
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 07 March 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1071030
This article is part of the Research Topic365 Days of Progress In Cancer Molecular Targets and TherapeuticsView all 23 articles
At present, studies have found that c-Met is mainly involved in epithelial-mesenchymal transition (EMT) of tumor tissues in urologic neoplasms. Hepatocyte growth factor (HGF) combined with c-Met promotes the mitosis of tumor cells, and then induces motility, angiogenesis, migration, invasion and drug resistance. Therefore, c-Met targeting therapy may have great potential in urologic neoplasms. Many strategies targeting c-Met have been widely used in the study of urologic neoplasms. Although the use of targeting c-Met therapy has a strong biological basis for the treatment of urologic neoplasms, the results of current clinical trials have not yielded significant results. To promote the application of c-Met targeting drugs in the clinical treatment of urologic neoplasms, it is very important to study the detailed mechanism of c-Met in urologic neoplasms and innovate c-Met targeted drugs. This paper firstly discussed the value of c-Met targeted therapy in urologic neoplasms, then summarized the related research progress, and finally explored the potential targets related to the HGF/c-Met signaling pathway. It may provide a new concept for the treatment of middle and late urologic neoplasms.
Renal cell carcinoma (RCC), prostate cancer (PCa) and bladder cancer (BCa) are the most common urologic neoplasms, which are a major tumor system threatening human health. RCC is the 12th most common cancer worldwide (1). The most common histological subtype is renal clear cell carcinoma(RCCC) (2). PCa is the second most common cancer and the fifth leading cause of cancer death in men. In recent years, the incidence of PCa is increasing year by year (3). BCa is the tenth most common cancer worldwide. It is more common in men than in women, with morbidity and mortality rates of 9.5 and 3.3 per 100,000 population, respectively. As a result, the disease is more prevalent in men, for whom it is the sixth most common cancer and the ninth leading cause of cancer death (3). Therefore, due to the high incidence of urinary tract tumors, which seriously affects human health, a large part of the world’s medical and health resources should be used for the prevention and treatment of urologic neoplasms.
In recent years, with the promotion of early screening, early detection of tumors and the development, application of tumor diagnostic markers, the diagnosis and treatment of urologic neoplasms have made rapid progress (4–6). Because the progression of urologic neoplasms is slower than that of other systems, it is easy to achieve radical curative effect when tumors are detected at an early stage. However, due to the unbalanced development of economic level, early cancer screening and early detection cannot be quickly and comprehensively popularized, which leads to a large number of patients with middle and advanced urologic neoplasms are still found in clinical practice. For these patients, recurrence and metastasis after surgery are the focus of treatment. Therefore, it is pressing to exploit new targeted therapies for the treatment of middle and advanced urologic neoplasms.
C-Met, as a tyrosine kinase receptor, is overexpressed in multiple tumors and exerts an active function in tumor progression as an oncogenic factor (7). Because c-Met activation occurs in combination with HGF or through ligand-independent mechanisms (8), c-Met is often dysregulated in solid tumors, including urologic neoplasms (9–11). It is significantly overexpressed in tumor metastasis sites due to its properties of promoting tumor proliferation, angiogenesis and metastasis (12). Besides, c-Met was found to mediate the resistance signaling axis of single-dose immunotherapies targeting PD-1 (13). This observation provides a theoretical basis for the combined anti-tumor effect of immune checkpoint inhibitors (ICIs) and c-Met inhibition. Furthermore, c-Met itself can act as a tumor specific antigen and can be used as a precise guidance for T cells to eliminate tumor cells in immunotherapy. Therefore, targeting c-Met has great potential in the treatment of urologic neoplasms. In this review, we reviewed the expression of c-Met in urologic neoplasms tissues and its clinical prognostic value. Then, the mechanism of c-Met in urologic neoplasms and the studies of c-Met targeted therapy in urologic neoplasms were summarized. Finally, the potential therapeutic targets related to HGF/c-Met signaling pathway were discussed.
Previous studies have demonstrated that c-Met overexpression exists in hepatobiliary tumors, so the treatment targeting c-Met has been carried out more frequently in the treatment of hepatobiliary tumors (14–16). However, recent studies have revealed that c-Met is also highly expressed in urologic neoplasms and is related with poor prognosis, indicating that c-Met is a potential target for urologic neoplasms.
In adult kidney, c-Met is expressed in renal tubular epithelial cells, and its main physiological function is to stimulate the growth of renal tubular cells (17, 18). Proper c-Met function is also crucial for inducing branching tubulogenesis during tubule repair after ischemia injury (19). Meanwhile, it has been shown that c-Met is involved in the progression of RCC as a proto-oncogene (20, 21).
Numerous studies have demonstrated that c-Met is overexpressed in RCC tissues and is closely related to pathological grade, stage and prognostic survival. It may have potential for prognostic assessment and targeted therapy (22–24). J. H. Kim et al. (25) conducted a study to evaluate the correlation between high c-Met expression and clinicopathologic factors and its impact on prognosis in RCC patients. Twelve studies involving 1724 patients were included. The results showed that compared with RCC with low c-Met expression, the tumor nuclear grade and pT stage were significantly higher with high c-Met expression. Besides, RCC patients with high c-Met levels had significantly lower overall survival (OS) than patients with low c-Met levels tumors. S. Macher-Goeppinger et al. (26) detected the expression of MET and the frequency of increased MET gene copies from the long-term follow-up data of patients with RCC based on a large hospital. The results showed that in 572 cases of RCC, 32% had high protein expression. High MET expression and increased MET copy number were also found to be related with an adverse patient outcome. These studies suggest that c-Met overexpression is present in RCC and overexpression is associated with significant malignant pathological features and poor survival. It also demonstrates that c-Met is a potential target for RCC treatment.
In addition, the expression of c-Met in chromophobe renal cell carcinoma (CRCC) and its prognostic significance have also been studied. F. Erlmeier et al. (27) evaluated the prevalence, distribution and prognostic impact of c-Met expression in CRCC. High expression of c-Met was found in 29.6% of patients, and there was a correlation between high expression of c-Met and lymph node metastasis. This suggests that the role and expression of c-Met in RCC progression may be universal and not limited by pathological types. This viewpoint also lays a theoretical foundation for the application of c-Met targeting in RCC.
C-Met expression can be detected in normal prostate basal epithelial cells, but it is generally not expressed in peripheral and transitional zone epithelial cells (28). C-Met protein overexpression was found in 84% of primary PCa and 100% of metastatic PCa (29). These studies indicate that c-Met overexpression is significantly related to high-grade adenocarcinoma and may exert a crucial function in tumor progression. Besides, K. Nakashiro et al. (30)demonstrated that HGF produced by prostate-derived stromal cells stimulated the growth of androgen-dependent PCa cells in vitro and in vivo. It was found that epithelial cells began to express c-Met protein with the development of tumor malignancy. Therefore, the study have shown that the stromal cells of PCa may form an autocrine c-Met loop, which may act together with HGF expressed by cancer cells to promote tumor progression.
To further explore the role of c-Met in the prognosis of PCa, D. Strohmeyer et al. (31) demonstrated that the expression of vascular endothelial growth factor(VEGF) and c-Met increased with the increase of tumor stage and grade. In addition to VEGF, c-Met exerts a significant function in the induction of angiogenesis in PCa and is related to clinical prognosis. Furthermore, S. Nishida et al. (32) also found that the expression of HGF in prostate tissues was correlated with the biochemical recurrence of PCa after surgery. The results showed that patients with HGF overexpression in PCa had significantly longer biochemical relapse-free survival. Survival risk analysis showed that HGF overexpression was an independent risk factor for postoperative biochemical recurrence.
