
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 31 March 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1064487
This article is part of the Research Topic Implementation of Genomic and Epigenomic Innovation in Clinical Cancer Diagnostics View all 8 articles
Background: Rapid profiling of the EGFR mutations is crucial to help clinicians choose the optimal treatment for patients with advanced/metastatic Non-Small Cell Lung Cancer (NSCLC). Unfortunately, current diagnostic techniques, including ARMS-PCR and NGS, generally require several days to deliver final results. This diagnostic delay may lead to treatment delays for patients who are worsening rapidly.
Methods: This study introduced the ultra-rapid Idylla™ system for rapid, sensitive and specific identification of the EGFR mutations among Chinese NSCLC patients. Idylla™ EGFR Assay, an integrated cartridge running on the Idylla™ system, which can detect 51 EGFR mutations directly from Formalin-Fixed, Paraffin-Embedded (FFPE) samples within 2.5 hours, was used in this study. The sensitivity and specificity of the Idylla™ system were evaluated in comparison with ARMS-PCR or NGS using 95 clinical samples.
Results: The Idylla™ system achieved a sensitivity of 97.6%, a specificity of 100%, and an overall concordance of 97.9% for 95 retrospective samples. When compared to ARMS-PCR, the Idylla™ system demonstrated high accuracy with an overall agreement of 97.1% (34/35), a sensitivity of 95.2% (20/21) (95% CI, 76.2% - 99.9%), and an estimated specificity of 100% (12/12) (95% CI, 76.8% - 100%) for 35 prospective samples.
Conclusions: This Idylla system provides a rapid, accurate and simple approach for screening EGFR mutations, which can guide Tyrosine Kinase Inhibitors (TKI) treatment for NSCLC patients in a timely manner.
Over the past decade, the discovery of oncogenic driver mutations has greatly facilitated the development of targeted drugs. The Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) remain the mainstay of targeted therapy for Non-Small Cell Lung Cancer (NSCLC) because EGFR mutations occur in 50% of patients with lung adenocarcinomas in the Asian population (1–3). Exon 19 deletions and the L858R point mutation in exon 21 account for 85% of all EGFR mutations, and some less common alterations including L861Q, G719X, and S768I make up the remaining 10% (4–7). These mutations can affect patients’ response to TKIs such as erlotinib, gefitinib, afatinib, osimertinib, or dacomitinib (8–13). It should be noted that patients with exon 20 insertions are not sensitive to the first or second generation of EGFR TKIs (14, 15). Similarly, approximately 60% patients treated with erolotinib, gefitinib, or afatinib eventually develop resistance due to the appearance of the T790M point mutation (16, 17). Therefore, the NCCN guidelines recommended EGFR mutation status be determined in NSCLC patients prior to initiating TKI therapy (18). Immunotherapy has been incorporated into the first- and second-line treatment strategies for NSCLC. However, NSCLC patients with EGFR mutations show a poor response to anti-PD-1/PD-L1 treatment, which suggests that EGFR is involved in regulating the tumour microenvironment and inhibiting immunotherapy (19). Immunotherapy is not currently recommended by NCCN guidelines for patients with EGFR-mutant NSCLC.
The Amplification Refractory Mutation System-Polymerase Chain Reaction (ARMS-PCR) and Next-Generation Sequencing (NGS) are widely used in Chinese patients to determine EGFR mutations from Formalin-Fixed Paraffin-Embedded (FFPE) tissue samples (20, 21). Both of these approaches suffer from labor intensive procedures, including DNA isolation and library preparation. These processes require considerable staff training in laboratory skills, data interpretation and reporting. Moreover, NGS testing is often outsourced to independent clinical laboratories due to the highly complex bioinformatics analyses. Generally, the typical turnaround time in clinical practices is three to five days for ARMS-PCR and more than two weeks for NGS. This inevitably leads to the significant delays in the delivery of result. Therefore, these approaches are not suitable for acutely deteriorating patients who can barely afford any treatment delays (22). Identification of EGFR mutation status within 24 hours could reduce the time between diagnosis and optimal treatment. It is urgent need to develop an ultra-rapid automated platform to test for EGFR mutation in the field, which would allow for faster diagnosis and treatment for patients with EGFR-mutant NSCLC. The Idylla™ EGFR automated real-time PCR assay provides an integrated solution by combining DNA extraction, thermal cycling and fluorescence detection. This approach streamlines the process and reduces the overall turnaround time for EGFR mutation testing. According to protocol, 51 EGFR mutations could be detected simultaneously from FFPE samples in 2.5 hours with <10 minutes of hands-on time. The automated workflow and compact size make it easy to deploy in any situation, which is particularly important for lower tier hospitals, that lack of the platform for high complexity molecular testing. The Idylla™ EGFR system has been extensively validated in Caucasian patients with lung adenocarcinoma patients and received European Community (CE)-marked approval in 2017 (23–26). In this study, we focus on validating of the performance of the Idylla™ EGFR system in Chinese NSCLC patients. We also optimize and discuss the molecular diagnosis of advanced NSCLC by combining the rapid EGFR characterization by Idylla™ assay with genomic profiling by NGS.
