteries. Patients underwent cisplatin-based TACE (2 mg/kg body weight) with a 1.7–2.4-F microcatheter (Progreat Lambda, Terumo, Tokyo, Japan; Renegade, Boston Scientific, Cork, Ireland; Carnelian, Tokai Medical Products, Aichi, Japan). A 1:1 emulsion of cisplatin and iodized oil (Lipiodol®, Laboratoire Guerbet) was infused into the feeding artery. Subsequent embolization was performed with gelatin particles (Upjohn, Kalamazoo, MI, USA) until arterial flow stasis was observed.
Patients were initially followed-up by contrast-enhanced CT or MR imaging and laboratory tests, 1 month after TACE. Subsequent follow-up examinations were repeated every 2–3 months during the first 2 years, and every 3–6 months thereafter until HCC recurrence. Repeated TACE was performed in patients with insufficient responses after a single session of TACE and those with recurrent tumors.
The primary outcomes were patient overall survival (OS, measured in months) and factors predictive of OS after TACE. OS was defined as the period between initial TACE and patient death. Pretreatment risk factors evaluated included the number of tumors (<4 vs. ≥4) (11), tumor size (≤10 cm vs. >10 cm) (12), morphological tumor type (nodular vs. infiltrative), tumor extent (unilobar vs. bilobar), presence or absence of portal hypertension, presence or absence of tumor rupture (13), Child–Pugh class (A vs. B), serum alpha-fetoprotein (AFP) concentration level (<400 ng/mL vs. ≥400 ng/mL) (14), presence or absence of underlying liver cirrhosis (LC), and presence or absence of bile duct invasion. Portal hypertension was diagnosed if the patient met any of the following criteria: noticeable portosystemic collaterals, ascites, esophageal varix, and splenomegaly with thrombocytopenia (platelet count <100,000/mm3) (15).
The secondary outcomes were progression-free survival (PFS), radiologic tumor response, and complications following TACE. PFS was defined as the time from initial TACE to tumor progression or death from any cause. Tumor response was evaluated according to the Modified Response Evaluation Criteria in Solid Tumors (m-RECIST), divided into four response categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (16). Patients with CR or PR were categorized as tumor responders, and patients with SD or PD were categorized as nonresponders. Initial tumor response 1 month after TACE and the best tumor response over the entire study period were evaluated (17). Complications were categorized using the Society of Interventional Radiology (SIR) clinical practice guidelines. Major complications were defined as marked escalation of care (ie, hospital admission or prolongation of existing hospital admission for > 24 hours, hospital admission that is atypical for the procedure, inpatient transfer from regular floor/telemetry to intensive care unit, or complex intervention performed requiring general anesthesia in previously nonintubated patient), life-threatening or disabling event, or patient death (18, 19). All other complications were considered minor.
Potential risk factors predicting OS after TACE were identified by univariable Cox-proportional hazards analyses. Variables with a p-value <0.05 were entered into a multivariate Cox-proportional hazards model using backward stepwise elimination and considering multicollinearity. A risk score was assigned to each variable based on its β regression coefficient in the multivariable Cox regression analyses, and the prediction model was designed according to risk scores. OS and PFS rates were estimated by the Kaplan–Meier method and compared using Log rank tests. Factors associated with major complications were evaluated by multivariable logistic regression analysis. Variables with p < 0.25 in the univariable analyses were entered into a multivariable analysis. All statistical analyses were performed using SPSS version 25 (IBM Corp.) and MedCalc Statistical Software version 20.1 (MedCalc Software Ltd).
Of the 1256 patients who underwent TACE as first-line treatment for advanced HCC during the study period, 251 had advanced HCC without MVI or EHM. Fifteen of these patients were excluded from this study, 11 because they were lost to follow-up and 4 because they had a prior or concurrent malignancy. The study population thus consisted of 236 patients, 200 men, and 36 women, of mean ± SD, age 59 ± 12 years. Most patients were Child–Pugh class A (85%), had an ECOG PS of 1 (89%), had underlying liver cirrhosis (LC) (81%), and had nodular type tumors (82%). The major etiology of HCC was chronic hepatitis B (78%). Of the 236 patients, 18 (8%) had bile duct invasion, 40 (17%) had portal hypertension, and 40 (17%) had tumor rupture with perihepatic hematoma. The baseline characteristics of the included patients are summarized in Table 1.
By the end of the follow-up period, 163 (69%) patients had died. The median overall survival (OS) time after TACE was 24 months (95% confidence interval [CI], 20–28 months). The cumulative OS rates at 1, 3, 5, and 10 years were 75%, 35%, 22%, and 16%, respectively.
Multivariate Cox regression analyses revealed that ≥4 (vs. <4) tumors (adjusted hazard ratio [HR], 2.96; p < 0.001), tumor size >10 cm (vs. ≤10 cm) (adjusted HR, 1.63; p = 0.003), Child–Pugh class B (vs. class A) (adjusted HR, 2.12; p < 0.001), AFP concentration ≥400 ng/mL (vs. <400 ng/mL) (adjusted HR, 1.62; p = 0.003), and tumor rupture before TACE (vs. no tumor rupture) (adjusted HR, 1.55; p = 0.037) were significantly associated with OS rates (Table 2). The Kaplan–Meier curves of OS relative to these five factors are shown in Figure 1.
