- 1Department of Thyroid Surgery, General Surgery Center, First Hospital of Jilin University, Changchun, China
- 2Department of Anesthesia, First Hospital of Jilin University, Changchun, China
Background: Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy, with an increasing incidence over the last decades. Human immunodeficiency virus (HIV)-induced immune deficiency was one of risk factors for cancer tumorigenesis and development. The aim of this study was to describe the clinicopathological features of PTC in HIV-infected patients and discuss possible connections between PTC and HIV infection.
Methods: A total of 17670 patients from September 2009 to April 2022 who underwent PTC surgery for the first time were analyzed retrospectively. At last, 10 patients of PTC with HIV infection (HIV-positive group) and 40 patients without HIV infection (HIV-negative group) were included. The differences in general data and clinicopathological characteristics between the HIV-positive group and the HIV-negative group were analyzed.
Results: There were statistically significant differences in age and gender between the HIV-positive group and the HIV-negative group (P<0.05), and males and <55 years old accounted for a higher proportion in the HIV-positive group. The differences in tumor diameter and capsular invasion between the HIV-positive group and HIV-negative group were statistically significant (P<0.05). Meanwhile, in terms of extrathyroid extension (ETE), lymph node metastasis and distant metastasis, the HIV-positive group were significantly higher than the HIV-negative group (P<0.001).
Conclusion: HIV infection was a risk factor for larger tumors, more severe ETE, more lymph node metastasis, and more distant metastasis. HIV infection could promote PTC proliferation and make PTC more aggressive. Many factors such as tumor immune escape, secondary infection, etc. may are responsible for these effects. More attention and more thorough treatment should be paid to these patients.
Introduction
Papillary thyroid carcinoma(PTC)is the most common endocrine malignancy, with an increasing incidence over the last decades (1). Usually, distant metastases from PTC are rare and the prognosis of PTC is relatively good (2). However, a few populations are considered to be at high risk of poor prognosis (3). Some factors have been associated with poor pathological features and clinical outcomes of PTC, such as BRAFV600E mutation (4), extrathyroidal invasion (5) and so on.
HIV/AIDS prevalence in China during more than a decade indicate that HIV/AIDS prevalence is getting more and more serious and the rapid spread of HIV exists with the characteristics of regional and age differences (6). Some research revealed that HIV-induced immune deficiency was one of risk factors for cancer tumorigenesis and development (7, 8). Other HIV-associated factors contribute to development of malignancies, including direct effect of HIV on various cellular processes, coinfection with oncogenic organisms, risk behaviors (e.g. alcohol or tobacco use), environmental oncogenic factors and possibly antiretroviral therapy (9).
The incidences of thyroid cancer in HIV-infected patients were 4%-8.5% according to previous reports (8, 10). With increased prevalence of PTC, PTC with concomitant HIV infection has become more common in recent years. It is well known that HIV infection can affect thyroid function (11), regulate the Hypothalamic-Pituitary-Thyroid Axis (12, 13) and cause papillomavirus infection (14). However, whether HIV infection is related to the occurrence and development of PTC and the impact of HIV infection on the clinicopathological characteristics of PTC are still unknown. The objective of this study is to describe the clinicopathological features of PTC in HIV-infected patients and discuss possible connections and effects between PTC and HIV infection.
Materials and methods
Patients
Data from a total of 17670 hospitalized patients undergoing PTC surgery for the first time from September 2009 to April 2022 at the first hospital of Jilin University were collected. Patients were enrolled if they met all of the following inclusion criteria (1): the patient had a complete documentation of his present and previous diseases (2); the patient underwent initial surgery treatment and the pathological result of the patient was clearly diagnosed as PTC by pathologists (3); without other pathologic types of thyroid cancer (4); there was no history of other malignant tumors. Tumor burden has been assessed preoperatively using imaging including color doppler ultrasonography and pulmonary CT scans as routine. If metastasis is suspected, Emission Computed Tomography (ECT) and PET/CT have been used to evaluate both the number and size of metastases as well as the number of organs involved. We collected ten cases of papillary thyroid carcinoma with HIV-infection and only one patient admitted that he was received systemic treatment for half a year before surgery. But the left nine patients were all the first time diagnosed HIV infection before surgery during screening of infection marker test and didn’t have any relative clinical features of HIV infection. In order to increase the test power of statistical analyses, we randomly matched HIV-positive group and HIV-negative group according to 1:4.
Surgery and clinicopathological characteristics
All patients underwent unilateral or total thyroidectomy (TT) with unilateral/bilateral central lymph node dissection (CLND), 1671 patients (9.46%) also received a unilateral/bilateral lateral lymph node dissection (LLND). Intraoperative nerve monitoring was done to evaluate recurrent laryngeal nerve (RLN) function and protect the nerve from disturbance. Thyroid function and several related proteins tests were performed. BRAF mutation was detected. The following clinical characteristics of PTC patients were recorded: gender, age at diagnosis of PTC and HIV infection, body mass index (BMI), and family history. The pathological features of PTC were as follows: maximum diameter of the tumor, multifocal lesions, bilateral lesions, capsular invasion only, extrathyroid extension (ETE), lymph node metastasis, distant metastasis, combined with Hashimoto’s thyroiditis (HT), pathological TNM stage (using the American Joint Committee on Cancer 8th edition TNM staging system).
