- 1Shenzhen Hospital of Guangzhou University of Chinese Medicine, Shenzhen, China
- 2Shenzhen Traditional Chinese Medicine Anorectal Hospital, Shenzhen, China
Proximal-type epithelioid sarcoma of the perineum is a rare soft-tissue malignancy, and only 55 cases have been reported in the English literature to date. This tumor has an indetectable early symptom and frequent recurrences. Here, we present the case of a 31-year-old man with proximal-type epithelioid sarcoma of the perineum who underwent wide excision. Further, we reviewed the current literature regarding differential diagnosis and management of this disease.
1 Introduction
Franz Enzinger described 62 cases of a peculiar sarcoma, initially named epithelioid sarcoma (ES) in 1970, which was frequently confused with chronic inflammation, squamous cell carcinoma, and necrotizing granuloma (1). ES is a rare, aggressive soft-tissue malignancy characterized by nodular aggregates of epithelioid cells.
This tumor accounts for less than 1% of adult soft-tissue sarcomas and 4%–8% of pediatric sarcomas (2, 3). It is most prevalent among young adults, with higher prevalence among men than among women. ES can be divided into classic and proximal-type variants.
ES of the perineum was first described in 1997 (4). PubMed and Embase were searched, and relevant literature published until January 30, 2022, were retrieved by LJ-Ma using the following search terms: “epithelioid sarcoma” OR “epithelioid sarcomas” AND “perineum.” A total of 55 cases were reported between 1997 and 2022 (Table 1). In this article, we report the 56th case of proximal-type ES of the perineum.
2 Case description
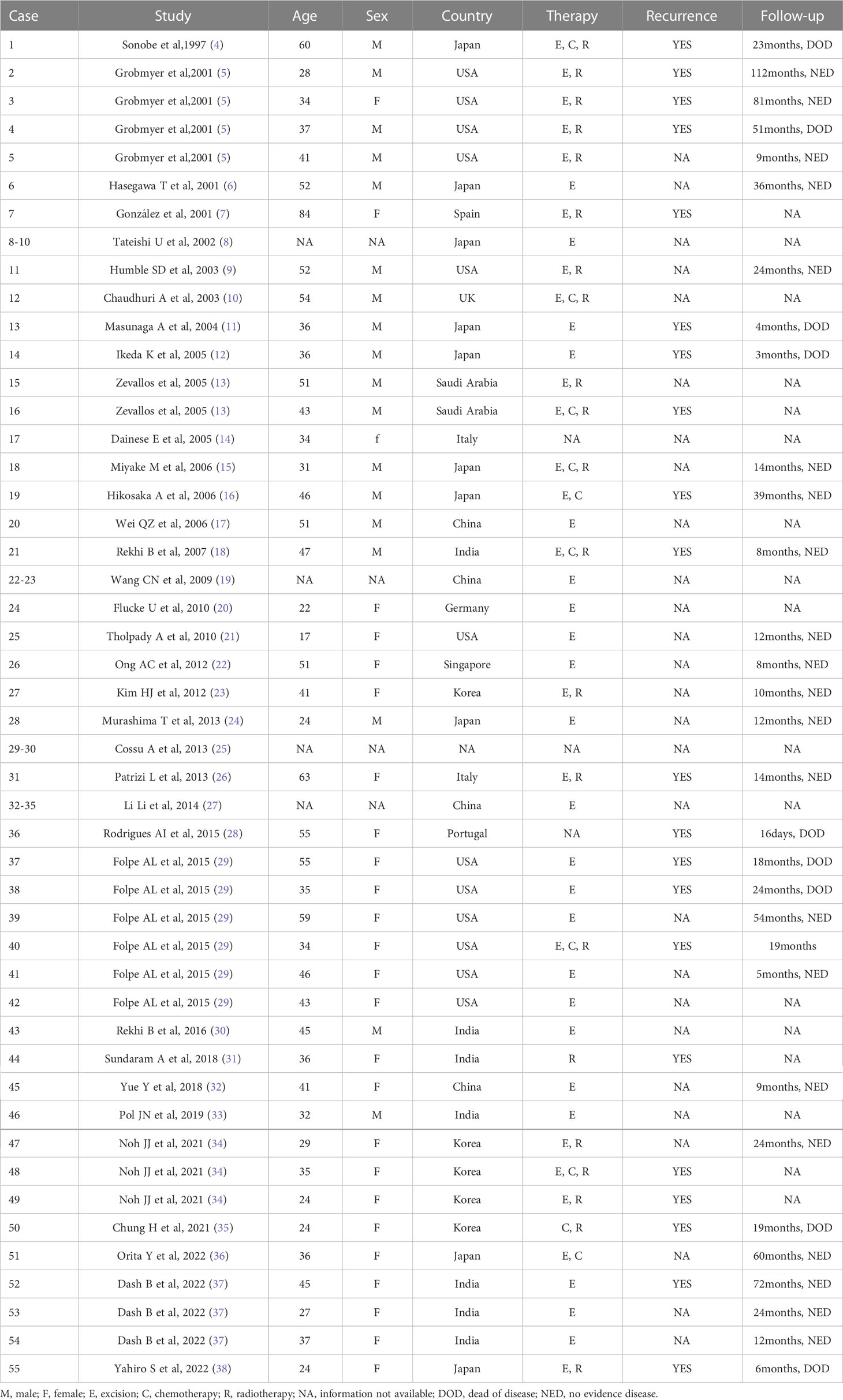
A 31-year-old man was admitted to a local hospital with a painless lump in the right perineum region, for which he received surgical incision and drainage. Five months after surgery (September 2019), he visited our hospital for further treatment because of poor healing of the incision accompanied by pain and swelling. Magnetic resonance imaging (MRI) revealed a well-circumscribed solid and homogeneous lump in the right perineum (Figure 1). Positron emission tomography-computed tomography (PET-CT) for initial staging showed a heterogeneous hypermetabolic mass in the right perineum and a fistula in the lump (Figure 2). However, no bone or skin involvement or lung or liver metastases were noted. His medical history was unremarkable. The patient underwent complete resection with negative margins of the tumor through a perianal incision and avoided damaging the rectum and anal sphincter, followed