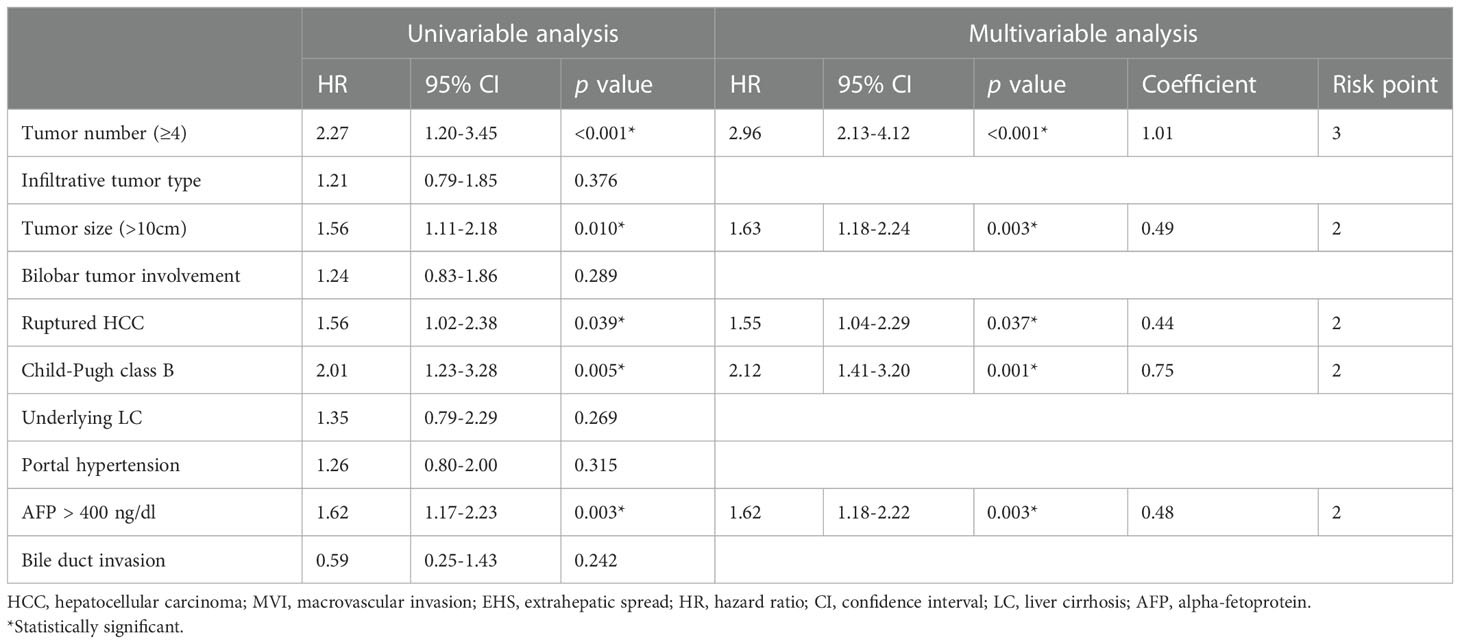
Table 2 Univariable and multivariable analyses of factors associated with overall survival after TACE in patients with advanced HCC without MVI and EHM.
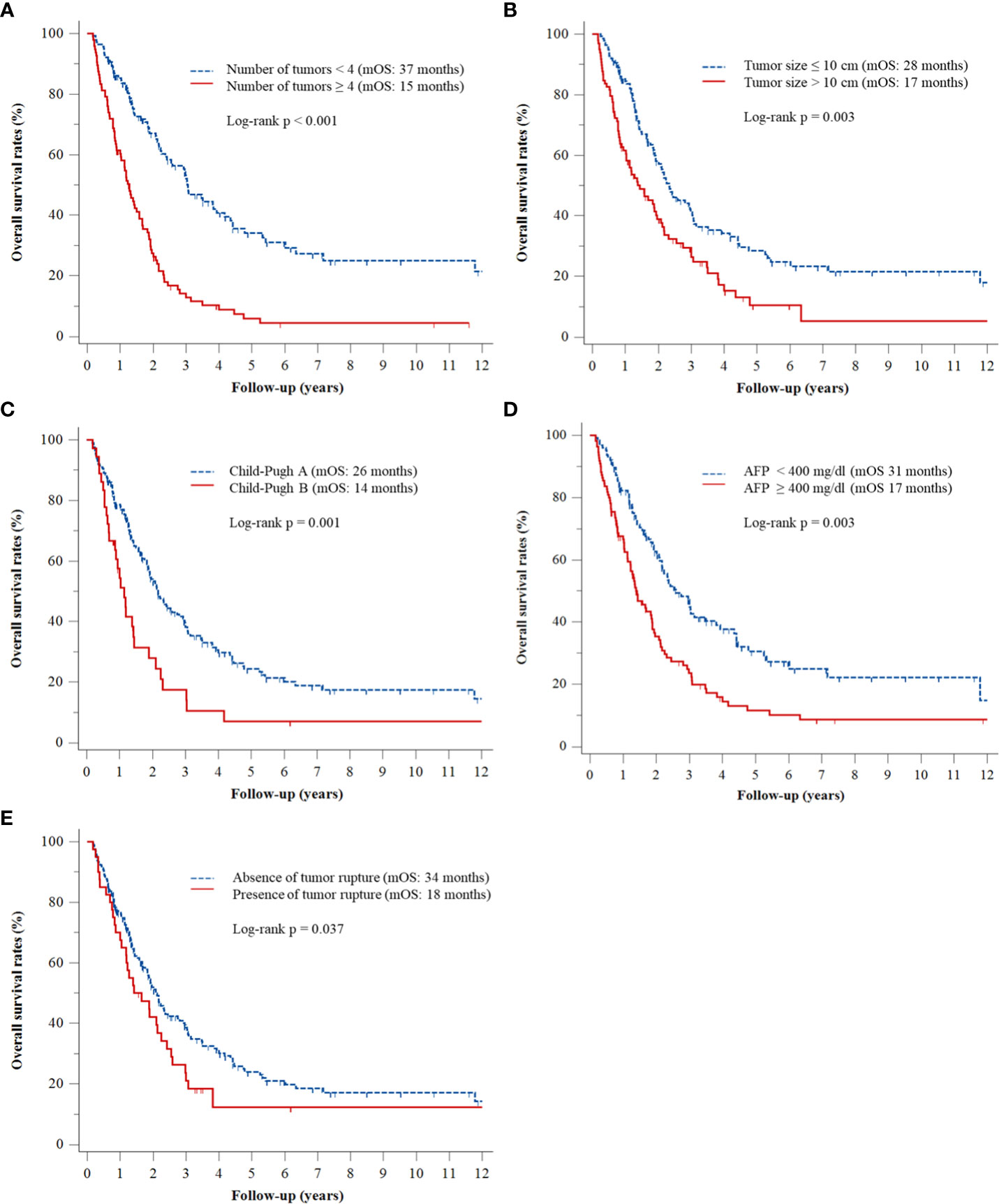
Figure 1 Kaplan–Meier analyses of OS rates according to (A) number of tumors, (B) tumor size, (C) Child–Pugh class, (D) serum AFP level, and (E) presence or absence of tumor rupture.
A predictive model was subsequently developed based on the five factors identified in the multivariable Cox analysis. The presence of ≥4 tumors was assigned three risk points, whereas tumor size ≥10 cm, Child–Pugh class B, serum AFP ≥400 