
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 16 March 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1072106
This article is part of the Research TopicInnovations in Surgical OncologyView all 20 articles
 Kosei Takagi1*
Kosei Takagi1* Kazuhiro Noma1
Kazuhiro Noma1 Yasuo Nagai1
Yasuo Nagai1 Satoru Kikuchi1
Satoru Kikuchi1 Yuzo Umeda1
Yuzo Umeda1 Ryuichi Yoshida1
Ryuichi Yoshida1 Tomokazu Fuji1
Tomokazu Fuji1 Kazuya Yasui1
Kazuya Yasui1 Takehiro Tanaka2
Takehiro Tanaka2 Hajime Kashima1
Hajime Kashima1 Takahito Yagi1
Takahito Yagi1 Toshiyoshi Fujiwara1
Toshiyoshi Fujiwara1Background: Cancer-associated fibroblasts (CAFs) reportedly enhance the progression of gastrointestinal surgery; however, the role of CAFs in ampullary carcinomas remains poorly examined. This study aimed to investigate the effect of CAFs on the survival of patients with ampullary carcinoma.
Materials and methods: A retrospective analysis of 67 patients who underwent pancreatoduodenectomy between January 2000 and December 2021 was performed. CAFs were defined as spindle-shaped cells that expressed α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP). The impact of CAFs on survival, including recurrence-free (RFS) and disease-specific survival (DSS), as well as prognostic factors associated with survival, was analyzed.
Results: The high-α-SMA group had significantly worse 5-year RFS (47.6% vs. 82.2%, p = 0.003) and 5-year DSS (67.5% vs. 93.3%, p = 0.01) than the low-α-SMA group. RFS (p = 0.04) and DSS (p = 0.02) in the high-FAP group were significantly worse than those in the low-FAP group. Multivariable analyses found that high α-SMA expression was an independent predictor of RFS [hazard ratio (HR): 3.68; 95% confidence intervals (CI): 1.21–12.4; p = 0.02] and DSS (HR: 8.54; 95% CI: 1.21–170; p = 0.03).
Conclusions: CAFs, particularly α-SMA, can be useful predictors of survival in patients undergoing radical resection for ampullary carcinomas.
Ampullary carcinomas of the duodenum are rare neoplasms that arise in the ampulla of Vater. Surgical resection is a standard treatment for ampullary carcinoma, and relatively good prognosis has been reported after radical resection (1, 2). Histologically, ampullary carcinoma can be divided into three subtypes: intestinal, and pancreatobiliary, and mixed types (3, 4). As several studies have reported the better prognosis and non-invasive nature of the intestinal type, the prognostic role of the histological subtypes has been currently recognized (5). However, the morphology and immunohistochemical features of ampullary carcinomas have been poorly investigated, owing to its rarity (3, 4).
The tumor microenvironment or stroma is a multicellular system consisting of mesenchymal, endothelial, and hematopoietic cells in the extracellular matrix (6). Cancer-associated fibroblasts (CAFs) are key components of the tumor microenvironment with various functions, including cancer initiation and progression (7). Markers of fibroblast subtypes include α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) (8). The clinical implications of CAFs as biomarkers and potential targets for prevention and treatment have been discussed in gastrointestinal oncology (9, 10). However, the role of CAFs in ampullary carcinoma has rarely been investigated.
This study aimed to investigate the presence of CAFs in patients with ampullary carcinoma. We also evaluated the effect of CAFs on the survival of patients with ampullary carcinoma.
We retrospectively reviewed 78 patients with ampullary carcinoma who underwent pancreatoduodenectomy at our institution between January 2000 and December 2021. The study protocol was approved by the Institutional ethics committee (approval no. 2110-003), and was conducted in accordance with the Declaration of Helsinki.
Clinicopathological data were extracted from our database: age, sex (male or female), body mass index, American Society of Anesthesiologists (ASA) physical status (grade 1, 2, or 3), hypertension, diabetes mellitus, preoperative biliary drainage, operative time, estimated blood loss, postoperative outcome [major complication defined as Clavien grade ≥3 (11) and mortality), pathological factors evaluated by the General Rules for Clinical and Pathological Studies on Cancer of the Biliary Tract of Japan (12) (T and N factors), histopathologic subtype evaluated by a pathologist (intestinal, pancreatobiliary, and mixed type) (13), recurrence (absence or presence), status at the last follow-up (survival or death), and cause of death (primary disease-related or others).