by 25 courses of adjuvant radiotherapy at a dose of 50 Gray targeting the right perineum. Specifically, the mass was excised with all margins free of tumor, and there was about a 1-cm distance between the tumor lesion and the excised margin in all directions. At the 18-month follow-up, the patient was still alive and free of disease.
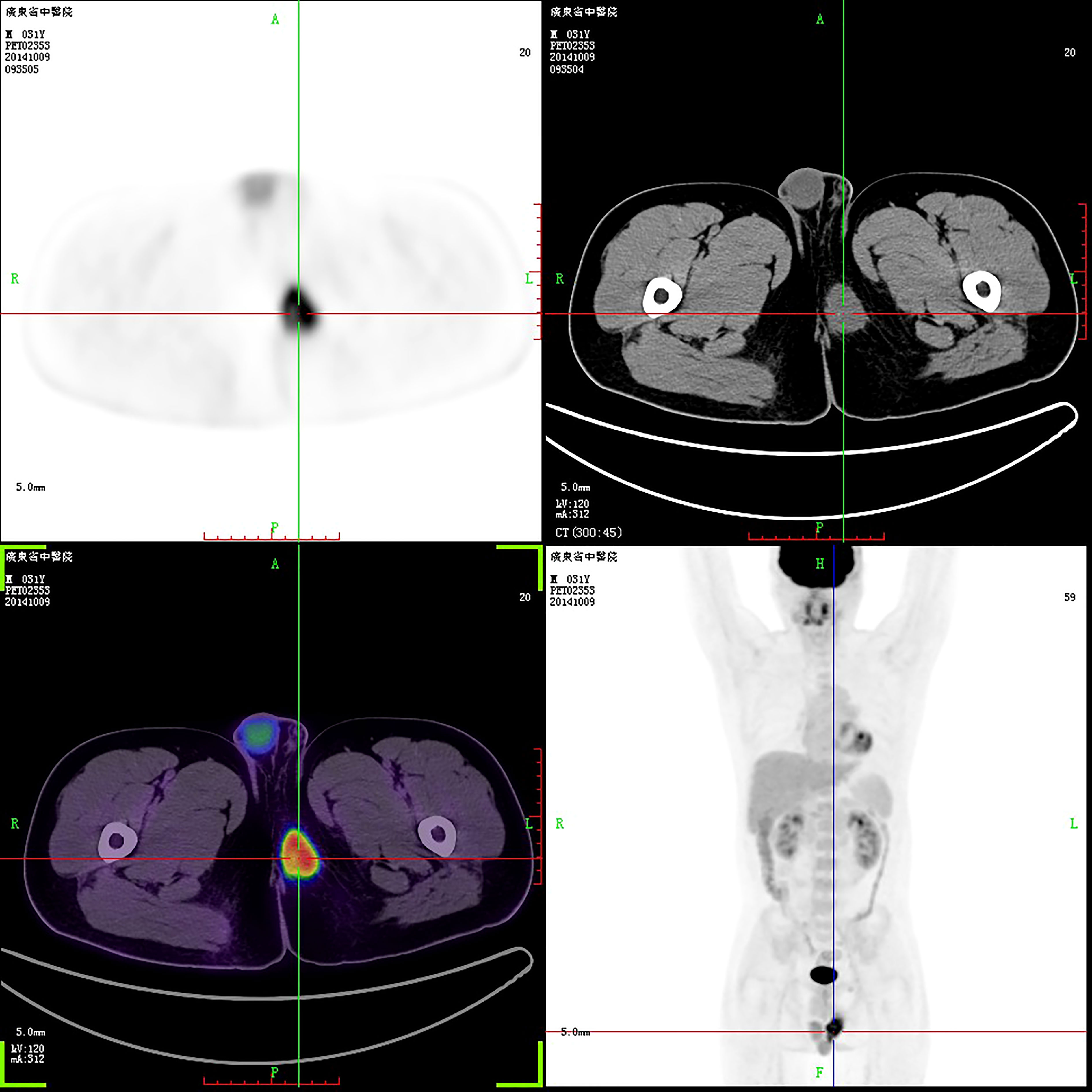
Figure 2 PET-CT revealed a perineal tumor. The purpose of uppercase letters is to mark the position.
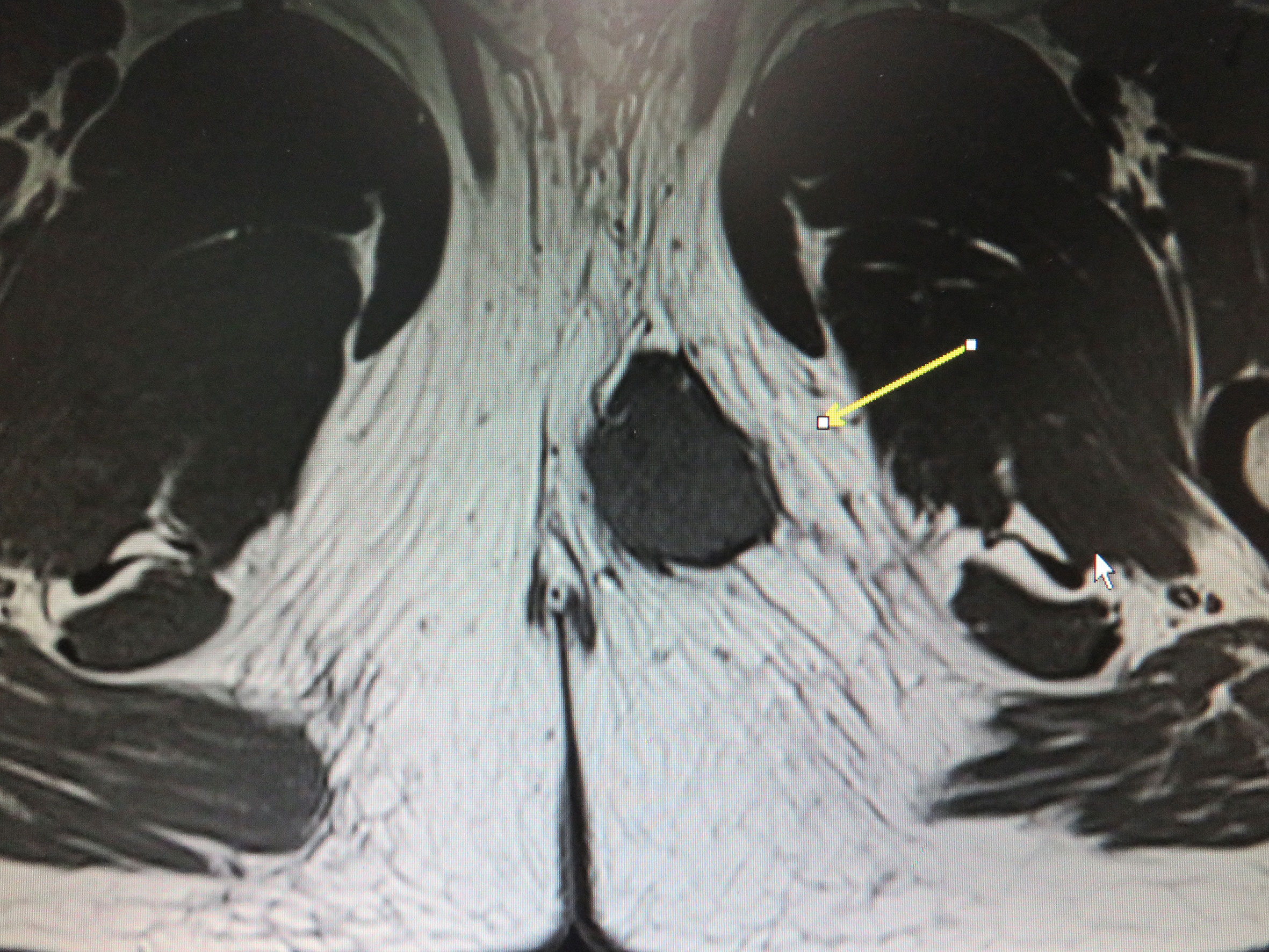
Macroscopically, the resected oval-shaped specimen measured 8 × 10 × 5 cm and was enclosed by a rim of fatty tissue (Figure 3). The incisal surface showed dispersive areas of necrosis and hemorrhage. Immunohistochemical staining indicated that the tumor cells were CK (portion +), EMA (+), Vimentin (+), CK7 (–), CK20 (–), HMB45 (–), MyoD1 (–), Myogenin (–), Desmin (–), SMA (+), Actin (–), CD34 (+), S-100 (–), Calretinin (–), CD31 (–), and Bcl-2 (–) (Figure 4).
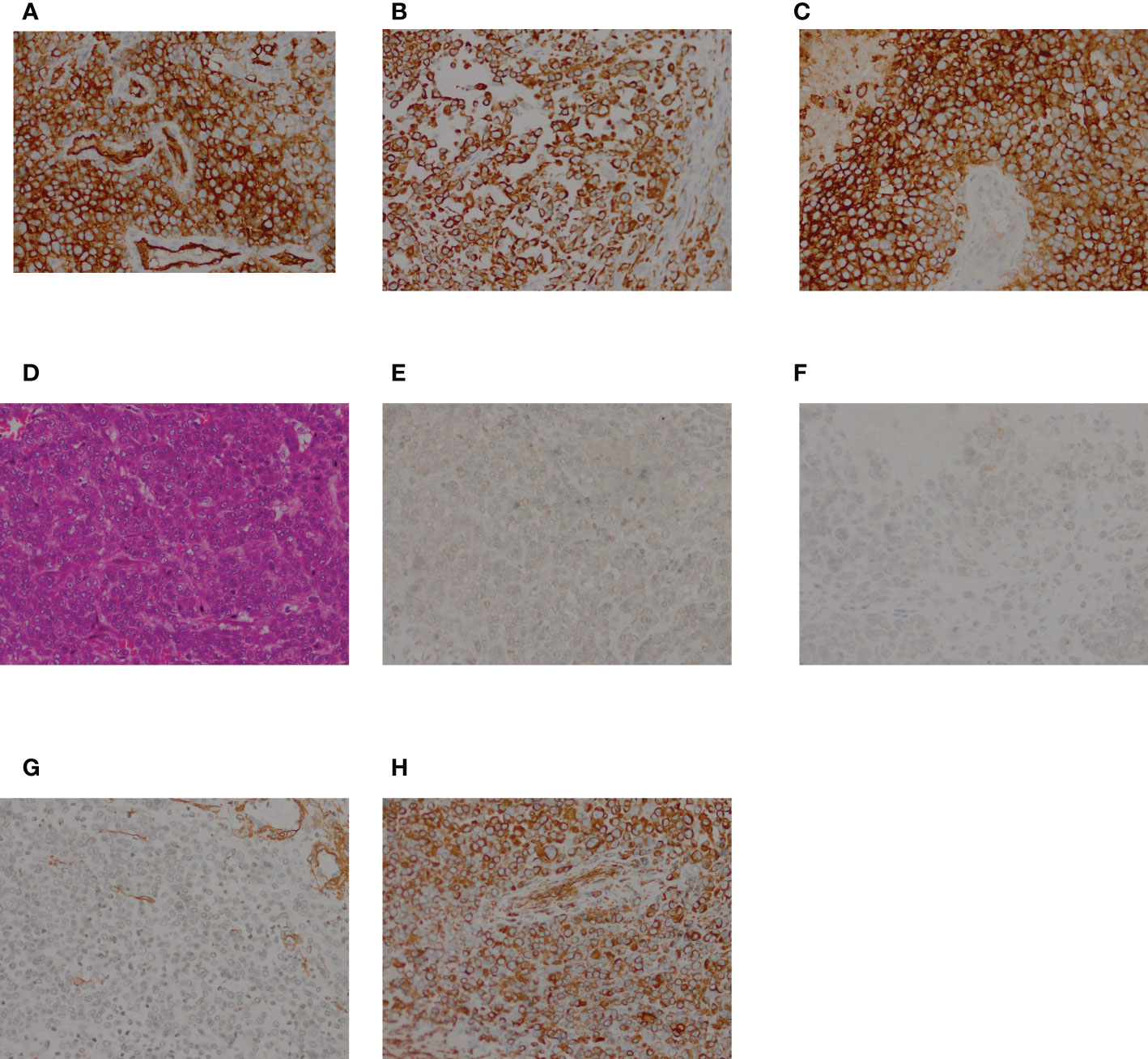
Figure 4 HE and immunohistochemical staining of the resected specimen. (A) HE staining showing polygonal tumor cells; (B–I) Immunohistochemical staining. The tumor cells were positive for CK (B), EMA (C), Vimentin (D), SMA (G), and CD34 (H), and negative forMyoD1 (E), and Myogenin (F).