ng/mL, and tumor rupture were each assigned two risk points (Table 3). Patients were stratified by number of risk points into three groups, defined as low- (score <2), intermediate- (score 2–4), and high- (score 5–9) risk groups. Median OS was 72 months (95% CI, 34–110 months) in the low-risk group, 29 months (95% CI, 21–38 months) in the intermediate-risk group, and 12 months (95% CI, 9–16 months) in the high-risk group (p < 0.001) (Figure 2).
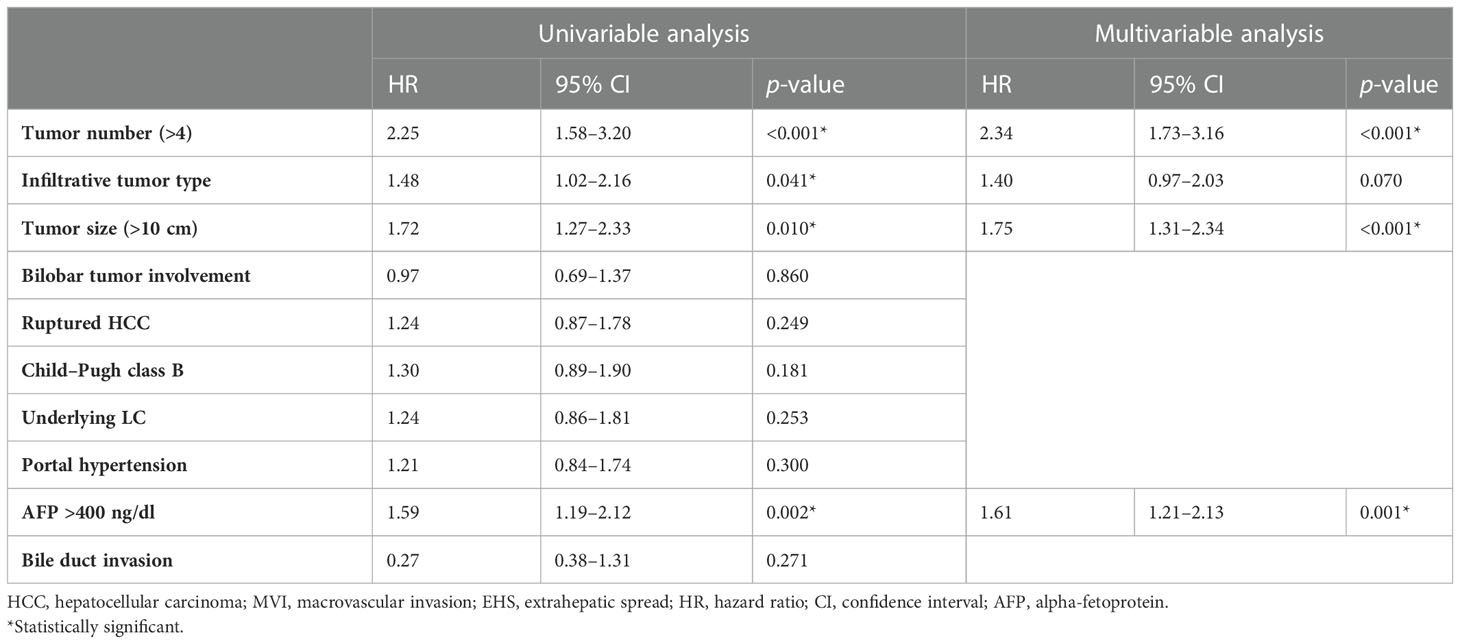
Table 3 Univariable and multivariable analyses of factors associated with progression-free survival after TACE in patients with advanced HCC without MVI and EHM.
