
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 18 April 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1070019
This article is part of the Research Topic Biomarkers, Functional Mechanisms, and Therapeutic Potentials in Gastrointestinal Cancers View all 40 articles
Background: The neutrophil to lymphocyte ratio (NLR) is a cost-effective and easily identifiable inflammatory biomarker that has been shown to be closely associated with tumor prognosis and predict survival in patients with multiple malignancies. However, the predictive value of NLR in patients with gastric cancer (GC) treated with immune checkpoint inhibitors (ICIs) has not been fully explored. Therefore, we conducted a meta-analysis to explore the potential of NLR as a predictor of survival in this population.
Methods: We systematically searched the PubMed, Cochrane Library, and EMBASE databases from inception to the present for observational researches on NLR and its relationship with progression or survival in GC patients receiving ICIs. To assess the prognostic significance of NLR on overall survival (OS) or progression-free survival (PFS), we used fixed or random-effect models to derive and combine hazard ratios (HRs) with 95% confidence intervals (CIs). We also examined the relationship between NLR and treatment efficacy by calculating relative risks (RRs) with 95% CIs for objective response rate (ORR) and disease control rate (DCR) in patients with GC receiving ICIs.
Results: Nine studies of 806 patients were eligible. OS and PFS data were obtained from 9 and 5 studies, respectively. In nine studies, NLR was associated with poor survival, the pooled HR was 1.98 (95% CI 1.67- 2.35, p < 0.001), indicating a significant association between high NLR and worse OS. We conducted subgroup analyses based on study characteristics to confirm the robustness of our findings. A relationship between NLR and PFS were reported in five studies with a HR of 1.49 (95% CI 0.99- 2.23, p = 0.056), which was not significantly associated. Pooling four studies that examined the correlation between NLR and ORR/DCR in GC patients, we observed a significant correlation between NLR and ORR (RR = 0.51, p = 0.003), but no significant correlation between NLR and DCR (RR = 0.48, p = 0.111).
Conclusion: In summary, this meta-analysis indicates that increased NLR is significantly linked to worse OS in patients with GC receiving ICIs. In addition, lowering NLR can improve ORR. Thus, NLR can serve as a predictor for prognosis and treatment response in GC patients treated with ICIs. Nevertheless, further high-quality prospective studies are required to verify our findings in the future.
According to projections from the International Agency for Research on Cancer (IARC), by 2020 there would be 1,089,103 new cases of gastric cancer (GC) globally, accounting for 5.6% of all diagnosed cancer cases, with 768,793 deaths attributable to the disease (1). GC remains the fourth most frequent type of cancer, with a high mortality rate (2). Conventional therapies have limited clinical efficacy, and the median overall survival rate for advanced GC is only approximately 8 months (3). Over the past decade, immune checkpoint inhibitors (ICIs) with monoclonal antibodies that suppress programmed cell death protein 1 (PD-1), PD-L1, and cytotoxic T lymphocyte antigen 4 (CTLA-4) has emerged as a promising therapeutic option for various cancers (4). After surgery, chemotherapy, radiation, and targeted therapy, immunotherapy has become an effective treatment technique and one of the breakthroughs in cancer treatment (5). ICIs can effectively interrupt the interaction of immune checkpoints, thereby disrupting tumor cells by activating the host’s immune system. Compared with traditional therapies, immune therapy has demonstrated potent efficacy and tolerable toxicity (6).
Chronic inflammation is linked to various steps of tumorigenesis, including cell transformation, invasion, proliferation, and angiogenesis (7). The systemic inflammatory response plays a significant role in the origin, progression, and metastasis of cancer and has a bearing on the clinical outcomes of cancer patients (8, 9). Tumor cells and associated inflammatory cells release large amounts of cytokines, chemokines, and other inflammatory factors at different stages of tumor development, invasion, and metastasis, promoting tumor cell growth (10). Proven tumor-induced systemic inflammatory responses have been found to be effective prognostic biomarkers in many cancers. For example, a low lymphocyte-to-monocyte ratio (LMR) before therapy is related to advanced clinicopathological characteristics and poor prognosis in individuals with pancreatic cancer (11). In numerous malignancies, including hepatocellular carcinoma and colorectal cancer, the neutrophil-to-lymphocyte ratio (NLR) in peripheral blood has been shown to have a prognostic relationship (12, 13). NLR is a simple and conveniently obtained biomarker that can measure the inflammatory status of the immune system.
