
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 09 May 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1066427
This article is part of the Research TopicTranslational Research for Better Diagnosis and Treatment of Endometrial CancerView all 18 articles
 Francesco Alessandrino1*
Francesco Alessandrino1* Nicole Goncalves2
Nicole Goncalves2 Sarah Wishnek Metalonis3
Sarah Wishnek Metalonis3 Cibele Luna1
Cibele Luna1 Matthew M. Mason2
Matthew M. Mason2 Jiangnan Lyu3
Jiangnan Lyu3 Marilyn Huang4
Marilyn Huang4Background: Uterine serous carcinoma (USC) is an aggressive subtype of endometrial carcinoma which has been increasing at alarming rates, particularly among Asian, Hispanic and Black women. USC has not been well characterized in terms of mutational status, pattern of metastases and survival.
Objective: To investigate the association between sites of recurrence and metastases of USC, mutational status, race, and overall survival (OS).
Methods: This single-center retrospective study evaluated patients with biopsy-proven USC that underwent genomic testing between January 2015 and July 2021. Association between genomic profile and sites of metastases or recurrence was performed using χ2 or Fisher’s exact test. Survival curves for ethnicity and race, mutations, sites of metastasis/recurrence were estimated using the Kaplan-Meier method and compared with log-rank test. Cox proportional hazard regression models were used to examine the association between OS with age, race, ethnicity, mutational status, and sites of metastasis/recurrence. Statistical analyses were performed using SAS Software Version 9.4.
Results: The study included 67 women (mean age 65.8 years, range 44-82) with 52 non-Hispanic women (78%) and 33 Black women (49%). The most common mutation was TP53 (55/58 women, 95%). The peritoneum was the most common site of metastasis (29/33, 88%) and recurrence (8/27, 30%). PR expression was more common in women with nodal metastases (p=0.02) and non-Hispanic women (p=0.01). ERBB2 alterations were more common in women with vaginal cuff recurrence (p=0.02), while PIK3CA mutation was more common in women with liver metastases (p=0.048). ARID1A mutation and presence of recurrence or metastases to the liver were associated with lower OS (Hazard Ratio (HR): 31.87; 95%CI: 3.21, 316.9; p<0.001 and HR: 5.66; 95%CI: 1.2, 26.79; p=0.01, respectively). In the bivariable Cox model, the presence of metastasis/recurrence to the liver and/or the peritoneum were both independent significant predictors of OS (HR: 9.8; 95%CI: 1.85-52.7; p=0.007 and HR: 2.7; 95%CI: 1.02-7.1; p=0.04, respectively).
Conclusions: TP53 is often mutated in USC, which most commonly metastasize and recur in the peritoneum. OS was shorter in women with ARID1A mutations and with metastasis/recurrence to the liver. The presence of metastasis/recurrence to liver and/or peritoneum were independently associated with shorter OS.
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States (1, 2) and is increasing at an alarming rate. Based on clinical and histological variables, EC have been divided into two types. Type I tumors, or endometrioid EC, represent approximately 85% of cases, and are usually low-grade with favorable outcomes. Type II tumors, or non-endometrioid EC, typically arise in postmenopausal patients, and are frequently of high-grade thus contributing to a relatively poor prognosis (3, 4). Type II EC is largely comprised of uterine serous carcinomas (USC), which represents less than 10% of all endometrial cancers, yet accounts for more than half of deaths attributed to EC (4–6). While type I are estrogen-dependent, the role of estrogens in type II EC is less clear, although studies have shown that the pathogenesis of type II EC may at least partially depend on the level of estrogens, differently from what was previously believed (7).
Pathological reporting of EC has limitations due to poor reproducibility of tumor typing and to accurately identify patients at risk for recurrence or metastatic disease (8). The identification of the underlying molecular background of EC has resulted in the development of new molecular based classifications of EC, namely The Cancer Genome Atlas (TCGA), which stratifies EC into four distinct clusters with prognostic significance: polymerase ε (POLE) ultramutated, copy-number low, and microsatellite instability (MSI) hypermutated, and copy-number high (9, 10).
The genomic characterization of USC is distinct from endometrioid EC, with USC exhibiting a high frequency of genetic aberrations involving TP53, FBXW7, PPP2R1A and ARID1A.USC are mostly classified as copy-number high according to TCGA (10, 11). In contrast, endometrioid EC typically demonstrates a higher frequency of microsatellite instability, frequent activation of WNT/CTNNB1 signaling, and mutations of POLE, KRAS, and CTNNB1. Endometrioid EC are mostly classified as copy-number low according to TCGA (9–11).
Although EC is typically diagnosed early and associated with a high 5-year survival of 80-90%, USCs have higher recurrence rates and carry a poor prognosis with significantly lower survival (4, 12). USCs have high risk of recurrence (up to 80%) and are associated with an increased incidence of extrauterine disease on presentation (5, 12–15). Hence, the ability to reduce mortality of EC largely depends on developing tailored therapy and management for recurrent and advanced USC (16).