By the end of the follow-up period, 204 patients (86%) died or experienced HCC progression. The median post-TACE PFS of the 236 patients was 7 months (95% CI, 6–8 months). Multivariate Cox regression analyses revealed that covariates significantly associated with PFS included ≥4 (vs. <4) tumors (adjusted HR, 2.34; p = 0.001), tumor size >10 cm (vs. ≤10 cm) (adjusted HR, 1.75; p < 0.001), and AFP concentration ≥400 ng/mL (vs. <400 ng/mL) (adjusted HR, 1.61; p = 0.001) (Table 2).
All 236 patients were evaluated by dynamic CT or MRI for tumor response 1 month after TACE, with 42 patients (18%) achieving CR, 126 (53%) achieving PR, 15 (6%) having PD, and 53 (22%) having SD. Thus, the initial tumor responder rate (CR plus PR) was 71%. During the follow-up period (median, 65 months; interquartile range, 31–114 months), 89 patients (38%) achieved CR, and 100 (42%) achieved PR, whereas 14 (6%) had PD, and 33 (14%) had SD as the best overall tumor response. The best overall tumor responder rate was 80%.
Major complications were observed in 26 (11%) patients after TACE. The major complications included acute renal failure in six patients; liver abscess in five; tumor lysis syndrome, acute cholecystitis, and persistent fever in three patients each; spontaneous bacterial peritonitis in two patients; and hepatic failure, allergic reaction to cisplatin, pleural effusion, and spinal infarction in one patient each. One patient died of tumor lysis syndrome (mortality rate, 0.04%). Major complication rates were significantly lower in patients with maximal tumor size ≤10 cm than in those with maximal tumor size >10 cm (4% [5/138] vs 21% [21/98], p = 0.001). Multivariate logistic regression analysis showed that tumor size >10 cm (odds ratio, 5.72; p = 0.001) was the only significant risk factor for major complication (Table 4).
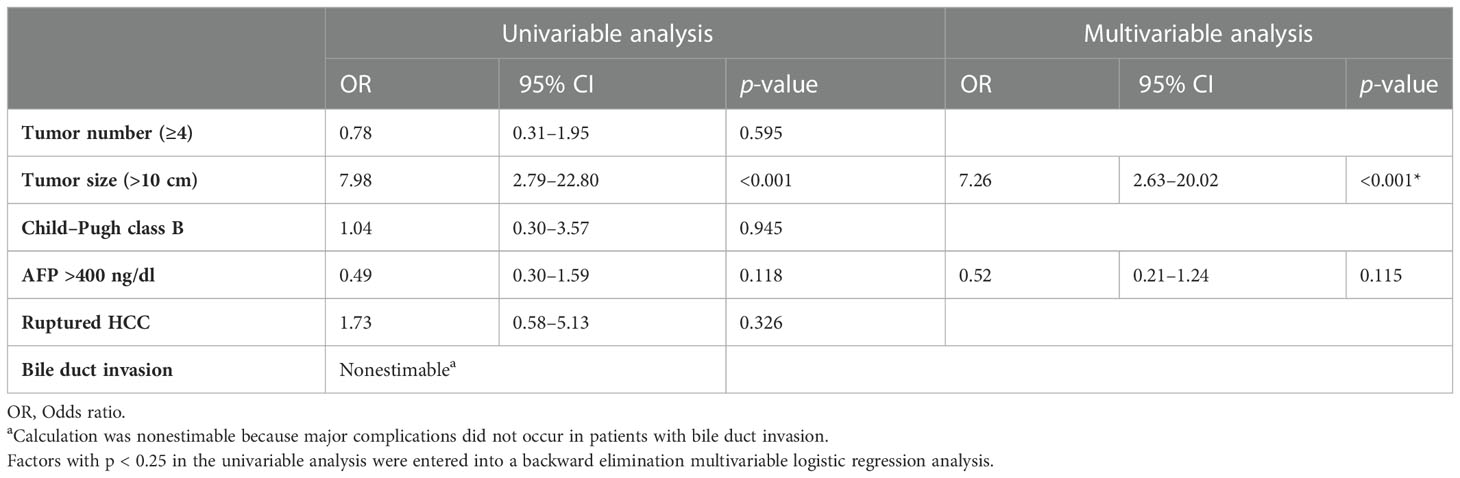
Table 4 Univariable and multivariable analyses of pretreatment factors associated with major complications.