In addition, other researchers have investigated the correlation between c-Met protein expression and Gleason grade. F. Jacobsen et al. (33) successfully examined the expression of c-Met in 3378 PCa tissues by immunohistochemistry and analyzed the follow-up data of patients. The results demonstrated that c-Met protein was often overexpressed in PCa, and the high expression of c-Met protein was significantly correlated with high Gleason grade. These results indicate that c-Met is not only expressed in PCa tissues, but also involved in tumor progression. It is enough to confirm that c-Met is a meaningful target for PCa treatment. The above points also indicate that targeting c-Met in the treatment of PCa is theoretically feasible.
As early as the 1990s, a study confirmed that the HGF/c-Met pathway was involved in the progression of BCa in animal models (34). Since then, the researchers have also compared HGF levels in the urine of BCa patients and healthy people, and found that HGF levels were significantly higher in BCa patients. Studies have shown that there seems to be a positive correlation between BCa progression and HGF levels (35). From then on, researchers have gradually begun to investigate the mechanism of HGF/c-Met signaling pathway in the progression of BCa.
K. Yamasaki et al. (36) retrospectively analyzed the expression of c-Met in tumor specimens of patients with invasive BCa and its relationship with prognosis. The results demonstrated that c-Met was highly expressed in 46% of cancer tissues. The overexpression of c-Met is significantly correlated with poor clinical prognosis, and the overexpression of c-Met indicates poor prognosis. Besides, X. Xu et al. (37) conducted a study to assess the pathological and prognostic role of c-Met status in BCa patients. Eight studies were eventually included, including 1,336 cases of BCa. The results showed that overexpression of c-Met in primary BCa was related to poor OS and was an independent risk factor for prognosis and survival. These studies suggest that c-Met is also involved in the progression of BCa and may be involved in the metastasis.
In addition, the correlation between c-Met and programmed death ligand 1 (PD-L1) in tumor tissues was investigated. Y. Mukae et al. (38) demonstrated that the high expression of c-Met was correlated with muscle invasion and metastasis of BCa, and c-Met exerted a vital function in invasion of tumor cell by regulating PD-L1. This study shows that c-Met is indeed involved in the invasion and metastasis of BCa, which again theoretically confirms that c-Met may affect the prognosis and survival of BCa patients. The above studies related to pathology and clinical prognosis also indicate that targeting c-Met may have great potential in the treatment of BCa.
C-Met is a transmembrane tyrosine kinase receptor that is activated by HGF to regulate the expression of related downstream genes. This process is essential for cell migration under normal and pathological conditions. Current studies have demonstrated that c-Met is mainly involved in EMT in many types of cancer. HGF combined with c-Met promotes the mitosis of various tumor cells, and then induces motility, angiogenesis, migration and invasion. In recent years, many studies have been conducted on the oncogenic mechanism of c-Met in urologic neoplasms (Table 1). Studies have shown that c-Met is also involved in the formation of various phenotypes of urologic neoplasms through relevant signaling pathways (Figure 1). It is also confirmed that c-Met is a prospective therapeutic target for urologic neoplasms from the perspective of basic biology.
At present, numerous studies have demonstrated that HGF can regulate the expression of VEGF and promote tumor angiogenesis through its receptor c-Met (39, 48, 49). Researchers found that Von Hippel-Lindau (VHL) mutation and hypoxia resulted in increased expression of HGF and c-Met in RCC (50, 51). Besides, hypoxia-inducible factor 1 (HIF-1) during hypoxia can regulate the expression of c-Met and VEGF (52). Therefore, c-Met is a crucial target for anti-tumor angiogenic therapy of RCC.
In recent years, researchers have conducted some studies on the specific mechanism of EMT in PCa. B. Yin et al. (40) confirmed that HGF/c-Met signaling pathway may be the main mechanism of EMT in PCa. This study also found that RON and c-Met promote tumor metastasis through ERK signaling pathway. Besides, Y. Han et al. (41) demonstrated that HGF could induce tumor EMT by activating ERK/MAPK and Zeb-1 signaling pathways, thereby increasing the invasion potential of PCa cells. They also investigated the role of c-Met in EMT of PCa (42). The results showed that c-Met enhanced the proliferation, migration and tumorigenicity of tumor cells by regulating E-cadherin/vimentin. These EMT translation is mediated through PI3K and MAPK signaling pathways. G. Davies et al. (46) also found that the correlation between E-cadherin/catenin and c-Met may regulate the adhesion between PCa cells. Further studies demonstrated that HGF enhanced the invasive potential of PCa cells by increasing the production of MMP-1, MMP-9, MT1-MMP, u-PA and uPAR (45). These studies indicated that HGF/c-Met signaling pathway enhanced tumor invasion and metastasis in the process of promoting tumor EMT.
PCa consists of secretory cells and immature cells. C-Met was found to be specifically expressed in immature prostate cells. G. J. van Leenders et al. (43) determined the role of immature cells in PCa by analyzing the HGF/c-Met pathway. The results of this study show that HGF induces a molecular signature associated with stem cells by upregulating the activation of the Notch pathway. The results indicate that c-Met activation in PCa cells can induce tumor stem cell-like phenotype, and c-Met may regulate tumor invasion in surrounding tissues through Notch pathway. Besides, changes in c-Met overexpression in PCa tissues were found to be associated with tumor-independent androgen progression. Activation of c-Met signaling may induce spontaneous mutations or genomic instability leading to tumor progression in an androgen-independent state (44). The above studies indicate that c-Met can compensate for the deficiency of androgen in the progression of PCa, so targeting c-Met has special significance in the treatment of PCa.
In addition, aberrant HGF/c-Met upregulation and activation were found to be frequently observed in BCa and correlated with cancer progression and invasion. W. J. Sim et al. (47) found that HGF stimulated TGF-β signaling through SMURF2 signal pathway, leading to enhanced stability of TGF-β receptor. Finally, upregulation of TGF-β pathway by HGF leads to tumor EMT and invasion. Therefore, the investigators found that the combination of TGF-β receptor inhibitors may be promising in the treatment of BCa.
Due to the role of c-Met in promoting the progression of urological neoplasms, c-Met targeting therapy have great potential in the treatment of urological neoplasms. Researchers have conducted a large number of preclinical studies in recent years, and preliminary results have been achieved (Table 2). Studies have shown that c-Met mediated signaling pathway exerts an important function in the progression of RCC. As an alternative pathway of VEGF, HGF/c-Met is emerging as a vital role in tumor angiogenesis and resistance to anti-VEGF therapy. The efficacy of simultaneous targeting of VEGF and c-Met in the treatment of RCC has been evaluated (56). The results demonstrated that the combination of axitinib and crizotinib could significantly improve the antitumor effect and prolong the survival time of the tumorigenic model. Honokiol (HNK) is a small molecule with antitumor effects. The researchers found that HNK exerts antitumor activity by inhibiting the c-Met-Ras axis (68). Besides, the antitumor effect of the combination of rapamycin and HNK in the treatment of RCC was also evaluated (54). The results show that the combination therapy can significantly inhibit the growth of RCC, which has significant therapeutic potential for the prevention of cancer after renal transplantation. In addition, other researchers have developed naturally occurring c-Met inhibitors for anti-tumor trials. K. Golovine et al. (55) studied the tumor killing activity of piperlongumine (PL) and found that PL rapidly reduced c-Met protein and RNA levels in RCC cells through a ROS-dependent mechanism. Therefore, PL has great potential in the adjuvant therapy of advanced RCC.