Statistical analysis
SPSS 24.0 statistical software was used for statistical analysis. Measurement data conforming to a normal distribution were expressed as χ ± SD, and the t-test was used for comparisons between groups. Nonparametric tests were used for rank data and continuous data with a nonnormal distribution. Statistical data were expressed by percentage or number of cases, and comparison between groups was performed by Student’s independent samples t test, χ2 test and Fisher’s exact test. Logistic regression analysis was used for multivariate analysis. All statistical tests were two-sided, and differences were considered statistically significant at a P value of less than 0.05.
Results
Clinicopathological features of the overall data
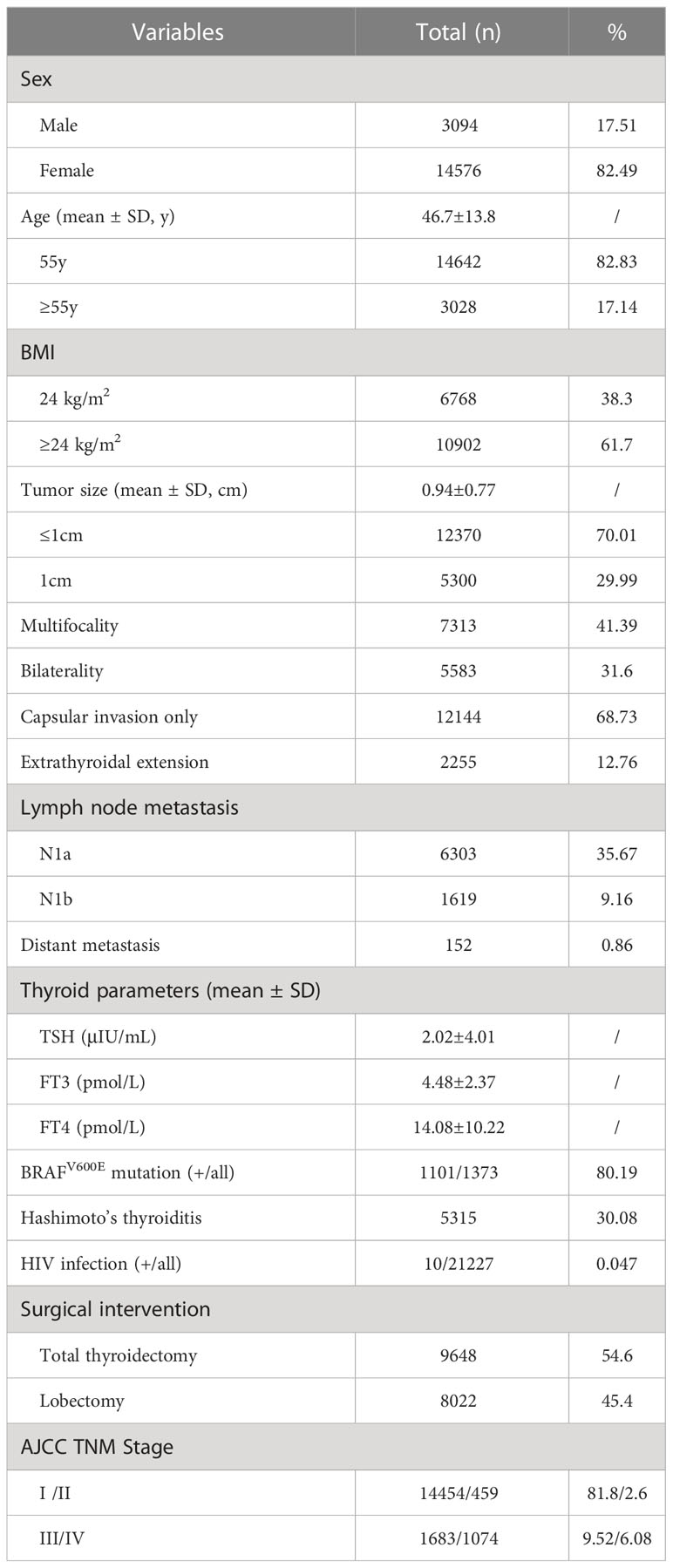
A detailed presentation of the clinicopathological features of enrolled patients of PTC can be accessed in Table 1. Our cohort comprised 14576 (82.49%) females and 3094 (17.51%) males among the 17670 patients, and the mean age of the patients was 46.7 ± 13.8 years. 3028 patients were > 55 years old (17.14%). The median tumor size was 0.94 ± 0.77 cm, and 12370 patients (70.01%) were papillary thyroid microcarcinoma (PTMC). Multifocal lesions were detected in 7313 cases (41.39%), bilateral lesions in 5583 cases (31.6%), thyroid capsule invasion in 12144 cases (68.73%), ETE in 2255 cases (12.76%), lymph node metastasis (N1a and N1b) in 6574 cases (37.2%), and distant metastasis in 152 cases (0.86%). The median TSH level was 2.02 (range: 0.35–4.94 μIU/mL), the median FT3 level was 4.48 (range: 2.43–6.01 pmol/L), and the median FT4 level was 14.08 (range: 9.01–19.05 pmol/L). BRAFV600E mutation test was performed in 1373 patients, of whom 1101 (80.19%) patients were positive. 10 patients were HIV infected. There were 8022 (45.4%) patients who underwent lobectomy, while the remaining 9648 (54.6%) underwent TT. Lateral neck node dissection for 1591 (9%). The numbers of patients in each TNM stage were as follows: 14454 (81.8%) in stage I, 459 (2.6%) in stage II, 1683 (9.52%) stage III, and 1074 (6.08%) stage IV.