ICIs are an important component of current GC treatment, particularly for advanced stage patients. Multiple studies have shown that both ICIs monotherapy and combined strategies with chemotherapy or other therapies significantly improve the survival of advanced GC patients (14–17). In patients who are Epstein-Barr virus (EBV) positive or microsatellite instability (MSI) positive, ICI has shown better response rates (18, 19). In Japan, nivolumab is now licensed for the treatment of patients with advanced stomach cancer who are resistant to standard chemotherapy. However, more inexpensive and convenient markers are needed to predict the efficacy and response to immunotherapy. Currently, there is no meta-analysis examining the predictive significance of NLR and its changes in GC patients treated with ICIs.
Therefore, we included retrospective or prospective cohort studies comparing the difference in prognosis and treatment response between high and low NLR for patients with advanced or locally advanced GC treated with ICIs to investigate the prognostic value of NLR for this group of patients.
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (20). Two independent researchers conducted a search of PubMed, Embase, and Cochrane Library to identify relevant papers on the prognosis of NLR in GC patients treated with immune checkpoint inhibitors, from inception to July 15, 2022. The following search terms were used to investigate the predictive significance of NLR and ICIs in patients with GC: (“neutrophil-lymphocyte ratio” OR “neutrophil-to-lymphocyte ratio” OR NLR) AND (“gastric cancer” OR “gastric adenocarcinoma”) AND (“PD-L1 inhibitor” OR “immune checkpoint inhibitor” OR “programmed death ligand-1 inhibitor” OR “immunotherapy”). The search terms were slightly modified for different databases. In addition, references of selected articles were screened to avoid missing any relevant studies.
Articles that met the following criteria were included (1): studies on patients with histopathologically confirmed advanced or locally advanced gastric cancer, (2) studies reporting long-term survival data, including overall survival (OS) or progression-free survival (PFS), objective response rate (ORR), or disease control rate (DCR), or provided data sufficient to calculate these outcomes, (3) studies published in English, and (4) studies reporting hazard ratios (HRs) or relative risks (RRs) with 95% confidence intervals (CI), either directly or obtained from the original research. The following exclusion criteria were applied: (1) studies reporting on the predictive significance of inflammatory markers without specific information on NLR, (2) studies without sufficient data, (3) conference abstracts, letters, editorials, expert opinions, reviews, and case reports.
Two researchers independently extracted the subsequent data from each article, and inconsistencies were resolved via discussion or consultation with a third researcher: first author, publication year, study country, study design, total number of cases and NLR value, subject age (mean or median), HR for OS and PFS with corresponding 95% CI, and ORR or DCR data.
The Newcastle-Ottawa Scale (NOS) was used to assess study quality. Two researchers independently scored eight questions each, on a scale of 0-9. Studies scoring more than 6 points were considered of high quality (21).
Statistical analysis was performed using Review Manager 5.3 and STATA 14.0 software. RR were used to assess the relationship between NLR and ORR or DCR in patients with gastric cancer. HR and their associated 95% CI were used to evaluate possible associations of NLR with OS and PFS. Heterogeneity between studies was assessed by the Cochran’s Q-test and I2, and appropriate effect models were selected based on them. Random effect models were used when I2 > 50% or p-value < 0.10 (for the Q-test) indicated significant heterogeneity. Otherwise, fixed effect models were used. We evaluated publication bias by observing the symmetry of the funnel plot, as well as by Begg regression and Egger’s linear regression methods, and p-values > 0.05 were deemed as indicative of no publication bias. We also conducted sensitivity analyses to determine the influence of each study on OS and PFS, and eventually, we calculated pooled statistics.
The process of literature selection was illustrated in Figure 1. At the outset, 113 studies were identified through database searches. Upon screening the titles and abstracts, 64 studies were excluded as they failed to meet the inclusion criteria, including duplicate reports, conference abstracts, reviews, and case reports. Five articles were also excluded as full text could not be obtained. Finally, nine observational cohort studies, including eight retrospective and one prospective study, were included in the meta-analysis, totaling 806 patients (22–30). The main features of the included studies were summarized in Table 1. The studies were published since 2014, with most conducted in Japan. The sample sizes ranged from 26 to 185, with seven studies using only Nivolumab and the remaining two studies using multiple ICIs that included Nivolumab. All included articles had NOS scores as shown in Table 2. Overall, the quality of the data was sufficient to explore the prognostic significance of NLR in patients with GC receiving ICIs therapy.