We employed a previously reported protocol for evaluating CAFs (14–16). First, the presence of tumor cells was confirmed by hematoxylin and eosin (H&E) staining. Sections on the microslides were deparaffinized with xylene, hydrated using a diluted alcohol series, and immersed in H2O2 with methanol to quench endogenous peroxidase activity. To reduce nonspecific staining, each section was blocked with a serum-free protein block (Dako, Agilent Technologies, Santa Clara, CA, USA) for 15 min. After heat-mediated antigen retrieval with Tris/EDTA buffer, the sections were incubated with anti-SMA antibody (1:1000 dilution; Sigma-Aldrich, St. Louis, MO, USA) or anti-FAP antibody (1:250 dilution; Abcam, Cambridge, UK) diluted in Dako REAL Antibody Diluent (Dako, Agilent Technologies, Santa Clara, CA, USA) and incubated overnight at 4°C. The sections were then incubated with Envision+ anti-mouse/rabbit antibodies (Dako, Agilent Technologies, Santa Clara, CA, USA) for 30 min at RT. The chromogen used was liquid DAB+ (Dako, Agilent Technologies, Santa Clara, CA, USA). The sections were visualized with a 3,3’-diaminobenzidine tetrahydrochloride solution, and nuclei were counterstained with Meyer’s hematoxylin. CAFs were defined as spindle-shaped cells expressing α-SMA or FAP, and evaluated using an area index calculated in low-magnification fields using ImageJ software (http://rsb.info.nih.gov/ij/). Three fields, including stromal cells per sample, were carefully selected to evaluate CAFs. The mean value obtained from each sectioned tissue sample was defined as the area index. The α-SMA and FAP positive rates were calculated as each area index. In this study, the median values of the area index for α-SMA and FAP were used as cut-off values to define the low and high groups.
The primary endpoint was the prognostic factors for survival after surgery. The secondary endpoint was survival after surgery, focusing on CAFs. The recurrence-free (RFS) and disease-specific (DSS) survival rates were analyzed.
RFS and DSS rates were investigated using the Kaplan–Meier method, and the log-rank test was used to evaluate differences between the groups. DSS was defined as the duration from surgery to the date of death as a result of the primary disease. Patients who died of causes unrelated to the primary disease were excluded.
The prognostic factors associated with RFS and DSS were investigated using a Cox proportional hazards model with hazard ratios (HR) and 95% confidence intervals (CI). As a significant correlation between α-SMA and FAP was found, the multivariable analyses were generated by including α-SMA and FAP separately. In Model 1, relevant factors, including α-SMA, were included in the multivariable analyses. In Model 2, the multivariable analyses included relevant factors, including FAP.
JMP version 11 software (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Of the 78 patients, 67 were available for immunohistochemical analysis. The characteristics of 67 patients are shown in Table 1. Pathological T factors included Tis (n = 10), T1 (n = 21), T2 (n = 22), and T3 (n = 17). Lymph node metastases were observed in 14 patients (21%). Histopathologic subtypes of ampullary carcinoma included the intestinal (n = 36, 54%), pancreatobiliary (n = 20, 30%), and mixed type (n = 11, 16%). Postoperative recurrence was observed in 17 patients (25%) during a mean follow-up period of 5.3 years.
CAFs were identified as stromal cells expressing α-SMA and FAP. The mean (standard deviation [SD]) area indices for α-SMA and FAP were 11.0 (10.5) and 6.9 (9.6), respectively (Figure 1). Using the median area indices for α-SMA and FAP, the cut-off values were set at 7.1 for α-SMA and 1.2 for FAP. Microscopic images of H&E, anti-α-SMA, and FAP staining, as well as the images generated using Image J, are shown in Figure 2. The area index of α-SMA significantly correlated with that of FAP (r2 = 0.55; p < 0.001), as shown in Figure 3.
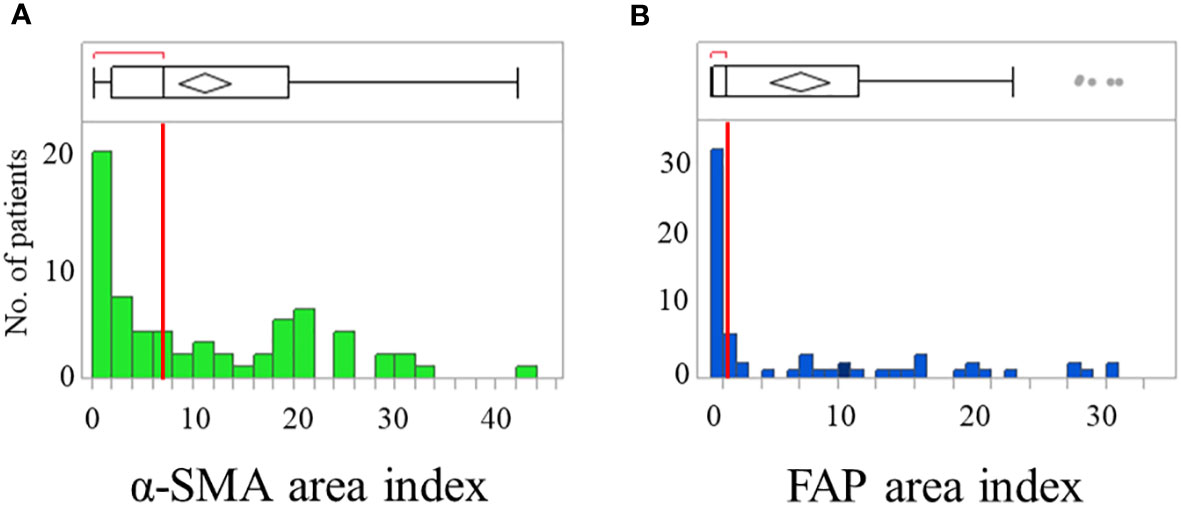
Figure 1 Distribution of patients showing α-smooth muscle actin (α-SMA) (A) and fibroblast activation protein (FAP) (B) area index. Box plots show median with the interquartile range; whiskers give the range.