A total of 96 restrospective FFPE samples were collected and assessed using the Idylla™ EGFR Assay at the Department of Pathology, Cancer Hospital, Chinese Academy of Medical Sciences (CAMS). Samples with a histological diagnosis of NSCLC and a tumor cell content of ≥10% were deemed eligible for inclusion in the study. EGFR mutational status of these samples were assessed between April 2017 and August 2018 using either ARMS-PCR or NGS (Illumina platform). Mutations detected by NGS that were beyond the scope of the Idylla™ EGFR Assay were not included in the analysis. In case of discordance, samples were retested by the Idylla™ assay, and if the results remained inconsistent, ARMS-PCR and NGS (Ion Torrent platform) were repeated for confirmation. Idylla™ tests were also repeated for the discordant cases by increasing tissue input or manual enrichment of tumor cell content via macro-dissection. Another 35 prospective samples were collected and screened for EGFR mutations with the Idylla™ system afterwards. The results were then compared with those obtained using ARMS-PCR and NGS between June 2020 and September 2020. The study was approved by the Institute Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The methods were carried out in accordance with approved guidelines. The written informed consent was obtained from all patients. This study followed the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical guidelines.
The Idylla™ EGFR Assay is an integrated cartridge with all sample processing buffers and PCR reagents pre-loaded. This assay is specifically designed to detect 51 mutations in exons 18–21 of the EGFR gene (Supplementary Table 1). For the 96 retrospective samples, a single 8 μm FFPE tissue section containing ≥10% neoplastic cells was added in the cartridge for each test, following the instruction for use of the Idylla™ EGFR Assay. For the 35 prospective samples, a single 8 μm FFPE tissue section was used for surgical samples, while three 8 μm FFPE sections were used separately for biopsy samples. In cases where the neoplastic cell content was lower than 10%, tissue sections were macro-dissected to enrich the sample. Tissue section was sandwiched between two layers of wetted filter paper and loaded directly into the cartridge. The cartridge was then inserted into the Idylla™ system. The system completes sample processing and real-time PCR automatically and reports result of mutations directly. In the Idylla™ EGFR Assay, the control is a wild-type EGFR sequence included in the assay cartridge, and the sample of interest is the DNA extracted from the patient’s tissue sample. The difference between the Cq (Cycle of Quantification) values of the control and the sample of interest (ΔCq) is used to determine the presence or absence of a mutation. If the ΔCq falls within the reference range, a mutant signal is considered valid and a mutation is identified, otherwise, the sample is considered EGFR mutation-negative.
DNA was isolated using the QIAamp DNA FFPE Tissue Kit (Qiagen, CA, USA) and was quantified by the Qubit double-stranded DNA (dsDNA) HS assay kit on the Qubit 3.0 fluorometer (Thermo Fisher Scientific, NH, USA) following the manufacturer’s instructions.
ARMS-PCR was carried out using the National Medical Product Administration (NMPA) approved Human EGFR Mutation Detection Kit (ACCB, Beijing, China). The kit is capable of detecting 44 mutations in EGFR exon 18-21, and some of the target mutations differ from those detected by Idylla™ EGFR Assay (Supplementary Table 1). In accordance with the Kit’s Instruction for Use, 15 ng of genomic DNA from each sample was used for each test. The PCR reaction was performed with the following parameters: initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 1 min. Mutation subtypes were determined by analyzing the threshold count (Ct) values of the samples, where mutations were identified when the Ct value was ≤36, following the manufacturer’s instructions. If the Ct value was between 36 and 39, the test was repeated, and if the result remained within this range, the sample was considered a possible EGFR mutant.
DNA-based hybrid capture sequencing was carried out following the protocol as previously reported (27). Genomic DNA was first fragmented using Covaris M220, and then subjected to end repair and adaptor ligation. DNA fragments ranging from 200 and 400 bp were isolated using beads hybridized with a capture-probe panel targeting all exons in 56 cancer-related genes. Subsequently, sequencing libraries were generated after PCR amplification. Indexed libraries were pooled together and then sequenced on a NextSeq N550 platform (Illumina, San Diego, USA). Sequencing data were analyzed by GATK 3.2.
Discrepancies in mutation status were resolved using the Ion Ampliseq Colon and Lung Cancer Panel on the Ion Torrent PGM platform (Thermo Fisher Scientific, NH, USA) following the protocol as previously described (28). Briefly, 10 ng of genomic DNA from each sample was PCR-amplified and then ligated to different barcodes to generate a library. The libraries were mixed and clonally amplified onto the IonSpheres (ISPs) for template preparation, and sequencing was carried out on a 318 chip using the Torrent Suite Software. Mutations were annotated through Torrent Variant Caller and viewed with Integrative Genomics Viewer. Mutations with a coverage depth of ≥1000 and a minor allele frequency (MAF) ≥5% were considered positive using the Torrent Variant Caller.
A total of 96 archival FFPE lung adenocarcinoma samples were included in this study for EGFR mutation analysis using the Idylla™ EGFR Assay as shown in Figure 1. Among the 96 samples, 79 were previously tested with AMRS-PCR, 11 with NGS, and 7 with both ARMS-PCR and NGS. Initially, 98.96% (95/96) of the sample were successfully tested by Idylla™ EGFR.(One result was invalid due to instrument error). Therefore, a total of 95 samples were included in the concordance analysis. Patients in the 95 samples had a median age of 61 years (interquartile range 37 to 81), with 62.1% (59/95) being female (Supplementary Table 2).