Patterns of recurrence and metastasis may provide prognostic information for EC. For example, patients with single-site local or nodal recurrence of EC have been associated with improved survival compared to those with pelvic recurrence or distant metastasis (17, 18), while patients with peritoneal carcinomatosis or multiple sites of recurrence have significantly worse post-relapse survival rates (18).
Comprehensive genomic analysis of USC provides a clearer understanding of the molecular pathways involved in oncogenesis (19). Knowledge of the somatic mutations may help predict patterns of metastases and recurrence in various cancers, including urothelial cancer, where patients with TP53 mutations are at a higher risk of lymph nodes metastases (12, 20–22).
The primary objective of this study is to investigate the association of somatic mutations occurring in a diverse patient population diagnosed with USC to the patterns of metastases, recurrence, overall survival (OS) and recurrence free survival (RFS).
This institutional review board (IRB)-approved, Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective study was performed at a single institution on consecutive patients diagnosed with USC who underwent somatic molecular testing between January 2015 and July 2021. Patients were excluded for 1) non-serous or mixed histology, or 2) data on recurrence or metastasis was not available in the electronic medical records (EMR).
Medical records in the EMR were reviewed to extract clinicopathologic data of the patients by (C.L.) (M.M.) (N.G.) (M.H.). The collected information included demographics; date and stage at diagnosis; histopathology and genomic testing results; initial treatment (systemic therapy, radiotherapy, or surgery); date and sites of metastatic and recurrent disease; treatment modality for recurrent disease; progression date; and date of death or last follow-up.
Cross-sectional images (Computed tomography (CT) of the chest, abdomen, and pelvis; FDG- Positron emission tomography (PET)/CT, and Magnetic resonance Imaging (MRI)) and reports were reviewed initially by a cancer imaging fellow (C.L.) and separately by a fellowship-trained cancer imaging radiologist with 5 years of experience (F.A.). Discrepancies were resolved by consensus between the two radiologists. The date of the first imaging showing metastasis or recurrence was recorded. Reports and images, when available, were analyzed to record sites of metastatic or recurrent disease, including pelvis, lung, liver, pleura, lymph nodes, peritoneum, bones, brain, and muscle for all patients. Lymph node involvement was determined by short-axis diameter ≥1.0 cm. Any new lesion identified at follow-up imaging after curative treatment was defined as recurrence based on pathologic confirmation whenever possible, or if it showed unequivocal growth on follow-up imaging, defined as >20% increase in the sum of diameters compared to baseline or nadir (with an absolute increase of at least 5 mm) according to RECIST 1.1 (23). Any extrauterine lesion present before curative treatment was performed was considered metastatic, based on pathologic confirmation whenever possible, or if it showed unequivocal growth on follow-up imaging exams according to RECIST 1.1 (23).
Molecular profiling was performed with immunohistochemistry (IHC) and next-generation sequencing (NGS) on either the primary tissue at diagnosis or from tissue obtained at recurrence. A board-certified gynecologic oncologist (M.H.) assigned a TCGA cluster based on the mutational profile (10). IHC and molecular testing was performed using either Caris Life Sciences (Caris Life Sciences, Phoenix, AZ, USA) or FoundationOne CDx (Foundation medicine inc., Cambridge, MA, USA) genomic profiling assays.
The IHC assays were performed using FDA-approved companion diagnostic or FDA-cleared tests consistent with the manufacturer’s instructions: ALK (Ventana ALK (D5F3) CDxAssay; ER (confirm anti-estrogen receptor ER, SP1, Ventana; FOLR1 (Ventana FOLR1-2.1 RxDx, Ventana; PR (confirm anti-progesterone PR (1E2), Ventana); HER2/neu (pathway anti-HER-2/neu (4B5), Ventana; PD-L1 22c3. pharmDx, Dako; Mismatch repair (MMR) proteins (MLH1, MSH2, MSH6, and PMS2; Ventana MMR RxDx Panel, Ventana). For ER/PR, staining intensity was classified as 0, 1+, 2+, 3+. The intensity thresholds for a positive test for ER were >/= 2+ with >/= 75% or >/=3+ with >/=50% of cells stained, for PR were >/=1+ and >/= 10% of cells stained.
Regarding molecular testing, details of specific NGS testing are available from Caris Life Sciences or FoundationOne CDx (24, 25). Among the genes covered, we focused our mutational analysis on the 12 genes most frequently mutated in this cohort.
Categorical variables were summarized using frequencies and percentages and the continuous variable, age, was summarized using mean and standard deviation. Different genetic aberrations involving the same gene were grouped under that gene. The association of mutational status with the location of metastases or recurrence was assessed using Chi-square tests or Fisher’s exact tests when 20% or more of the frequency cells had expected counts less than 5.
OS was measured from the date of initial diagnosis of USC to death from any cause, censored at the date of the last follow-up in alive patients. Stratified Kaplan-Meier survival curves were generated, and univariable Cox proportional hazards regression was used to examine the association of ethnicity, race, mutational status, sites of recurrence or metastases with OS. OS curves were computed in all patients. Statistical significance was determined through the log-rank test. A p-value <0.05 was considered statistically significant.