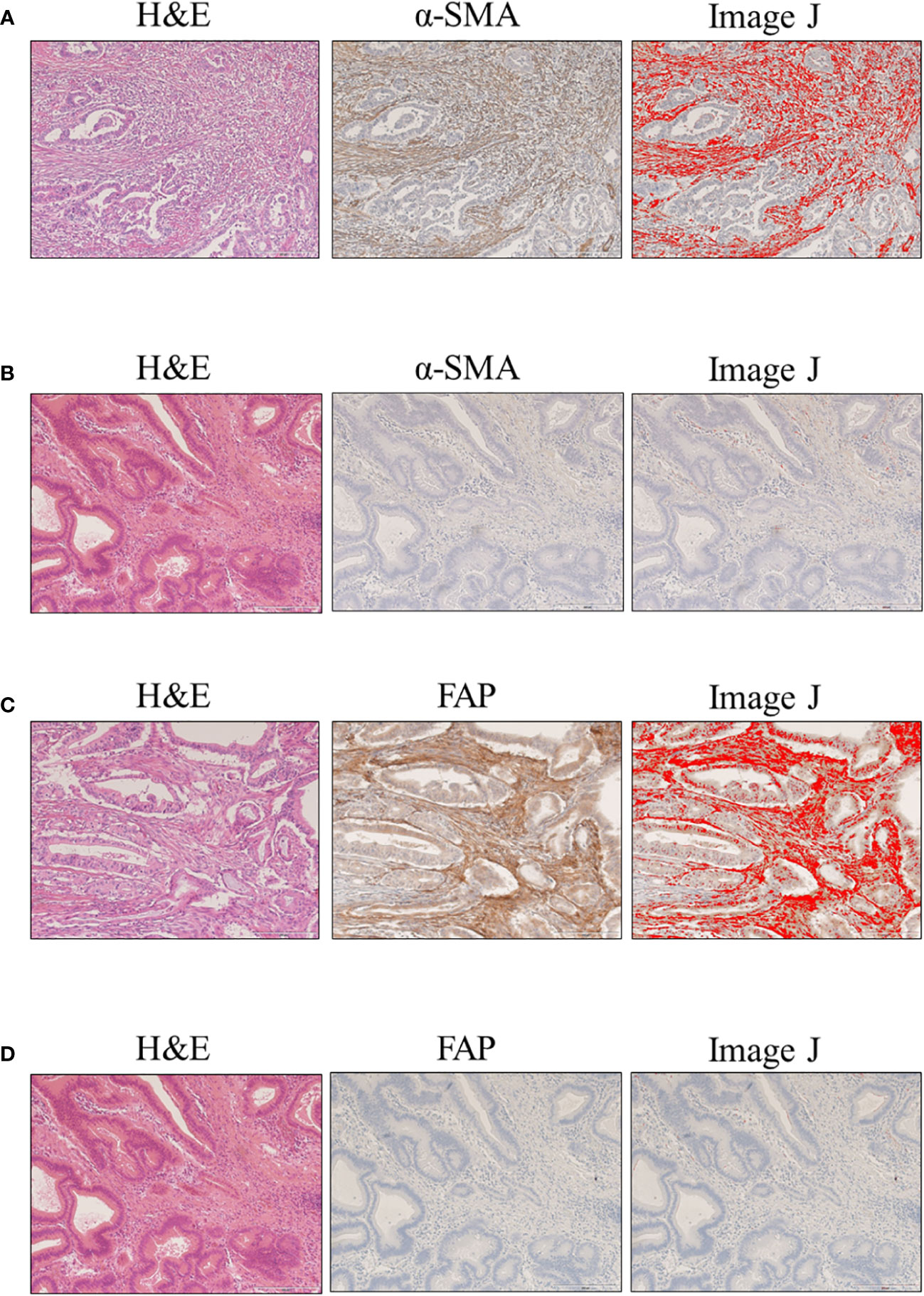
Figure 2 Evaluation of α-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) expression in clinical samples of ampullary carcinoma. Microscopic images with hematoxylin and eosin (H&E), cancer-associated fibroblasts (CAF) staining, and Image J: (A) high α-SMA expression; (B) low α-SMA expression; (C) high FAP expression; and (D) low FAP expression.