Comparison of clinicpathological features between the HIV-positive and HIV-negative groups
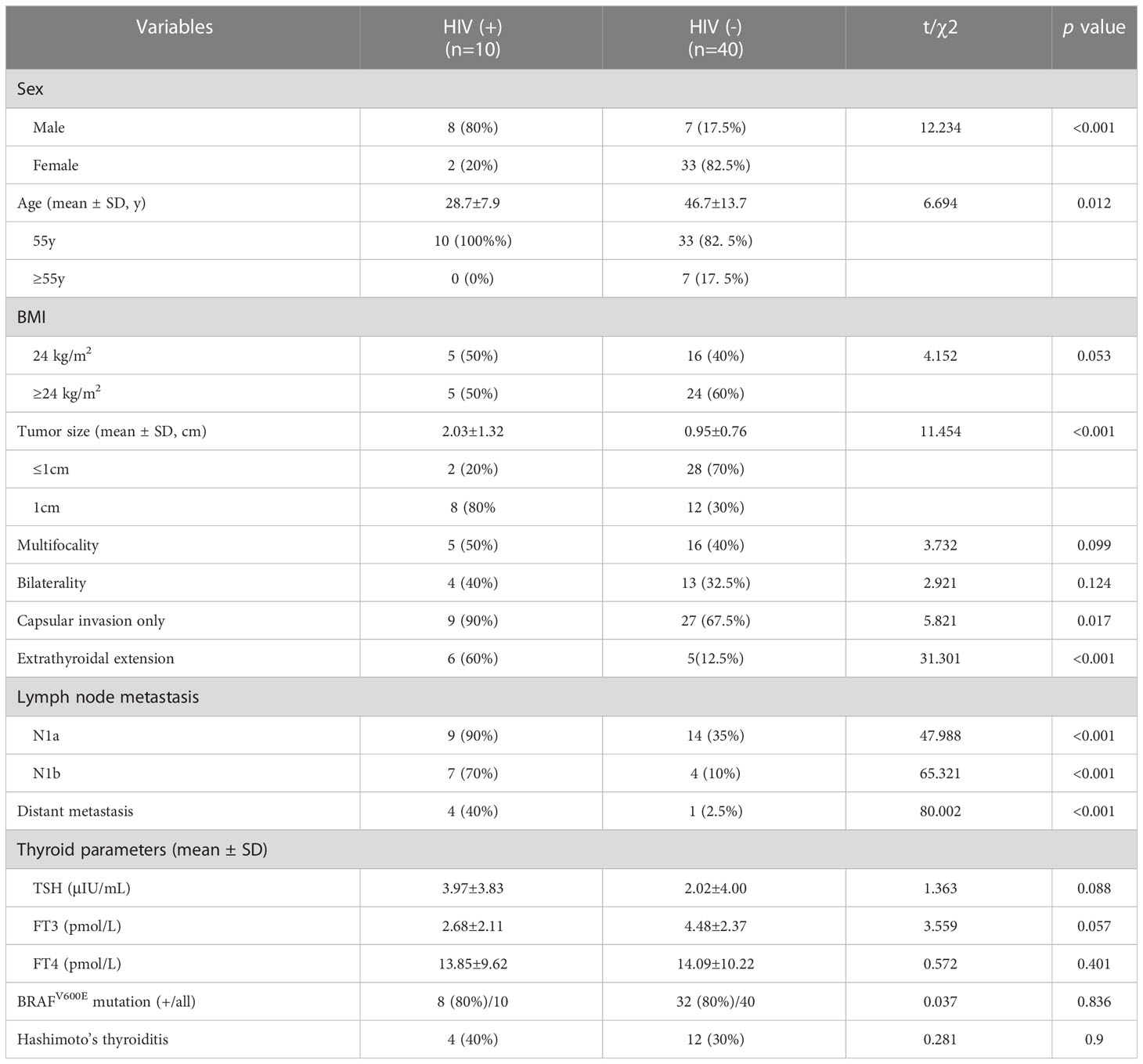
There were statistically significant differences in age and gender between the HIV-positive group and the HIV-negative group (P<0.05), and males and <55 years old accounted for a higher proportion in the HIV-positive group. The HIV-positive group had a lower BMI than the HIV-negative group, though differences were not statistically significant. The differences in tumor diameter and capsular invasion between the HIV-positive group and HIV-negative group were statistically significant (P<0.05). Meanwhile, in terms of ETE, the HIV-positive group was significantly higher than the HIV-negative group (P<0.001). In terms of lymph node metastasis and distant metastasis, the proportions of the HIV-positive group were higher than those of the HIV-negative group, differences were statistically significant (P<0.001) (Table 2, Figures 1, 2). In addition, there were no significant differences in Hashimoto’s thyroiditis, multifocal lesions, bilateral lesions, BRAFV600E mutation, TSH, FT3 and FT4 between patients with and without HIV infection (Table 2).

Figure 1 Distant metastasis of PTC in a HIV-infected patient (SPECT/CT). (A) Multiple lung metastases, (B) Right rib metastasis.