Multivariable Cox proportional hazard regression with backward stepwise selection was performed with OS as response. The variables that had log-rank p-values<0.20 and sample size > 50 were used as the predictors in the model. Likelihood Ratio tests were used to test model predictability and test significance for additional individual and/or collection of variables. Hazards ratios and 95% CIs were calculated in each model to determine association and significant predictors of survival. Statistical computations were performed, and output was generated using SAS Software Version 9.4 (The SAS Institute, Cary, NC).
From a total of 134 patients with EC who underwent genomic profiling, 67 patients with USC were included in the final study analysis. Fifty-eight patients with endometrioid histology, 5 patients with clear cell histology, 3 patients with mixed histology, and 1 patient with no data on recurrence or metastasis were excluded. Characteristics of the included subjects are summarized in Table 1. The average age was 65.8 years (Standard Deviation=8.3 years). Fifty-two patients were non-Hispanic (78%), 33 patients were Black (49%), 32 were White (48%), 1 was Asian (1.5%), and 1 patient self-reported multi-racial (1.5%). Thirty-three patients had evidence of metastases at time of diagnosis (49.2%), 27 patients had evidence of recurrence during follow-up (40.3%), and 7 patients did not show evidence of recurrence or metastasis during follow-up (10.5%). Median follow up was 21 months (range 20 days-10.2 years).
Molecular profiling was performed on primary tissue at diagnosis on 45/67 patients (67%), on tissue obtained at recurrence in 20/67 patients (30%). In 2/67 patients (3%), molecular profiling was not performed. The most frequently mutated genes identified on NGS were TP53 (55/58 patients; 94.8%), followed by PIK3CA (14/61 patients; 22.9%), and ERBB2 (12/37 patients; 32%) (Table 1). A high tumor mutational burden (TMB) was identified in 14/51 patients (27.5%). On IHC, PTEN was expressed in 51/56 patients (91.1%), ER was expressed in 43/65 patients (66.2%), PR in 24/65 patients (36.9%). None of the patients expressed MMR gene mutation or microsatellite instability (MSI) (Table 1).
Fifty-three patients were classified as TCGA copy-number high (79%), six patients as TCGA copy-number low (9%), and 8 patients could not be classified (12%) based on mutational profile.
Thirty-three patients (49.2%) had evidence of metastases at time of diagnosis (7 on biopsy, 18 on CT, 1 on MRI and 7 on PET/CT), 27 patients (40.3%) had evidence of recurrence during follow-up (3 on biopsy, 13 on CT, and 11 on PET/CT), and 7 patients (10.5%) did not show evidence of metastasis or recurrence during follow-up (Table 1).
The sites of metastasis and recurrence are summarized in Figure 1. The most common sites of metastasis were the peritoneum (29/33, 88%), followed by lymph nodes (18/33, 55%) and in the pelvis (14/33, 42%). The most common site of recurrence was lymph nodes (18/27, 67%), followed by peritoneal implants (8/27, 30%) and vaginal cuff (6/27, 22%).
PR was expressed on IHC more commonly in patients with lymph node metastases (p=0.02), and in non-Hispanic patients (p=0.01). ERBB2 was more commonly mutated in patients with vaginal cuff recurrence (p=0.02), while PIK3CA was more commonly mutated in patients with liver metastases (p=0.048). Peritoneal metastases were significantly more common in Non-Hispanic patients (p=0.04). No other associations between mutations, ethnicity, sites of metastatic and recurrent disease were identified.
ARID1A mutation was associated with lower OS (mean OS: 10.2 months vs 60.9 months; Hazard Ratio (HR): 31.87; (95% CI: 3.21, 316.9)). Presence of recurrence or metastases to the liver was associated with lower OS (mean OS: 48.8 months vs 58.4 months; HR: 5.66; (95% CI: 1.2, 26.79)) (Figures 2, 3).