For the past few years, chimeric antigen receptor T cells (CAR-T) therapy has revealed remarkable efficacy in cancer immunotherapy, especially in the treatment of B-cell malignancies. To apply this technique to RCC, J. I. Mori et al. (53) developed c-Met targeting CAR-T cells for the treatment of papillary renal cell carcinoma (PRCC) and studied the anti-tumor efficacy of CAR-T cells. The results showed that a large number of c-Met targeting CAR-T cells infiltrated into tumor tissues and significantly inhibited tumor growth. Besides, the antitumor efficacy of CAR-T cells was synergistically enhanced when combined with axitinib. Due to the specific expression of c-Met in renal cancer tissues, CAR-T cells therapy targeting c-Met in renal cancer is expected.
Studies have shown that hormone-independent PCa is highly resistant to most conventional therapies, including radiation therapy, which is a major obstacle in the treatment of such patients. H. Yu et al. (62) shown that the inhibition of c-Met by SU11274 could significantly inhibit the survival and proliferation of DU145 cells and enhance their radiosensitivity. The potential mechanism may include inhibition of c-Met signaling, damage of DNA repair function and enhancement of cell death. This study is the first to demonstrate the efficacy of combining c-Met inhibition with ionizing radiation in the treatment of hormone-independent PCa. In addition, c-Met was found to be abnormally activated in the absence of HGF in many solid tumors (69). Y. Dai et al. (63) studied the reaction of PC-3 cells against HGF neutralizing antibody or small molecule c-Met kinase inhibitor (BMS-777607). The findings suggest that targeting c-Met in the absence of functional HGF remains a viable therapeutic option to halt cancer progression. These studies suggest that the antitumor activity of tyrosine kinase inhibitors (TKIs) targeting c-Met against PCa can be independent of the presence of HGF.
In addition, cabozantinib has been found in preclinical studies to reduce PCa growth in bone and has been shown to inhibit osteoblast activity. C. Lee et al. (59) found that the use of cabozantinib in vivo could inhibit c-Met and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) in osteoblasts, thereby reducing the expression of RANKL and M-CSF, and was associated with tumor-induced reduction of osteolysis. Other researchers have found similar results, J. Eswaraka et al. (61) tested the efficacy of axitinib combined with crizotinib in the treatment of castration resistant prostate cancer (CRPC) with bone metastases in a mouse model. The results showed that combined inhibitions of c-Met and VEGFR were helpful in the treatment of CRPC with bone metastases. Furthermore, molecular signal complementarity between RON and c-Met has been found (70), and some scholars have studied the anti-tumor efficacy of simultaneously targeting RON and c-Met. B. Yin et al. (40)demonstrated that foretinib (GSK1363089) inhibited the metastasis of PCa cells and promoted the reversal of EMT of PCa cells through the inhibition of RON and c-Met. Therefore, Foretinib with its broad tyrosine kinase inhibitory activity may hold promise in the treatment of metastatic PCa.
At present, the study of c-Met monoclonal antibody in urologic neoplasms is less. Only Y. Yu et al. (67) have developed anti-HGF rabmab, which can both block HGF/c-Met interaction and inhibit c-Met phosphorylation. The study confirmed the efficacy and potency of anti-HGF RabMAb in a mouse model of tumor transplantation. These results suggest that monoclonal antibodies targeting HGF may be a new therapeutic approach for advanced PCa. In addition, researchers have also found that curcumin (66), Heteronemin (60), heterocyclic compound (57) and Evodiamine(EVO) (64) can inhibit the progression of PCa by inhibiting HGF/c-Met pathway signaling. Quercetin can reverse doxorubicin resistance in PCa cells by down-regulating c-Met expression (65). These drugs are potential strategies in the treatment of PCa, and the specific efficacy needs to be confirmed in future clinical studies.
It is well known that patients with advanced urologic neoplasms have few treatment options. While these treatments may slow the progression of the disease, none is a complete cure. Therefore, it is necessary to continue to investigate other treatments for advanced urologic neoplasms. TKIs have been widely studied as a therapeutic approach for a variety of malignant tumors. As shown in Table 3, numerous clinical studies have been carried out in the treatment of urologic neoplasms with TKIs, some of which have been completed and some of which are being recruited. Most of the research was conducted in the United States, indicating that American institutions contributed significantly to the research. Published studies have shown that most TKIs targeting c-Met have good tolerability and safety in the treatment of urologic neoplasms. However, studies have found that most of the multi-target c-Met TKIs and combined with multi-target therapy have a good clinical response rate and clinical prognosis.
Cabozantinib is a TKI that inhibits both VEGFR and c-Met. Therefore, the antiangiogenic properties of cabozantinib have led to its use as a second-line therapy for metastatic Renal Cell Carcinoma (mRCC) and as a first-line treatment option when ICIs are contraindicated (71). Cabozantinib has been evaluated for safety and activity. G. Procopio et al. (72) conducted a multicenter study of Cabozantinib in the treatment of mRCC patients in Italy. Only 5 patients (5.0%) stopped treatment due to adverse events. Partial responses were observed in 35 patients (36%), stable disease in 33 patients (34%), and progression in 28 patients (30%). The median progression-free survival (PFS) was 8.0 months. This study have shown that Cabozantinib exhibits acceptable tolerability and antitumor activity. Furthermore, K. J. Peltola et al. (73) evaluated the expression of c-Met in mRCC patients treated with sunitinib. The results showed that patients with low c-Met expression had longer PFS and OS. Survival risk analysis showed that high c-Met expression was an independent risk factor for adverse PFS. Studies have demonstrated that targeting c-Met can provide a survival benefit for patients with mRCC.
The progression of PCa requires androgen support, and this characteristic determines that the treatment of PCa should not be without anti-androgen therapy. Therefore, most of the clinical trials of TKIs targeting c-Met are combination therapy. A. Tripathi et al. (74) conducted a phase I trial of crizotinib and enzalutamide (androgen receptor antagonist) in the treatment of CRPC, with the main purpose of investigating its safety and pharmacokinetics. The study found that concurrent administration of enzalutamide and crizotinib resulted in a clinically significant reduction of systemic crizotinib exposure of 74%. Meanwhile, P. G. Corn et al. (75) evaluated cabozantinib combined with androgen deprivation therapy (ADT) for metastatic PCa. The results showed that cabotinib combined with ADT had better clinical activity in the treatment of metastatic PCa. In addition, Tivantinib has been found to be mildly toxic and improve PFS in patients with asymptomatic or minimally symptomatic mCRPC (76). The above studies indicate that TKIs combined with special treatment determined by the characteristics of the tumor itself may produce better efficacy, which also proves that TKIs is only suitable for adjuvant treatment of advanced tumors.
At present, olaparib has been clinically approved for the treatment of PCa, but cytotoxicity and DNA damage limit its clinical application. Z. Wang et al. (58) found that the combined inhibition of c-Met and poly ADP-ribose polymerase(PARP) had a synergistic effect on blocking the growth of PCa cell lines. When the two drugs were combined, tumor invasion and migration were prominently inhibited. This study suggests that targeting both c-Met and PARP may be a valuable strategy for PCa treatment.
Previous studies have shown that HGF/c-Met signaling pathway exerts a crucial function in the progression of urologic neoplasms. Therefore, targeting HGF/c-Met signaling pathway is a hopeful approach for the treatment of urologic neoplasms. Besides, more and more studies have confirmed that HGF/c-Met signaling is also regulated by other targets. Some studies have also confirmed that tumor progression can be inhibited by inhibiting these targets. Therefore, targets that regulate the HGF/c-Met signaling pathway may also have potential value for targeted therapy (Figure 2).