All nine studies reported on the relationship between NLR levels and OS in GC patients treated with ICIs. After conducting a heterogeneity test, the results showed no heterogeneity (P = 0.275 > 0.1, I2 = 18.9% < 50%), indicating that a fixed-effects model was appropriate for the meta-analysis. The pooled HR was 1.98 (95% CI: 1.67 to 2.35, P < 0.001), suggesting that higher NLR values were associated with worse OS in GC patients (Figure 2A).
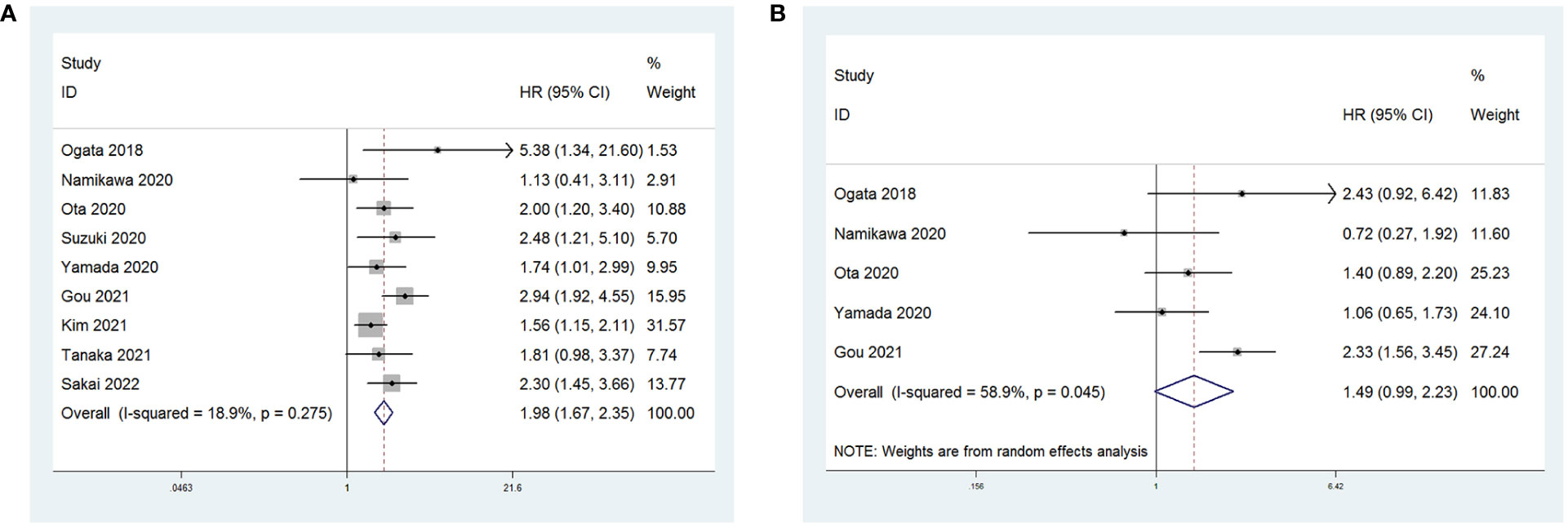
Figure 2 Forest plot for the association between NLR and (A) overall survival (OS) and (B) progression-free survival (PFS).
Five studies reported on the relationship between NLR levels and PFS in GC patients receiving ICIs. A random-effects model was used due to considerable heterogeneity among the included studies (P = 0.045 < 0.1, I2 = 58.9%). The combined HR was 1.49 (95% CI: 0.99 to 2.23, P = 0.056). However, the association between an increase in NLR and PFS in patients receiving ICIs was not statistically significant (Figure 2B).
To assess publication bias, HRs and their associated 95% CIs for OS and PFS were aggregated and evaluated using a funnel plot and the Begg and Egger tests. The funnel plots for both OS and PFS showed good symmetry (Figure 3A for OS and Figure 3B for PFS). The Begg test (p = 1.0 for OS, p = 0.462 for PFS) and Egger test (p = 0.412 for OS, p = 0.597 for PFS) indicated that there was no significant publication bias for OS (Figures 4A, B) and PFS (Figures 4C, D).