3 Discussion
Proximal-type ES is an uncommon but aggressive soft-tissue malignancy with a poor prognosis. However, deficient information is available in the literature regarding the clinical aspects and management of proximal-type ES of the perineum. Table 1 summarizes cases of proximal-type ES of the perineum reported since 1997. Based on the available data from these reports, the average age of the 55 patients was 40.8 years. Among them, 18 patients (40.9%) were men, 26 were women (59.1%), and the sex was not specified for the remaining 11. In subgroups stratified by ethnicity, Asians had a higher incidence than other ethnic groups. Our patient is the 66th reported case of proximal-type ES of the perineum.
Seeking immediate treatment is uncommon in these patients because the tumor is often painless. Therefore, a proximal-type ES located deep in the perineum may become quite large at the initial diagnosis. Plain radiography, MRI, CT, and PET can help estimate the volume of the soft tissue mass and determine its metastaticity. Moreover, histological diagnosis of the soft tissue mass is essential.
Unlike most soft-tissue sarcomas that are classified according to the presumptive tissue of origin, such as liposarcoma, synovial sarcoma, or leiomyosarcoma, the histogenesis of ES remains unclear. Immunohistochemical staining, however, may help identify the presumptive tissue of origin in patients with ES. ES is typically positive for vimentin, CK, and EMA.
Surgical excision with negative gross margins is the mainstay of therapy for patients with primary sarcoma. Generally, there is no evidence of distant metastasis at the initial diagnosis. For localized disease, adjuvant radiotherapy after wide surgical excision is necessary to reduce the risk of local recurrence. In a retrospective review of 1,170 patients with newly diagnosed soft tissue sarcoma during a 7.5-year period, the incidence of distant metastases at diagnosis was 10%, 83% of which were in the lungs (39). Hematogenous metastasis is the most common spread in patients with soft tissue sarcoma. Compared to the classical type, proximal-type ES is a more dreaded type with a poorer prognosis and trend of distant metastasis (40). Therefore, the 5-year overall survival rate of proximal- and classical types are 57% and 77%, respectively. Treatment options for proximal-type ES with distant metastasis usually involve surgical treatment, adjuvant radiotherapy, and/or adjuvant chemotherapy. For patients with local recurrence or metastatic disease, re-excision may be warranted.