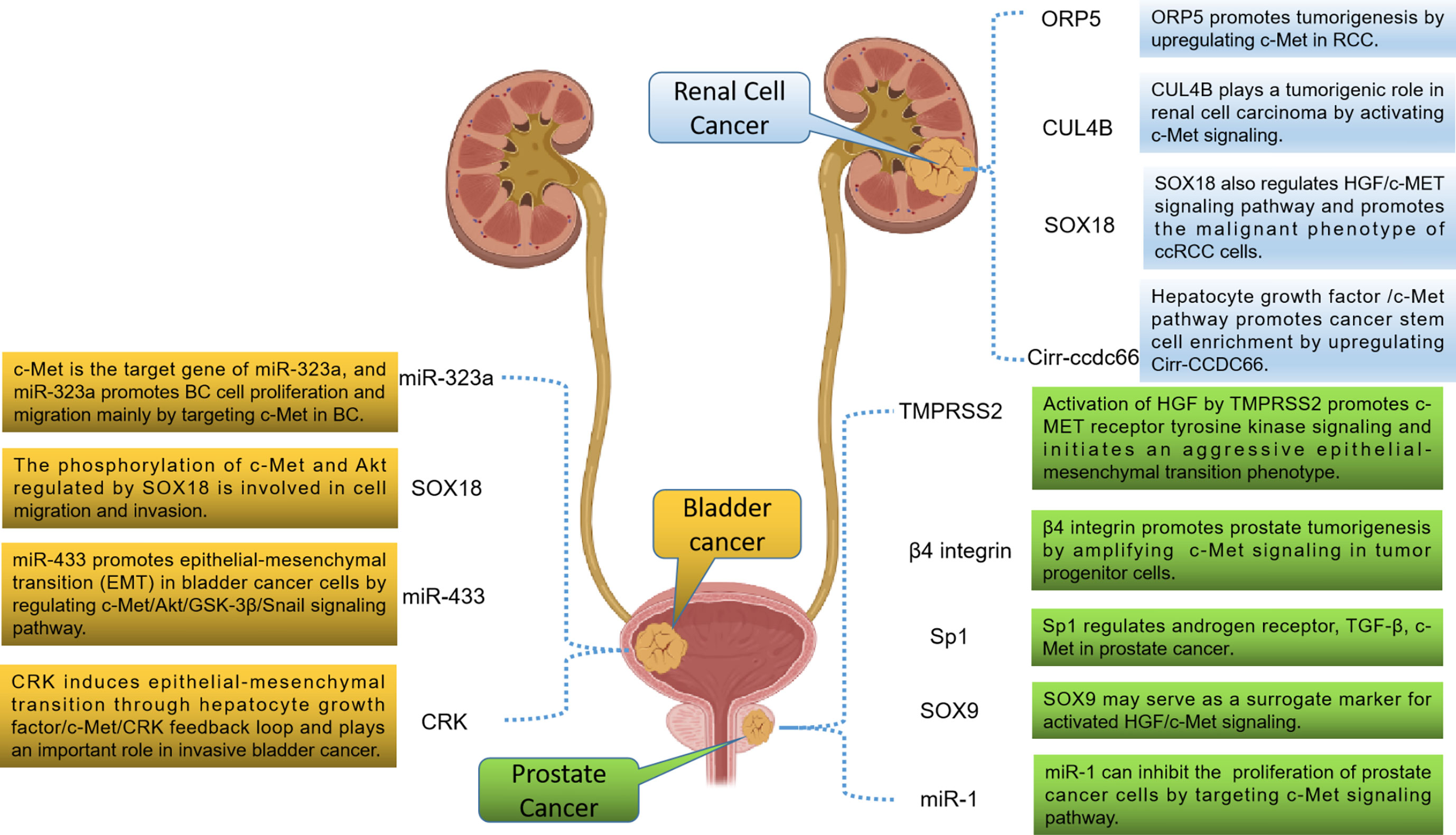
Figure 2 Potential therapeutic targets related to the HGF/c-Met signaling pathway in urologic neoplasms.
Cullin 4B (CUL4B) is a structural protein encoding ubiquitin ligase complex, which is normally involved in physiological and developmental processes of the body. In recent years, it has been found that it is overexpressed as an oncogene in various solid tumors (77–79). S. Chen et al. (80) found that the expression of CUL4B in RCC cells and tissues was positively associated with the expression of c-Met. Further studies have demonstrated that CUL4B exerts a function in promoting tumor progression by activating c-Met signaling in RCC. Therefore, CUL4B may have potential value in the treatment of RCC. Besides, the transcription factor SOX18 has now been implicated in malignant tumor phenotype, angiogenesis, and lymphangiogenesis. Y. Huaqi et al. (81) found that activated SOX18 could induce HGF/c-Met signaling pathway both in vitro and in vivo in RCC. These results suggest that SOX18 may be a valuable target for the treatment of RCC. Previous studies have shown that circRNAs are involved in the occurrence and development of many cancers. J. Yang et al. (82) found that the HGF/c-Met pathway was activated during the enrichment of cancer stem cells and was responsible for the upregulation of circ-CCDC66. Inhibition of HGF/c-Met blocked circ-CCDC66-induced enrichment of cancer stem cells. It was confirmed that circ-CCDC66 may be also a therapeutic target for RCC. In addition, ORP5 is a lipid transporter that increases metastasis in a variety of cancers (83). L. Song et al. (84) also found that ORP5 promoted tumorigenesis by upregulating c-Met in RCC. These studies suggest that molecules that regulate HGF/c-Met signaling may be developed as therapeutic targets for RCC in the future.
TMPRSS2 is an androgen-regulated serine protease that has been found to be highly expressed in most metastatic PCa. J. M. Lucas et al. (85) found that TMPRSS2 initiated invasive EMT through activated HGF/c-Met signaling. The researchers also screened a potent TMPRSS2 inhibitor for in vivo studies and found that TMPRSS2 inhibitors inhibited PCa metastasis in vivo. Meanwhile, T. Yoshioka et al. (86) found that a large number of advanced PCa and CRPC expressed high levels of β4 integrin. Further studies revealed that β4 integrin is often co-expressed with c-Met and ErbB2 in PCa, and TKIs that simultaneously target these targets have shown significant ability to inhibit tumor progression in an in vivo model of PCa. These results suggest that β4 integrin, ErbB2 and c-Met may be involved in the occurrence and development of PCa through interaction. Besides, the specific protein (Sp) family has been shown to be involved in tumorigenesis. Studies have shown that Sp1 can regulate TGF-β, c-Met, PSA and α-integrin in PCa. These results indicate that Sp1 has potential value in targeted therapy for PCa because of its important role in PCa progression (87). Other researchers have investigated the mechanism of microRNA-1 (miR-1) in PCa cells (88). The results revealed that miR-1 promoted the proliferation of PCa cells by activating the c-Met/Akt/mTOR signaling pathway. Therefore, targeting MiR-1 may be used to treat PCa. In addition, H. Qin et al. (89) found that PCa cells regulate SOX9 molecules through the HGF/c-Met-ERK axis. SOX9 may serve as a surrogate marker for activated HGF/c-Met signaling to recruit optimal PCa patients for HGF/c-Met inhibitory therapy because it is more stable and easier to detect.