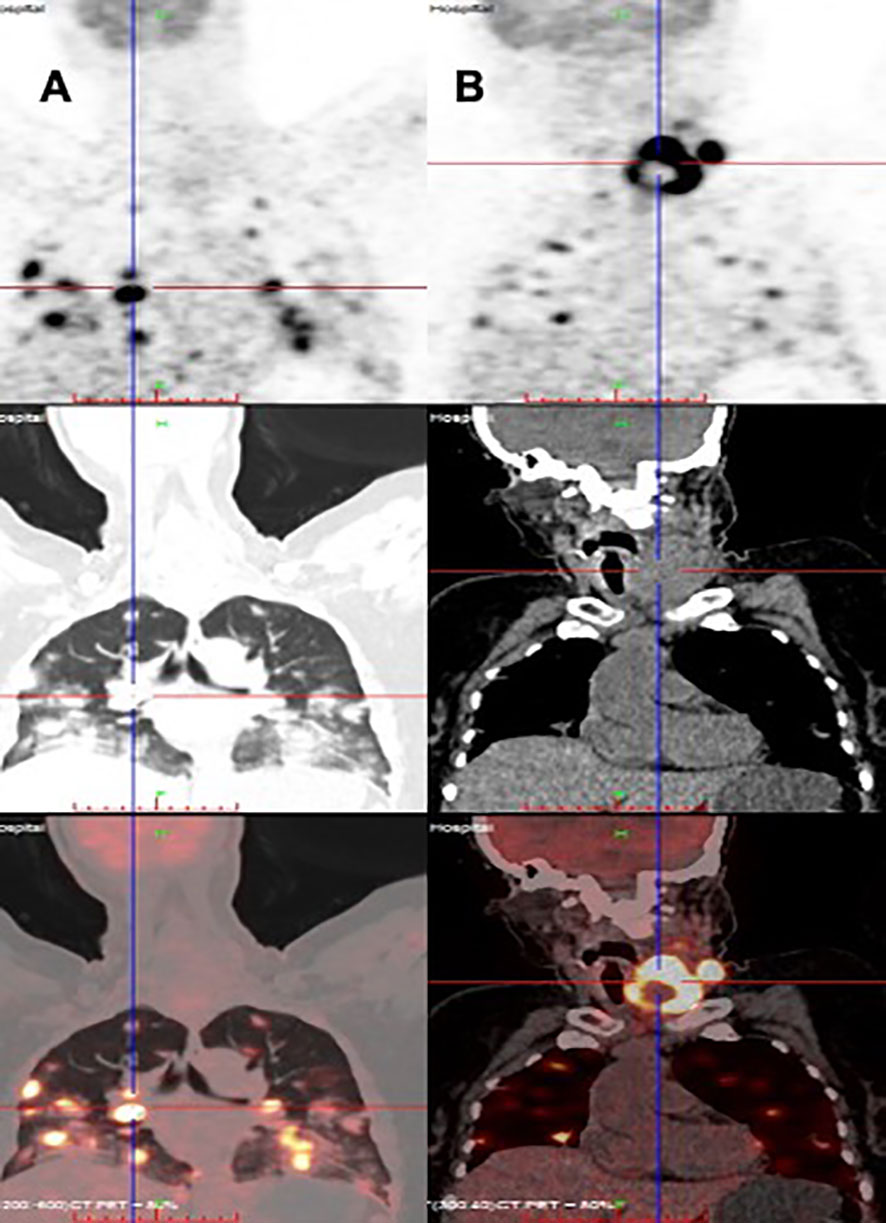
Figure 2 Distant metastasis of PTC in a HIV-infected patient (PET/CT). (A) Multiple lung metastases, (B) papillary thyroid carcinoma.
Association between HIV infection and clinicopathological features of PTC
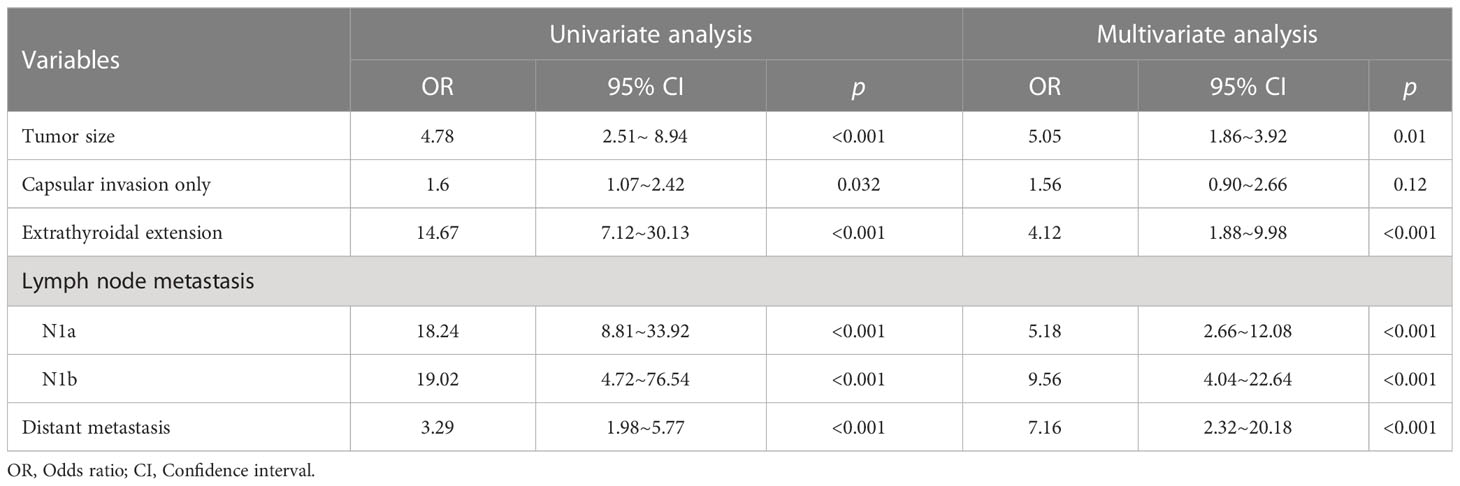
Univariate and multivariate analyses were performed to detect the correlation between clinicopathological features of PTC and HIV infection. As shown in Table 3, in univariate analysis, larger tumor (OR=4.78, 95% CI:2.51–8.94), more severe capsular invasion (OR=1.6, 95% CI:1.07–2.42) and ETE (OR=14.67, 95% CI:7.12–30.13), more lymph node metastasis (N1a: OR=18.24, 95% CI:8.81–33.92; N1b: OR=19.02, 95% CI:4.72–76.54), and more distant metastasis (OR=3.29, 95% CI:1.98–5.77) were associated with HIV infection. Furthermore, after correction for age, sex, TSH, FT3, FT4 and BMI, HIV infection was still a risk factor for larger tumors (OR=5.05, 95% CI:1.86–3.92), more severe ETE (OR=4.12, 95% CI:1.88–9.98), more lymph node metastasis (N1a: OR=5.18, 95% CI:2.66–12.08; N1b: OR=9.56, 95% CI:4.04–22.64), and more distant metastasis (OR=7.16, 95% CI:2.32–20.18).