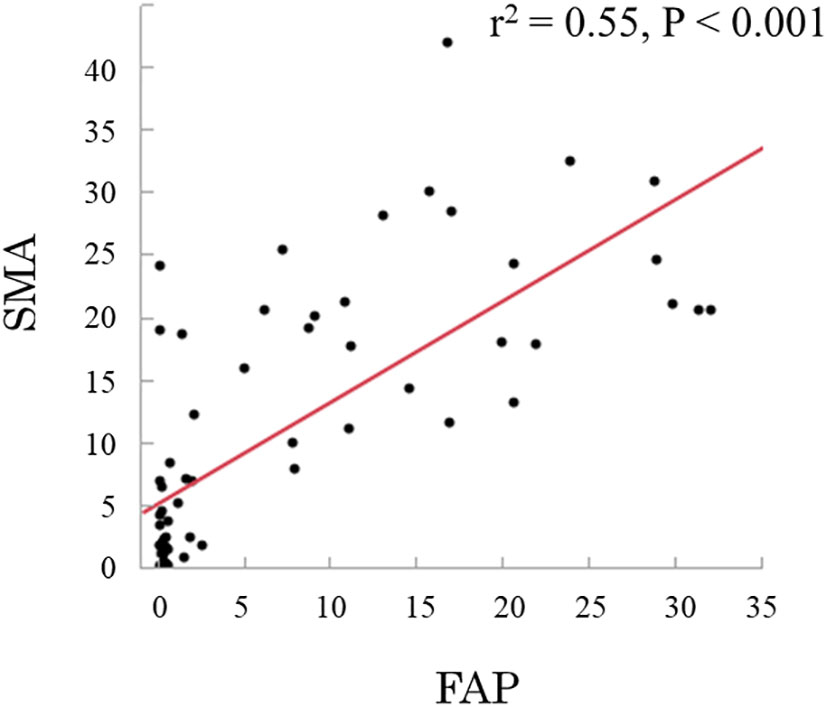
Figure 3 Relationship between smooth muscle actin (α-SMA) and fibroblast activation protein (FAP), showing a linear correlation (r2 = 0.55; p < 0.001).
Relationship between CAF expression and histopathologic subtype of ampullary carcinoma is depicted in Table 2. A significant difference was found in the mean (SD) area index for α-SMA and FAP between three groups: 4.8 (5.9) and 2.2 (5.8) in the intestinal type; 19.1 (9.9) and 12.8 (10.1) in the pancreatobiliary type; and 16.0 (10.8) and 11.1 (11.0) in the mixed type. The high-α-SMA and high-FAP was 25% and 19% in the intestinal type, 90% and 80% in the pancreatobiliary type, and 73% and 91% in the mixed type, respectively.
The 5-year RFS and DSS rates were 63.9% and 78.5%, respectively. RFS and DSS curves stratified by α-SMA and FAP are shown in Figure 4. Patients with high α-SMA expression had a significantly worse RFS than those with low α-SMA expression (5-year RFS, 47.6% vs. 82.2%; p = 0.003; Figure 4A). Furthermore, the 5-year DSS rates in the low- and high-α-SMA groups were 93.3% and 67.5%, respectively, with a significant difference between the groups (p = 0.01; Figure 4B). Regarding the effect of FAP on survival, the high-FAP group had significantly worse RFS (5-year RFS, 53.5% vs. 73.7%; p = 0.04; Figure 4C) and DSS (5-year DSS, 65.7% vs. 94.4%; p = 0.02; Figure 4D) than the low-FAP group.