Idylla™ detected mutations in 82 out of the 95 samples as presented in Supplementary Table 3 and Figure 2. Of these mutated samples, 66 had a single mutation while 16 had two mutations, resulting in a total of 98 mutations being discovered by Idylla™. Among the 66 samples with a single mutation, there were 21 with Exon 19 deletion, 25 with L858R, and 4 with Exon 20 insertion mutations, accounting for 75.8% (50/66) of all mutations. For the remaining 16 samples, 10 had L861Q, 3 had G719X, and 3 had S768I mutations. The 16 samples with two mutations comprised 6 with G719X and S768I, 6 with L858R and T790M, 2 with L858R and S768I, 1 with G719X and L861Q, and 1 with T790M and S768I mutations. The frequency of different types of mutations, from high to low, is as follows: L858R at 33.7% (33/98), Exon 19 del at 21.4% (21/97), L861Q at 11.2% (11/98), G719X and S768I both at 10.2% (10/98), T790M at 6.1% (6/98), and Exon 20 ins at 4.1% (4/98). Among these 95 samples, only two exhibited inconsistent results between the Idylla™ EGFR and the reference method. In one sample (Sample No. 91#), Idylla failed to detect any mutation, whereas the reference method identified G719X + S768I mutations (NGS also detected E709K in this sample, which falls outside the detection range of the Idylla™). In the other sample (Sample No. 88#), Idylla detected only the L858R mutation, while the reference method revealed the presence of both L858R and L861Q mutations. Among all 101 mutations detected by the reference method, Idylla™ missed a total of 3 mutations in 2 samples. The Idylla™ system achieved a sensitivity of 97.6%, a specificity of 100%, and an overall concordance of 97.9%.
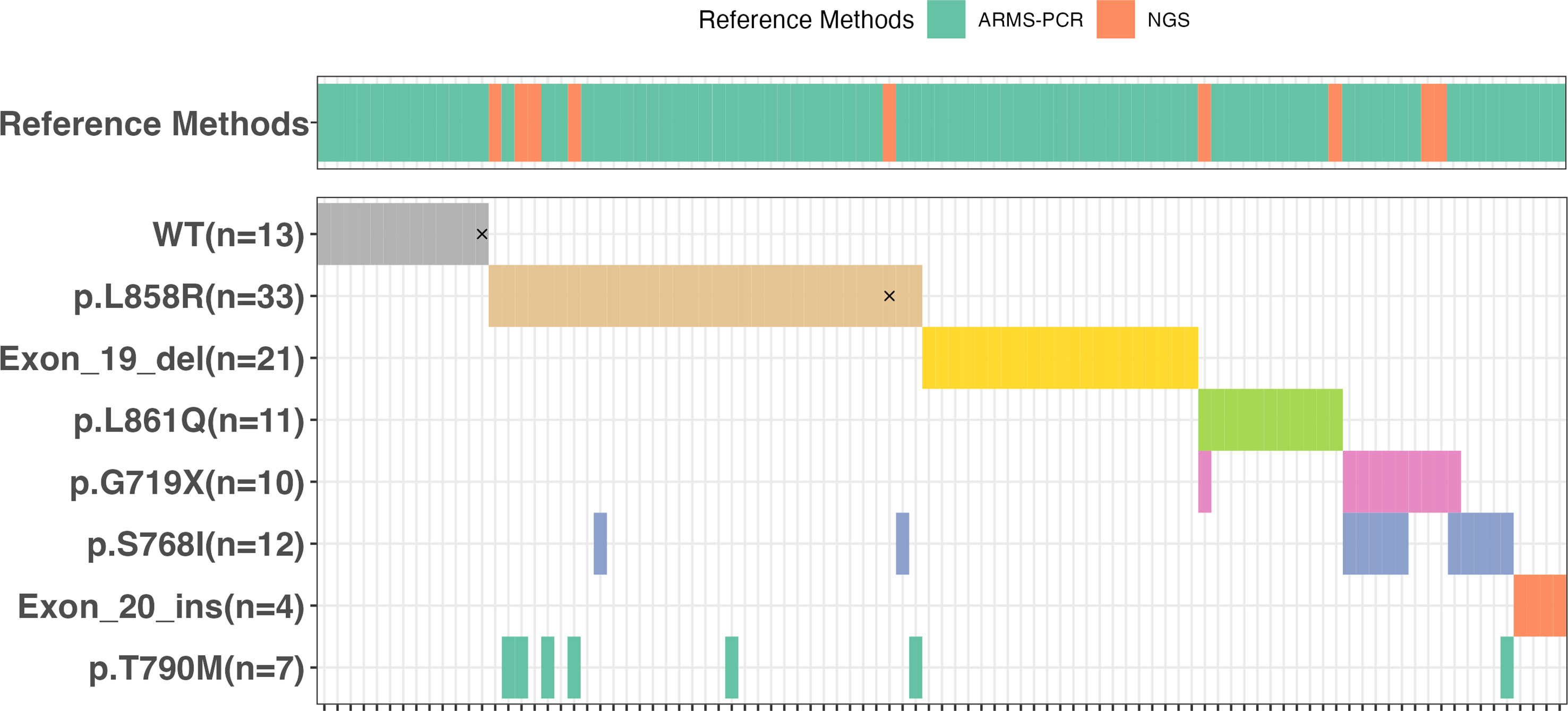
Figure 2 The oncoplot corresponding to EGFR mutations identified by Idylla™ assay in the 95 samples that were subjected to the reference methods ARMS-PCR or NGS. The discordances were marked by “×”.
The two discordant cases (91# and 88#) were re-examined using the Idylla™ assay, followed by Ion Torrent NGS and ARMS-PCR (Table 1) The H&E photos of samples 88 and 91 were displayed in Figure 3C, F. Sample 91# was wild-type when re-tested with Idylla™ using only one FFPE section. However, when the number of FFPE sections was increased to two, S768I mutation was detected. Further increasing the number of sections to three or four, both G719X and S768I mutations were identified as shown in Table 2. In the second Idylla™ test of Sample 88#, the same result was obtained as in the first test, with only L858R mutation detected. Subsequently, the neoplastic cell content of the FFPE sections was enriched through macro-dissection, and 2-4 sections were re-tested with Idylla™. However, the result remained the same for 88#, with only the L858R mutation being detected. As illustrated in Figure 3D, the median Cq value for sample 91# was 27.04, indicating that the amount of amplifiable DNA in the cartridge was less than 1.584 ng according to the manufacturer’s instructions. For sample 88#, the median Cq value for the EGFR control was 24.73 (Figure 3A), which suggested that the amount of amplifiable DNA in the cartridge was between 7.92 ng and 15.84 ng. However, most of the other samples had a median Cq value of less than 20.00 for the EGFR control, which corresponded to more than 396 ng of amplifiable DNA in the cartridge. The Ct values for samples 88# and 91# by ARMS-PCR were 29.21 and 29.87, respectively, which were close to the upper limit of detection of the assay. Moreover, the Ct values of samples 88# and 91# by ARMS-PCR were 37.04 for L858R (Figure 3B), 37.28 for G719X, and 36.62 for S768I (Figure 3E), suggesting that the discordance was caused by low DNA input or low mutational allele frequency.