Discussion
HIV infection is associated with dysfunction of many endocrine organs and their axis, including thyroid gland (15, 16). Various types of thyroid disorders have been described in HIV-infected patients, such as Graves’ disease, hypothyroidism, euthyroid sick syndrome, etc (17–19). Meanwhile, some researches revealed that HIV-induced immune deficiency was the most common risk factor to develop malignancies (8–10).
During more than a decade, AIDS-defining cancers remain frequent in China, and non-AIDS-defining cancers play an important role in morbidity and mortality of HIV positive population in China (8). However, less is known about the impact of HIV infection on PTC so far. Here, we investigate the clinicopathological features of PTC in patients with HIV and discuss possible connections between PTC and HIV infection.
In this study, age and gender distribution had a significant difference in both groups. There were more males and the patients were younger in HIV-positive group. It was consistent with the background of the increasing proportion of young homosexual men in China in recent years. These people have been the fastest-growing risk group for HIV epidemic (20, 21). Moreover, the impact of HIV infection on life expectancy is another reason for the younger age of HIV-positive patients (22). Some researchers believed that HIV infection leads to altered metabolism, poor oral intake and increased prevalence of weight loss (23, 24). BMI was used as a major measurement in most studies. Epidemiology studies have suggested that higher BMI was associated with an increased risk of PTC (25). However, the BMI of the HIV-positive group was slightly lower than that of the HIV-negative group according to our data, but the difference was not significant. This means that HIV infection is unlikely to affect PTC through changes in BMI.
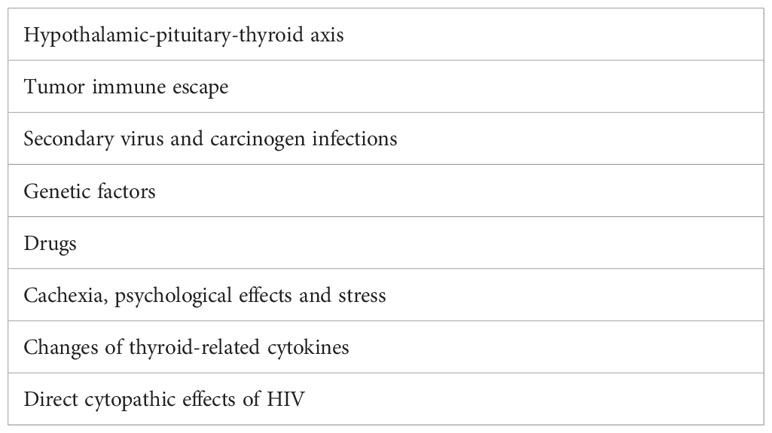
We found that the tumor was larger in the HIV-positive group. It suggested HIV infection could promote PTC proliferation. In addition to, HIV infection also makes PTC more aggressive. Capsular invasion, ETE, lymph node metastasis, and distant metastasis were significantly more common in HIV-infected PTC patients. Several possible reasons are as follows (Table 4) (1). Numerous studies have confirmed that hypothroidism and mildly elevated TSH level were more frequently observed in HIV-positive population as compared to HIV-negative group (26–29). In our study, mean TSH value was indeed higher in the HIV-infected patients than in the control group, although the difference was not statistically significant. This agrees with previous findings. TSH is released from the anterior pituitary under positive regulation from TSH-releasing hormone (which is released from the hypothalamus) and negative feedback from the thyroid hormones tri-iodothyronine (T3) and thyroxine (T4). HIV infection frequently results in early and protracted disturbances of hypothalamus/pituitary and thyroid dysfunction, which could change TSH concentrations in serum (30). Higher TSH has a proliferative effect on PTC growth that is most likely mediated by TSH receptors on tumor cells (31) (2); The immune system is no longer functioning effectively after HIV infection, which enable cancer cells to escape from immune surveillance and develop rapidly (32, 33) (3); Secondary virus and carcinogen infections are common in patients with HIV. For example, some viruses, such as human papillomavirus, cytomegalovirus, Epstein-Barr virus, herpes virus, etc., promote tumorigenesis and development of PTC (34–36) (4); Genetic factors may play roles in the pathogenesis of PTC in HIV-positive patients. Yahong Chen et al. had identified nearly 40 HIV-related genes from widely biological pathways (37). Molecular biology has discovered several pathways that play major roles in the occurrence and development of PTC. For example, VHL is a well-known tumor suppressor that is deregulated in the majority of PTC tissues (38). STAT1 promotes cell proliferation and inhibits apoptosis via AMPK signaling pathway in PTC (39). NCOR2 accelerates PTC progression by upregulating metastasis-associated protein 2 expression (40) (5); Highly active anti-retroviral therapy (HAART) may alter clinicopathological features of PTC by drug interactions or effects on the immune system (41) (6); Cachexia, psychological effects and stress with HIV infection are also the important clinical risk factors of PTC (42) (7); Changes of thyroid-related cytokines after HIV infection, such as thyroxine-binding globulin (TBG), rT3 and anti-TPO antibody may also play roles. But further studies are needed here (43, 44) (8); Direct cytopathic effects of HIV on the thyroid gland. Here we could put forward such hypotheses: HIV can affect the normal physiological status of the thyroid gland by altering CD4 and CD8 (45, 46), or HIV affects thyroid hormone metabolism through the interaction with peripheral T3 receptors, and then affects the status of thyroid follicular cells (47).