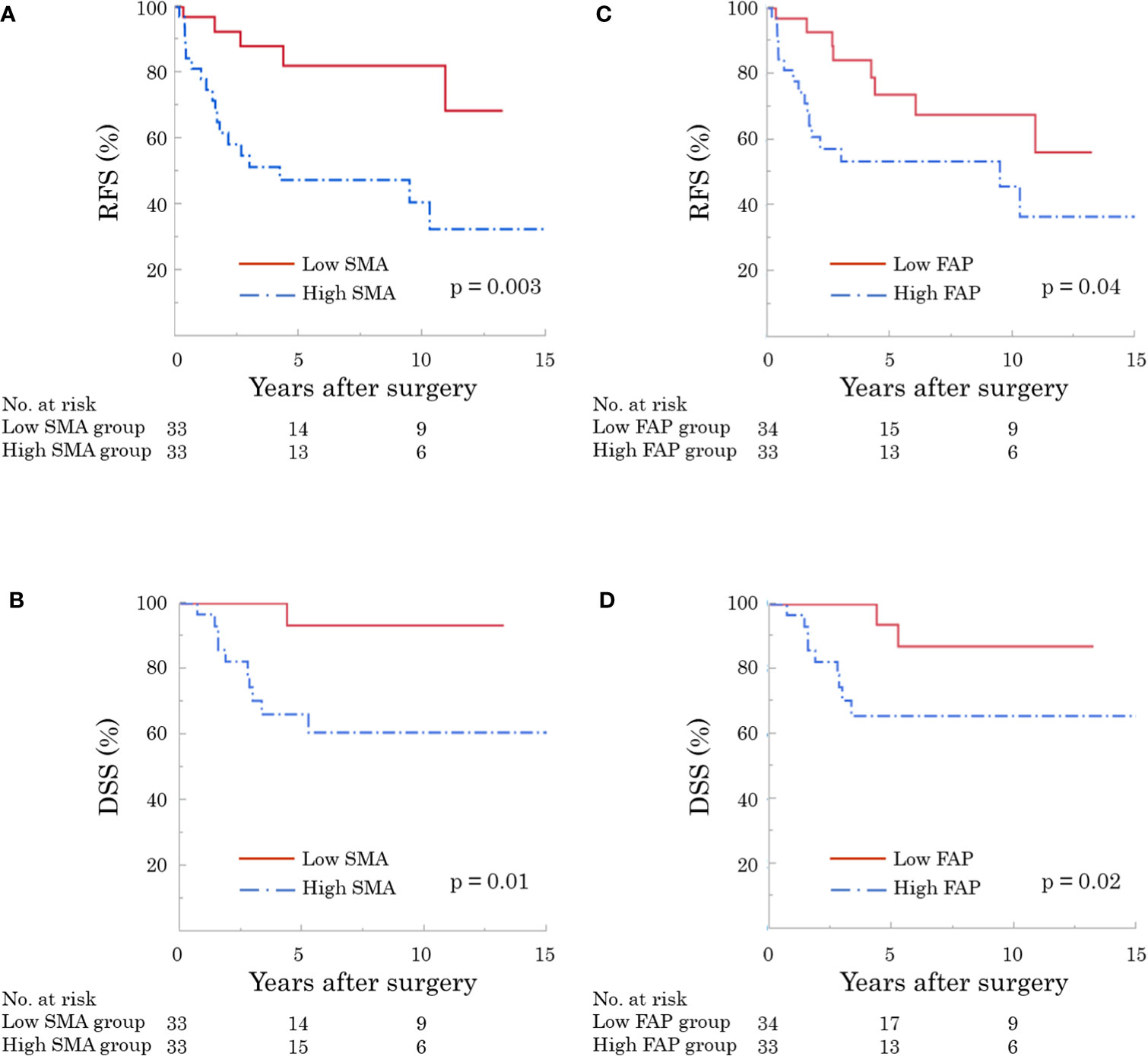
Figure 4 Recurrence-free (RFS) and disease-specific survival (DSS) according to expression of cancer-associated fibroblasts (CAF). The high α-smooth muscle actin (α-SMA) group had significantly worse RFS [(A) p = 0.003] and DSS [(B) p = 0.01]. In addition, patients with high fibroblast activation protein (FAP) showed significantly worse RFS [(C) p = 0.04] and DSS [(D) p = 0.02].
The results of the univariate and multivariable analyses for investigating the prognostic factors of RFS are shown in Table 3. Univariate analyses revealed that α-SMA, FAP, and lymph node metastasis were significant factors, but histopathologic subtypes were not an independent factor. In model 1, multivariable analysis revealed that high α-SMA expression was an independent predictor (HR: 3.68; 95% CI: 1.21–12.4; p = 0.02). In contrast, high FAP was not an independent index for RFS in model 2.
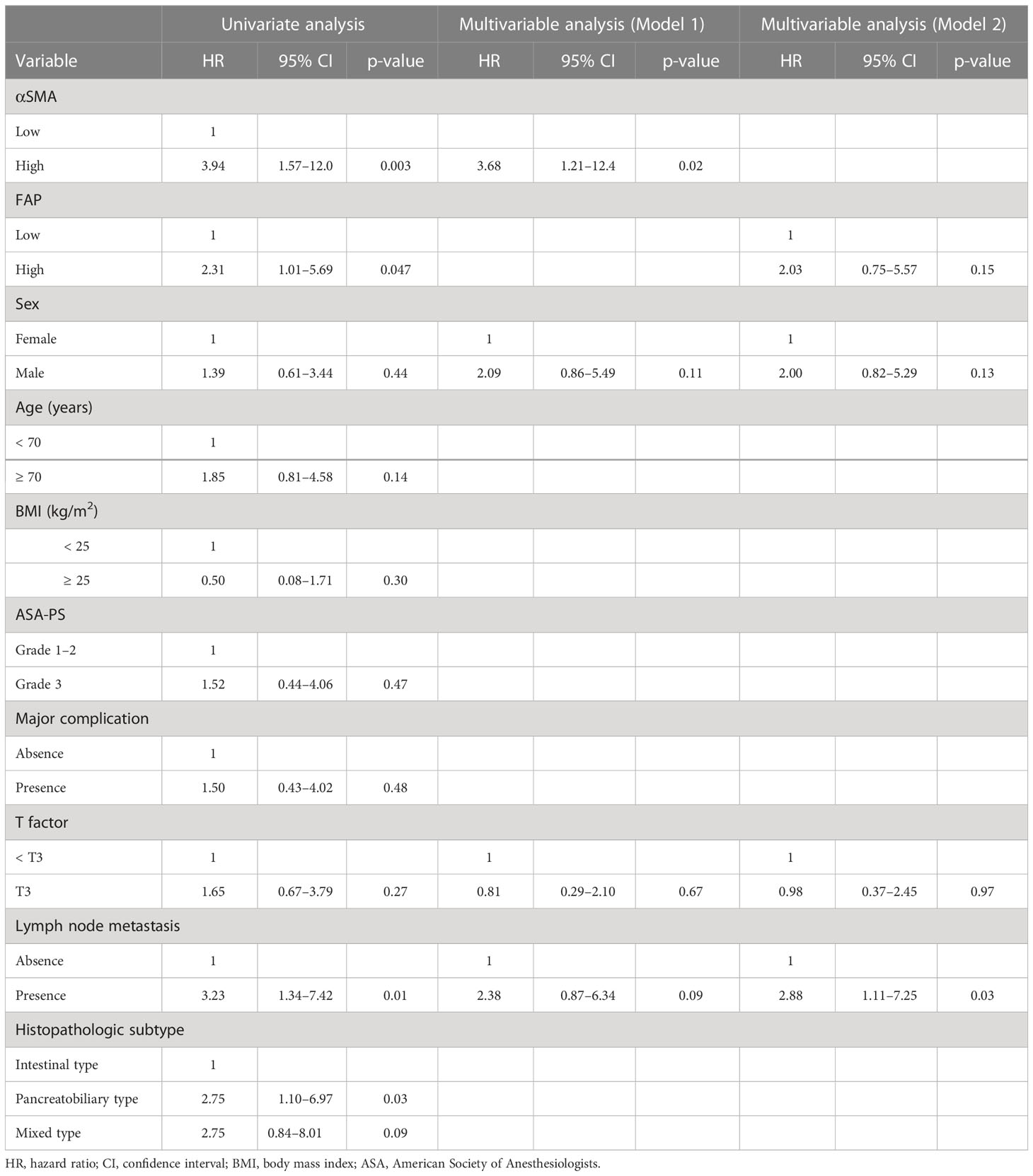
Table 3 Univariate and multivariable analyses of prognostic factors associated with recurrence-free survival.