Table 1 Discordant cases between the Idylla™ EGFR assay and reference methods in the 95 retrospective samples.
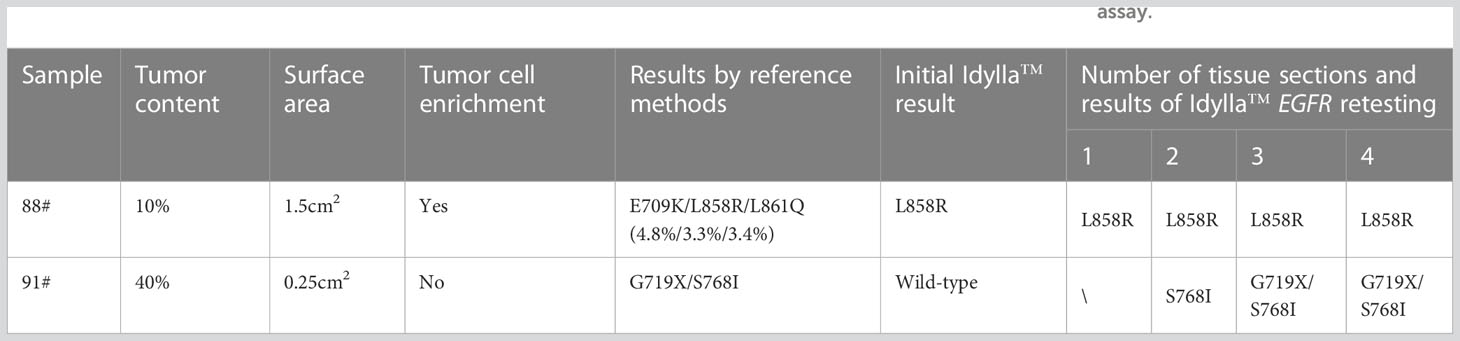
Table 2 The impact of tumor cell enrichment and increasing sample input on the performance of Idylla™ EGFR assay.
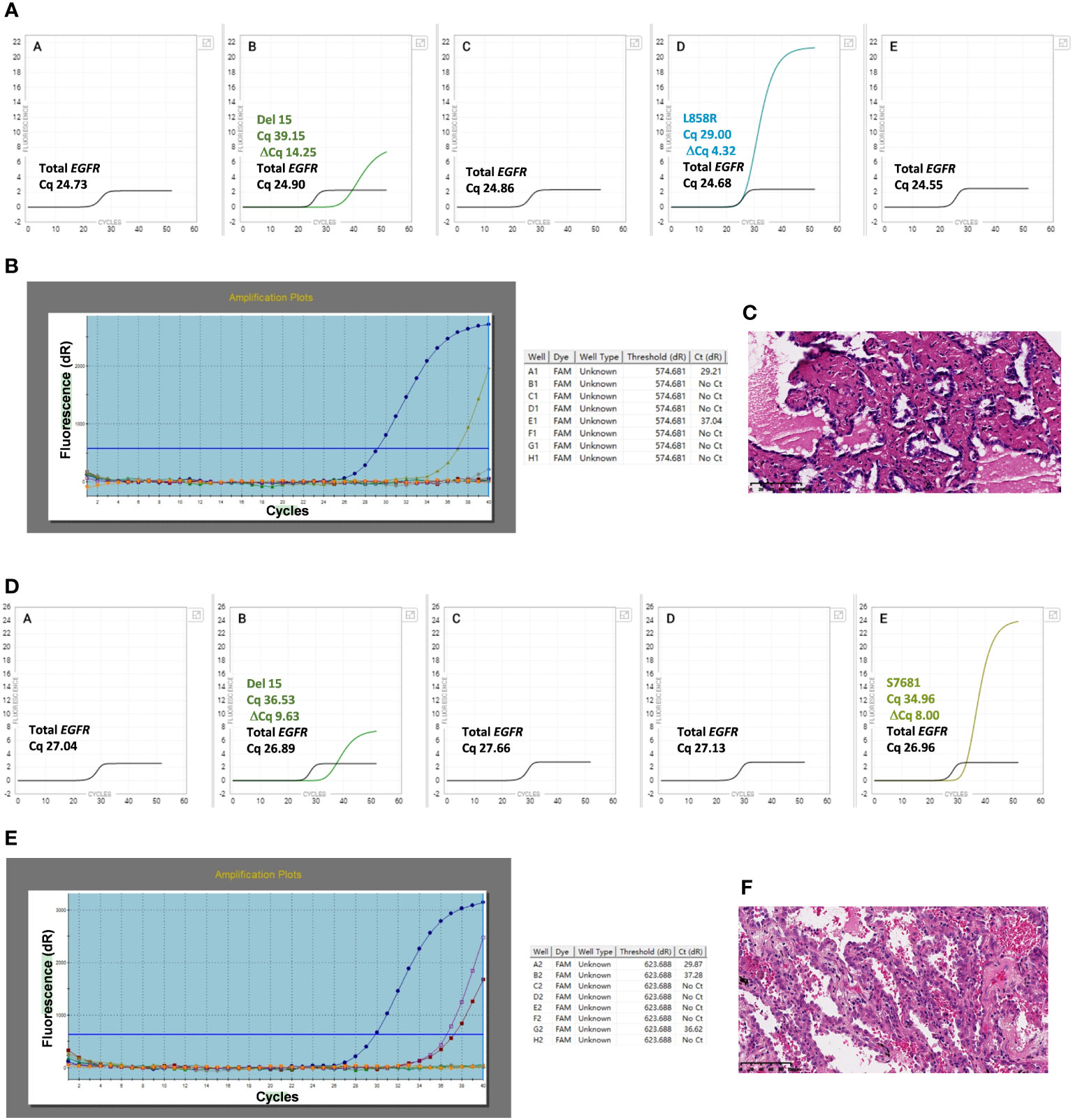
Figure 3 Idylla™ Explore version 2.5.1294.1 (Biocartis, Mechelen, Belgium) default display showing the detail of PCR curves and cycle of quantification (Cq) values of sample 88# (3A) and samples 91# (3D). Cq values are label for the sample processing controls, EGFR Total, and the target for which a signal has been detected (default view). Panels A to E in the image represent the five PCR chambers in the Idylla™ cartridge. Details of PCR curves and Cycle threshold (Ct) values by ARMS-PCR for samples 88# (3B) and 91# (3E). Ct value of well A is available for the quality control of DNA input, and Ct values of the other wells are for the target sequences detected. The H&E photos of samples 88 and 91 are displayed in (3C, 3F).