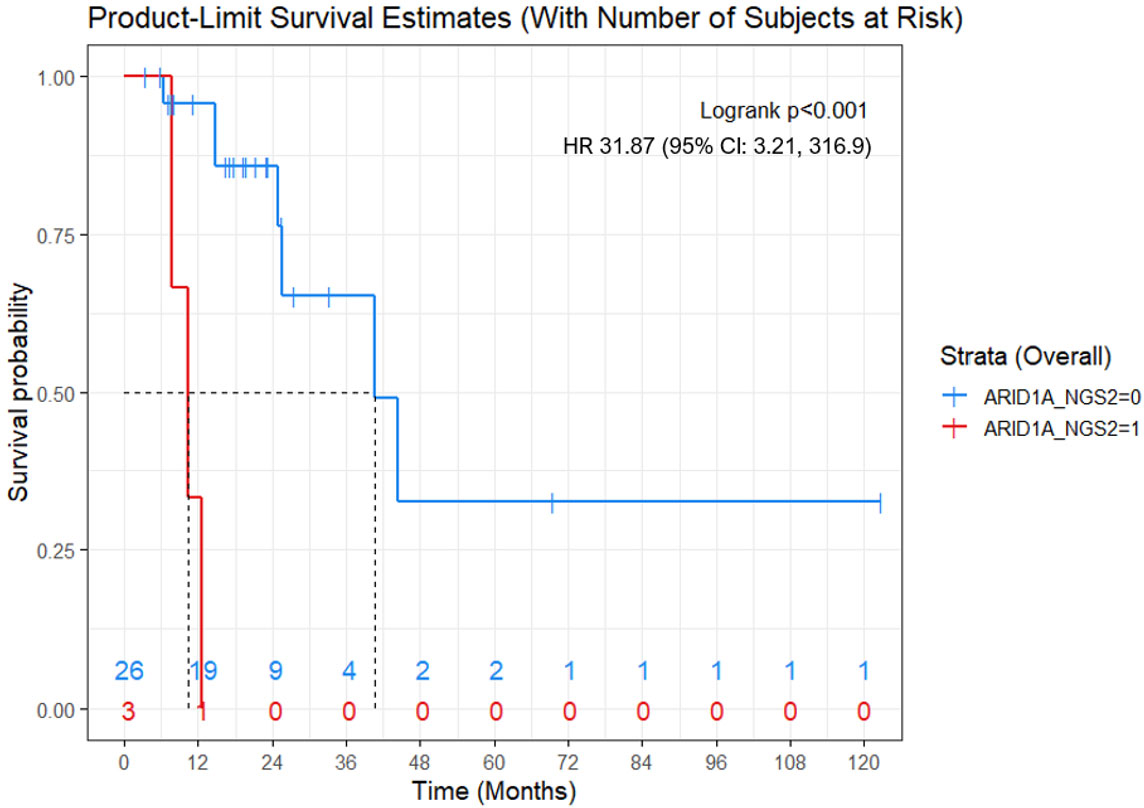
Figure 2 Kaplan-Meier OS curve for women with ARID1A mutations (red) versus women without ARID1A mutation (blue). ARID1A mutation was associated with shorter OS (mean, 10.2 months vs 60.9 months; Cox proportional hazard model - Hazard Ratio: 31.87; 95% CI: 3.21, 316.9; p<0.001). Numbers at the bottom of the graph refers to the number of women with ARID1A mutations (red) versus women without ARID1A mutation (blue) at risk at a determinate timepoint.
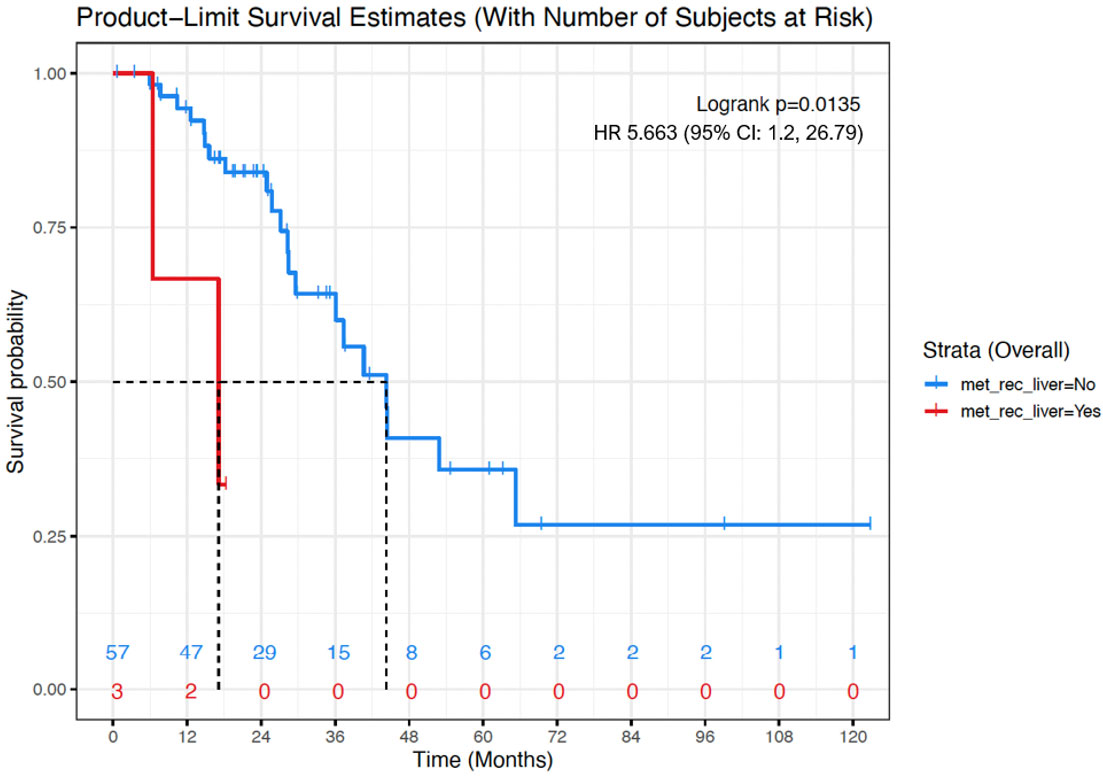
Figure 3 Kaplan-Meier OS curve for women with evidence of liver metastases/recurrence (red) versus women with no evidence or liver metastases or recurrence (blue). Presence of liver metastases/recurrence was associated with shorter OS (mean, 48.8 months vs 58.4 months; Cox proportional hazard model - Hazard Ratio: 5.66; 95% CI: 1.2, 26.79; p=0.01). Numbers at the bottom of the graph refers to the number of women with evidence of liver metastases/recurrence (red) versus women with no evidence or liver metastases or recurrence (blue) at risk at a determinate timepoint.