SOX18 is also a transcription factor that exerts a crucial function in regulating cell differentiation, lymphatic and vascular development. Y. Huaqi et al. (90) found that SOX18 promotes the migration and invasion of tumor cells by regulating c-Met and Akt, indicating that SOX18 plays a crucial role in BCa metastasis. There is growing evidence that dysregulation of certain microRNAs (miR) may contribute to tumor progression and metastasis. Xu X et al. (91) found that miR-409-3p was down-regulated in human bladder cancer tissues and cell lines. Overexpression of miR-409-3p in bladder cancer cells significantly reduced its migration and invasion. Further studies showed that miR-409-3p inhibited the expression of c-Met by binding to the 3 ‘ untranslated region of c-Met. These findings suggest that miR-409-3p may inhibit the progression of bladder cancer via the c-Met pathway. Meanwhile, J. Qiu et al. (92) studied and evaluated the expression and role of miR-323a in BCa progression. Studies have revealed that the reduced expression of miR-323a in BCa promotes the proliferation and migration of BCa cells mainly by targeting c-Met. Besides, X. Xu et al. (93) found that miR-433 promoted BCa EMT by regulating the c-Met/Akt/GSK-3β/Snail signaling pathway. Targeting miR-433 may be a new method to inhibit the progression and metastasis of BCa. In addition, CRK is an adaptor protein that plays a crucial role in the malignant potential of various invasive human cancers. R. Matsumoto et al. (94) demonstrated that CRK induced EMT through HGF/c-Met/CRK feedback loop in invasive BCa. Therefore, CRK may also be a significant target for BCa, especially in preventing metastasis.
This review have revealed that c-Met is highly expressed in urologic neoplasms and plays an significant role in tumor progression. Numerous studies have been conducted on c-Met targeted therapy in urologic neoplasms, and preclinical studies have shown obvious tumor suppressive activity. At present, published clinical studies have shown that most TKIs targeting c-Met have good tolerability and safety, and the combined multi-target treatment strategy has shown acceptable clinical response rate and prognostic survival in cancer treatment. Meanwhile, studies have also shown that TKIs targeting c-Met combined with VEGF, RON inhibitors or specific tumor treatment strategies (such as anti-androgen therapy for PCa) can achieve better clinical efficacy. Therefore, it also indicates that the current TKIs targeting c-Met are more suitable for adjuvant therapy of advanced urologic neoplasms.
C-Met interacts with other oncogenic molecules (such as EGFR and RON) to activate downstream pathways, thereby mediating tumor progression and drug resistance (69). Similarly, there is also signal interaction between c-Met and VEGF and RON molecules in urologic neoplasms (40, 56). This may also be a reason why c-Met targeting therapy has not achieved significant breakthroughs in the treatment of urologic neoplasms. Studies have also shown that targeting both c-Met and VEGF can achieve better tumor killing efficacy (59, 61). However, RON and VEGF are certainly not the only tumorigenic factors that interact with c-Met to promote tumor progression or generate drug resistance. Therefore, it is particularly crucial to study the mechanism of c-Met signaling pathway interaction in urologic neoplasms. Only by comprehensively mastering the signaling pathways and interaction mechanisms related to c-Met and tumor, we can better select the c-Met targeting therapy.
For the past few years, monoclonal antibodies against PD-1/PD-L1 and VEGF have been approved for clinical application in anti-tumor therapy. Besides, monoclonal antibodies against c-Met, such as Onartuzumab and Emibetuzumab, have been applied to digestive system tumors, and have shown good tolerance and clinical response rate (95, 96). However, there are few studies on these antibodies in urinary tract tumors. In the future, the monoclonal antibody against c-Met should be developed for the study of urologic neoplasms, or the targeted therapy of c-Met combined with the monoclonal antibody of PD-1/PD-L1 and VEGF should be used for the killing test of urologic neoplasms.
In addition, tumor killing by CAR-T cells technology depends only on the targeting of the target, not on the mechanism of the target. As a membrane protein specifically expressed in tumor cells, c-Met is suitable for tumor therapy using CAR T cell technology. CAR-T cells targeting c-Met have been studied in gastric cancer (97, 98), liver cancer (99, 100) and breast cancer (101), and good tumor killing activity has been achieved. Previous studies have demonstrated that c-Met plays a vital role in the progression of urologic neoplasms, and c-Met is highly expressed in tumor tissues. Therefore, CAR-T cells technology targeting c-Met has the potential for application in urologic neoplasms. However, only one c-Met-CAR-T cells study has been conducted in PRCC (53). In the future, c-Met-CAR-T cells technology should be widely studied in the treatment of urologic neoplasms to achieve good results.
In conclusion, c-Met is involved in the progression of urologic neoplasms. It is highly expressed in tumor tissues and is associated with poor clinical prognosis. Due to the interaction mechanism between c-Met and other molecular signals, the use of targeting c-Met alone has limitations, while the combination of other antitumor methods showed better tumor killing efficacy. In the future, with the in-depth research on the mechanism of c-Met in urologic neoplasms and the optimization of CAR-T cells technology, it is believed that the targeted therapy of c-Met in the treatment of urologic neoplasms will surely make breakthrough progress.
PS and XK also performed literature management and produced tables and graphs. All authors contributed to the article and approved the submitted version.
Thanks to www.clinicaltrials.gov database for data support and to Honghui Hospital for their convenience.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Gray RE, Harris GT. Renal cell carcinoma: Diagnosis and management. Am Fam Physician (2019) 99:179–84.
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
4. Jeong SH, Ku JH. Urinary markers for bladder cancer diagnosis and monitoring. Front Cell Dev Biol (2022) 10:892067. doi: 10.3389/fcell.2022.892067
5. Ma SJ, Oladeru OT, Wang K, Attwood K, Singh AK, Haas-Kogan DA, et al. Prostate cancer screening patterns among sexual and gender minority individuals. Eur Urol (2021) 79:588–92. doi: 10.1016/j.eururo.2020.11.009
6. Usher-Smith J, Simmons RK, Rossi SH, Stewart GD. Current evidence on screening for renal cancer. Nat Rev Urol (2020) 17:637–42. doi: 10.1038/s41585-020-0363-3
7. Fu J, Su X, Li Z, Deng L, Liu X, Feng X, et al. HGF/c-MET pathway in cancer: from molecular characterization to clinical evidence. Oncogene (2021) 40:4625–51. doi: 10.1038/s41388-021-01863-w
8. Papaccio F, Della Corte CM, Viscardi G, Di Liello R, Esposito G, Sparano F, et al. HGF/MET and the immune system: Relevance for cancer immunotherapy. Int J Mol Sci (2018) 19:3595. doi: 10.3390/ijms19113595
9. Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature (2015) 522:349–53. doi: 10.1038/nature14407
10. Mohareb RM, Elmetwally AM, Mohamed AA. Multi-component reactions of cyclohexan-1,3-dione: Synthesis of fused pyran, pyridine, thiophene and pyrazole derivatives with c-met, anti-proliferative activities. Anticancer Agents Med Chem (2021) 21:2443–63. doi: 10.2174/1871520621666210112115128
11. Feng Y, Yang Z, Xu X. C-met: A promising therapeutic target in bladder cancer. Cancer Manag Res (2022) 14:2379–88. doi: 10.2147/CMAR.S369175
12. Lalani AA, Gray KP, Albiges L, Callea M, Pignon JC, Pal S, et al. Differential expression of c-met between primary and metastatic sites in clear-cell renal cell carcinoma and its association with PD-L1 expression. Oncotarget (2017) 8:103428–36. doi: 10.18632/oncotarget.21952
13. Razzak M. Targeted therapies: hepatocyte growth factor-a culprit of drug resistance. Nat Rev Clin Oncol (2012) 9:429. doi: 10.1038/nrclinonc.2012.124
14. Shang R, Song X, Wang P, Zhou Y, Lu X, Wang J, et al. Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut (2021) 70:1746–57. doi: 10.1136/gutjnl-2020-320716
15. Qiu W, Chang Y, Liu J, Yang X, Yu Y, Li J, et al. Identification of p-Rex1 in the regulation of liver cancer cell proliferation and migration via HGF/c-Met/Akt pathway. Onco Targets Ther (2020) 13:9481–95. doi: 10.2147/OTT.S265592
16. Altaf S, Saleem F, Sher AA, Ali A. Potential therapeutic strategies to combat HCC. Curr Mol Pharmacol (2022) 15:929–42. doi: 10.2174/1874467215666220103111009
17. Di Renzo MF, Narsimhan RP, Olivero M, Bretti S, Giordano S, Medico E, et al. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene (1991) 6:1997–2003.
18. Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol (1993) 123:223–35. doi: 10.1083/jcb.123.1.223
19. Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA (1994) 91:4357–61. doi: 10.1073/pnas.91.10.4357
20. Natali PG, Prat M, Nicotra MR, Bigotti A, Olivero M, Comoglio PM, et al. Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer (1996) 69:212–7. doi: 10.1002/(SICI)1097-0215(19960621)69:3<212::AID-IJC11>3.0.CO;2-9
21. Miyata Y, Kanetake H, Kanda S. Presence of phosphorylated hepatocyte growth factor receptor/c-met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res (2006) 12:4876–81. doi: 10.1158/1078-0432.CCR-06-0362
22. Chen S, Zhu Y, Cui J, Wang Y, Xia Y, Song J, et al. The role of c-met in prognosis and clinicopathology of renal cell carcinoma: Results from a single-centre study and systematic review. Urol Oncol (2017) 35:532.e15–23. doi: 10.1016/j.urolonc
23. Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR, et al. C-met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol (2013) 24:343–9. doi: 10.1093/annonc/mds463
24. Jung M, Lee S, Moon KC. C-met and EPHA7 receptor tyrosine kinases are related to prognosis in clear cell renal cell carcinoma: Focusing on the association with myoferlin expression. Cancers (Basel) (2022) 14:1095. doi: 10.3390/cancers14041095
25. Kim JH, Kim BJ, Kim HS. Clinicopathological impacts of high c-met expression in renal cell carcinoma: a meta-analysis and review. Oncotarget (2017) 8:75478–87. doi: 10.18632/oncotarget.20796
26. Macher-Goeppinger S, Keith M, Endris V, Penzel R, Tagscherer KE, Pahernik S, et al. MET expression and copy number status in clear-cell renal cell carcinoma: prognostic value and potential predictive marker. Oncotarget (2017) 8:1046–57. doi: 10.18632/oncotarget.13540
27. Erlmeier F, Ivanyi P, Hartmann A, Autenrieth M, Wiedemann M, Weichert W, et al. C-met in chromophobe renal cell carcinoma. Med Oncol (2017) 34:15. doi: 10.1007/s12032-016-0874-1
28. Knudsen BS, Edlund M. Prostate cancer and the met hepatocyte growth factor receptor. Adv Cancer Res (2004) 91:31–67. doi: 10.1016/S0065-230X(04)91002-0
29. Pisters LL, Troncoso P, Zhau HE, Li W, von Eschenbach AC, Chung LW. C-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol (1995) 154:293–8. doi: 10.1016/S0022-5347(01)67297-5
30. Nakashiro K, Hayashi Y, Oyasu R. Immunohistochemical expression of hepatocyte growth factor and c-Met/HGF receptor in benign and malignant human prostate tissue. Oncol Rep (2003) 10:1149–53. doi: 10.3892/or.10.5.1149
31. Strohmeyer D, Strauss F, Rössing C, Roberts C, Kaufmann O, Bartsch G, et al. Expression of bFGF, VEGF and c-met and their correlation with microvessel density and progression in prostate carcinoma. Anticancer Res (2004) 24:1797–804.
32. Nishida S, Hirohashi Y, Torigoe T, Nojima M, Inoue R, Kitamura H, et al. Expression of hepatocyte growth factor in prostate cancer may indicate a biochemical recurrence after radical prostatectomy. Anticancer Res (2015) 35:413–8. doi: 10.1016/S1569-9056(15)60513-9
33. Jacobsen F, Ashtiani SN, Tennstedt P, Heinzer H, Simon R, Sauter G, et al. High c-MET expression is frequent but not associated with early PSA recurrence in prostate cancer. Exp Ther Med (2013) 5:102–6. doi: 10.3892/etm.2012.764
34. Inui M, Nishi N, Yasumoto A, Takenaka I, Miyanaka H, Matsumoto K, et al. Enhanced gene expression of transforming growth factor-alpha and c-met in rat urinary bladder cancer. Urol Res (1996) 24:55–60. doi: 10.1007/BF00296735
35. Rosen EM, Joseph A, Jin L, Yao Y, Chau MH, Fuchs A, et al. Urinary and tissue levels of scatter factor in transitional cell carcinoma of bladder. J Urol (1997) 157:72–8. doi: 10.1016/S0022-5347(01)65286-8
36. Yamasaki K, Mukai S, Nagai T, Nakahara K, Fujii M, Terada N, et al. Matriptase-induced phosphorylation of MET is significantly associated with poor prognosis in invasive bladder cancer; an immunohistochemical analysis. Int J Mol Sci (2018) 19:3708. doi: 10.3390/ijms19123708
37. Xu X, Zhang G, He L, Zhu Y. Clinicopathological impacts of c-met overexpression in bladder cancer: evidence from 1,336 cases. Onco Targets Ther (2019) 12:2695–702. doi: 10.2147/OTT.S197540
38. Mukae Y, Miyata Y, Nakamura Y, Araki K, Otsubo A, Yuno T, et al. Pathological roles of c-met in bladder cancer: Association with cyclooxygenase-2, heme oxygenase-1, vascular endothelial growth factor-a and programmed death ligand 1. Oncol Lett (2020) 20:135–44. doi: 10.3892/ol.2020.11540
39. Matsumura A, Kubota T, Taiyoh H, Fujiwara H, Okamoto K, Ichikawa D, et al. HGF regulates VEGF expression via the c-met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int J Oncol (2013) 42:535–42. doi: 10.3892/ijo.2012.1728
40. Yin B, Liu Z, Wang Y, Wang X, Liu W, Yu P, et al. Et al: RON and c-met facilitate metastasis through the ERK signaling pathway in prostate cancer cells. Oncol Rep (2017) 37:3209–18. doi: 10.3892/or.2017.5585
41. Han Y, Luo Y, Wang Y, Chen Y, Li M, Jiang Y. Hepatocyte growth factor increases the invasive potential of PC-3 human prostate cancer cells via an ERK/MAPK and zeb-1 signaling pathway. Oncol Lett (2016) 11:753–9. doi: 10.3892/ol.2015.3943
42. Han Y, Luo Y, Zhao J, Li M, Jiang Y. Overexpression of c-met increases the tumor invasion of human prostate LNCaP cancer cells in vitro and in vivo. Oncol Lett (2014) 8:1618–24. doi: 10.3892/ol.2014.2390
43. van Leenders GJ, Sookhlall R, Teubel WJ, de Ridder CM, Reneman S, Sacchetti A, et al. Activation of c-MET induces a stem-like phenotype in human prostate cancer. PloS One (2011) 6:e26753. doi: 10.1371/journal.pone.0026753
44. Maeda A, Nakashiro K, Hara S, Sasaki T, Miwa Y, Tanji N, et al. Inactivation of AR activates HGF/c-met system in human prostatic carcinoma cells. Biochem Biophys Res Commun (2006) 347:1158–65. doi: 10.1016/j.bbrc.2006.07.040
45. Fujiuchi Y, Nagakawa O, Murakami K, Fuse H, Saiki I. Effect of hepatocyte growth factor on invasion of prostate cancer cell lines. Oncol Rep (2003) 10:1001–6. doi: 10.3892/or.10.4.1001
46. Davies G, Jiang WG, Mason MD. HGF/SF modifies the interaction between its receptor c-met, and the e-cadherin/catenin complex in prostate cancer cells. Int J Mol Med (2001) 7:385–8. doi: 10.3892/ijmm.7.4.385
47. Sim WJ, Iyengar PV, Lama D, Lui SKL, Ng HC, Haviv-Shapira L, et al. C-met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat Commun (2019) 10:4349. doi: 10.1038/s41467-019-12241-2