Table 4 shows the results of the univariate and multivariable analyses for DSS. Univariate analyses identified high α-SMA (HR: 9.48; p = 0.005) and FAP (HR: 4.50; p = 0.03) as independent predictors of DSS. However, only high α-SMA level (HR: 8.54; 95% CI: 1.21–170; p = 0.03) was a significant factor associated with DSS in the multivariable analyses.
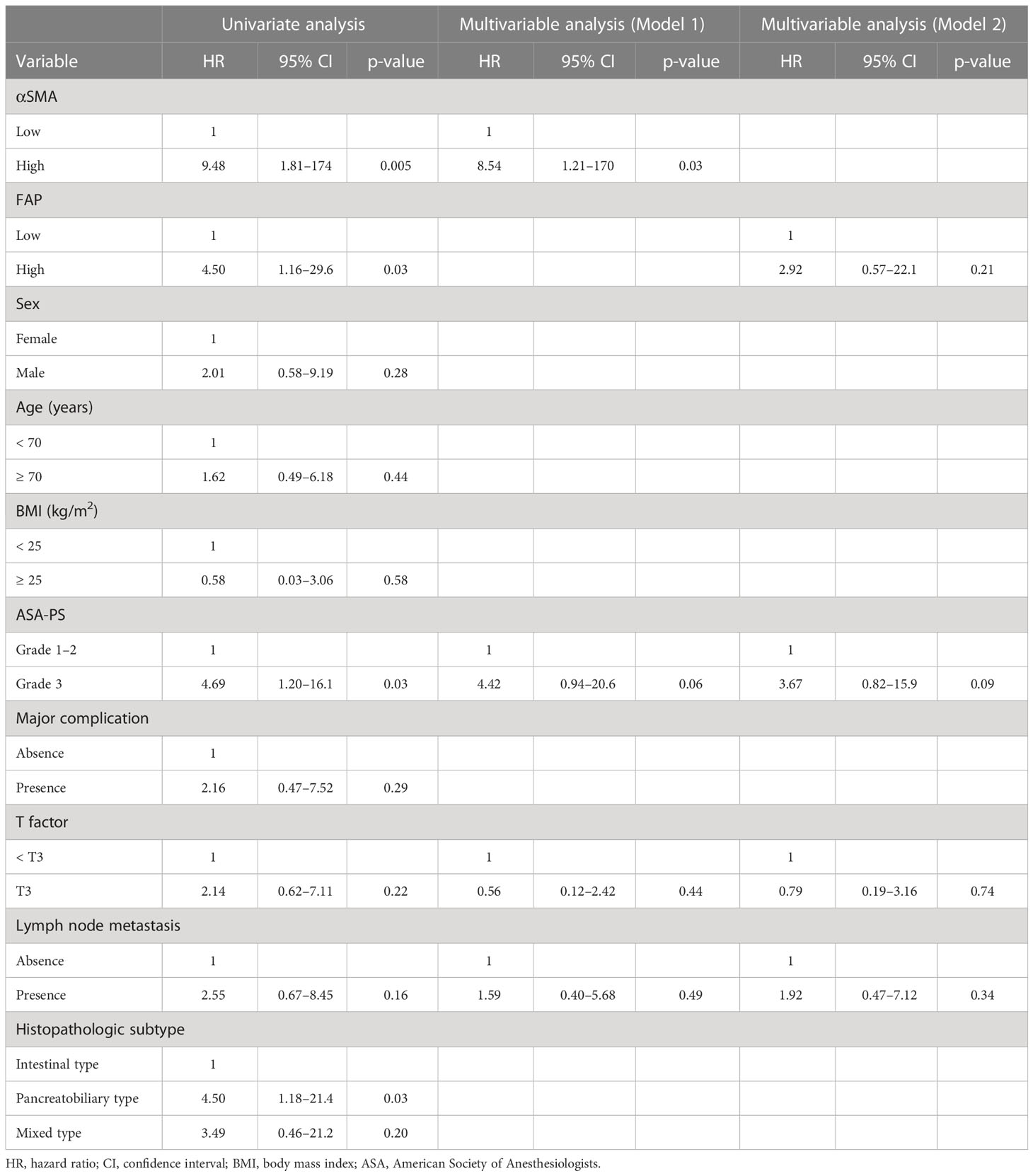
Table 4 Univariate and multivariable analyses of prognostic factors associated with disease-specific survival.