To our knowledge, this study is the first to specifically investigate the survival outcomes and safety of first-line TACE in treatment-naïve, symptomatic HCC patients without MVI or EHS. First-line TACE showed acceptable efficacy in prolonging survival in these patients, with a median OS after TACE of 24 months. This median OS was longer than that observed in patients with advanced HCC treated with systemic treatment in the recent randomized phase III trials (median OS; 15 – 16 months) (20, 21). The median OS of patients with advanced HCC in the present study was similar to that of patients with intermediate HCC receiving TACE, which has been reported to range 25–30 months (22–25). Based on the extended indications for TACE and the suboptimal responsive to systemic treatment in this patient group, appropriate locoregional tumor control with TACE may be a better option than systemic treatment. In addition, the determination of tumor-related symptoms is frequently subjective as it is dependent on several confounding factors, including advanced age, LC, extrahepatic comorbidities, and individual sensitivity to pain (26). Thus, classifying patients as having advanced HCC based only on clinical findings, despite favorable oncological features, may be a limitation of the BCLC staging system. The results of this study indicate that symptomatic HCC patients without MVI or EHM should not be regarded as having advanced-stage tumors as it may limit any potential locoregional treatment.
Multivariable Cox regression analyses showed that pretreatment oncological features, including ≥4 tumors, tumor size >10 cm, and tumor rupture, as well as clinical factors, such as decompensated liver function and AFP ≥400 ng/mL, were statistically significant predictors of poorer OS. Because this study included a relatively large number of patients (n = 236), these five parameters could be used to develop a prediction model. Three risk groups were identified and classified as low-, intermediate-, and high-risk groups, with median OS of 72, 29, and 12 months, respectively. Based on this prediction model, the low-risk group, consisting of patients with <4 tumors, tumor size ≤10 cm, absence of tumor rupture, compensated liver function, and AFP <400 ng/mL, could benefit most from TACE. Therefore, TACE may be actively recommended as a preferable treatment option for this group.
The present study also showed that tumor rupture was associated with poor prognosis after TACE. Previous studies have yielded conflicting results on whether tumor rupture is a poor prognostic factor and on the role of TACE in patients with tumor rupture (27, 28). Based on recently reported data on equivalent survival outcomes in patients with ruptured HCC, the Sixth edition of the Liver Cancer Study Group of Japan (LCSGJ) guidelines does not consider tumor rupture in T staging. In contrast, the Eighth edition of the American Joint Committee on Cancer guidelines has classified HCC rupture as T4 stage (29). The results of the present study indicate that ruptured HCC may have prognostic significance in predicting a poorer OS. However, based on the management of ruptured HCC, TACE may be the best option for obtaining hemostasis for bleeding from a ruptured HCC as well as for antitumor treatment of the HCC itself. A recent meta-analysis (30) involving 21 studies reported that TACE was significantly superior to emergency surgery in reducing complication rates, with comparable 1 year survival rates. Therefore, TACE should not be precluded for the treatment of patients with ruptured HCC.
Although bile duct invasion (BDI) is regarded as an indicator of poorer prognosis in patients with HCC, few studies to date have specifically focused on HCC patients with BDI. Moreover, of the various HCC staging systems currently used, only the LCSGJ staging system regards BDI as having an impact comparable to that of MVI (31, 32). Interestingly, the present study found that BDI was not significantly associated with OS after TACE. Theoretically, HCC with BDI may be accompanied by portal vein tumor thrombus, which is considered prognostic of poorer OS. Because the present study excluded the features of portal vein invasion, BDI itself was not associated with survival outcomes. These findings suggest that TACE can be recommended to treat HCC patients with BDI without portal vein tumor thrombus.
In the present study, evaluation of safety profiles showed that only large tumor size (>10 cm) was significantly associated with the occurrence of major complications, with a relatively high rate (21/98; 21%). TACE has been considered a relative contraindication for the treatment of huge HCC (> 10 cm) due to the increased risk of peri-procedural adverse effects and the suboptimal treatment response (33, 34), and recent studies reported that hepatic arterial infusion chemotherapy (HAIC) was found to prolong OS compared with TACE in patients with huge HCC (35–37). A recent randomized phase III trial showed that the use of combinations of chemotherapy fluorouracil/leucovorin/oxaliplatin in HAIC group had a better OS (HR 0.57 [95% CI, 0.44 to 0.74]; P <.001) and a lower incidence of serious adverse events (19% vs. 30%, P = .03) compared with the TACE group for patients with large unresectable HCC without vascular invasion or extrahepatic metastasis (38). Thus, due to the high risk of major complications, TACE should not be recommended as a primary therapeutic option for patients with huge HCC. Additional randomized controlled trials are needed to determine a safe and effective option for the treatment of huge HCCs.