48. Kummar S, Srivastava AK, Navas T, Cecchi F, Lee YH, Bottaro DP, et al. Combination therapy with pazopanib and tivantinib modulates VEGF and c-MET levels in refractory advanced solid tumors. Invest New Drugs (2021) 39:1577–86. doi: 10.1007/s10637-021-01138-x
49. Martorana A, La Monica G, Lauria A. Quinoline-based molecules targeting c-met, EGF, and VEGF receptors and the proteins involved in related carcinogenic pathways. Molecules (2020) 25:4279. doi: 10.3390/molecules25184279
50. Nakaigawa N, Yao M, Baba M, Kato S, Kishida T, Hattori K, et al. Inactivation of von hippel-lindau gene induces constitutive phosphorylation of MET protein in clear cell renal carcinoma. Cancer Res (2006) 66:3699–705. doi: 10.1158/0008-5472.CAN-05-0617
51. Oh RR, Park JY, Lee JH, Shin MS, Kim HS, Lee SK, et al. Expression of HGF/SF and met protein is associated with genetic alterations of VHL gene in primary renal cell carcinomas. Apmis (2002) 110:229–38. doi: 10.1034/j.1600-0463.2002.100305.x
52. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer (2003) 3:721–32. doi: 10.1038/nrc1187
53. Mori JI, Adachi K, Sakoda Y, Sasaki T, Goto S, Matsumoto H, et al. Anti-tumor efficacy of human anti-c-met CAR-T cells against papillary renal cell carcinoma in an orthotopic model. Cancer Sci (2021) 112:1417–28. doi: 10.1111/cas.14835
54. Sabarwal A, Chakraborty S, Mahanta S, Banerjee S, Balan M, Pal S. A novel combination treatment with honokiol and rapamycin effectively restricts c-Met-Induced growth of renal cancer cells, and also inhibits the expression of tumor cell PD-L1 involved in immune escape. Cancers (Basel) (2020) 12:1782. doi: 10.3390/cancers12071782
55. Golovine K, Makhov P, Naito S, Raiyani H, Tomaszewski J, Mehrazin R, et al. Piperlongumine and its analogs down-regulate expression of c-met in renal cell carcinoma. Cancer Biol Ther (2015) 16:743–9. doi: 10.1080/15384047.2015.1026511
56. Ciamporcero E, Miles KM, Adelaiye R, Ramakrishnan S, Shen L, Ku S, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Mol Cancer Ther (2015) 14:101–10. doi: 10.1158/1535-7163.MCT-14-0094
57. Mohareb RM, Helal MHE, Mohamed SS, Abdallah AEM. New approaches for the synthesis of 2,3,5,6-tetrahydrobenzo[d]thiazole derivatives and their anti-proliferative, c-met enzymatic activity and tyrosine kinases inhibitions. Anticancer Agents Med Chem (2022) 22:2327–39. doi: 10.2174/1871520622666211224102301
58. Wang Z, Dai Z, Wang B, Gao Y, Gao X, Wang L, et al. Targeting c-MET to enhance the efficacy of olaparib in prostate cancer. Onco Targets Ther (2021) 14:4383–9. doi: 10.2147/OTT.S291267
59. Lee C, Whang YM, Campbell P, Mulcrone PL, Elefteriou F, Cho SW, et al. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett (2018) 414:205–13. doi: 10.1016/j.canlet.2017.11.016
60. Wu JC, Wang CT, Hung HC, Wu WJ, Wu DC, Chang MC, et al. Heteronemin is a novel c-Met/STAT3 inhibitor against advanced prostate cancer cells. Prostate (2016) 76:1469–83. doi: 10.1002/pros.23230
61. Eswaraka J, Giddabasappa A, Han G, Lalwani K, Eisele K, Feng Z, et al. Axitinib and crizotinib combination therapy inhibits bone loss in a mouse model of castration resistant prostate cancer. BMC Cancer (2014) 14:742. doi: 10.1186/1471-2407-14-742
62. Yu H, Li X, Sun S, Gao X, Zhou D. C-met inhibitor SU11274 enhances the response of the prostate cancer cell line DU145 to ionizing radiation. Biochem Biophys Res Commun (2012) 427:659–65. doi: 10.1016/j.bbrc.2012.09.117
63. Dai Y, Siemann DW. Constitutively active c-met kinase in PC-3 cells is autocrine-independent and can be blocked by the met kinase inhibitor BMS-777607. BMC Cancer (2012) 12:198. doi: 10.1186/1471-2407-12-198
64. Hwang ST, Um JY, Chinnathambi A, Alharbi SA, Narula AS, Namjoshi OA, et al. Evodiamine mitigates cellular growth and promotes apoptosis by targeting the c-met pathway in prostate cancer cells. Molecules (2020) 25:1320. doi: 10.3390/molecules25061320
65. Shu Y, Xie B, Liang Z, Chen J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol Lett (2018) 15:2252–8. doi: 10.3892/ol.2017.7561
66. Hu HJ, Lin XL, Liu MH, Fan XJ, Zou WW. Curcumin mediates reversion of HGF-induced epithelial-mesenchymal transition via inhibition of c-met expression in DU145 cells. Oncol Lett (2016) 11:1499–505. doi: 10.3892/ol.2015.4063
67. Yu Y, Chen Y, Ding G, Wang M, Wu H, Xu L, et al. A novel rabbit anti-hepatocyte growth factor monoclonal neutralizing antibody inhibits tumor growth in prostate cancer cells and mouse xenografts. Biochem Biophys Res Commun (2015) 464:154–60. doi: 10.1016/j.bbrc.2015.06.107
68. Balan M, Chakraborty S, Flynn E, Zurakowski D, Pal S. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci Rep (2017) 7:5900. doi: 10.1038/s41598-017-05455-1
69. Zhang Z, Li D, Yun H, Tong J, Liu W, Chai K, et al. Opportunities and challenges of targeting c-met in the treatment of digestive tumors. Front Oncol (2022) 12:923260. doi: 10.3389/fonc.2022.923260
70. Zhao S, Cao L, Freeman JW. Knockdown of RON receptor kinase delays but does not prevent tumor progression while enhancing HGF/MET signaling in pancreatic cancer cell lines. Oncogenesis (2013) 2:e76. doi: 10.1038/oncsis.2013.36
71. Iaxx R, Lefort F, Domblides C, Ravaud A, Bernhard JC, Gross-Goupil M. An evaluation of cabozantinib for the treatment of renal cell carcinoma: Focus on patient selection and perspectives. Ther Clin Risk Manag (2022) 18:619–32. doi: 10.2147/TCRM.S251673
72. Procopio G, Prisciandaro M, Iacovelli R, Cortesi E, Fornarini G, Facchini G, et al. Safety and efficacy of cabozantinib in metastatic renal-cell carcinoma: Real-world data from an Italian managed access program. Clin Genitourin Cancer (2018) 16:e945–51. doi: 10.1016/j.clgc.2018.03.014
73. Peltola KJ, Penttilä P, Rautiola J, Joensuu H, Hänninen E, Ristimäki A, et al. Correlation of c-met expression and outcome in patients with renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer (2017) 15:487–94. doi: 10.1016/j.clgc.2017.01.021
74. Tripathi A, Supko JG, Gray KP, Melnick ZJ, Regan MM, Taplin ME, et al. Dual blockade of c-MET and the androgen receptor in metastatic castration-resistant prostate cancer: A phase I study of concurrent enzalutamide and crizotinib. Clin Cancer Res (2020) 26:6122–31. doi: 10.1158/1078-0432.CCR-20-2306