Owing to the rarity of such tumors of the perineum, published treatment recommendations are unavailable. At present, total surgical resection combined with postoperative chemotherapy and radiotherapy is the major treatment modality for primary proximal-type ES. However, EZH2 inhibitors and immunotherapy may represent potential treatments for such tumors (41). New features of proximal-type ES and differences in responses to various treatments must be further explored to accumulate high-quality evidence for treatment suggestions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SX: analysis and interpretation of data, drafting the article or revising it critically for important intellectual content; WW: final approval of the version to be published; LM: acquisition of data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer (1970) 26(5):1029–41. doi: 10.1002/1097-0142(197011)26:5<1029::aid-cncr2820260510>3.0.co;2-r
2. Casanova M, Ferrari A, Collini P, Bisogno G, Alaggio R, Cecchetto G, et al. Epithelioid sarcoma in children and adolescents: a report from the Italian soft tissue sarcoma committee. Cancer (2006) 106(3):708–17. doi: 10.1002/cncr.21630
3. Jawad M, Extein J, Min E, Scully SP. Prognostic factors for survival in patients with epithelioid sarcoma: 441 cases from the SEER database. Clin Orthop Relat Res (2009) 467(11):2939–48. doi: 10.1007/s11999-009-0749-2
4. Sonobe H, Ohtsuki Y, Ido E, Furihata M, Iwata J, Enzan H, et al. Epithelioid sarcoma producing granulocyte colony-stimulating factor. Hum Pathol (1997) 28(12):1433–5. doi: 10.1016/S0046-8177(97)90236-7
5. Grobmyer SR, Clary B, Lewis JJ, Delgado R, Woodruff JM, Brennan MF, et al. Adult perineal sarcomas. J Surg Oncol (2001) 77(2):101–4. doi: 10.1002/jso.1078
6. Hasegawa T, Matsuno Y, Shimoda T, Umeda T, Yokoyama R, Hirohashi S, et al. Proximal-type epithelioid sarcoma: a clinicopathologic study of 20 cases. Mod Pathol (2001) 14(7):655–63. doi: 10.1038/modpathol.3880368
7. González-Peramato P, Jiménez-Heffernan JA, Cuevas J. Fine-needle aspiration cytology of "proximal-type" epithelioid sarcoma. Diagn Cytopathol (2001) 25(2):122–5. doi: 10.1002/dc.2018
8. Tateishi U, Hasegawa T, Kusumoto M, Yokoyama R, Moriyama N. Radiologic manifestations of proximal-type epithelioid sarcoma of the soft tissues. AJR Am J Roentgenol (2002) 179(4):973–7. doi: 10.2214/ajr.179.4.1790973
9. Humble Scott D, Prieto Victor G. Horenstein marcelo GCytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol (2003) 30(4):242–6. doi: 10.1046/j.0303-6987.2003.047.x
10. Chaudhuri A. Harris M D'Proximal-type' epithelioid sarcoma: Is agent orange still at large? Ann R Coll Surg Engl (2003) 85(6):410–2. doi: 10.1308/003588403322520816
11. Masunaga A, Ikeda K, Suzuki T, Fukumori N, Ishibashi K, Tajiri T, et al. Proximal-type epithelioid sarcoma in a 36-year-old man: Closer immunoelectron-microscopic resemblance of the tumor cells to epithelial cells than to mesenchymal cells. Pathol Int (2004) 54(8):616–22. doi: 10.1111/j.1440-1827.2004.01671.x
12. Ikeda K, Tate G, Suzuki T, Mitsuya T. Fine needle aspiration cytology of primary proximal-type epithelioid sarcoma of the perineum: a case report. Acta Cytol (2005) 49(3):314–8. doi: 10.1159/000326155
13. Zevallos-Giampietri E-A, Barrionuevo C. Proximal-type epithelioid sarcoma: Report of two cases in the perineum: differential diagnosis and review of soft tissue tumors with epithelioid and/or rhabdoid features. Appl Immunohistochem Mol Morphol (2005) 13(3):221–30. doi: 10.1097/01.pai.0000145131.80060.6c
14. Dainese E, Sessa F, Riva C, Placidi C, Capella C. Sarcoma epitelioide di tipo prossimale della vulva: Diagnosi differenziale con altri tumori rabdoidi extrarenali ["Proximal type" epithelioid sarcoma of the vulva: differential diagnosis with other extrarenal rhabdoid tumors]. Pathologica (2005) 97(3):133–6.