A Cox proportional hazards model was selected using a backward stepwise selection process starting with a fully adjusted model. The fully adjusted model included AKT1,2,3 mutations, presence of recurrence or metastases to the liver, peritoneum, lymph nodes, lungs and a race dummy variable defined as Black or not Black. The predicters in the full model all had log rank p-values<0.20 except for race (p-value=0.59). The Full model was reduced 4 times based on the Wald test statistics for individual variables and the Likelihood Ratio tests (-2 Log Likelihood) resulting in a bivariable Cox model with a total of 60 cases. The final model included presence of recurrence or metastases to the liver, and presence of recurrence or metastases to the peritoneum as predictors (HR: 9.8; 95% CI: 1.85, 52.7; p=0.007 and HR: 2.7; 95% CI: 1.02, 7.1; p=0.04, respectively) (Table 2).
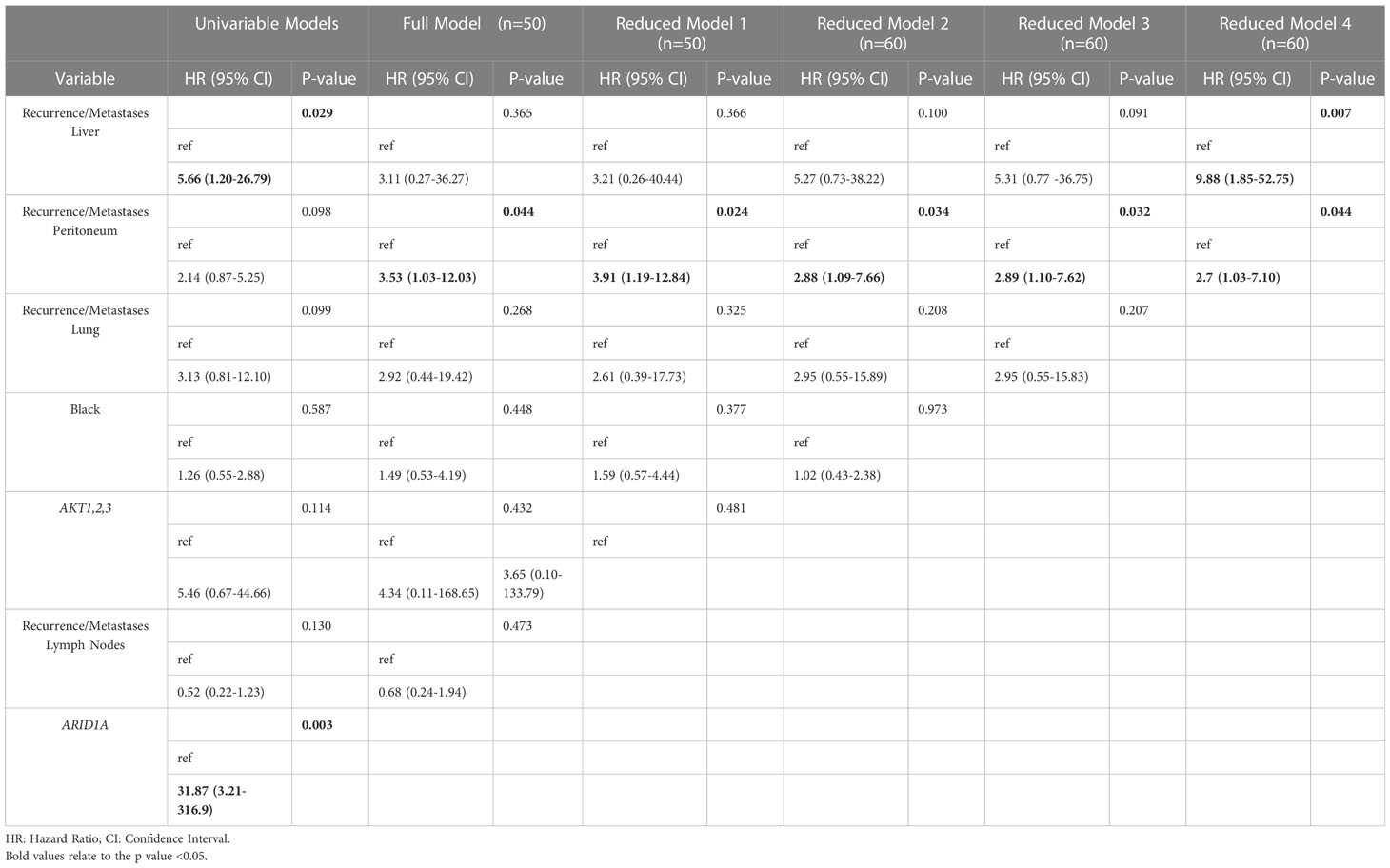
Table 2 Univariable and multivariable Cox proportional hazard regression analysis of survival in patients with uterine serous carcinoma.