The relationship between α-SMA expression and clinicopathological parameters is shown in Table 5. No significant differences were found between the low- and high-α-SMA groups in terms of the demographic variables. High α-SMA expression was significantly associated with advanced T stage as well as higher incidences of lymph node metastases and recurrence. In fact, the low α-SMA group had lymph node metastasis in only one patient (3%) and no recurrence after surgery.
This study is the first to investigate the significance of CAFs in patients undergoing pancreatoduodenectomy for ampullary carcinoma. We found CAFs in ampullary carcinoma. Furthermore, our results revealed that CAFs, especially α-SMA, are significantly associated with survival after radical resection.
Interesting association of CAF expression with histopathologic subtype of ampullary carcinoma was detected in this study. A novel finding included that the intestinal type was associated with a lower area index of α-SMA as well as FAP compared to those of the pancreatobiliary and mixed types (Table 2). Similar to previous reports (5), prognosis in the intestinal type was better than those in the pancreatobiliary and mixed types (Supplementary Figure 1). Moreover, multivariable analyses revealed that CAF expression was a stronger predictor of survival than histopathologic subtypes. Further research would be required to examine the interaction between CAF and histopathologic subtypes.
The present study reveals a strong relationship between CAFs and survival. Patients with high α-SMA and FAP expression had significantly worse RFS and DSS (Figure 4), in line with previous reports in gastrointestinal surgical oncology (14, 15). Moreover, our multivariable analyses suggested that α-SMA was an independent predictor of RFS and DSS after surgery (Tables 3, 4). There was a significant association between α-SMA expression and pathological factors (Table 5). Based on the relationship between α-SMA expression and advanced tumor stages, the findings of multivariable analyses can be explained. Briefly, patients with high α-SMA expression had more advanced tumors and a higher incidence of lymph node metastases, leading to worse RFS and DSS.
The role of CAFs has recently gained widespread attention in the field of cancer biology. CAF biology is mediated through direct and paracrine interactions of cellular and acellular compartments (9). The role of CAFs in cancer invasion and metastasis has been investigated over the past few years. A recent review reported the association of CAFs with cancer invasion and metastasis that occurs through extracellular matrix deposition and remodeling, epithelial-mesenchymal transition in cancer cells, and secretion of growth factors supporting cancer cells (17). Furthermore, potential CAF-targeted therapeutic strategies have been suggested (6, 14–17). There are ongoing clinical trials investigating the efficacy of CAF-targeted therapies combined with existing therapies (8, 18–20).
Current extensive research has demonstrated subtypes of CAFs including pro-tumor or anti-tumor characteristics. Recent studies have supported the evidence for CAF heterogeneity in pancreatic ductal adenocarcinoma, showing several subpopulations of CAFs such as myofibroblastic CAFs (myCAFs), inflammatory CAFs (iCAFs), and antigen-presenting CAFs (apCAFs) (21, 22). However, the role of CAFs subtypes of ampullary carcinoma has not yet been investigated.
Translating the results of this study into clinical practice is important. The assessment of CAF expression can be easy and useful for detecting high-risk patients who could have poor long-term outcomes. Although the utility of adjuvant therapy for high-risk patients with ampullary carcinoma has been suggested (23), further studies are required to understand its biological features and histological characteristics and to develop an optimal therapeutic strategy to treat ampullary carcinoma (4). Therefore, evaluation of CAFs could be regarded as a novel treatment strategy in decision making for the introduction of adjuvant or first-line chemotherapy.
This study has several limitations, given that was a retrospective single-center study. The sample size was relatively small because of the rarity of the disease. Further studies with larger sample sizes are needed to clarify the role and efficacy of CAFs in ampullary carcinoma. The detailed mechanisms underlying the interaction between CAFs and prognosis are unknown. We suggest that CAFs promote epithelial-mesenchymal transition as well as cancer invasion and metastasis, including lymph node metastasis (17), leading to worse prognosis in patients with ampullary carcinoma. However, further studies should be performed to identify and delineate the interactions among CAFs, epithelial-mesenchymal transition, and cancer invasion.
The present study indicates that the assessment of CAFs can be helpful in evaluating cancer progression as well as in estimating survival after radical resection in patients with ampullary carcinoma.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Okayama University Hospital. The ethics committee waived the requirement of written informed consent for participation.
Concept and study design: KT, KN, and SK; Acquisition of data: KT, YN, YU, RY, TomF, KY, and HK; Pathological evaluation: TT; Drafting of the manuscript: KT; Critical revision of the manuscript for important intellectual content: KN, SK, YU, RY, TY and TosF. All authors contributed to the article and approved the final version of the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1072106/full#supplementary-material
Supplementary Figure 1 | Recurrence-free (RFS) and disease-specific survival (DSS) by histopathologic subtypes. The 5-year RFS showed 79.0% in the intestinal group, 43.6% in the pancreatobiliary group, and 53.9% in the mixed group (A; p = 0.046). The 5-year DSS included 91.8% in the intestinal group, 60.5% in the pancreatobiliary group, and 71.4% in the mixed group (B; p = 0.07).