Thirty-five prospective samples were tested using the Idylla™ EGFR Assay in parallel with ARMS-PCR and NGS. Of the 35 samples, 23 were biopsy tissue samples and 12 were surgical tissue samples. Out of the 23 biopsy tissue samples, 2 had a neoplastic cell content of 10%, which is at the minimum threshold required for the Idylla sample input. Patients in the 35 samples had a median age of 58 years (interquartile range 42 to 84), with 54.3% (19/35) being female (Supplementary Table 2). Eleven patients were untreated, four had undergone chemotherapy, and three had received or were currently undergoing EGFR-TKIs. The treatment status of the remaining seventeen patients was unknown. Idylla™ detected mutations in 21 out of the 35 samples, resulting in positive rate of 60% (21/35) (Supplementary Table 4). Of these mutated samples, 15 had a single mutation while 5 had two mutations, resulting in a total of 25 mutations being discovered by Idylla™. Among the 15 samples with a single mutation, there were 6 with Exon 19 deletion, 8 with L858R, and 1 with G719X mutations. Each of the remaining 6 samples had a distinct combination of two mutations. The frequency of different types of mutations, from high to low, is as follows: L858R at 44.0% (11/25), Exon 19 del at 32.0% (8/25), G719X, S768I and T790M each at 8.0% (2/27). Among these 35 samples, only 1(Sample No. 2#) exhibited inconsistent results between the Idylla™ EGFR and ARMS-PCR. Idylla detected only the L858R mutation in 2#, while ARMS-PCR revealed the presence of both L858R and T790M mutations. The Cq value of 2# in Idylla was above 26(data not shown). Two samples were found to have the 19Del variant according to NGS, but they were reported as wild-type by both Idylla™ and ARMS-PCR (Table 3), as both variant types fell outside the detection range of the two methods. Additionally, the presence of C797S in cis with T790M mutation was identified by NGS in one sample. Compared to ARMS-PCR, the Idylla™ system demonstrated high accuracy with an overall agreement of 97.1% (34/35), a sensitivity of 95.2% (20/21) (95% CI, 76.2%-99.9%), and an estimated specificity of 100% (12/12) (95% CI, 76.8%-100%). When compared to NGS, including the two rare 19Del variations, the overall accuracy was 91.4% (32/35), with a sensitivity of 87% (20/23) (95% CI, 66.4%-97.2%), and a specificity of 100% (12/12) (95% CI, 73.5%-100%).
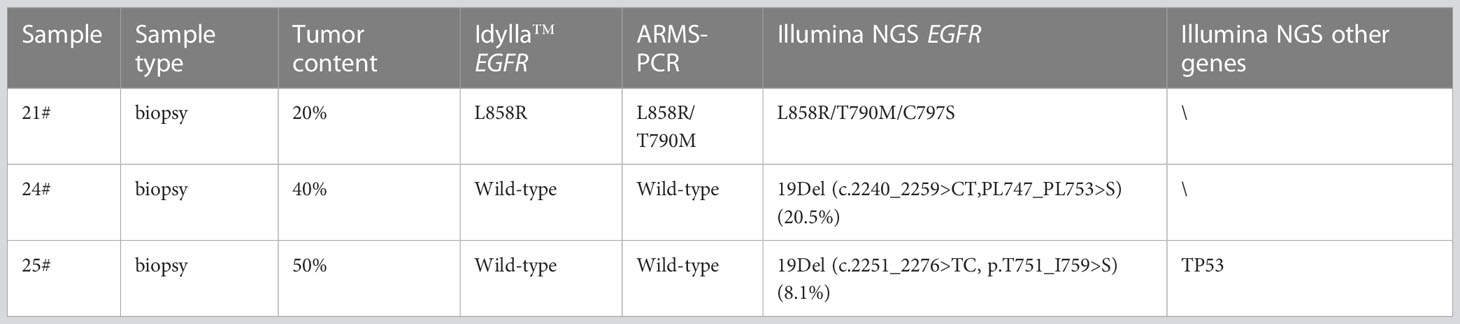
Table 3 Discordant cases between the Idylla™ EGFR assay and reference methods in the 35 routine clinical samples.