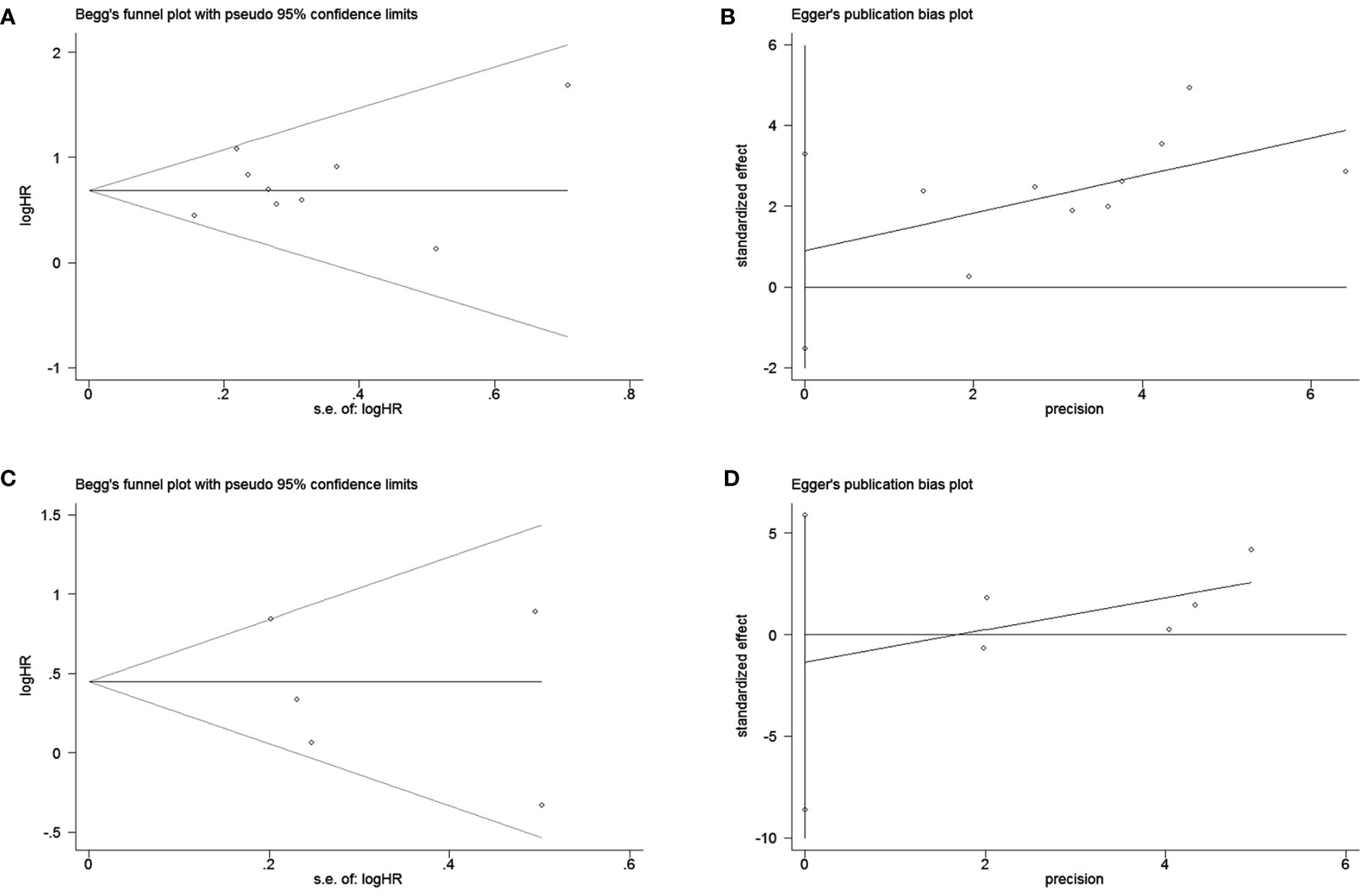
Figure 4 Begg and egger test for overall survival (OS) (A, B); Begg and egger test for progression-free survival (PFS) (C, D).
Sensitivity analyses showed no significant effect of any study on the observed effect size for the association between NLR and OS and PFS. Furthermore, no significant change occurred by removing any of the articles in this study, which indicates that the random-effects model used above was stable. (Figure 5A for OS and Figure 5B for PFS).