As a preliminary study, there are still some limitations in this study. Firstly, the sample size of the study is relatively small in HIV-positive group. We only collected 10 patients of PTC with HIV infection (HIV-positive group) from total of 17670 patients who underwent PTC surgery for the first time during the past 13 years in our department. Perhaps the reasons for the great difference are the lower incidence of HIV infection and higher incidence of thyroid deceases in northeast of China. Secondly, there is only one patient who received anti-virus therapy for a short time before surgery and we can’t get more favorable information to compare the different clinicopathological features of papillary thyroid carcinoma of performing HIV therapy or not. The potential biases of the study may because more diagnosed HIV (+) patients were transferred to hospital for infectious diseases to performing surgery and systematic anti-virus therapy, which result in less cases collected. Thirdly, Serum-based biomarkers, such as lactate dehydrogenase, can also reflect tumor burden and are often also correlated with a poor response to immune-checkpoint inhibitors. Other circulating markers (such as circulating free tumor DNA and/or circulating tumor cells) are also attracting research interest as surrogate markers of tumor burden. Herein, we didn’t compare the serum-based biomarkers and hope more complete data can be collected and analyzed to enrich the clinicopathological features in the follow-up study.
Conclusion
Our study described that the clinicopathological features of PTC in patients with HIV. HIV infection could promote PTC proliferation and make PTC more aggressive. Many factors such as tumor immune escape, secondary infection, etc. may are responsible for these effects. More attention and more thorough treatment should be paid to these patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of first hospital of jilin university. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JL wrote the article. JZ and DW collected data. SD analyzed data and gave administration support. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
National Natural Science Foundation of JiLin Province (20210101353JC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Borazan H, Kececioglu A, Okesli S, Otelcioglu S. Oral magnesium lozenge reduces postoperative sore throat: a randomized, prospective, placebo-controlled study. Anesthesiology (2012) 117(3):512–8. doi: 10.1097/ALN.0b013e3182639d5f
2. Zhang C, Li B, Zhang L, Chen F, Zhang Y, Cheng W. Clinicopathological and ultrasound features as risk stratification predictors of clinical and pathological nodal status in papillary thyroid carcinoma: a study of 748 patients. BMC Cancer (2022) 22(1):354. doi: 10.1186/s12885-022-09474-8
3. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
4. Yan C, Huang M, Li X, Wang T, Ling R. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr Connect (2019) 8(7):988–96. doi: 10.1530/EC-19-0246
5. Zhao J, Zhang Y, Zheng X. Clinicopathological characteristics of papillary thyroid cancer located in the isthmus with delphian lymph node metastasis. Br J Oral Maxillofac Surg (2021) 60(5):635–638. doi: 10.1016/j.bjoms.2021.11.016
6. Qiao YC, Xu Y, Jiang DX, Wang X, Wang F, Yang J, et al. Epidemiological analyses of regional and age differences of HIV/AIDS prevalence in China, 2004-2016. Int J Infect Dis (2019) 81:215–20. doi: 10.1016/j.ijid.2019.02.016
7. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet (2007) 370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2
8. Wang F, Xiang P, Zhao H, Gao G, Yang D, Xiao J, et al. A retrospective study of distribution of HIV associated malignancies among inpatients from 2007 to 2020 in China. Sci Rep (2021) 11(1):24353. doi: 10.1038/s41598-021-03672-3
9. Proulx J, Ghaly M, Park IW, Borgmann K. HIV-1-Mediated acceleration of oncovirus-related non-AIDS-Defining cancers. Biomedicines (2022) 10(4):768. doi: 10.3390/biomedicines10040768
10. Basilio-De-Oliveira CA. Infectious and neoplastic disorders of the thyroid in AIDS patients: an autopsy study. Braz J Infect Dis (2000) 4(2):67–75.