75. Corn PG, Zhang M, Nogueras-Gonzalez GM, Xiao L, Zurita AJ, Subudhi SK, et al. A phase II study of cabozantinib and androgen ablation in patients with hormone-naïve metastatic prostate cancer. Clin Cancer Res (2020) 26:990–9. doi: 10.1158/1078-0432.CCR-19-2389
76. Monk P, Liu G, Stadler WM, Geyer S, Huang Y, Wright J, et al. Phase II randomized, double-blind, placebo-controlled study of tivantinib in men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC). Invest New Drugs (2018) 36:919–26. doi: 10.1007/s10637-018-0630-9
77. Wang Y, Pan X, Li Y, Wang R, Yang Y, Jiang B, et al. CUL4B renders breast cancer cells tamoxifen-resistant via miR-32-5p/ER-α36 axis. J Pathol (2021) 254:185–98. doi: 10.1002/path.5657
78. Zhao M, Qi M, Li X, Hu J, Zhang J, Jiao M, et al. CUL4B/miR-33b/C-MYC axis promotes prostate cancer progression. Prostate (2019) 79:480–8. doi: 10.1002/pros.23754
79. Li Y, Wang X. The role of cullin4B in human cancers. Exp Hematol Oncol (2017) 6:17. doi: 10.1186/s40164-017-0077-2
80. Chen S, Wang Y, Chen L, Xia Y, Cui J, Wang W, et al. CUL4B promotes aggressive phenotypes of renal cell carcinoma via upregulating c-met expression. Int J Biochem Cell Biol (2021) 130:105887. doi: 10.1016/j.biocel.2020.105887
81. Huaqi Y, Caipeng Q, Qiang W, Yiqing D, Xiang D, Xu T, et al. Transcription factor SOX18 promotes clear cell renal cell carcinoma progression and alleviates cabozantinib-mediated inhibitory effects. Mol Cancer Ther (2019) 18:2433–45. doi: 10.1158/1535-7163.MCT-19-0043
82. Yang J, Yang L, Li S, Hu N. HGF/c-met promote renal carcinoma cancer stem cells enrichment through upregulation of cir-CCDC66. Technol Cancer Res Treat (2020) 19:1533033819901114. doi: 10.1177/1533033819901114
83. Du X, Zadoorian A, Lukmantara IE, Qi Y, Brown AJ, Yang H. Oxysterol-binding protein-related protein 5 (ORP5) promotes cell proliferation by activation of mTORC1 signaling. J Biol Chem (2018) 293:3806–18. doi: 10.1074/jbc.RA117.001558
84. Song L, Zhang L, Zhou Y, Shao X, Xu Y, Pei D, et al. ORP5 promotes tumor metastasis via stabilizing c-met in renal cell carcinoma. Cell Death Discov (2022) 8:219. doi: 10.1038/s41420-022-01023-3
85. Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discovery (2014) 4:1310–25. doi: 10.1158/2159-8290.CD-13-1010
86. Yoshioka T, Otero J, Chen Y, Kim YM, Koutcher JA, Satagopan J, et al. β4 integrin signaling induces expansion of prostate tumor progenitors. J Clin Invest (2013) 123:682–99. doi: 10.1172/JCI60720
87. Sankpal UT, Goodison S, Abdelrahim M, Basha R. Targeting Sp1 transcription factors in prostate cancer therapy. Med Chem (2011) 7:518–25. doi: 10.2174/157340611796799203
88. Gao S, Zhao Z, Wu R, Wu L, Tian X, Zhang Z. MiR-1 inhibits prostate cancer PC3 cells proliferation through the Akt/mTOR signaling pathway by binding to c-met. BioMed Pharmacother (2019) 109:1406–10. doi: 10.1016/j.biopha.2018.10.098
89. Qin H, Yang Y, Jiang B, Pan C, Chen W, Diao W, et al. Et al: SOX9 in prostate cancer is upregulated by cancer-associated fibroblasts to promote tumor progression through HGF/c-Met-FRA1 signaling. FEBS J (2021) 288:5406–29. doi: 10.1111/febs.15816
90. Huaqi Y, Caipeng Q, Qiang W, Yiqing D, Tao X. The role of SOX18 in bladder cancer and its underlying mechanism in mediating cellular functions. Life Sci (2019) 232:116614. doi: 10.1016/j.lfs.2019.116614
91. Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, et al. MicroRNA-409-3p inhibits migration and invasion of bladder cancer cells via targeting c-met. Mol Cells (2013) 36:62–8. doi: 10.1007/s10059-013-0044-7
92. Qiu J, Zeng FR, Fang Y, Li J, Xiao SY. Increased miR-323a induces bladder cancer cell apoptosis by suppressing c-met. Kaohsiung J Med Sci (2019) 35:542–9. doi: 10.1002/kjm2.12091
93. Xu X, Zhu Y, Liang Z, Li S, Xu X, Wang X, et al. C-met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis (2016) 7:e2088. doi: 10.1038/cddis.2015.274
94. Matsumoto R, Tsuda M, Wang L, Maishi N, Abe T, Kimura T, et al. Adaptor protein CRK induces epithelial-mesenchymal transition and metastasis of bladder cancer cells through HGF/c-met feedback loop. Cancer Sci (2015) 106:709–17. doi: 10.1111/cas.12662
95. Sakai D, Chung HC, Oh DY, Park SH, Kadowaki S, Kim YH, et al. A non-randomized, open-label, single-arm, phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer. Cancer Chemother Pharmacol (2017) 80:1197–207. doi: 10.1007/s00280-017-3445-z
96. Morley R, Cardenas A, Hawkins P, Suzuki Y, Paton V, Phan SC, et al. Et al: Safety of onartuzumab in patients with solid tumors: Experience to date from the onartuzumab clinical trial program. PloS One (2015) 10:e0139679. doi: 10.1371/journal.pone.0139679
97. Kang CH, Kim Y, Lee DY, Choi SU, Lee HK, Park CH. C-Met-Specific chimeric antigen receptor T cells demonstrate anti-tumor effect in c-met positive gastric cancer. Cancers (Basel) (2021) 13:5738. doi: 10.3390/cancers13225738
98. Chen C, Gu YM, Zhang F, Zhang ZC, Zhang YT, He YD, et al. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-met CAR-T in gastric cancer. Oncoimmunology (2021) 10:1901434. doi: 10.1080/2162402X.2021.1901434
99. Liu B, Liu ZZ, Zhou ML, Lin JW, Chen XM, Li Z, et al. Development of c−MET−specific chimeric antigen receptor−engineered natural killer cells with cytotoxic effects on human liver cancer HepG2 cells. Mol Med Rep (2019) 20:2823–31. doi: 10.3892/mmr.2019.10529
100. Jiang W, Li T, Guo J, Wang J, Jia L, Shi X, et al. Bispecific c-Met/PD-L1 CAR-T cells have enhanced therapeutic effects on hepatocellular carcinoma. Front Oncol (2021) 11:546586. doi: 10.3389/fonc.2021.546586
Keywords: c-Met, HGF, urologic neoplasms, renal cell carcinoma, prostate cancer, bladder cancer, tyrosine kinase inhibitors, CAR-T
Citation: Su P, Zhang M and Kang X (2023) Targeting c-Met in the treatment of urologic neoplasms: Current status and challenges. Front. Oncol. 13:1071030. doi: 10.3389/fonc.2023.1071030
Received: 15 October 2022; Accepted: 23 February 2023;
Published: 07 March 2023.
Edited by:
Massimo Broggini, Mario Negri Institute for Pharmacological Research, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyCopyright © 2023 Su, Zhang and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Kang, aG9uZ2h1aWthbmd4aW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.