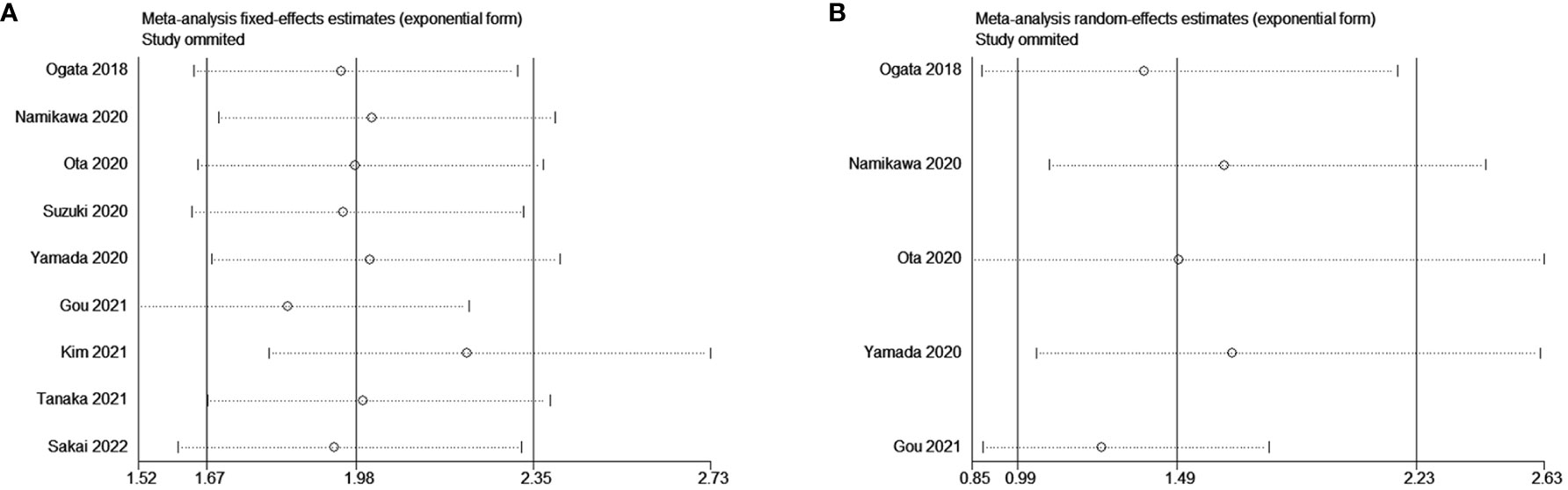
Figure 5 Sensitivity analysis for the association between NLR and (A) overall survival (OS) and (B) progression-free survival (PFS).
To ascertain the origin of OS heterogeneity, we conducted a subgroup analysis. Our findings indicate that high-NLR indicated worse OS among patients, regardless of publication country (China, Japan, or Korea), sample size (≥ 100 or < 100), cut-off value (> 3 or ≤3), or analytical model (multivariate or univariate) (Table 3).
In four of these studies, shown in Figure 6, the relationship between NLR and therapy effectiveness (ORR or DCR) in patients with GC receiving ICIs was investigated. NLR and ORR had a significant relationship (RR = 0.51; p = 0.003). However, NLR and DCR had no significant relationship (RR = 0.48; p = 0.111).
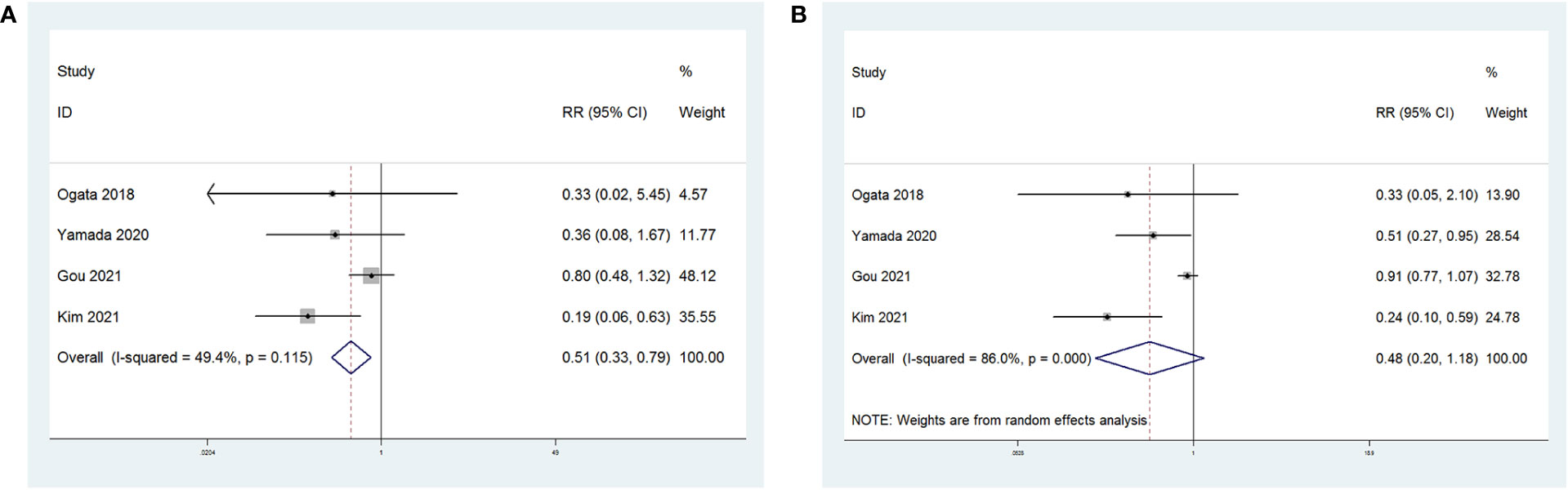
Figure 6 Forest plot for the association between NLR and (A) objective regression rate (ORR) and (B) disease control rate (DCR).
Gastric cancer is the fourth most common cancer worldwide and the second leading cause of cancer-related deaths (31, 32). In addition to genetic factors, the incidence of GC can also be attributed to various pathogenic infections, resulting in high morbidity and mortality (33, 34). Despite the progress made in multimodal therapy for GC, recurrence of the disease is still common (35). Furthermore, owing to the absence of early diagnostic markers, GC is frequently diagnosed in its advanced stages, significantly diminishing the likelihood of survival, dependable biomarkers are critically necessary to facilitate early detection and survival forecasting.
Thus, many studies have investigated related inflammatory factors and tumor prognosis to use biological indicators to predict survival outcomes after a certain treatment and provide timely intervention to improve patient survival rates (36, 37). Among the inflammatory factors, NLR has been widely studied by scholars because of its low cost and easy availability. Some previous studies have researched whether NLR can predict the prognosis of GC. Han et