The optimal use of TACE as a treatment option for patients with Child-Pugh class B patients who are not eligible for liver transplantation remains uncertain. In real-world allocation, there is variation in the application of TACE due to variable angiographical techniques (lobar, selective, and super selective) and different chemo therapeutic drugs. According to the American
Association for the Study of Liver Diseases guidelines, TACE is recommended for asymptomatic patients with Child–Pugh class A. On the other hand, the European Association for the Study of the Liver guidelines expand the indication to include Child–Pugh class B7 patients without ascites while the Japan Society of Hepatology guidelines also extend the indication to Child–Pugh class B patients (39–41). In the present study, of 36 child-Pugh class B patients, majority of patients had a B7 score (n = 22, 61%), whereas 7 (19%) and 7 (19%) patients had B8 and B9 scores. Although indirect comparisons should be undertaken with caution, the median OS of Child-Pugh class B patients after TACE in this study was longer compared with results of a recent multicenter phase I/II trial investigating of immunotherapy in patients with Child-Pugh class B advanced HCC (14 months vs. 7.6 months, respectively) (42). However, the findings of the present study showed that Child-Pugh class B patients may have prognostic significance in predicting a worse OS outcome. Moreover, subgroup analysis for the Child-Pugh class B group was not feasible due to small size. Therefore, further studies are necessary to provide reliable data on the appropriate indications of TACE in patients with Child-Pugh class B patients
In this study, 29% (69/236) of patients were nonresponders. Close monitoring of liver function is mandatory for this group of patients because recent studies suggest that patients without a radiological response after TACE are likely to be ineffective and harmed by additional local therapies and may also be at risk of cumulative liver injury (43). This could lead to a deterioration of liver function and make patients ineligible for subsequent systemic treatment (4, 24, 44–46). Therefore, it is crucial not to miss the appropriate time for switching to therapy in patients with refractoriness to TACE.
The present study had several limitations. First, its retrospective, single-institution design may have a potential for selection bias. However, efforts were made to minimize potential bias by performing multivariate analyses with relatively a large sample size (n = 236). Second, we examined patients underwent only TACE as first-line treatment. Nowadays a lot of research has been made in adjuvant immuno therapy after TACE in advanced-stage HCC patients (47–52). Combining TACE with immunotherapeutic agents hypothesized that prolonged survival could be achieved because adjuvant immunotherapy can control TACE-induced neo-angiogenesis and reduce the risk of tumor recurrence and metastatic growth. So, prospective, multicenter randomized controlled trials comparing several therapeutic modalities are needed to optimize treatment in this specific group of patients.
In conclusion, TACE may be safe and effective in selected patients with advanced HCC without MVI or EHS, with a median OS of 24 months. Patients with a limited tumor burden, defined as <4 tumors and maximal tumor size ≤10 cm, compensated liver function, absence of HCC rupture, and AFP concentration <400 ng/mL, were found to benefit most from TACE. Because of its high rate of major complications (21%), TACE should not be recommended for patients with huge (>10 cm) HCCs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization, JHK. Methodology, JHK. Validation, JHK and JS. Formal analysis, JK, and JHK. Data curation, JK, JHK, H-KK, HC, SK, JS, DG, G-YK, H-KY, YK, GK, and SA. Writing—original draft preparation, JK. Writing—review and editing, JHK, H-KK, HC, SK, JS, DG, G-YK, H-KY, YK, GK, and SA. Visualization, JK. Supervision, JHK. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. Bclc strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
2. (2020). Available at: https://Oncologypro.Esmo.Org/Oncology-in-Practice/Practice-Tools/Performance-Scales (Accessed 6 January 2022).
3. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: asco guideline. J Clin Oncol (2020) 38(36):4317–45. doi: 10.1200/jco.20.02672
4. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet (2022) 400(10360):1345–62. doi: 10.1016/s0140-6736(22)01200-4
5. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
6. Yen YH, Cheng YF, Wang JH, Lin CC, Chen YY, Yong CC, et al. Real world clinical practice in treating advanced hepatocellular carcinoma: when East meets West. PloS One (2020) 15(3):e0230005. doi: 10.1371/journal.pone.0230005
7. Lucatelli P, Guiu B. 2022 Update of bclc treatment algorithm of hcc: what's new for interventional radiologists? Cardiovasc Intervent Radiol (2022) 45(3):275–6. doi: 10.1007/s00270-021-03047-1
8. Matsumoto MM, Mouli S, Saxena P, Gabr A, Riaz A, Kulik L, et al. Comparing real world, personalized, multidisciplinary tumor board recommendations with bclc algorithm: 321-patient analysis. Cardiovasc Intervent Radiol (2021) 44(7):1070–80. doi: 10.1007/s00270-021-02810-8