With advances in genomic testing and improved understanding of cancer biology, an increasing number of cancer patients undergo mutational testing to identify potential targeted treatment options. As we gain more insight into the impact of the mutational make-up of cancer on prognosis, understanding its associations to known clinicopathologic factors is crucial. Our study cohort was largely comprised of minority Hispanic and Black women that were poorly represented in prior TCGA data and in the PanCancer Atlas (26). Furthermore, 40% of our patients had evidence of recurrence during follow-up while 49% of patients had metastatic disease at diagnosis. This is significantly higher than the TCGA cohort, where 32% presented with recurrence and only 11% had metastatic disease at diagnosis (10).
We showed that TP53, PIK3CA and ERBB2 are often mutated in USC, consistent with TGCA data. In our cohort, 79% of cases were classified as copy-number high, consistent with TCGA data, where 77% of cases with serous histology were classified as such (10). In a recent study by Watanabe et al. on 100 EC cases, TP53 mutations were associated with non-endometrioid histology (12). Several studies have demonstrated that human epidermal growth factor receptor 2 (HER2) a tyrosine kinase increasing cell proliferation encoded by ERBB2, is frequently overexpressed in USC (27–29). HER2 overexpression has also been associated with advanced-stage disease, and poorer survival outcomes in USC (25). Few ARID1A gene mutations in were identified in our cohort, consistent with the findings of the PanCancer Atlas, where ARID1A gene mutations were identified in 13% of cases (26, 30). Mutations in ARID1A in EC have been associated with promoting tumor invasion and metastasis, which may shed light on the significant mortality among women diagnosed with USCs (30).
In our cohort, ER and PTEN are expressed in the majority of USC on IHC. A study on 1054 women with EC showed that ER, though more common in type I EC, was expressed in 72% of type II EC (31). A study on 56 high grade EC showed that majority of USC were positive for PTEN on IHC (32). A study on 221 women with EC, showed that loss of PTEN expression was associated with endometrioid histology and favorable survival (33).
In our study, FBXW7 was mutated in 15% of USC. No association between FBXW7 mutations, ethnicity and race was identified in our study. FBXW7 was mutated in 29 out of 109 (27%) USC included in the PanCancer Atlas. In a study on 66 USC, FBXW7 mutations were identified in 18.2% of cases (11). On the study by Watanabe et al., FBWX7 mutations were associated with late-stage, vascular invasion, and lymph node metastasis (12).
In our cohort, USC most commonly metastasized to lymph nodes, peritoneum and pelvic organs, while recurrences were mostly nodal, peritoneal and at the vaginal cuff. These findings are similar to two prior studies: a study on 841 EC, which showed that the most common sites of recurrence of USC was the abdomen, including ascites, followed by the vaginal cuff and a study on 50 USC, which showed that the most common sites of metastases of USC were the lymph nodes, pelvic organs and peritoneum/omentum (13, 14).
In our study, PR expression was associated with non-Hispanic ethnicity and nodal metastases. A study on 99 endometrioid EC showed that nodal metastases correlated with negative PR status on IHC (34). This discrepancy may be related to the different histologies. The association between non-Hispanic ethnicity and PR expression is unclear.
We found that ERBB2 alterations were more common in patients with vaginal cuff recurrence and PIK3CA mutation was more common in patients with liver metastases. ERBB2 amplifications are associated with higher stage, chemoresistance, and lower survival, especially in Black patients (35, 36). Trastuzumab, a HER2/neu receptor inhibitor monoclonal antibody, demonstrated increased progression free survival when added to carboplatin paclitaxel, in patients with USC and ERBB2 overexpression (37). This highlights the significance of somatic tumor testing either at diagnosis or at recurrence to aid in prioritizing treatment options.
Women with presence of recurrence or metastases to the liver and ARID1A mutations had lower OS. A study on 86 recurrent EC, showed that recurrence to the liver was associated with lower OS, with an HR of 10 [3.72–26.81 95% CI] (14). A metanalysis on the prognostic significance of ARID1A in endometrium-related gynecological cancers showed that negative ARID1A expression predicted shorter progression free survival (38). ARID1A mutations affects multiple pathways, and may mediate resistance to platinum chemotherapy, possibly explaining the lower OS observed in our study (39). Therapies targeting the pathways affected by ARID1A mutations, such as poly(ADP-ribose) polymerase inhibitors, immune checkpoint inhibitors and mTOR inhibitors, have shown activity in preclinical models and in patient, and could be implemented in patients with ARID1A-mutated USC (40, 41).
Finally, on multivariable Cox proportional hazards regression analysis, the presence of recurrence or metastases to the liver and/or the peritoneum were independently associated with shorter OS regardless of their mutational status, race, ethnicity or other sites of recurrence or metastases. Various studies attempted to build predictive survival models for USC, including a study by Chen et al. on 110 women with USC which showed that a combination of mutated genes, a 4-gene signature, was predictive of OS (42). Differently from our study, their model did not include sites of metastases or survival as potential predictors. Knowledge of the associations between survival data and sites of recurrence or metastases of USC is valuable: it allows for a more accurate risk stratification and helps the oncologists and radiologists to potentially formulate more appropriate follow-up strategies (43).