al. demonstrated that pre-operative NLR is an independent prognostic factor in patients with GC and were associated with worse survival (38). Hirahara et al. found that the outcome of treatment and prognosis in patients with advanced gastric cancer can be predicted by the combination of NLR and platelet-to-lymphocyte ratio (PLR) (39). Wang et al. demonstrated that pretreatment NLR could be a prognostic factor for survival in locally advanced gastric cancer receiving adjuvant chemotherapy after D2 resection (40).
While several studies have evaluated the predictive value of NLR in patients, to our knowledge, no meta-analysis has yet comprehensively examined whether NLR predicts survival outcomes in GC patients receiving ICIs. To address this gap in knowledge, we conducted a meta-analysis of data from nine relevant trials involving 806 patients from three countries to determine whether survival outcomes could be predicted by NLR values in gastric cancer patients treated with ICIs. Our analysis found that higher NLR values were associated with a lower survival rate and a significant correlation existed between high NLR and poor OS, with the combined HR of NLR and OS being 1.98. In addition, reducing NLR increased ORR, while high NLR played a negative role in ORR in patients treated with ICIs. However, the relationship between NLR and PFS was not statistically significant (p = 0.056).
It is important to note that our study has several limitations. First, all studies included in this analysis were conducted on gastric cancer patients in Asian countries, with seven of the studies coming from Japan. Although subgroup analyses did not reveal significant differences between the studies in the three countries, the regionalization of the studies suggests caution should be applied in extrapolating the results to Western countries due to potential differences in biology. Second, the majority of the included studies were retrospective, which would have resulted in a reduced level of evidence. Finally, some studies with relatively small sample sizes may have introduced selection bias.