9. Goyal P, Salem R, Mouli SK. Role of interventional oncology in hepatocellular carcinoma: future best practice beyond current guidelines. Br J Radiol (2022) 95(1138):20220379. doi: 10.1259/bjr.20220379
10. Li Y, Zhu D, Ren W, Gu J, Ji W, Zhang H, et al. Curative analysis of patients with hepatocellular carcinoma using transcatheter arterial chemoembolization combined with radiofrequency ablation. Med Sci Monit (2022) 28:e936246. doi: 10.12659/msm.936246
11. Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-pacific primary liver cancer expert consensus statements. Liver Cancer (2020) 9(3):245–60. doi: 10.1159/000507370
12. Wang ZX, Li J, Wang EX, Xia DD, Bai W, Wang QH, et al. Validation of the six-and-Twelve criteria among patients with hepatocellular carcinoma and performance score 1 receiving transarterial chemoembolization. World J Gastroenterol (2020) 26(15):1805–19. doi: 10.3748/wjg.v26.i15.1805
13. Xia F, Ndhlovu E, Zhang M, Chen X, Zhang B, Zhu P. Ruptured hepatocellular carcinoma: current status of research. Front Oncol (2022) 12:848903. doi: 10.3389/fonc.2022.848903
14. Yen YH, Liu YW, Li WF, Wang CC, Yong CC, Lin CC, et al. Alpha-fetoprotein combined with radiographic tumor burden score to predict overall survival after liver resection in hepatocellular carcinoma. Cancers (Basel) (2023) 15(4). doi: 10.3390/cancers15041203
15. Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in child-pugh b cirrhosis. J Hepatol (2020) 72(1):75–84. doi: 10.1016/j.jhep.2019.08.032
16. Llovet JM, Lencioni R. Mrecist for hcc: performance and novel refinements. J Hepatol (2020) 72(2):288–306. doi: 10.1016/j.jhep.2019.09.026
17. Park C, Chu HH, Kim JH, Kim SY, Alrashidi I, Gwon DI, et al. Clinical significance of the initial and best responses after chemoembolization in the treatment of intermediate-stage hepatocellular carcinoma with preserved liver function. J Vasc Interv Radiol (2020) 31(12):1998–2006.e1. doi: 10.1016/j.jvir.2020.04.017
18. Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol (2017) 28(10):1432–7.e3. doi: 10.1016/j.jvir.2017.06.019
19. Lucatelli P, Burrel M, Guiu B, de Rubeis G, van Delden O, Helmberger T. Cirse standards of practice on hepatic transarterial chemoembolisation. Cardiovasc Intervent Radiol (2021) 44(12):1851–67. doi: 10.1007/s00270-021-02968-1
20. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence (2022) 1(8):EVIDoa2100070. doi: 10.1056/EVIDoa2100070
21. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (Cosmic-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(8):995–1008. doi: 10.1016/s1470-2045(22)00326-6
22. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
23. Llovet JM, Vogel A, Madoff DC, Finn RS, Ogasawara S, Ren Z, et al. Randomized phase 3 leap-012 study: transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma not amenable to curative treatment. Cardiovasc Intervent Radiol (2022) 45(4):405–12. doi: 10.1007/s00270-021-03031-9
24. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2021) 18(5):293–313. doi: 10.1038/s41575-020-00395-0
25. Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: aasld consensus conference. Hepatology (2021) 73(Suppl 1):158–91. doi: 10.1002/hep.31327
26. Park J-W. KLCA-NCC Korea Practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol (2022) 28(4):583–705. doi: 10.3350/cmh.2022.0294
27. Kwon JH, Song GW, Hwang S, Kim KH, Ahn CS, Moon DB, et al. Surgical outcomes of spontaneously ruptured hepatocellular carcinoma. J Gastrointest Surg (2021) 25(4):941–53. doi: 10.1007/s11605-020-04555-0
28. Yan J, Li T, Deng M, Fan H. Ruptured hepatocellular carcinoma: what do interventional radiologists need to know? Front Oncol (2022) 12:927123. doi: 10.3389/fonc.2022.927123
29. Xia F, Huang Z, Zhang Q, Ndhlovu E, Zhang M, Chen X, et al. Early-stage ruptured hepatocellular carcinoma with different tumor diameters: small tumors have a better prognosis. Front Oncol (2022) 12:865696. doi: 10.3389/fonc.2022.865696
30. Xu X, Chen C, Liu Q, Huang X. A meta-analysis of Tae/Tace versus emergency surgery in the treatment of ruptured hcc. Cardiovasc Intervent Radiol (2020) 43(9):1263–76. doi: 10.1007/s00270-020-02514-5
31. Huang Q, Chen Y, Lin K, Sun C, Zheng S, Chen J, et al. Redefining hepatocellular carcinoma staging systems based on the bile duct invasion status: a multicenter study. Front Oncol (2021) 11:673285. doi: 10.3389/fonc.2021.673285
32. Feng JK, Chen ZH, Wu YX, Wang K, Sun JX, Chai ZT, et al. Comparison of different surgical interventions for hepatocellular carcinoma with bile duct tumor thrombus: a systematic review and meta-analysis. Ann Transl Med (2020) 8(23):1567. doi: 10.21037/atm-20-3935
33. Kim GH, Kim JH, Shim JH, Ko HK, Chu HH, Shin JH, et al. Chemoembolization for single Large hepatocellular carcinoma with preserved liver function: analysis of factors predicting clinical outcomes in a 302 patient cohort. Life (Basel) (2021) 11(8). doi: 10.3390/life11080840
34. Yu SCH, Hui JW, Li L, Cho CC, Hui EP, Chan SL, et al. Comparison of chemoembolization, radioembolization, and transarterial ethanol ablation for huge hepatocellular carcinoma (≥ 10 cm) in tumour response and long-term survival outcome. Cardiovasc Intervent Radiol (2022) 45(2):172–81. doi: 10.1007/s00270-021-02777-6