11. Dev N, Sahoo R, Kulshreshtha B, Gadpayle AK, Sharma SC. Prevalence of thyroid dysfunction and its correlation with CD4 count in newly-diagnosed HIV-positive adults–a cross-sectional study. Int J STD AIDS (2015) 26(13):965–70. doi: 10.1177/0956462414563776
12. Pekic S, Miljic D, Popovic V, Feingold KR, Anawalt B, Blackman MR, et al. Infections of the hypothalamic-pituitary region. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. South Dartmouth (MA): Endotext [Internet]. MDText.com, Inc.; (2000).
13. Merenich JA. Hypothalamic and pituitary function in AIDS. Baillieres Clin Endocrinol Metab (1994) 8(4):757–67. doi: 10.1016/S0950-351X(05)80298-8
14. Dona MG, Di Bonito P, Chiantore MV, Amici C, Accardi L. Targeting human papillomavirus-associated cancer by oncoprotein-specific recombinant antibodies. Int J Mol Sci (2021) 22(17):9143. doi: 10.3390/ijms22179143
15. Zandman-Goddard G, Shoenfeld Y. HIV And autoimmunity. Autoimmun Rev (2002) 1(6):329–37. doi: 10.1016/S1568-9972(02)00086-1
16. Pommier JD, Laouenan C, Michard F, Papot E, Urios P, Boutten A, et al. Metabolic syndrome and endocrine status in HIV-infected transwomen. AIDS (2019) 33(5):855–65. doi: 10.1097/QAD.0000000000002152
17. Parsa AA, Bhangoo A. HIV And thyroid dysfunction. Rev Endocr Metab Disord (2013) 14(2):127–31. doi: 10.1007/s11154-013-9248-6
18. Ugwueze CV, Young EE, Unachukwu CN, Onyenekwe BM, Nwatu CB, Okafor CI, et al. The prevalence and pattern of thyroid dysfunction in HAART-naive HIV patients in enugu, Nigeria: a cross-sectional comparative study. West Afr J Med (2021) 38(12):1200–5. doi: 10.55891/wajm.v38i12.49
19. Jariyawattanarat V, Sungkanuparph S, Sriphrapradang C. Characteristics of graves disease in hiv-infected patients on antiretroviral therapy. Endocr Pract (2020) 26(6):612–8. doi: 10.4158/EP-2019-0514
20. Delaugerre C, Gallien S, Flandre P, Mathez D, Amarsy R, Ferret S, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PloS One (2012) 7(5):e36673. doi: 10.1371/journal.pone.0036673
21. Xu J, Luo Y, Dong H, Zhao G. The effects of Internet exposure on sexual risk behavior among sexually experienced Male college students in China: cross-sectional study. JMIR Public Health Surveill (2022) 8(5):e31847. doi: 10.2196/31847
22. Morales DR, Moreno-Martos D, Matin N, McGettigan P. Health conditions in adults with HIV compared with the general population: a population-based cross-sectional analysis. EClinicalMedicine (2022) 47:101392. doi: 10.1016/j.eclinm.2022.101392
23. Harmooshi NN, Abeshtan A, Zakerkish M, Mirmomeni G, Rahim F. The effect of metformin on body mass index and metabolic parameters in non-diabetic HIV-positive patients: a meta-analysis. J Diabetes Metab Disord (2021) 20(2):1901–11. doi: 10.1007/s40200-021-00869-1
24. Njoku PO, Ejim EC, Anisiuba BC, Ike SO, Onwubere BJ. Clinical and echocardiographic findings in a cross-sectional study of HIV-infected adults in enugu, Nigeria. Cardiovasc J Afr (2021) 32(6):320–6.
25. Zhao S, Jia X, Fan X, Zhao L, Pang P, Wang Y, et al. Association of obesity with the clinicopathological features of thyroid cancer in a large, operative population: a retrospective case-control study. Med (Baltimore) (2019) 98(50):e18213. doi: 10.1097/MD.0000000000018213
26. Thaimuta ZL, Sekadde-Kigondu C, Makawiti DW. Thyroid function among HIV/AIDS patients on highly active anti-retroviral therapy. East Afr Med J (2010) 87(12):474–80.
27. Amadi K, Sabo AM, Ogunkeye OO, Oluwole FS. Thyroid hormone: a "prime suspect" in human immunodeficiency virus (HIV/AIDS) patients? Niger J Physiol Sci (2008) 23(1-2):61–6. doi: 10.4314/njps.v23i1-2.54927
28. Wang JJ, Zhou JJ, Yuan XL, Li CY, Sheng H, Su B, et al. Hyperthyroidism caused by acquired immune deficiency syndrome. Eur Rev Med Pharmacol Sci (2014) 18(6):875–9.