15. Miyake M, Tanaka N, Matsushita C, Tanaka M, Tanaka M, Fujimoto K, et al. [Case of proximal-type epithelioid sarcoma of the perineum]. Nihon Hinyokika Gakkai Zasshi (2006) 97(3):602–6. doi: 10.5980/jpnjurol1989.97.602
16. Hikosaka A, Yamada K, Fujita K, Iwase Y, Tada T. Proximal-type epithelioid sarcoma of the perineum: Calling attention of urologists. Int J Urol (2006) 13(12):1542–4. doi: 10.1111/j.1442-2042.2006.01581.x
17. Wei Q-z, Liu Y-h, Zhuang H-g, Luo DL, Luo XL. [Proximal epithelioid sarcoma: A case report]. Zhonghua Bing Li Xue Za Zhi (2006) 35(10):638–9.
18. Bharat R, Gorad Biru D. Chinoy roshni FProximal-type epithelioid sarcoma–a rare, aggressive subtype of epithelioid sarcoma presenting as a recurrent perineal mass in a middle-aged male. World J Surg Oncol (2007) 5:28. doi: 10.1186/1477-7819-5-28
19. Wang CN, An XJ, Shi QL, Xu Y, Zhou XJ, Li NY, et al. [Clinicopathological study of 5 cases of proximal-type epithelioid sarcoma]. Zhonghua Bing Li Xue Za Zhi (2009) 38(5):298–301.
20. Flucke U, Hulsebos Theo JM, Van Krieken J Han JM, Mentzel T. Myxoid epithelioid sarcoma: a diagnostic Challenge. a report on six cases. Histopathology (2010) 57(5):753–9. doi: 10.1111/j.1365-2559.2010.03688.x
21. Tholpady A, Lonergan CL, Wick MR. Proximal-type epithelioid sarcoma of the vulva: Relationship to malignant extrarenal rhabdoid tumor. Int J Gynecol Pathol (2010) 29(6):600–4. doi: 10.1097/PGP.0b013e3181e31f94
22. Ong AC, Lim TY, Tan TC, Wang S, Raju GC. Proximal epithelioid sarcoma of the vulva: A case report and review of current medical literature. J Obstet Gynaecol Res (2012) 38(7):1032–5. doi: 10.1111/j.1447-0756.2011.01819.x
23. Kim HJ, Kim MH, Kwon J, Kim JY, Park K, Ro JY. Proximal-type epithelioid sarcoma of the vulva with INI1 diagnostic utility. Ann Diagn Pathol (2012) 16(5):411–5. doi: 10.1016/j.anndiagpath.2011.04.002
24. Murashima T, Kamibeppu T, Hiromasa T, Mukai S, Kamoto T. A case of proximal type epithelioid sarcoma of the perineum. Hinyokika Kiyo (2013) 59(11):759–63.
25. Cossu A, Paliogiannis P, Capobianco G, Sini MC, Dessole M, Dessole S, et al. Immunoreactivity for Ca 125 and INI 1 loss of expression are useful markers in the diagnosis of vulvar proximal-type epithelioid sarcomas: report of two cases. Eur J Gynaecol Oncol (2013) 34(5):469–72.
26. Patrizi L, Corrado G, Saltari M, Perracchio L, Scelzo C, Piccione E, et al. Vulvar "proximal-type" epithelioid sarcoma: report of a case and review of the literature. Diagn Pathol (2013) 8:122. doi: 10.1186/1746-1596-8-122
27. Li L, Xia Q, Rao Q, Liu B, Wu B, Shi S, et al. [Molecular genetics and immunophenotype of INI1/SMARCB in epithelioid sarcoma]. Zhonghua Bing Li Xue Za Zhi (2014) 43(6):389–93.