35. Li S, Lyu N, Han X, Li J, Lai J, He M, et al. Hepatic artery infusion chemotherapy using fluorouracil, leucovorin, and oxaliplatin versus transarterial chemoembolization as initial treatment for locally advanced hepatocellular carcinoma: a propensity score-matching analysis. J Vasc Interv Radiol (2021) 32(9):1267–76.e1. doi: 10.1016/j.jvir.2021.06.008
36. Tsai WL, Sun WC, Chen WC, Chiang CL, Lin HS, Liang HL, et al. Hepatic arterial infusion chemotherapy vs transcatheter arterial embolization for patients with huge unresectable hepatocellular carcinoma. Med (Baltimore) (2020) 99(32):e21489. doi: 10.1097/md.0000000000021489
37. Kong SY, Song JJ, Jin YQ, Deng MJ, Yan JX. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization for patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Acta Clin Belg (2023) 78(2):171–9. doi: 10.1080/17843286.2022.2076791
38. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for Large hepatocellular carcinoma: a randomized phase iii trial. J Clin Oncol (2022) 40(2):150–60. doi: 10.1200/jco.21.00608
39. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: jsh consensus statements and recommendations 2021 update. Liver Cancer (2021) 10(3):181–223. doi: 10.1159/000514174
40. Tampaki M, Papatheodoridis GV, Cholongitas E. Management of hepatocellular carcinoma in decompensated cirrhotic patients: a comprehensive overview. Cancers (Basel) (2023) 15(4). doi: 10.3390/cancers15041310
41. Kudo M. A paradigm change in the treatment strategy for hepatocellular carcinoma. Liver Cancer (2020) 9(4):367–77. doi: 10.1159/000507934
42. Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, Acosta-Rivera M, et al. Checkmate 040 cohort 5: a phase I/Ii study of nivolumab in patients with advanced hepatocellular carcinoma and child-pugh b cirrhosis. J Hepatol (2021) 75(3):600–9. doi: 10.1016/j.jhep.2021.04.047
43. Han G, Berhane S, Toyoda H, Bettinger D, Elshaarawy O, Chan AWH, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology (2020) 72(1):198–212. doi: 10.1002/hep.31022
44. Granito A, Forgione A, Marinelli S, Renzulli M, Ielasi L, Sansone V, et al. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol (2021) 14:17562848211016959. doi: 10.1177/17562848211016959
45. Ogasawara S, Ooka Y, Koroki K, Maruta S, Kanzaki H, Kanayama K, et al. Switching to systemic therapy after locoregional treatment failure: definition and best timing. Clin Mol Hepatol (2020) 26(2):155–62. doi: 10.3350/cmh.2019.0021n
46. Palmer DH, Malagari K, Kulik LM. Role of locoregional therapies in the wake of systemic therapy. J Hepatol (2020) 72(2):277–87. doi: 10.1016/j.jhep.2019.09.023
47. Hatzidakis A, Müller L, Krokidis M, Kloeckner R. Local and regional therapies for hepatocellular carcinoma and future combinations. Cancers (Basel) (2022) 14(10). doi: 10.3390/cancers14102469
48. Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The significance of transarterial Chemo(Embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: a systematic review. Front Immunol (2022) 13:913464. doi: 10.3389/fimmu.2022.913464
49. Chami P, Jarnagin W, Abou-Alfa GK, Harding J, Kim N, Lin H, et al. Non-surgical locoregional therapies alone or in combination with systemic therapy in patients with hepatocellular carcinoma. Cancers (Basel) (2023) 15(6). doi: 10.3390/cancers15061748
50. Sun T, Ren Y, Kan X, Chen L, Zhang W, Yang F, et al. Advanced hepatocellular carcinoma with hepatic arterioportal shunts: combination treatment of transarterial chemoembolization with apatinib. Front Mol Biosci (2020) 7:607520. doi: 10.3389/fmolb.2020.607520
51. Zou X, Fan W, Xue M, Li J. Evaluation of the benefits of tace combined with sorafenib for hepatocellular carcinoma based on untreatable tace (Untaceable) progression. Cancer Manag Res (2021) 13:4013–29. doi: 10.2147/cmar.S304591
52. Shen L, Chen S, Qiu Z, Qi H, Yuan H, Cao F, et al. Transarterial chemoembolization combined with apatinib versus transarterial chemoembolization alone for hepatocellular carcinoma with macroscopic vascular invasion: a propensity score matching analysis. J Cancer Res Ther (2020) 16(5):1063–8. doi: 10.4103/jcrt.JCRT_801_19
Keywords: hepatocellular carcinoma, chemoembolization, ethiodized oil, treatment outcome, Barcelona clinic liver cancer (BCLC) staging
Citation: Kim JH, Kim JH, Yoon H-K, Ko G-Y, Shin JH, Gwon DI, Ko H-K, Chu HH, Kim SH, Kim GH, Kim Y and Aljerdah S (2023) Transarterial chemoembolization for advanced hepatocellular carcinoma without macrovascular invasion or extrahepatic metastasis: analysis of factors prognostic of clinical outcomes. Front. Oncol. 13:1072922. doi: 10.3389/fonc.2023.1072922
Received: 18 October 2022; Accepted: 09 May 2023;
Published: 06 June 2023.
Edited by:
Hong-Tao Hu, Henan Provincial Cancer Hospital, ChinaReviewed by:
Pil Soo Sung, The Catholic University of Korea, Republic of KoreaCopyright © 2023 Kim, Kim, Yoon, Ko, Shin, Gwon, Ko, Chu, Kim, Kim, Kim and Aljerdah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Hyoung Kim, amhraW1yYWRAYW1jLnNlb3VsLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.