In this study, we evaluated the performance of the ultra-rapid Idylla™ system for the rapid, sensitive, and specific identification of EGFR mutations in Chinese NSCLC patients. The Idylla™ system exhibited a sensitivity of 97.6%, a specificity of 100%, and an overall concordance of 97.9% for 95 retrospective samples. Additionally, when compared to ARMS-PCR, the Idylla™ system demonstrated high accuracy with an overall agreement of 97.1% (34/35), a sensitivity of 95.2% (20/21) (95% CI, 76.2% - 99.9%), and an estimated specificity of 100% (12/12) (95% CI, 76.8% - 100%) for 35 prospective samples.
Out of the 95 retrospective samples, only two samples showed discordant results between the Idylla™ EGFR and the reference method. One of the discrepancies (91#) was resolved by increasing the sample input with additional tissue sections. Further analysis revealed that the tissue area of 91# was only 0.25 cm2, and the Cq value analysis showed that the amount of amplifiable DNA in the sample after extraction was only 1.584 ng, indicating that insufficient sample volume was the main reason for the inconsistent result. The Cq value of the only discrepant result among the 35 prospective samples also indicated the same. The Idylla EGFR Assay does not specify the minimum tissue area for loading, but only requires the tumor cell proportion and the maximum tissue area for loading. However, the lack of a minimum tissue area requirement may lead to missed or erroneous results. Despite enriching the neoplastic cell content and increasing the tissue sections, sample 88# still exhibited discordant results. Further analysis revealed that allele frequency of the L861Q mutation, missed in the Idylla assay, was 3.4% according to NGS result. This indicates that Idylla has lower sensitivity than NGS for detecting L861Q mutations with low allele frequency.
Among the 95 retrospective samples, NGS detected an additional E709K mutation in one sample. In the 35 prospective samples, NGS detected two rare 19 deletion mutations in two samples and an additional C797S cis mutation in one sample. In addition, in five samples with EGFR mutations, NGS detected PIK3CA mutations in two samples and TP53 mutations in three samples (Figure 4). In 12 samples with wild-type EGFR analyzed in this study, NGS detected nine samples with mutations in other genes related to tumorigenesis, including four with KRAS mutations, two with HER2 mutations, one with an EML4-ALK fusion mutation, and three with TP53 mutations (data not shown). This indicates that NGS has a significant advantage over traditional fluorescence-based quantitative PCR methods in terms of panel size. This may provide additional benefits to patients, such as those with HER2 exon 20 mutations and KRAS G12C mutations. Despite this, AMRS-PCR and Idylla EGFR still detect the majority of clinically validated EGFR mutations that can provide clinical benefits to patients. Compared to AMRS-PCR, Idylla EGFR can detect more types of EGFR mutations (51 versus 44).
The Idylla system is a fully automated PCR testing system that follows a “sample in, result out” approach, offering advantages such as speed, low sample volume requirement, and standardized testing process. The use of this system eliminates the need for sample pooling and enables on-demand testing, leading to improved efficiency of testing equipment utilization. In this study, 93 out of 95 retrospective samples yielded consistent results in the first test using a single FFPE slice. Among the 35 prospective samples, 12 surgical samples yielded consistent results in the first test using a single FFPE slice. Only one out of 21 biopsy samples using three FFPE slices showed inconsistent results in the first test. The low sample volume requirement expands the accessibility of the Idylla EGFR assay and benefits more patients. The hands-on time of the Idylla EGFR assay is less than 2 minutes, and the turnaround time from sample input to result output is less than 2.5 hours, with automatic report sending. In the prospective study, the turnaround time for different testing methods was compared. The average time from detection to report sending was 3-5 working days for ARMS-PCR, 10-15 working days for NGS, and 2 working days for Idylla EGFR. Based on this, we proposed an optimized flow for non-small cell lung cancer molecular diagnosis (Figure 5) as a supplement to routine molecular diagnosis. In this flow, Idylla EGFR is first deployed to test emergency patients first. If the result is negative, NGS is used to detect other potential gene mutations that may benefit the patient. If the result is positive, based on the patient’s pathology and staging diagnosis, first- or second-generation EGFR TKIs such as gefitinib, erlotinib, dacomitinib, and osimertinib can be used. If the tissue or biopsy sample is insufficient, liquid biopsy can be used for testing. Based on the mutation detection results of the 130 cases (95 retrospective and 35 prospective) in this study, this flow enabled 97.7% (127/130) of patients to receive timely treatment after the first use of Idylla EGFR.