28. Rodrigues AI, Lopes HI, Lima O, Marta S. Proximal-type epithelioid sarcoma-unusual presentation: Unilateral vulvar mass. BMJ Case Rep (2015) 2015:bcr2014208488. doi: 10.1136/bcr-2014-208488
29. Folpe AL, Schoolmeester JK, McCluggage WG, Sullivan LM, Castagna K, Ahrens WA, et al. SMARCB1-deficient vulvar neoplasms: A clinicopathologic, immunohistochemical, and molecular genetic study of 14 cases. Am J Surg Pathol (2015) 39(6):836–49. doi: 10.1097/PAS.0000000000000397
30. Rekhi B, Verma A, Jambhekar NA, Menon S, Laskar S, Merchant N, et al. Osteoclast-rich, proximal-type epithelioid sarcoma: clinicopathologic features of 3 unusual cases expanding the histomorphological spectrum. Ann Diagn Pathol (2016) 21:39–43. doi: 10.1016/j.anndiagpath.2015.12.003
31. Sundaram A, Elangovan A, Rajwanshi A, Srinivasan R, Kapoor R. Proximal-type epithelioid sarcoma of the vulva: Cytopathological diagnosis of a rare neoplasm. Cytopathol (2018) 29(5):471–3. doi: 10.1111/cyt.12561
32. Yue Y, Lu Y, Ma X, Tang Z, Cheng Y. Ultrasonography findings of proximal-type epithelioid sarcoma of the vulva: A case report. Mol Clin Oncol (2018) 9(5):507–10. doi: 10.3892/mco.2018.1708
33. Pol JN, Nisar Z, Phadke MD, Kadkol GA. Epithelioid sarcoma of paratesticular region: A case report of an unusual case. Indian J Pathol Microbiol (2019) 62(2):306–9. doi: 10.4103/IJPM.IJPM_431_18
34. Noh JJ, Jeon JE, Jung H, Kim HS, Lee YY, Choi CH, et al. Vulvar epithelioid sarcoma: A case report with literature review. Taiwan J Obstet Gynecol (2021) 60(1):132–5. doi: 10.1016/j.tjog.2020.11.020
35. Chung H, Jang TK, Kwon SY, Ha J, Shin SJ. A proximal type epithelioid sarcoma of the vulva with multiple distant metastases: A case report and review of the literature. Gynecol Oncol Rep (2021) 37:100835. doi: 10.1016/j.gore.2021.100835
36. Orita Y, Kamio M, Tokudome A, Kitazono I, Ichihara F, Kobayashi H. Successful management of vulvar proximal-type epithelioid sarcoma in pregnancy. Gynecol Oncol Rep (2022) 39:100933. doi: 10.1016/j.gore.2022.100933
37. Dash B, Rekhi B, Shylasree TS, Maheshwari A, Bajpai J. Proximal-type epithelioid sarcoma of vulva - case series of a rare tumor. Gynecol Oncol Rep (2022) 39:100921. doi: 10.1016/j.gore.2022.100921
38. Yahiro S, Fujimoto T, Fujita I, Takai T, Sakuma T, Sudo T, et al. Proximal-type epithelioid sarcoma in pubic region expressing l-type amino acid transporter 1: A case report. SAGE Open Med Case Rep (2022) 10:2050313X211067917. doi: 10.1177/2050313X211067917
39. Christie-Large M, James SL, Tiessen L, Tiessen L, Davies AM, Grimer RJ, et al. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur J Cancer (2008) 44:1841. doi: 10.1016/j.ejca.2008.06.004
40. Frezza AM, Sbaraglia M, Lo Vullo S, Baldi GG, Simeone N, Frenos F, et al. The natural history of epithelioid sarcoma. a retrospective multicentre case-series within the Italian sarcoma group. Eur J Surg Oncol (2020) 46(7):1320–6. doi: 10.1016/j.ejso.2020.03.215
Keywords: proximal-type, epithelioid sarcoma, perineum, diagnosis, resect
Citation: Xia S, Wu W and Ma L (2023) Proximal-type epithelioid sarcoma of the perineum: A case report and literature review. Front. Oncol. 13:1057466. doi: 10.3389/fonc.2023.1057466
Received: 29 September 2022; Accepted: 16 February 2023;
Published: 06 March 2023.
Edited by:
Giuseppe Zimmitti, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Jasper Sijberden, Amsterdam University Medical Center, NetherlandsLuigi Della Corte, University of Naples Federico II, Italy
Copyright © 2023 Xia, Wu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjiang Wu, 1053660645@qq.com
 Shijun Xia
Shijun Xia Wenjiang Wu1*
Wenjiang Wu1*