Some limitations of this study should be noted. This is a retrospective study performed at a single institution with inherent selection bias. The relatively small patient cohort and the widely variable follow-up period may have limited the power in detection of some of the associations between mutations and patterns of metastases and recurrence, as well as the prognostic values of mutations. Furthermore, our sample included only patients with USC selected from a tertiary cancer center and may not be representative of patients treated at a community hospital. We grouped different genetic aberrations involving the same gene under that gene to facilitate analysis until additional data are available. This methodology has also been previously utilized and reported (11, 21).
One of the significant strengths of this study is the cohort of Hispanic and Black women diagnosed with USC with genomic testing. Our study showed that mutational status of USC had implications on pattern of metastases and survival, and that sites of recurrence and metastases influence survival. These findings should be assessed in larger studies, to confirm our findings and may be valuable for future trial design.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Miami. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MH: Supervision, ideation, and draft editing. SW: statistical analysis and draft editing. FA: ideation, statistical analysis, draft writing, and editing. CL: data collection and draft editing. NG: data collection MM: data collection and draft editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. US Surveillance, Epidemiology, and End Results (SEER). Cancer stat facts - uterine cancer (2022). Available at: https://www.seer.cancer.gov/statfacts/html/corp.html (Accessed January 5, 2022).
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: A Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet (2016) 387(10023):1094–108. doi: 10.1016/S0140-6736(15)00130-0
4. Lu KH, Broaddus RR. Endometrial cancer. New Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
5. del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: a review of the literature. Gynecol Oncol (2012) 127(3):651–61. doi: 10.1016/j.ygyno.2012.09.012
6. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA: A Cancer J Clin (2019) 69(4):258–79. doi: 10.3322/caac.21561
7. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol: Off J Am Soc Clin Oncol (2013) 31(20):2607–18. doi: 10.1200/JCO.2012.48.2596
8. Luna C, Balcacer P, Castillo P, Huang M, Alessandrino F. Endometrial cancer from early to advanced-stage disease: an update for radiologists. Abdominal Radiol (New York) (2021) 46(11):5325–36. doi: 10.1007/s00261-021-03220-7
9. Arciuolo D, Travaglino A, Raffone A, Raimondo D, Santoro A, Russo D, et al. TCGA molecular prognostic groups of endometrial carcinoma: current knowledge and future perspectives. Int J Mol Sci (2022) 23(19):11684. doi: 10.3390/ijms231911684
10. Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12113
11. Kuhn E, Wu RC, Guan B, Wu G, Zhang J, Wang Y, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst (2012) 104(19):1503–13. doi: 10.1093/jnci/djs345
12. Watanabe T, Nanamiya H, Kojima M, Nomura S, Furukawa S, Soeda S, et al. Clinical relevance of oncogenic driver mutations identified in endometrial carcinoma. Trans Oncol (2021) 14(3):101010. doi: 10.1016/j.tranon.2021.101010
13. Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol (1994) 54(3):264–8. doi: 10.1006/gyno.1994.1208
14. Rosenberg P, Blom R, Högberg T, Simonsen E. Death rate and recurrence pattern among 841 clinical stage I endometrial cancer patients with special reference to uterine papillary serous carcinoma. Gynecol Oncol (1993) 51(3):311–5. doi: 10.1006/gyno.1993.1296
15. Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol (2007) 62(1):28–34. doi: 10.1016/j.crad.2006.06.015
16. Sorbe B, Juresta C, Ahlin C. Natural history of recurrences in endometrial carcinoma. Oncol Lett (2014) 8(4):1800–6. doi: 10.3892/ol.2014.2362
17. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. post operative radiation therapy in endometrial carcinoma. Lancet (2000) 355(9213):1404–11. doi: 10.1016/S0140-6736(00)02139-5
18. Legge F, Restaino S, Leone L, Carone V, Ronsini C, Di Fiore GLM, et al. Clinical outcome of recurrent endometrial cancer: analysis of post-relapse survival by pattern of recurrence and secondary treatment. Int J Gynecol Cancer (2019) 30(2):193–200. doi: 10.1136/ijgc-2019-000822
19. Ferriss JS, Erickson BK, Shih IM, Fader AN. Uterine serous carcinoma: key advances and novel treatment approaches. Int J Gynecol Cancer (2021) 31(8):1165–74. doi: 10.1136/ijgc-2021-002753
20. Uygur MC, Yaman I, Kutluay L, Altuğ U, Erol D. The relation between p53 overexpression and lymph node metastases in clinical stage t2 and t3a transitional cell bladder carcinoma. J Exp Clin Cancer Res (1999) 18(3):391–5.
21. Park DS, Lee YT, Lee JM. Prediction of lymph node metastasis based on p53 and nm23-H1 expression in muscle invasive grade III transitional cell carcinoma of bladder. Adv Exp Med Biol (2003) 539(Pt A):67–85. doi: 10.1007/978-1-4419-8889-8_6
22. Alessandrino F, Williams K, Nassar AH, Silverman SG, Sonpavde G, Shinagare AB. Muscle-invasive urothelial cancer: association of mutational status with metastatic pattern and survival. Radiology (2020) 295(3):572–80. doi: 10.1148/radiol.2020191770
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
24. U.S. Food and Drug Administration. FoundationOne CDx– P170019/S014 technical information. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019S006C.pdf (Accessed December 20, 2022).