In conclusion, our meta-analysis suggests that a higher NLR is significantly correlated with worse OS and adverse ORR in GC patients treated with ICIs. NLR may serve as a promising biomarker for predicting prognosis and treatment response. However, more large-scale, multicenter, high-quality prospective trials are required to validate our findings.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
XX, CQ and YX contributed to the research concept and design. CQ and HY retrieved and filtered articles, CQ and SZ extracted data. CQ and SZ analyzed the data. SZ, CQ, XX, and YX explained the data. CQ and SZ drafted manuscript. SZ, XX and YX contributed to critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grant 2021KY628 (Medicine and Health Care in Zhejiang Province Science and Technology Plan).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer (2021) 1876(2):188615. doi: 10.1016/j.bbcan.2021.188615
4. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657
5. Hogner A, Moehler M. Immunotherapy in gastric cancer. Curr Oncol (2022) 29(3):1559–74. doi: 10.3390/curroncol29030131
6. Jin X, Liu Z, Yang D, Yin K, Chang X. Recent progress and future perspectives of immunotherapy in advanced gastric cancer. Front Immunol (2022) 13:948647. doi: 10.3389/fimmu.2022.948647
7. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med (2019) 18(3):121–6. doi: 10.4103/aam.aam_56_18
8. Stoiber D, Assinger A. Platelet-leukocyte interplay in cancer development and progression. Cells (2020) 9(4):855. doi: 10.3390/cells9040855
9. Ohno Y. Role of systemic inflammatory response markers in urological malignancy. Int J Urol (2019) 26(1):31–47. doi: 10.1111/iju.13801
10. Aguilar-Cazares D, Chavez-Dominguez R, Marroquin-Mucino M, Perez-Medina M, Benito-Lopez JJ, Camarena A, et al. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front Endocrinol (Lausanne) (2022) 13:929572. doi: 10.3389/fendo.2022.929572
11. Hu RJ, Ma JY, Hu G. Lymphocyte-to-Monocyte ratio in pancreatic cancer: prognostic significance and meta-analysis. Clin Chim Acta (2018) 481:142–6. doi: 10.1016/j.cca.2018.03.008
12. Yang XG, Huang YC, Wang CH, Sun YY, Huang Z, Xu GH. Predictive value of preoperative neutrophil-to-Lymphocyte ratio in patients with hepatocellular carcinoma after transarterial chemoembolization combined with radiofrequency ablation. Cancer Invest (2022) 40(6):494–504. doi: 10.1080/07357907.2022.2065508
13. Tominaga T, Nonaka T, Oyama S, Takamura Y, Hashimoto S, Shiraishi T, et al. Efficacy of neutrophil-to-Lymphocyte ratio for cancer-specific survival in elderly patients with localized colon cancer: a single center propensity score-matched analysis. Clin Exp Gastroenterol (2023) 16:1–9. doi: 10.2147/CEG.S385207
14. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (Ono-4538-12, attraction-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5
15. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical keynote-059 trial. JAMA Oncol (2018) 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013
16. Vafaei S, Zekiy AO, Khanamir RA, Zaman BA, Ghayourvahdat A, Azimizonuzi H, et al. Combination therapy with immune checkpoint inhibitors (Icis); a new frontier. Cancer Cell Int (2022) 22(1):2. doi: 10.1186/s12935-021-02407-8
17. Varayathu H, Sarathy V, Thomas BE, Mufti SS, Naik R. Combination strategies to augment immune check point inhibitors efficacy - implications for translational research. Front Oncol (2021) 11:559161. doi: 10.3389/fonc.2021.559161
18. Pietrantonio F, Randon G, Di Bartolomeo M, Luciani A, Chao J, Smyth EC, et al. Predictive role of microsatellite instability for pd-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open (2021) 6(1):100036. doi: 10.1016/j.esmoop.2020.100036
19. Lima A, Sousa H, Medeiros R, Nobre A, Machado M. Pd-L1 expression in ebv associated gastric cancer: a systematic review and meta-analysis. Discov Oncol (2022) 13(1):19. doi: 10.1007/s12672-022-00479-0
20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
21. Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol (2020) 120:94–103. doi: 10.1016/j.jclinepi.2019.12.013
22. Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, et al. Neutrophil-to-Lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget (2018) 9(77):34520–7. doi: 10.18632/oncotarget.26145