29. Otieno CF. Thyroid dysfunction in HIV/AIDS–cause or consequence? East Afr Med J (2010) 87(12):473.
30. Collazos J, Ibarra S, Mayo J. Thyroid hormones in HIV-infected patients in the highly active antiretroviral therapy era: evidence of an interrelation between the thyroid axis and the immune system. AIDS (2003) 17(5):763–5. doi: 10.1097/00002030-200303280-00019
31. Demircioglu ZG, Demircioglu MK, Aygun N, Akgun IE, Unlu MT, Kostek M, et al. Relationship between thyroid-stimulating hormone level and aggressive pathological features of papillary thyroid cancer. Sisli Etfal Hastan Tip Bul (2022) 56(1):126–31. doi: 10.14744/SEMB.2022.14554
32. Shindiapina P, Ahmed EH, Mozhenkova A, Abebe T, Baiocchi RA. Immunology of EBV-related lymphoproliferative disease in HIV-positive individuals. Front Oncol (2020) 10:1723. doi: 10.3389/fonc.2020.01723
33. Castle PE, Burk RD, Massad LS, Eltoum IE, Hall CB, Hessol NA, et al. Epidemiological evidence that common HPV types may be common because of their ability to evade immune surveillance: results from the women's interagency HIV study. Int J Cancer (2020) 146(12):3320–8. doi: 10.1002/ijc.32693
34. Ortiz-Gutierrez F, Sanchez-Minutti L, Martinez-Herrera JF, Torres-Escobar ID, Pezzat-Said EB, Marquez-Dominguez L, et al. Identification of genetic variants of human papillomavirus in a group of Mexican HIV/AIDS patients and their possible association with cervical cancer. Pol J Microbiol (2021) 70(4):501–9. doi: 10.33073/pjm-2021-047
35. Gernert M, Kiesel M, Frohlich M, Renner R, Strunz PP, Portegys J, et al. High prevalence of genital human papillomavirus infection in patients with primary immunodeficiencies. Front Immunol (2021) 12:789345. doi: 10.3389/fimmu.2021.789345
36. Pereira LMS, Franca EDS, Costa IB, Lima IT, Freire ABC, Ramos FLP, et al. Epstein-Barr Virus (EBV) genotypes associated with the immunopathological profile of people living with HIV-1: immunological aspects of primary EBV infection. Viruses (2022) 14(2):168. doi: 10.3390/v14020168
37. Chen Y, Yuan J, Han X, Liu X, Han X, Ye H. Coexpression analysis of transcriptome on AIDS and other human disease pathways by canonical correlation analysis. Int J Genomics (2017) 2017:9163719. doi: 10.1155/2017/9163719
38. Baldini E, Tuccilli C, Arlot-Bonnemains Y, Chesnel F, Sorrenti S, De Vito C, et al. Deregulated expression of VHL mRNA variants in papillary thyroid cancer. Mol Cell Endocrinol (2017) 443:121–7. doi: 10.1016/j.mce.2017.01.019
39. Li HP, Yang TZ, Wei L, Ge YF, Meng QS. STAT1-induced upregulation of lncRNA LINP1 promotes cell proliferation and inhibits apoptosis via AMPK signaling pathway in papillary thyroid cancer. Eur Rev Med Pharmacol Sci (2020) 24(17):8911–7.
40. Luan S, Fu P, Wang X, Gao Y, Shi K, Guo Y. Circular RNA circ-NCOR2 accelerates papillary thyroid cancer progression by sponging miR-516a-5p to upregulate metastasis-associated protein 2 expression. J Int Med Res (2020) 48(9):300060520934659. doi: 10.1177/0300060520934659
41. Marima R, Hull R, Lolas G, Syrigos KN, Kgoebane-Maseko M, Kaufmann AM, et al. The catastrophic HPV/HIV dual viral oncogenomics in concert with dysregulated alternative splicing in cervical cancer. Int J Mol Sci (2021) 22(18):10115. doi: 10.3390/ijms221810115
42. Possel P, Mitchell AM, Harbison B, Fernandez-Botran GR. Association of cancer caregiver stress and negative attribution style with depressive symptoms and cortisol: a cross-sectional study. Support Care Cancer (2022) 30(6):4945–52. doi: 10.1007/s00520-022-06866-1
43. Hommes MJ, Romijn JA, Endert E, Adriaanse R, Brabant G, Eeftinck Schattenkerk JK, et al. Hypothyroid-like regulation of the pituitary-thyroid axis in stable human immunodeficiency virus infection. Metabolism (1993) 42(5):556–61. doi: 10.1016/0026-0495(93)90212-7
44. Sharma N, Sharma LK, Dutta D, Gadpayle AK, Anand A, Gaurav K, et al. Prevalence and predictors of thyroid dysfunction in patients with HIV infection and acquired immunodeficiency syndrome: an Indian perspective. J Thyroid Res (2015) 2015:517173. doi: 10.1155/2015/517173
45. Akinsete A, Oyenusi E, Odugbemi B, Odugbemi T, Temiye E. Spectrum of thyroid abnormalities among children living with HIV in Lagos, Nigeria. J Thyroid Res (2019) 2019:1096739. doi: 10.1155/2019/1096739
46. Emokpae MA, Akinnuoye IM. Asymptomatic thyroid dysfunction in human immunodeficiency virus-1-infected subjects. J Lab Physicians (2018) 10(2):130–4. doi: 10.4103/JLP.JLP_172_16
Keywords: papillary thyroid carcinoma, thyroid cancer, clinicopathological features, HIV, infection
Citation: Liu J, Wu D, Zhu J and Dong S (2023) Clinicopathological features of papillary thyroid carcinoma in HIV-infected patients. Front. Oncol. 13:1071923. doi: 10.3389/fonc.2023.1071923
Received: 17 October 2022; Accepted: 24 April 2023;
Published: 05 May 2023.
Edited by:
Andy Bertolin, Ospedale Civile di Vittorio Veneto, ItalyCopyright © 2023 Liu, Wu, Zhu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Dong, ZG9uZ19zdUBqbHUuZWR1LmNu
 Jia Liu
Jia Liu Deqian Wu1
Deqian Wu1 Su Dong
Su Dong