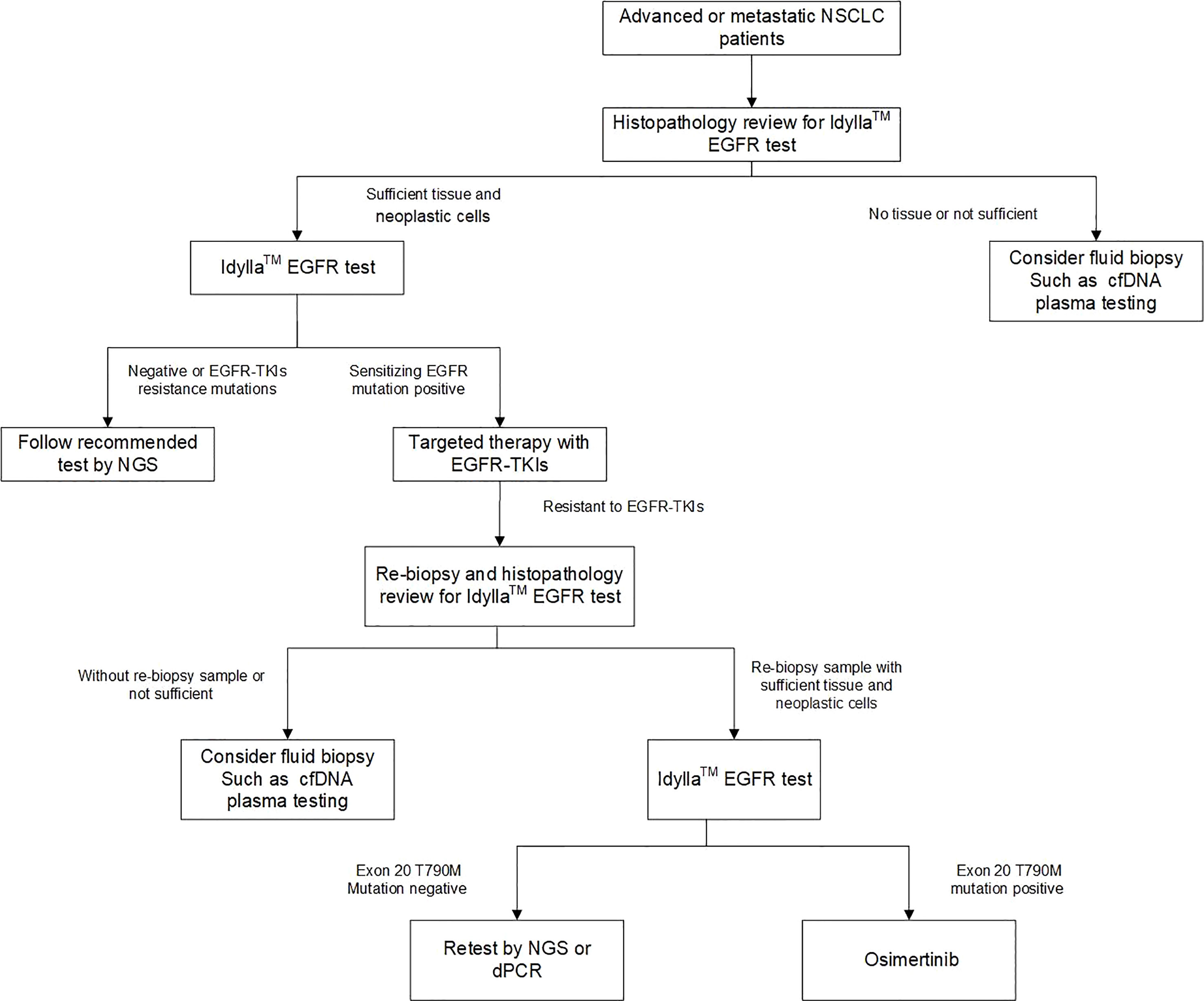
Figure 5 An optimized workflow for molecular diagnosis of NSCLC by combining rapid EGFR detection with genomic profiling by NGS.
In conclusion, the Idylla™ EGFR mutation system provides an ultra-rapid, accurate, and easy to-use automated solution for molecular genotyping. Integrating this ultra-rapid detection system as a critical screening step with NGS could provide timely and comprehensive benefits to patients, ultimately leading to better treatment outcomes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: BioProject, accession number PRJNA923137.
The studies involving human participants were reviewed and approved by the Institute Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The patients/participants provided their written informed consent to participate in this study.
(I) Conception and design: TQ, JY. (II) Administrative support: JY. (III) Provision of study materials or patients: TQ, FZ. (IV) Collection and assembly of data: TQ, FZ. (V) Data analysis and interpretation: TQ, FZ, BZ, ZF, WL, HZ, LC. (VI) Manuscript writing: All authors. All authors contributed to the article and approved the submitted version.
This work was supported by grants from Beijing Hope Run Special Fund of Cancer Foundation of China, China (LC2020A36, LC2019L04, LC2019B05), the Fundamental Research Funds for the Central Universities (3332021031) and the National Natural Science Foundation of China, China (21703290, 22272203).
We thank Guangzhou Wondfo-Cartis Biotech Co., Ltd. for providing the Idylla™ system and the Idylla™ EGFR Mutations Assay cartridges.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1064487/full#supplementary-material
1. Nokin MJ, Ambrogio C, Nadal E, Santamaria D. Targeting infrequent driver alterations in non-small cell lung cancer. Trends Cancer (2021) 7(5):410–29. doi: 10.1016/j.trecan.2020.11.005
2. Hirsch FR, Bunn PA Jr. Egfr testing in lung cancer is ready for prime time. Lancet Oncol (2009) 10(5):432–3. doi: 10.1016/S1470-2045(09)70110-X
3. Midha A, Dearden S, McCormack R. Egfr mutation incidence in non-Small-Cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (Mutmapii). Am J Cancer Res (2015) 5(9):2892–911.