23. Yamada T, Hayashi T, Inokuchi Y, Hayashi K, Watanabe H, Komori K, et al. Impact of the neutrophil-to-Lymphocyte ratio on the survival of patients with gastric cancer treated with nivolumab monotherapy. Target Oncol (2020) 15(3):317–25. doi: 10.1007/s11523-020-00716-y
24. Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, et al. Changes in the neutrophil-to-Lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol (2020) 85(2):265–72. doi: 10.1007/s00280-019-04023-w
25. Gou M, Qu T, Wang Z, Yan H, Si Y, Zhang Y, et al. Neutrophil-to-Lymphocyte ratio (Nlr) predicts pd-1 inhibitor survival in patients with metastatic gastric cancer. J Immunol Res (2021) 2021:2549295. doi: 10.1155/2021/2549295
26. Tanaka K, Tanabe H, Sato H, Ishikawa C, Goto M, Yanagida N, et al. Prognostic factors to predict the survival in patients with advanced gastric cancer who receive later-line nivolumab monotherapy-the asahikawa gastric cancer cohort study (Agcc). Cancer Med (2022) 11(2):406–16. doi: 10.1002/cam4.4461
27. Kim N, Yu JI, Lim DH, Lee J, Kim ST, Hong JY, et al. Prognostic impact of sarcopenia and radiotherapy in patients with advanced gastric cancer treated with anti-Pd-1 antibody. Front Immunol (2021) 12:701668. doi: 10.3389/fimmu.2021.701668
28. Sakai D, Omori T, Fumita S, Fujita J, Kawabata R, Matsuyama J, et al. Real-world effectiveness of third- or later-line treatment in Japanese patients with Her2-positive, unresectable, recurrent or metastatic gastric cancer: a retrospective observational study. Int J Clin Oncol (2022) 27(7):1154–63. doi: 10.1007/s10147-022-02162-4
29. Suzuki H, Yamada T, Sugaya A, Ueyama S, Yamamoto Y, Moriwaki T, et al. Retrospective analysis for the efficacy and safety of nivolumab in advanced gastric cancer patients according to ascites burden. Int J Clin Oncol (2021) 26(2):370–7. doi: 10.1007/s10147-020-01810-x
30. Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today (2020) 50(11):1486–95. doi: 10.1007/s00595-020-02048-w
31. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65(1):5–29. doi: 10.3322/caac.21254
32. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (2016) 388(10060):2654–64. doi: 10.1016/S0140-6736(16)30354-3
33. O'Connor A, O'Morain CA, Ford AC. Population screening and treatment of helicobacter pylori infection. Nat Rev Gastroenterol Hepatol (2017) 14(4):230–40. doi: 10.1038/nrgastro.2016.195
34. O'Connor A, O'Morain C. Helicobacter pylori infection in Europe: current perspectives. Expert Rev Gastroenterol Hepatol (2013) 7(6):541–8. doi: 10.1586/17474124.2013.824707
35. Cann C, Ciombor KK. Systemic therapy for gastric cancer: perioperative strategies and beyond. J Surg Oncol (2022) 125(7):1151–60. doi: 10.1002/jso.26834
36. Winther-Larsen A, Aggerholm-Pedersen N, Sandfeld-Paulsen B. Inflammation scores as prognostic biomarkers in small cell lung cancer: a systematic review and meta-analysis. Syst Rev (2021) 10(1):40. doi: 10.1186/s13643-021-01585-w
37. Hayama T, Ozawa T, Tsukamoto M, Fukushima Y, Shimada R, Nozawa K, et al. Predicting overall survival using preoperative nutritional and inflammation status for colorectal cancer. In Vivo (2022) 36(1):450–7. doi: 10.21873/invivo.12724
38. Han QY, Zhang X, Zhang JG, Zhou WJ, Chen QY, Chen YY, et al. Pre-operative neutrophil-to-Lymphocyte ratio is an independent prognostic factor in patients with gastric cancer. Int Immunopharmacol (2022) 113(Pt A):109371. doi: 10.1016/j.intimp.2022.109371
39. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer (2019) 19(1):672. doi: 10.1186/s12885-019-5903-y
Keywords: gastric cancer, meta-analysis, neutrophil to lymphocyte ratio (NLR), biomarker, immune checkpoint inhibitors (ICIs)
Citation: Zhang S, Qiu C, Yu H, Xu Y and Xu X (2023) Prognostic value of neutrophil to lymphocyte ratio in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Oncol. 13:1070019. doi: 10.3389/fonc.2023.1070019
Received: 14 October 2022; Accepted: 03 April 2023;
Published: 18 April 2023.
Edited by:
Kui Zhang, The University of Chicago, United StatesReviewed by:
Zhanjun Guo, Fourth Hospital of Hebei Medical University, ChinaCopyright © 2023 Zhang, Qiu, Yu, Xu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Xu, Y2RjeHV5YW5AeWVhaC5uZXQ=; Xiaoming Xu, WHV4bTEwMDJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.