25. Caris Life Sciences. Comprehensive molecular profiling. Available at: https://www.carislifesciences.com/products-and-services/molecular-profiling/ (Accessed December 20, 2022).
26. Uterine corpus endometrial carcinoma (TCGA, PanCancer atlas). Available at: https://www.cbioportal.org/study/summary?id=ucec_tcga_pan_can_atlas_2018 (Accessed July 30, 2022).
27. Singh P, Smith CL, Cheetham G, Dodd TJ, Davy MLJ. Serous carcinoma of the uterus–determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: its significance and clinical correlation. Int J Gynecol Cancer (2008) 18(6):1344–51. doi: 10.1111/j.1525-1438.2007.01181.x
28. Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol (2004) 22(15):3126–32. doi: 10.1200/JCO.2004.11.154
29. Villella JA, Cohen S, Smith DH, Hibshoosh H, Hershman D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int J Gynecol Cancer (2006) 16(5):1897–902. doi: 10.1111/j.1525-1438.2006.00664.x
30. Suryo Rahmanto Y, Shen W, Shi X, Chen X, Yu Y, Yu ZC, et al. Inactivation of Arid1a in the endometrium is associated with endometrioid tumorigenesis through transcriptional reprogramming. Nat Commun (2020) 11(1):2717. doi: 10.1038/s41467-020-16416-0
31. Shen F, Gao Y, Ding J, Chen Q. Is the positivity of estrogen receptor or progesterone receptor different between type 1 and type 2 endometrial cancer? Oncotarget (2016) 8(1):506–11. doi: 10.18632/oncotarget.13471
32. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol (2013) 37(6):874–81. doi: 10.1097/PAS.0b013e31827f576a
33. Akiyama-Abe A, Minaguchi T, Nakamura Y, Michikami H, Shikama A, Nakao S, et al. Loss of PTEN expression is an independent predictor of favourable survival in endometrial carcinomas. Br J Cancer (2013) 109(6):1703–10. doi: 10.1038/bjc.2013.455
34. Iwai. K, Fukuda. K, Hachisuga. T, Mori. M, Uchiyama. M, Iwasaka. T, et al. Prognostic significance of progesterone receptor immunohistochemistry for lymph node metastases in endometrial carcinoma. Gynecol Oncol (1999) 72(3):351–9. doi: 10.1006/gyno.1998.5286
35. Santin AD, Bellone S, Siegel ER, Palmieri M, Thomas M, Cannon MJ, et al. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/neu): a major prognostic indicator in uterine serous papillary cancer. Am J Obstet Gynecol (2005) 192(3):813–8. doi: 10.1016/j.ajog.2004.10.605
36. Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, et al. Amplification of c-erbB2 oncogene. Cancer (2005) 104(7):1391–7. doi: 10.1002/cncr.21308
37. Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-Paclitaxel-Trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol: Off J Am Soc Clin Oncol (2018) 36(20):2044–51. doi: 10.1200/JCO.2017.76.5966
38. Liu G, Xu P, Fu Z, Hua X, Liu X, Li W, et al. Prognostic and clinicopathological significance of ARID1A in endometrium-related gynecological cancers: a meta-analysis. J Cell Biochem (2017) 118(12):4517–25. doi: 10.1002/jcb.26109
39. Mullen J, Kato S, Sicklick JK, Kurzrock R. Targeting ARID1A mutations in cancer. Cancer Treat Rev (2021) 100:102287. doi: 10.1016/j.ctrv.2021.102287
40. Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci (2013) 14(9):18824–49. doi: 10.3390/ijms140918824
41. Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer (2020) 8(1):e000438. doi: 10.1136/jitc-2019-000438
42. Chen H, Li L, Qin P, Xiong H, Chen R, Zhang M, et al. A 4-gene signature predicts prognosis of uterine serous carcinoma. BMC Cancer (2021) 21(1). doi: 10.1186/s12885-021-07834-4
Keywords: uterine serous cancer, next generation sequencing, endometrial cancer, somatic mutations, recurrence, metastases
Citation: Alessandrino F, Goncalves N, Metalonis SW, Luna C, Mason MM, Lyu J and Huang M (2023) Uterine serous carcinoma: assessing association between genomics and patterns of metastasis. Front. Oncol. 13:1066427. doi: 10.3389/fonc.2023.1066427
Received: 10 October 2022; Accepted: 20 April 2023;
Published: 09 May 2023.
Edited by:
Tea Lanisnik Rizner, University of Ljubljana, SloveniaReviewed by:
Emanuele Perrone, (IRCCS), ItalyCopyright © 2023 Alessandrino, Goncalves, Metalonis, Luna, Mason, Lyu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Alessandrino, ZmFsZXNzYW5kcmlub0BtZWQubWlhbWkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.