4. O'Kane GM, Bradbury PA, Feld R, Leighl NB, Liu G, Pisters KM, et al. Uncommon egfr mutations in advanced non-small cell lung cancer. Lung Cancer (2017) 109:137–44. doi: 10.1016/j.lungcan.2017.04.016
5. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-Small-Cell lung cancer harbouring uncommon egfr mutations: A combined post-hoc analysis of lux-lung 2, lux-lung 3, and lux-lung 6. Lancet Oncol (2015) 16(7):830–8. doi: 10.1016/S1470-2045(15)00026-1
6. Tu HY, Ke EE, Yang JJ, Sun YL, Yan HH, Zheng MY, et al. A comprehensive review of uncommon egfr mutations in patients with non-small cell lung cancer. Lung Cancer (2017) 114:96–102. doi: 10.1016/j.lungcan.2017.11.005
7. Fang S, Wang Z. Egfr mutations as a prognostic and predictive marker in non-Small-Cell lung cancer. Drug Des Devel Ther (2014) 8:1595–611. doi: 10.2147/DDDT.S69690
8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced egfr mutation-positive non-Small-Cell lung cancer (Eurtac): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X
9. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
10. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase iii study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with egfr mutations. J Clin Oncol Off J Am Soc Clin Oncol (2013) 31(27):3327–34. doi: 10.1200/JCO.2012.44.2806
11. Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, et al. Osimertinib as first-line treatment of egfr mutation-positive advanced non-Small-Cell lung cancer. J Clin Oncol (2018) 36(9):841–9. doi: 10.1200/JCO.2017.74.7576
12. Ahn MJ, Tsai CM, Shepherd FA, Bazhenova L, Sequist LV, Hida T, et al. Osimertinib in patients with T790m mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer (2019) 125(6):892–901. doi: 10.1002/cncr.31891
13. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with egfr-Mutation-Positive non-Small-Cell lung cancer (Archer 1050): A randomised, open-label, phase 3 trial. Lancet Oncol (2017) 18(11):1454–66. doi: 10.1016/S1470-2045(17)30608-3
14. Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, et al. Natural history and molecular characteristics of lung cancers harboring egfr exon 20 insertions. J Thorac Oncol (2013) 8(2):179–84. doi: 10.1097/JTO.0b013e3182779d18
15. Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (Egfr) exon 20 insertion mutations in lung cancer. Sci Transl Med (2013) 5(216):216ra177. doi: 10.1126/scitranslmed.3007205
16. Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: Increased technology, increased complexity. J Clin Oncol (2015) 33(31):3533–4. doi: 10.1200/JCO.2015.63.3628
17. Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol (2013) 31(31):3987–96. doi: 10.1200/JCO.2012.45.2029
18. NCCN. NCCN clinical practice guidelines in Oncology-Non–small cell lung cancer (2020 version 6) [Db/Ol] (2020). Available at: http://www.nccn.org.
19. Lin A, Wei T, Meng H, Luo P, Zhang J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (Nsclc) with egfr mutations. Mol Cancer (2019) 18(1):139. doi: 10.1186/s12943-019-1062-7
20. Liu J, Zhao R, Zhang J, Zhang J. Arms for egfr mutation analysis of cytologic and corresponding lung adenocarcinoma histologic specimens. J Cancer Res Clin Oncol (2015) 141(2):221–7. doi: 10.1007/s00432-014-1807-z
21. Han JY, Kim SH, Lee YS, Lee SY, Hwang JA, Kim JY, et al. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (Egfr-tki) therapy in never-smokers with lung adenocarcinoma. Lung Cancer (2014) 85(2):161–7. doi: 10.1016/j.lungcan.2014.04.009
22. Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for egfr and alk tyrosine kinase inhibitors: Guideline from the college of american pathologists, international association for the study of lung cancer, and association for molecular pathology. J Thorac Oncol (2013) 8(7):823–59. doi: 10.1097/JTO.0b013e318290868f
23. Evrard SM, Taranchon-Clermont E, Rouquette I, Murray S, Dintner S, Nam-Apostolopoulos YC, et al. Multicenter evaluation of the fully automated pcr-based idylla egfr mutation assay on formalin-fixed, paraffin-embedded tissue of human lung cancer. J Mol Diagn (2019) 21(6):1010–24. doi: 10.1016/j.jmoldx.2019.06.010
24. De Luca C, Rappa AG, Gragnano G, Malapelle U, Troncone G, Barberis M. Idylla assay and next generation sequencing: An integrated egfr mutational testing algorithm. J Clin Pathol (2018) 71(8):745–50. doi: 10.1136/jclinpath-2018-205197
25. Thomas De Montpreville V, Ghigna MR, Lacroix L, Lemoine A, Besse B, Mercier O, et al. Egfr and kras molecular genotyping for pulmonary carcinomas: Feasibility of a simple and rapid technique implementable in any department of pathology. Pathol Res Pract (2017) 213(7):793–8. doi: 10.1016/j.prp.2017.03.011
26. De Luca C, Conticelli F, Leone A, Gragnano G, Salatiello M, Galasso P, et al. Is the idylla egfr mutation assay feasible on archival stained cytological smears? a pilot study. J Clin Pathol (2019) 72(9):609–14. doi: 10.1136/jclinpath-2019-205863
27. Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling Y, et al. Concurrence of egfr amplification and sensitizing mutations indicate a better survival benefit from egfr-tki therapy in lung adenocarcinoma patients. Lung Cancer (2015) 89(3):337–42. doi: 10.1016/j.lungcan.2015.06.008
Keywords: rapid detection, epidermal growth factor receptor, IdyllaTMEGFR assay, Chinese NSCLC patients, molecular diagnosis
Citation: Qiu T, Zhang F, Zheng B, Feng Z, Li W, Zeng H, Chu L and Ying J (2023) Ultra-rapid Idylla™ EGFR mutation screening followed by next-generation sequencing: An integrated solution to molecular diagnosis of non-small cell lung cancer. Front. Oncol. 13:1064487. doi: 10.3389/fonc.2023.1064487
Received: 08 October 2022; Accepted: 21 March 2023;
Published: 31 March 2023.
Edited by:
Francesco Pepe, University of Naples Federico II, ItalyReviewed by:
Alessandro Russo, A.O. Papardo, ItalyCopyright © 2023 Qiu, Zhang, Zheng, Feng, Li, Zeng, Chu and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Qiu, cWl1dGlhbkBjaWNhbXMuYWMuY24=; Jianming Ying, am15aW5nQGNpY2Ftcy5hYy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.