1. Takagi K, Nagai Y, Umeda Y, Yoshida R, Yoshida K, Fuji T, et al. Prognostic value of the regional lymph node station in pancreatoduodenectomy for ampullary carcinoma. In Vivo (2022) 36(2):973–8. doi: 10.21873/invivo.12789
2. Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg (2010) 14(2):379–87. doi: 10.1007/s11605-009-1080-7
3. Okano K, Oshima M, Suto H, Ando Y, Asano E, Kamada H, et al. Ampullary carcinoma of the duodenum: current clinical issues and genomic overview. Surg Today (2022) 52(2):189–97. doi: 10.1007/s00595-021-02270-0
4. Rizzo A, Dadduzio V, Lombardi L, Ricci AD, Gadaleta-Caldarola G. Ampullary carcinoma: An overview of a rare entity and discussion of current and future therapeutic challenges. Curr Oncol (2021) 28(5):3393–402. doi: 10.3390/curroncol28050293
5. Shin DW, Kim S, Jung K, Jung JH, Kim B, Ahn J, et al. Impact of histopathological type on the prognosis of ampullary carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol (2023) 49(2):306–15. doi: 10.1016/j.ejso.2022.10.001
6. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol (2019) 12(1):86. doi: 10.1186/s13045-019-0770-1
7. Noma K, Smalley KS, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, et al. The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology (2008) 134(7):1981–93. doi: 10.1053/j.gastro.2008.02.061
8. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi: 10.1038/s41568-019-0238-1
9. Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol (2019) 16(5):282–95. doi: 10.1038/s41575-019-0115-0
10. Sunami Y, Häußler J, Zourelidis A, Kleeff J. Cancer-associated fibroblasts and tumor cells in pancreatic cancer microenvironment and metastasis: Paracrine regulators, reciprocation and exosomes. Cancers (Basel) (2022) 14(3):744. doi: 10.3390/cancers14030744
11. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
12. Surgery JSoH-B-P. Japanese Society of Hepato-Biliary-Pancreatic Surgery: General Rules for Clinical and Pathological Studies on Cancer of the Biliary Tract (7th edition). Japan, Kanehiora Publ Corp., (2021).
13. Quero G, Laterza V, Fiorillo C, Menghi R, De Sio D, Schena CA, et al. The impact of the histological classification of ampullary carcinomas on long-term outcomes after pancreaticoduodenectomy: a single tertiary referral center evaluation. Langenbecks Arch Surg (2022) 407(7):2811–21. doi: 10.1007/s00423-022-02563-z
14. Ogawa T, Kikuchi S, Tabuchi M, Mitsui E, Une Y, Tazawa H, et al. Modulation of p53 expression in cancer-associated fibroblasts prevents peritoneal metastasis of gastric cancer. Mol Ther Oncol (2022) 25:249–61. doi: 10.1016/j.omto.2022.04.009
15. Kashima H, Noma K, Ohara T, Kato T, Katsura Y, Komoto S, et al. Cancer-associated fibroblasts (CAFs) promote the lymph node metastasis of esophageal squamous cell carcinoma. Int J Cancer (2019) 144(4):828–40. doi: 10.1002/ijc.31953
16. Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, et al. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells Via IL6 in the tumor microenvironment. Clin Cancer Res (2018) 24(19):4820–33. doi: 10.1158/1078-0432.CCR-18-0205
17. Asif PJ, Longobardi C, Hahne M, Medema JP. The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers (Basel) (2021) 13(18):4720. doi: 10.3390/cancers13184720
18. Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncol (2019) 5(7):1020–7. doi: 10.1001/jamaoncol.2019.0892
19. Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, et al. HALO 202: Randomized phase II study of PEGPH20 plus nab-Paclitaxel/Gemcitabine versus nab-Paclitaxel/Gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol (2018) 36(4):359–66. doi: 10.1200/JCO.2017.74.9564
20. Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie (2003) 26(1):44–8. doi: 10.1159/000069863
21. Geng X, Chen H, Zhao L, Hu J, Yang W, Li G, et al. Cancer-associated fibroblast (CAF) heterogeneity and targeting therapy of CAFs in pancreatic cancer. Front Cell Dev Biol (2021) 9:655152. doi: 10.3389/fcell.2021.655152
22. Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med (2017) 214(3):579–96. doi: 10.1084/jem.20162024
Keywords: ampullary carcinoma, carcinomas of the papilla of Vater, cancer-associated fibroblast, outcome, survival, recurrence
Citation: Takagi K, Noma K, Nagai Y, Kikuchi S, Umeda Y, Yoshida R, Fuji T, Yasui K, Tanaka T, Kashima H, Yagi T and Fujiwara T (2023) Impact of cancer-associated fibroblasts on survival of patients with ampullary carcinoma. Front. Oncol. 13:1072106. doi: 10.3389/fonc.2023.1072106
Received: 17 October 2022; Accepted: 06 March 2023;
Published: 16 March 2023.
Edited by:
Beatrice Aramini, University of Bologna, ItalyReviewed by:
Hao Liu, University of Pittsburgh Medical Center, United StatesCopyright © 2023 Takagi, Noma, Nagai, Kikuchi, Umeda, Yoshida, Fuji, Yasui, Tanaka, Kashima, Yagi and Fujiwara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kosei Takagi, a290YWthZ2kxNUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.